Abstract
Pancreatic fibrosis (PF) is an essential component of the pathobiology of chronic pancreatitis (CP) and pancreatic ductal adenocarcinoma (PDAC). Activated pancreatic myofibroblasts (PMFs) are crucial for the deposition of the extracellular matrix, and fibrotic reaction in response to sustained signaling. Consequently, understanding of the molecular mechanisms of PMF activation is not only critical for understanding CP and PDAC biology but is also a fertile area of research for the development of novel therapeutic strategies for pancreatic pathologies. This review analyzes the key signaling events that drive PMF activation including, initiating signals from transforming growth factor-β1, platelet derived growth factor, as well as other microenvironmental cues, like hypoxia and extracellular matrix rigidity. Further, we discussed the intracellular signal events contributing to PMF activation, and crosstalk with different components of tumor microenvironment. Additionally, association of epidemiologically established risk factors for CP and PDAC, like alcohol intake, tobacco exposure, and metabolic factors with PMF activation, is discussed to comprehend the role of lifestyle factors on pancreatic pathologies. Overall, this analysis provides insight into the biology of PMF activation and highlights salient features of this process, which offer promising therapeutic targets.
Keywords: Pancreatic fibrosis, Chronic pancreatitis, Pancreatic cancer, Myofibroblast, Cell signaling
Introduction
Chronic pancreatitis (CP) is a condition characterized by recurrent episodes of pancreatic inflammation leading to the development of fibroinflammatory histology. While episodes of acute pancreatitis are a frequent element of CP patient histories, the development of CP itself is multifactorial with numerous risk factors playing significant contributory roles [1]. In western countries, the incidence and prevalence of clinical CP range from 4.03 to 14 per 100,000 person-years [2–5] and from 13.5 to 143 per 100,000 at risk [2, 4, 6–8], respectively. Recently, the incidence of CP has increased, indicating a growing burden of this disease [3]. Beyond the clinical presentation, 26–29% of patients without lifestyle risk factors for CP and 42–47% of smokers and alcoholics have pancreatic fibrosis (PF) on autopsy [9–11]. Compared to the general population, CP patients have a 35.8% increase in death rate over a 20-year observation period [12]. Moreover, CP patients are predisposed to pain [13], bile duct strictures, pseudocysts, pancreatic ascites, pancreatic-exocrine- [14], and endocrine insufficiencies leading to type 3C diabetes mellitus [15, 16]. Importantly, CP is a risk factor for pancreatic ductal adenocarcinoma (PDAC) with a relative risk of PDAC of 13.3 compared to the general population [17]. Like CP, the incidence of PDAC has increased in the U.S. from 11 to 13.1 per 100,000 person-years between 2002 and 2017 [18]. Despite modest incidence, PDAC is the third leading cause of cancer-related mortality, accounting for ~ 47,050 cancer-related deaths in 2020 in the U.S. [19]. Due to increasing incidence and dismal prognosis, PDAC is projected to be the second leading cause of cancer-related mortality by 2030 [20, 21]. Overall, CP and PDAC represent significant burdens in modern healthcare systems and are growing causes of morbidity and mortality.
PF is a histological change accompanying chronic or recurring acute episodes of pancreatic injury and inflammation. Notably, PF is a pathological hallmark of CP and PDAC. Regarding CP, the presence of PF is arguably necessary for the diagnosis of CP, especially in late-stage disease, and replacement of normal pancreatic parenchyma with fibrosis is a critical component of endocrine and exocrine pancreatic insufficiency, suggesting that PF is a critical contributor to the pathobiology of CP. Similarly, the roles of PF, especially activated pancreatic myofibroblast (PMF) in PDAC biology (desmoplasia) have begun to be appreciated, and the data from early studies elucidated the ability of desmoplasia to drive pancreatic cancer cell proliferation, promote their metastatic potential through induction of stem cell and EMT phenotypes, and dampen anti-tumor immune responses (see reference 22 by Apte et al.) [22]. Moreover, pharmacologic suppression or depletion of desmoplasia inhibited PDAC progression in animals, indicating the potential of desmoplasia-targeted therapies [23–25]. However, spontaneous PDAC models that lack crucial elements of the desmoplastic reaction had accelerated PDAC progression [26]. The interaction of PMFs with PDAC cells is complex, and our understanding of the subject continues to evolve. Recent work has highlighted the contributions of several potential subset of PMFs. Irrespective of this ongoing controversy, it is clear that desmoplasia is a key factor in PDAC biology. Because of the critical role of activated PMFs in PF, the mechanisms governing their activation are fundamental to CP and PDAC biology and represent potential therapeutic targets. This review summarizes the current understanding of the molecular mechanisms of PMF activation as it pertains to CP and PDAC.
Characterization of pancreatic fibroblast populations
Early studies of the distinct cell populations in the pancreas revealed a pancreas-resident, quiescent fibroblast population interspersed between interacinar and interlobular spaces, stored retinoid-rich cytoplasmic lipid droplets, and expressed desmin similar to hepatic stellate cells (HSCs); thus, they were dubbed pancreatic stellate cells (PSCs) [27, 28]. Upon culture, PSCs lost their cytoplasmic lipids and began expressing α-smooth muscle actin (SMA) along with extracellular matrix (ECM) proteins (including collagen I and III, fibronectin and laminin), which mirrors HSC activation during hepatic fibrosis [27–29]. Because of this similarity and the contributions of HSCs to cirrhosis, PSCs were hypothesized to drive PF. Supporting this hypothesis, SMA-positive cells expressing pro-collagens show marked proliferation in fibrotic regions of inflamed pancreas, surrounding pancreatic intraepithelial neoplasm (PanIN) lesions, and adjacent to PDAC glands in human and murine PDAC, suggesting that PSC-like cells are responsible for the ECM deposition and fibrosis in CP and PDAC [30–32]. While it is widely believed that PSCs are the primary contributor to PF, recent studies demonstrate that bone marrow-derived cells and monocytes are recruited to chronically inflamed pancreas [33–36] and PDAC tumors [37] and differentiate into fibroblasts that express PSC markers. Because the origin of the fibroblasts present in pathological conditions is uncertain, we utilize the term PMF to refer to cells expressing PSC markers to describe this population accurately irrespective of their origin.
Extracellular signals regulating PMF activation
At the outset of a pancreatic fibrotic pathology, activation of PMFs is an essential part of the fibrotic process, as quiescent PSCs are neither proliferative nor secretory. The molecular processes underlying the activation of PSCs/PMFs/cancer-associated fibroblasts (CAFs) are highly diverse. Broadly, these mechanisms can be categorized as those involved in initial activation of PSCs and those that contribute specifically to the perpetual activation of fibroblasts, as observed in CP and PDAC.
Extracellular signals involved in early activation of PMFs
Regarding the initial activation of PMFs, critical roles for transforming growth factor-β1 (TGF-β1), platelet-derived growth factor (PDGF), sonic hedgehog (SHH), and connective tissue growth factor (CTGF) have been characterized. TGF-β1 is a crucial cytokine for damaged tissue repair and is abundantly expressed in CP and PDAC. Transgenic mice expressing TGF-β1 under an insulin promoter demonstrate spontaneous PF, suggesting that TGF-β1 initiates de novo PMF activation [38]. PMFs express both TGF-β receptor1 (TGF-βR1) and receptor2 (TGF-βR2) at significant levels [39]. TGF-β1 treatment augmented α-SMA, procollagens I and III, laminin, and fibronectin expression in PMF both under in vitro and in vivo conditions [29, 38, 40, 41]. Moreover, transfection of a dominant-negative TGF-βR2 diminished ECM protein synthesis in cultured PMFs, further implicating TGF-βR2 in PF [42]. Interestingly, TGF-β1 did not increase the proliferation of PMFs, which is another hallmark of activated PMFs. A separate study showed that neutralization of TGF-β1 increased PMF proliferation [39], suggesting that PMF proliferation in CP and PDAC is not directly promoted by TGF-β1. Indirectly, TGF-β1 increased expression of PDGF receptors on PMFs [29] as well as additional pro-fibrotic cytokines, including CTGF and fibroblast growth factors 1/2 (FGF1/2) [38].
In rodent CP models, overexpression of TGF-βR2 extracellular domain suppressed PF and the number of activated PMFs following cerulein injection [43]. In the setting of PDAC, neutralization of TGF-β1 abrogated the production of fibronectin induced by PDAC cell line-conditioned media [44]. Neutralization of FGF2 also suppressed the production of fibronectin in pancreatic fibroblasts stimulated with PDAC cell-conditioned media. Importantly, neutralization of either TGF-β1 or FGF2 caused nearly complete abrogation of fibronectin production, except in the PANC1-based pancreatic cancer cell model in which only FGF2 neutralization was effective. A possible explanation for the latter finding is that PANC-1 lacks appreciable TGF-β1 expression [44, 45]. These observations indicate that TGF-β1 and FGF2 are required for stimulation of ECM synthesis in CAFs, depending on the unique secretome of the underlying cancer cells. Furthermore, ectopic overexpression of TGF-β1 in PANC-1 augmented the fibrosis in orthotopic tumors compared to tumors derived from mock-transfected PANC-1 [45]. Cumulatively, these findings suggest that TGF-β1 is a master regulator of desmoplasia and PF in general; and drives PMF ECM synthesis, and indirectly promotes fibroblast proliferation through increasing sensitivity of PMFs to PDGF (Fig. 1).
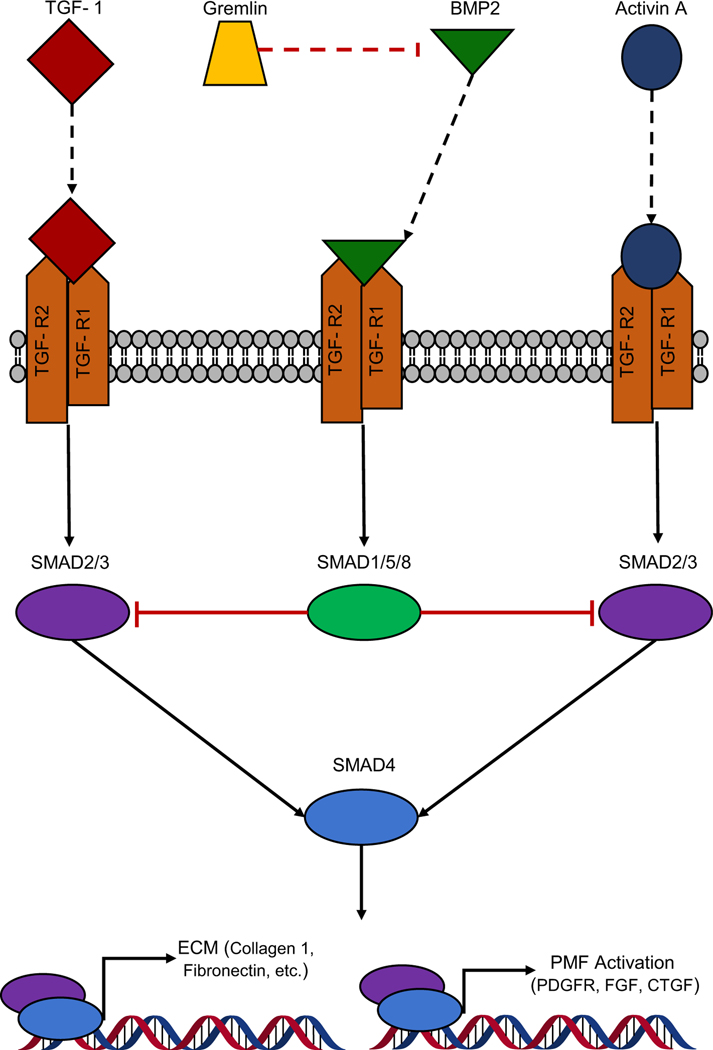
Fig. 1.
Schematic representation of the regulation and function of TGF-β1 and SMAD2/3 signaling in the PMFs. TGF-β1, Activin A, and BMP2 activate TGF-β receptor, while Gremlin acts as an antagonist to BMP2-mediated TGF-β1 signaling by binding BMP2 directly. Downstream, TGF-β1 and Activin A favor activation of SMAD2/3, resulting in the expression of genes classically associated with PMF activation. In contrast, BMP2-mediated activation favors SMAD1/5/8 activation in PMFs, which competes with SMAD2/3 for SMAD4, thereby abrogating PMF activation signatures
While TGF-β1 is critical for promoting ECM deposition by PMFs, it fails to induce PMF proliferation, a vital component of PMF activation and a prominent feature of fibrosis in CP and PDAC. Unlike TGF-β1, PDGF treatment drove proliferation in PMFs isolated from CP patients [29, 40] (Fig. 2). PDGF also stimulated the production of ECM by PMFs; however, comparison of TGF-β1 and PDGF concerning fibronectin synthesis demonstrated decreased potency of PDGF [41]. Consistently, conditioned media of PDAC cell lines MiaPaCa-2, PANC-1, and SW850 stimulated the proliferation and synthesis of collagen I and fibronectin in cultured PMFs from PDAC and CP patients [44]. Subsequent studies showed that neutralization of PDGF-A and -B abrogated the ability of conditioned media to induce PMF proliferation, further demonstrating the prominence of PDGF in tumor-stroma crosstalk [44]. Notably, PDGF neutralization resulted in variable inhibition of fibronectin synthesis across cell line models and was insignificant in two out of three models [44]. In addition to its effects on proliferation, PDGF also induced the migration of PMFs in culture largely through the PI3K/AKT pathway [46, 47]. More interestingly, PDGF treatment of quiescent and activated PMFs demonstrated that quiescent cells had a 48-h delay in their response to PDGF, indicating that activation of PMF is a prerequisite to respond to PDGF and that PDGF itself is not capable of initial activation [46]. Based on these findings, animal models with forced pancreatic overexpression of PDGF, with and without experimental pancreatic injury, may yield important insights into the temporal regulation of PF by PDGF.
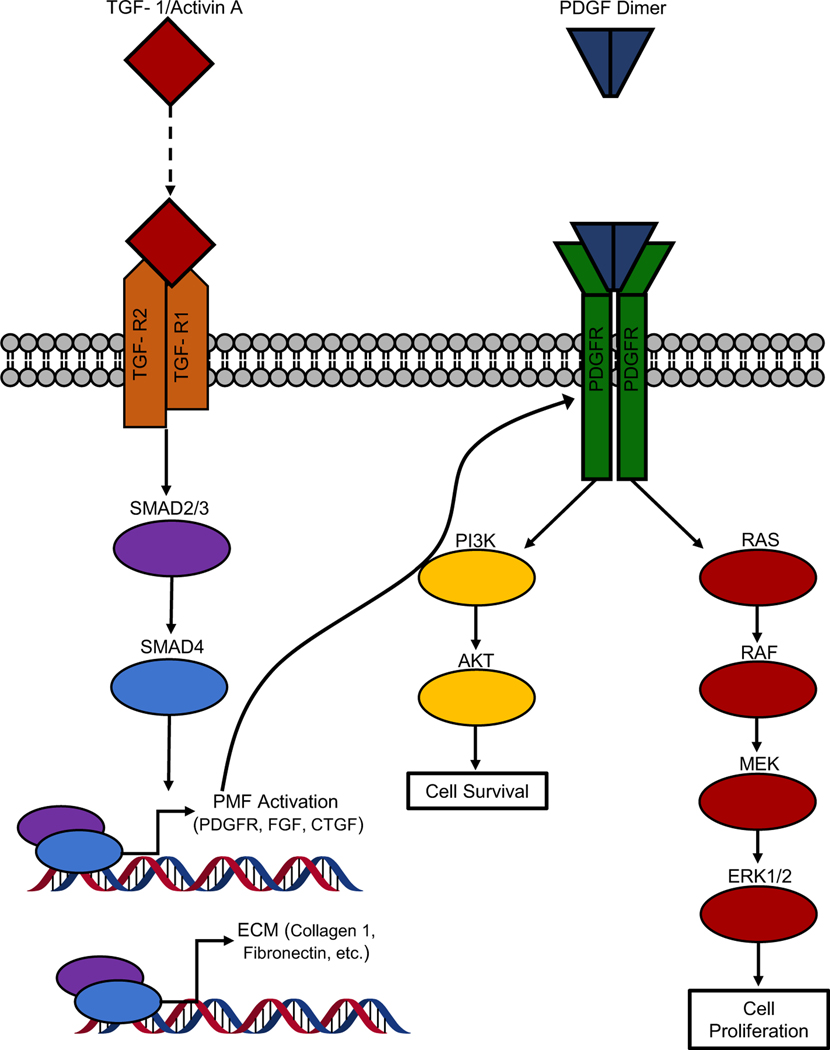
Fig. 2.
Comparison of roles of TGF-β1 and PDGF in PMF activation. TGF-β1 signaling acts through SMAD2/3/4, to promote an ECM deposition and prime PMFs for further activation, in part through upregulation of PDGFR’s. Upon upregulation of PDGFR PMFs are sensitized to PDGF. The ensuing signaling cascade through PI3K/AKT and RAS/RAF/MEK/ERK1/2 signaling results in augmented cell survival and proliferation
SHH, a molecule essential to the embryonic development of the pancreas, is also involved in PMF activation. In PDAC, SHH is derived from neoplastic epithelium and acts on activated PMFs, which express the necessary components for SHH signaling [48, 49]. In murine PDAC models, pancreas-specific knockout of SHH sharply reduced the ECM deposition and activated PMFs in the tumor microenvironment (TME) [50, 51]. Using Capan-2-derived, orthotopic PDAC tumors, Bailey et al. demonstrated that SHH neutralization profoundly suppressed the appearance of desmoplasia, as measured by Hematoxylin and Eosin, α-SMA, collagen I and fibronectin staining [49]. Further, in transformed human pancreatic normal epithelium (T-HPNE) -derived tumors, which lack natural SHH expression, ectopic SHH expression profoundly augmented the desmoplasia induced by T-HPNE cells, as assessed by the similar criteria as were used for the Capan-2 model [49]. In vitro, SHH augmented CAF proliferation, α-SMA expression, and migration of CAFs toward SHH-secreting cancer cells [49]. Despite these data that demonstrate a clear role of SHH in PF, the detailed mechanism still remains elusive.
CTGF is yet another factor in PMF activation and PF. Notably, CTGF expression in the pancreas is regulated by TGF-β1, Activin-A, PDGF, and tumor necrosis factor-α (TNF-α) [52, 53], and in CP specimens, CTGF and TGF-β1 were upregulated by greater than 20-fold [54]. Furthermore, in vitro studies revealed that CTGF stimulates proliferation, migration, matrix adhesion, and collagen I synthesis in rat PSCs [52, 53]. Additional analyses revealed that CTGF interacts with integrin α5β1 through a novel motif in the fourth module of CTGF [55]. Moreover, inhibition of integrin α5β1 suppressed the ability of CTGF to enhance the activated phenotype in PMFs [52, 55]. Downstream, CTGF activates extracellular signal related kinase 1/2 (ERK1/2), signal transducer and activator of transcription 3 (STAT3), and nuclear factor kappa beta (NFκB) pathways, resulting in increased expression of matrix metalloproteinase 9 (MMP-9), tissue inhibitor of metalloproteinases-1 (TIMP-1), TGF-β1, Interleukin-1β (IL-1β ), and Interleukin-6 (IL-6) [53]. The profibrogenic role of CTGF is also implicated in the PDAC desmoplasia, and CTGF is upregulated in PDAC-associated CAFs. Moreover, the murine homologue of CTGF (Fisp12) was induced upon orthotopic implantation of human PDAC cell lines in mice [56]. While these data suggest a role in the desmoplastic response, additional neutralization or genetic ablation studies of CTGF in CP and PDAC will be useful to differentiate the contributions of this axis to PMFs and epithelial cells’ biology.
Given the inflammatory nature of CP and PDAC, the impact of cellular and molecular mediators of inflammation on PMF activation is paramount to the PF that occurs in these two conditions. As demonstrated by co-cultures, activated macrophages secrete several PMF-activating factors, including TGF-β1, thereby augmenting the activation and subsequent PF [57, 58]. Similarly, co-culture of PMFs with lymphocytes increased PMF proliferation and collagen expression [59]. Finally, depletion of neutrophils from murine models of PDAC in obese mice decreased the number of activated PMFs as well as the expression of genes associated with PF [60].
At a molecular level, significant roles for TNF-α, IL-1β, and Cyclooxygenase-2 (COX2, the rate-limiting enzyme in prostaglandin biosynthesis) in PMF activation have been described. Treatment of PMFs with TNF-α increased α-SMA expression [41, 61, 62], collagen synthesis [61], and PMF proliferation [61, 62]. Along with TNF-α, IL-1β may also activate PMFs; as murine PDAC models lacking IL-1β showed drastic reductions in the number of α-SMA-positive PMFs in tumors [63]. Under in vitro conditions, Mews and colleagues demonstrated that IL-1 increased α-SMA expression in PMF without altering collagen synthesis or cell proliferation [61]. Moreover, the treatment of PSCs with IL-1β increased the expression of TGF-β1 in PMFs [64]. These multiple studies suggests that IL-1β has both direct and indirect effects on PMF activation; however, the contributions of these roles remain to be parsed fully.
The role of prostaglandins in pancreatic fibroblast activation was initially proposed from the studies showing that PANC-1-conditioned media and culture-mediated PMF activation upregulate cyclooxygenase-2 (COX-2) expression in PMFs [65], and inhibition of COX2 suppressed PMF proliferation and α-SMA expression upregulated by PANC-1-conditioned media and culture conditions [65, 66]. A subsequent investigation supported these results by demonstrating that prostaglandin E2, a product downstream of COX-2, stimulated PMF proliferation, migration, and expression of ECM proteins and matrix metalloproteinases in a prostaglandin EP4 receptor-dependent manner [67]. Though, it should be noted that these effects may be dependent on the specific prostaglandin receptor involved, activation of the EP2 receptor inhibits PMF activation [68]. In addition to the direct impact on activation of PMFs, COX-2 potentiated the effects of other factors on PMF biology. Here, COX-2 inhibition with NS-398 suppressed the activation of quiescent PSCs by prolonged culture, TGF-β1, and IL-1β [64]. Further supporting the profibrogenic role of COX-2, knockout of Cox-2 from the exocrine pancreas delayed progression to PDAC and completely abrogated associated desmoplasia in K-ras-driven PDAC models [69]. Similarly, ectopic expression of COX-2 in the acinar compartment of mouse pancreas resulted in a spontaneous phenotype resembling CP, including the presence of inflammatory cells, acinar-to-ductal metaplasia, and progressive deposition of ECM proteins [70]. However, it must be noted that the effects of COX-2 knockout and overexpression in these models on desmoplasia may be indirect due to reduced neoplastic insult and modulation of immune infiltrate.
Finally, PMFs are sensitive to damage-associated molecular patterns (DAMPs). Masamune et al. demonstrated that rat PMFs express toll-like receptors (TLRs) 2, 3, 4, and 5 along with co-receptors, CD14 and MD2 [71]. Of these, TLR2 and 4 are known receptors of high mobility group box 1 (HMGB1), heat shock protein 70 (HSP70), and fibrinogen. Moreover, treatment of PMFs with microbe-derived ligands of these TLRs promoted the expression of pro-inflammatory cytokines monocyte chemoattractant protein 1 (MCP-1) and the rat homologue of CXCL8 [71]. While these ligands failed to induce proliferation or collagen synthesis in response to bacterial ligands [71], treatment with fibrinogen, a mammalian ligand of TLR4, increased expression of the inflammatory cytokines along with collagen 1, suggesting that fibrinogen, as a DAMP, can activate PMFs [72]. Furthermore, signaling downstream of fibrinogen activates central signaling cascades in PMF activation, including ERK1/2, c-Jun N-terminal Kinase, and p38 MAPK. Similarly, PMFs express high levels of P2 purinergic receptors, allowing them to respond to nucleotides released as a result of tissue damage [73]. Additionally, mice null for CD39 (an enzyme involved in maintaining the sensitivity of P2 receptors) have diminished fibrosis compared to wild-type (WT) counterparts when treated with cerulein. In vitro, loss of CD39 expression from PMFs results in severe deficits in PMF proliferation in response to PDGF and collagen synthesis in response to TGF-β1 [73]. Finally, PMFs treated with DNA derived from neutrophil extracellular traps showed increased α-SMA expression and proliferation, suggesting that mammalian DNA is another vital cue for PMF activation [74]. Cumulatively, these findings demonstrate that PMFs are activated by products of inflammation in CP and PDAC and are sensitive to signals that initiate inflammatory responses.
Signals involved in the propagation of PMF activation
Initial activation of PMFs is crucial for the formation of PF; however, it is insufficient as sustained PMF activation is also required for proliferation and ECM protein synthesis. While the molecules discussed in the previous section likely contribute to initial and sustained activation of PMFs, additional environmental cues associated with CP and PDAC have salient effects on prolonged PMF activation; including hypoxia and ECM stiffness. Notably, both of these environmental features are products of PF. In cultured PMFs, hypoxia induced a migratory phenotype and increased expression of collagen I by augmenting vascular endothelial growth factor (VEGF) expression [75, 76]. In addition to VEGF, hypoxia augments CTGF expression in PMFs, thereby indirectly contributing to sustaining PMF activation [77]. These changes are further associated with decreased microvessel density in PDAC and CP [76]. Interestingly, another study found that hypoxia not only regulates the amount of collagen produced but also the orientation of the collagen that is deposited, which has implications for the migration and invasion of cancer cells [78], suggesting that hypoxia may contribute to multiple aspects of PMF biology, and tumor microenvironment.
Interactions of PSCs with a rigid growth substrate is another classic means of PMF activation. To support these findings from the initial characterization of PSCs/PMFs, PMFs from CP patients grown on Matrigel showed early morphological changes indicative of quiescence and, over time, showed increased cytoplasmic lipid accumulation compared to cells cultured on plastic [79]. While culture of pancreatic fibroblasts in Matrigel resulted in decreased expression of α-SMA, Collagen I, TGF-β1, and CTGF, it did not reduce the proliferation of PMFs nor the expression of fibronectin [79]. Another study utilizing polyacrylamide gels to modulate growth surface rigidity (thereby avoiding confounding influences of the biological activity of Matrigel) found that PMFs cultured on less rigid gels had decreased α-SMA and vimentin expression as well as increased cytoplasmic lipid content [80].
There are three possible mechanisms proposed by which the rigidity of the attachment substrate might regulate PMF activation in vivo: The first is that mature TGF-β is secreted bound to both latency-associated peptide (LAP) and latent TGF-β binding protein (LTBP), which cumulatively is known as the large latency complex. This complex interacts with fibrillin in the ECM and is not available for signaling activity. To produce active TGF-β, a cell must apply tension to fibronectin fibrils, dissociating TGF-β from LTBP-1 and LAP [81]. In this process, both cellular contractility and the ECM are critical for the release of TGF-β [82, 83]. Inhibition of PMF activation [by use of all-trans retinoic acid (ATRA)], as well as inhibition of activated fibroblast contraction (by blebbistatin), impaired the release of TGF-β1 from LTBP-1, thereby demonstrating that this mechanism of TGF-β1 release may be necessary for TGF-β1 signaling in PMFs, specifically when the cells are attached to ECM-based scaffolds [84].
While the second potential mechanism has not explicitly been investigated in PMFs associated with CP or PDAC, Calvo and colleagues showed that inhibition of ECM remodeling by CAFs blocked the activation of yes-associated protein-1 (YAP1), which was, in turn, required for vimentin expression, collagen production, and collagen disc contraction [85]. The observation that, in different mouse models of PDAC, the level of Yap1 activation parallels the degree of matricellular tension present within the TME [86] supports these findings. It is consistent with a mechanical mechanism of YAP1 activation [87]. Furthermore, YAP1 expression is induced by PDAC and CP in PMFs, and knockdown or inhibition of YAP1 in PMFs suppresses the activated phenotype [88–90]. Based on these studies, it is likely that YAP1 plays a critical role in PMF activation in PF, and that YAP1 activation is, at least in part, mediated by substrate rigidity.
Finally, recent evidence indicates that ion channels may also be critical for the response of PMFs to a rigid growth substrate through TRPC1 and 3, KCa3.1, and Piezo-1 mediated mechanisms [91–93]. Activation of these ion channels either with mechanical stress or a small molecule activator enhanced PMF migration in vitro. Mechanistically, each ion channel augmented calcium influx in PMFs upon activation, suggesting that intracellular Ca2+ is central to the response of PMFs to mechanical stress [91–93]. Despite the associations between Ca2+ signaling, activation of mechanosensitive ion channels, and PMF activation, additional studies inhibiting calcium signaling are required to concretely demonstrate the role of Ca2+ in the response of PMFs to mechanical stress.
Negative regulators of PMF activation
ATRA, vitamin D, and interferons have prominent antifibrotic roles in CP and PDAC. Initial characterization of PSCs demonstrated that quiescent PSCs store retinoids in cytoplasmic lipid droplets, which are lost upon activation [27, 28]. Retinol (vitamin A) is acquired through the diet and enters cells through interaction with retinol-binding protein [94]. Inside the cell, retinol is either metabolized to the biologically active retinoic acid by two enzymes, retinol dehydrogenase (RolDH) and retinaldehyde dehydrogenase (RALDH), or converted to retinol esters for storage [95]. Retinoic acids serve as ligands for the retinoic acid receptors (RARs) and retinoid X receptors (RXRs) [96]. Importantly, PSCs express RolDHII as well as members of both retinoic acid receptor families, suggesting that PSCs express the necessary components for active retinoic acid signaling [97]. Under in vivo conditions, ATRA treatment reduced the amount of PF and α-SMA-positive PMFs induced by cerulein treatment [98]. Mechanistically, cultured rat PSCs treated with exogenous ATRA had reduced proliferation, collagen synthesis, and MMP2 expression [99]. However, α-SMA, PDGF-induced phosphorylated ERK 1/2, and MMP9 were not reduced by ATRA treatment [99]. ATRA functions as a transrepressor of the AP-1 complex without affecting upstream activating signals or AP-1 complex binding to DNA [99]. Additional studies demonstrated that retinol, 9-Cis RA, and ATRA suppressed PMF proliferation as well as collagen and fibronectin expression [97, 98]. Further, α-SMA expression, phospho-ERK1/2, phospho-p38, and phospho-JNK-2 were abrogated by retinoid after prolonged treatment [97]. This delay in the observed changes likely reflects indirect effects of retinoid signaling, including the suppression of TGF-βR2 and PDGF-Rβ expression in PMFs [98]. To further support the role of ATRA as a suppressor of PMF activation, forced expression of albumin (which is critical for the storage of retinol and is downregulated upon PMF activation) suppresses the activated PMF phenotype [100]. Finally, treatment of PMFs with ATRA inhibited activation mediated by the stiffness of the growth substrate; ATRA-treated PMFs lost the ability to sense strain, and apply force to, their attachment surface. The inability to sense substrate stiffness resulted in decreased polyacrylamide gel contraction and α-SMA and vimentin expression [101].
Like ATRA, vitamin D and its analogs suppress the activity of PMFs. Sherman et al. identified vitamin D receptor (VDR) expression in mouse PMFs and human CAFs. Activation of VDR using calcipotriol (a vitamin D derivative) in isolated PMFs suppressed the activation-associated gene signature and phenotype of the fibroblasts, including downregulation of collagen I, MMP2 and IL6, and increased accumulation of cytoplasmic lipids [23]. Transient knockdown of VDR abrogated calcipotriol-induced quiescence, indicating that VDR was required for the inactivation of fibroblasts and that the mechanism likely involves competition of VDR for mothers against decapentaplegic homolog 3 (SMAD3) binding at the promoters of profibrotic genes. Moreover, in mice, calcipotriol abrogated PF in response to cerulein-induced pancreatitis and vdr−/− mice showed spontaneous fibrosis in the pancreas [23]. While these studies demonstrate the ability of VDR to reprogram already activated pancreatic fibroblasts and suggests that loss of VDR activity, in the form of receptor knockdown, leads to PF, it remains unclear if and how this fibrosis-suppressing pathway is altered during pancreatic pathologies and how these underlying alterations lead to PF.
Surprisingly, interferons also have a prominent role in the suppression of PMF activation. In rat PSCs, IFN-β and IFN-γ treatment reduced proliferation and collagen synthesis compared to control, whereas IFN-γ also reduced α-SMA expression. Consistent with the known signaling mechanisms of interferons, treatment with IFN-β and -γ induce STAT1 and 3 phosphorylation, and the inhibition of STAT1 expressly abrogated the effects of IFNs on PSC proliferation [102]. Further investigation of the inhibitory effects of IFN-mediated STAT1 signaling revealed that STAT1 overexpression suppresses TGF-β1-mediated upregulation of CTGF and endothelin-1, which promote PMF proliferation [103]. Mathematical models of PMF response to IFN-γ treatment concerning STAT1 activation, STAT1 expression, and inhibitory suppressor of cytokine signaling (SOCS-1) expression demonstrate that high concentrations of IFN-γ robustly activate STAT1 expression and signaling, which is significant and long-lived compared to competing SOCS-1 upregulation [104]. Finally, IFN-γ, specifically, may suppress PF through its regulation of CXCR3 ligands. A study demonstrated that administration of CXCL9 to mice with pancreatitis attenuated fibrosis, whereas neutralization of CXCL9 worsened the PF [105]. While the relevance of these inhibitory signals to PF in CP and PDAC remains to be thoroughly described, these findings suggest that lymphoid subsets in the pancreas may have more balanced effects on PMFs than myeloid subset mediated mechanisms.
Signaling cascades central to PMF activation
Despite the presence of numerous extracellular factors that regulate the activation of PSCs/PMFs, the list of intracellular signaling mechanisms leading to PMF activation is surprisingly succinct. The essential roles of ERK-, Rho kinase-, YAP1-, and SMAD-mediated signaling mechanisms have been described in Table 1. The initial observation implicating MAPK signaling in PSC activation was derived from the spontaneous increase in ERK phosphorylation with progressive, culture-mediated PMF activation [106]. This study also demonstrated that PDGF induced ERK phosphorylation in PSCs and that inhibition of ERK signaling with trapidil or PD98059 suppressed basal and PDGF-stimulated PMF proliferation (Fig. 2) [106, 107]. Furthermore, ERK signaling was shown to mediate the effect of TGF-β1 on PSC expression of TGF-β1 and α-SMA [108, 109]. Similarly, inhibition of ERK signaling in PMFs treated with PANC1-conditioned media abrogated the expression of TIMP-1, thereby supporting the role of ERK signaling in both the proliferative and fibrotic phenotype of pancreatic fibroblasts [110]. Interestingly, using lovastatin (a hydroxymethylglutaryl coenzyme A reductase inhibitor), Jaster and colleagues inhibited ERK activation in PMF, resulting in loss of proliferation and increased apoptosis [111]. Likewise, inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMGCoA) reductase was shown to interfere with isoprenylation of Rho and Ras family members, causing reduced membrane localization and suppression of ERK signaling [111]. Finally, ERK signaling is implicated in angiotensin II-mediated activation of PSCs. Here Angiotensin II treatment activates epidermal growth factor receptor (EGFR) through non-canonical heterotrimeric G-protein-mediated transactivation of EGFR, resulting in ERK phosphorylation [112]. Importantly, inhibition of EGFR and ERK signaling in this setting abrogated the proliferation of pancreatic fibroblasts in response to angiotensin II [112].
Table 1.
Summary of key intracellular signaling pathways in PMF activation
| Signaling pathway | Positive regulationa | Negative regulationa | Primary cellular effectsb | Secondary cellular effectsb |
|---|---|---|---|---|
|
| ||||
| ERK 1/2 | PDGF, CTGF, PAR-2, ROS, Angiotensin II | ATRA | PMF proliferation | Collagen expression |
| JNK | PDGF, PAR-2, ROS | ATRA | PMF proliferation | Collagen expression |
| p38 | PDGF, PAR-2, ROS | ATRA | PMF proliferation | Collagen expression |
| RhoA/ROCK1 | PDGF | NA | Potentiate PDGF-induced proliferation and migration | Collagen and SMA expression |
| YAP1 | Matricellular stress | ATRA | Potentiate TGF-β1 and PDGF signaling | NA |
| SMAD2/3 | TGF-β1, Activin A, matricellular stress | BMP2, SMAD1/5/8, STAT1 | ECM deposition | Expression of PDGFR, and CTGF |
Positive and negative regulators refer to factors that activate and suppress a given signaling pathway
Primary and secondary cellular effects refer to the results of the activation of that pathway in PMFs
The role of MAPK signaling in PMF activation extends beyond the functions of ERK1/2 to include p38 and c-Jun N-terminal kinase (JNK). The inhibition of p38 suppressed PMF proliferation in response to PDGF, as well as collagen and α-SMA expression under basal conditions [113]. Still, it failed to alter α-SMA and fibronectin expression in the presence of TGF-β1 [109]. Critically, loss of p38 signaling blocked the conversion of quiescent PSCs to the activated form after prolonged culture. Together, these findings indicate that p38 is not universally involved in PMF activation but may mediate specific effects downstream of PDGF and signaling involved in substrate-mediated PMF activation. Like p38, JNK inhibition abrogated PDGF-mediated proliferation, as well as collagen expression, upon serum exposure [114]. In contrast to p38, inhibition of JNK also suppressed TGF-β1-stimulated expression of α-SMA and fibronectin, which is similar to the effect of ERK inhibition in PMFs [109]. As a final note, these three signaling axes are essential components downstream of several PMF-activating stimuli, including protease activated receptor 2 (PAR-2) [115] and reactive oxygen species (ROS) [116–118]. The sheer diversity of the signals that proceed through MAPK pathways in route to PMF activation is a testament to the central roles of these pathways in PMF biology.
Along similar lines, Rho kinases have also been implicated in pancreatic fibroblast activation. Pancreatic fibroblasts isolated from male Wistar rats demonstrated expression of RhoA as well as ROCK-1 and −2 [119]. Furthermore, the inhibition of ROCK using Y-27632 and HA-1077 suppressed stress fiber formation (characteristic of PSC activation), α-SMA and collagen expression, and PMF proliferation and migration in response to serum or PDGF [119]. Importantly, these changes in pancreatic fibroblast activation occurred independently of ERK activation, suggesting independent functions of RhoA and ROCK in PMF activation [119]. Furthermore, inhibition of ROCK kinases in PDAC mouse models decreased collagen density within tumors, providing further evidence that this pathway is important in vivo [120].
The parallel changes in stress fiber formation and the activation state of PMFs are indicative that mechanical factors may be important for PMF activation. As previously discussed, YAP1 signaling can be stimulated by mechanically transduced signals. In cultured mouse and human PMFs, knockdown of YAP1 inhibited the activation of PMFs induced by both PDGF and TGF-β1 treatment. Interestingly, mechanical activation of YAP1 is independent of phosphorylation of serine127, a known negative regulatory marker [87]. These results indicate the possibility that specifically mechanotransduction dependent on RhoA/ROCK signaling results in the non-canonical activation of YAP1, which in turn cooperates with PDGF and TGF-β1 signaling to promote PMF activation.
ROS appears to play an important intracellular role in PMF activation. Early studies by Kikuta et al. demonstrated that treatment of PMFs with 4-hydroxy-2, 3 nonenol and hydrogen peroxide both resulted in the activation of AP-1 and MAPK protein [116, 117]. Subsequently, this group demonstrated that PMFs express functional components of NADPH oxidase [121]. Moreover, PDGF-BB, IL-1β, and angiotensin II stimulated ROS production in vitro. Importantly, inhibition of NADPH oxidase with diphylene iodonium (DPI) and ROS scavenging with N-acetyl-cysteine, suppressed PDGF-mediated fibroblast proliferation, IL-1β-mediated cytokine production,α-SMA, collagen, and TGF-β1 expression in vitro [116, 117, 122]. In Wistar rat and dibutyltin dichloride-induced models of CP, treatment with DPI inhibited the development of PF [121]. Subsequent studies using NADPH oxidase deficient mice showed decreased histologic evidence of CP following repeated cerulein injection [123]. Finally, in the setting of hyperglycemia-mediated PMF activation, treatment with antioxidants suppressed transition to the activated PMF phenotype [124].
Given the prominent role of TGF-β1 in PSC activation, it is not surprising that SMADs play a prominent role in the intracellular signaling leading to PMF activation. However, the role of SMADs in PMF activation extends beyond TGF-β1 signaling, as Activin-A also activates SMAD2/3 resulting in PMF activation [125]. Using a dominant-negative form of Smad3, which inhibits Smad2 and 3 signaling, along with co-expression of either Smad2 or Smad3, Ohnishi and colleagues studied the differential effects of these Smads in response to TGF-β1 treatment in PMFs [108]. Upon TGF-β1 treatment, Smad2 and 3 translocated to the nucleus of PMFs. Further, expression of the dominant-negative form of Smad3 suppressed the expression of α-SMA and augmented PMF proliferation [108]. Co-expression of Smad2 with the dominant-negative Smad3 did not suppress the increased proliferation of fibroblasts that was stimulated by the dominant-negative Smad. In contrast, co-expression of Smad3 suppressed the increased proliferation, indicating that Smad3 signaling may be responsible for the decreased proliferation observed with TGF-β1 treatment. Later, dominant-negative Smad2/3 was used to show that Smad3 also promoted the expression of IL-1β and IL-6 [126, 127]. To extend the role of SMADs to PF, He et al. used cerulein to induce CP in mice with pancreas-specific, transgenic Smad7 expression, an inhibitor of receptor/co-SMAD signaling. In this model, the expression of Smad7 reduced cerulein-induced PF along with the number of α-SMA-positive stromal cells [128]. However, it must be noted that expression of Smad7 in this model is under control of the elastase promoter, and thus expression of Smad7 in PSCs/PMFs and subsequent modulation of TGF-β1 signaling in this population by Smad7 is questionable [128, 129]. Another cerulein-induced model of CP comparing wild type and haploinsufficient BMP2 mice, showed that loss of BMP2 exacerbated fibrotic response to recurrent cerulein treatment. Interestingly, the loss of Smad1/5/8 signaling in BMP2 haploinsufficient mice increased Smad2/3 signaling and worsened the PF [130]. These findings were confirmed in vitro by demonstrating that BMP2 treatment suppressed TGF-β1-mediated PMF activation in a SMAD1-dependent manner [131]. Gremlin acts as an additional regulator of SMAD-mediated signaling in PMFs. Here Gremlin suppresses BMP2-mediated activation of SMAD1/5, thereby allowing augmented SMAD2/3 signaling (Figs. 1 and 2) [132]. Cumulatively, the diverse mechanism of activation and multiple layers of regulation of SMAD signaling point towards this pathway being central to PMF activation.
Molecular correlates of clinical CP and PDAC risk factors
Alcohol consumption, tobacco use, diabetes, and obesity are risk factors for the development of CP and PDAC. This section explores the underlying molecular mechanisms of PMF activation in light of these risk factors (Table 2). Ethanol and its metabolite acetaldehyde augment α-SMA and collagen 1 expression in cultured PMFs and accelerate the conversion of quiescent PSCs to activated PMFs [133]. Importantly, this effect of ethanol was dependent on the activity of alcohol dehydrogenase, indicating that the metabolites of ethanol rather than ethanol itself are important for PMF activation. Mechanistically, ethanol and acetaldehyde activated ERK1/2, p38, and JNK in PMFs [134]. Subsequent studies demonstrated that N-acetyl cysteine blocked both MAPK signaling and PMF activation downstream of ethanol and acetaldehyde, suggesting that ROS, potentially produced by NADPH oxidase, is an important intermediate in ethanol-mediated PMF activation [134, 135]. Interestingly, analysis of ERK, p38, and JNK independently revealed that p38 inhibition suppressed the activity of ethanol and acetaldehyde in PMFs, whereas ERK and JNK were dispensable for the ethanol-related PMF activation [134, 136]. Under in vivo conditions, the effects of alcohol on pancreatic biology are more complicated. Alcohol mediates rapid acinar cell injury and both direct and indirect mechanisms of PMF activation [137]. Moreover, in murine models of CP, the persistence of ethanol exposure prolongs the duration of active fibrotic response by suppressing PMF apoptosis [138]. In sum, these data suggest that alcohol and its metabolites contribute directly and indirectly to the activation of PMFs, thereby contributing to the PF observed in CP and PDAC.
Table 2.
Summary of the molecular correlates of CP and PDAC risk factors and their effects on PMF activation
| Risk factor | Component, metabolite, or feature | Signaling effects | Cellular effect |
|---|---|---|---|
|
| |||
| Alcohol | Ethanol/ acetaldehyde | ERK, JNK, and p38 | Increased rate of PMF activation and α-SMA and Collagen expression |
| Tobacco | Nicotine | α7nAChR, JAK 2 STAT3 activation | Increased PMF proliferation and α-SMA and Collagen expression |
| Aryl hydrocarbons | Increased IL-22 expression | Collagen 1 and Fibronectin expression | |
| Obesity | Obesity (obese vs. non-obese mice) | IL-1β, AT2R1, MAPK, decreased vitamin A content | Augmented PMF proliferation and activation |
| VLDL | Not studied | Increased Collagen and Fibronectin expression; Augmented PMF proliferation | |
| Oleic acid | Not studied | Increased PMF proliferation, TNF-α expression and Fibronectin synthesis | |
| Diabetes | High glucose | Angiotensin II, ERK, p38 | Increased PMF proliferation and expression of TGF-β1, CTGF, and α-SMA |
Several compounds in tobacco products or generated by tobacco use also play roles in PMF activation. Nicotine treatment induces PMF proliferation, induces α-SMA and collagen expression, and suppresses apoptosis in cultured PMFs. The α-Bungarotoxin inhibited nicotine-mediated PMF proliferation and α-SMA expression, suggesting the involvement of α7n-acetylcholine receptor (AChR). This study further implicated the involvement of JAK2/STAT3 signaling in nicotine-mediated activation, though further studies are required to validate the involvement of this signaling axis [139]. In addition to nicotine, aryl hydrocarbons derived from cigarette smoke play indirect roles in PMF activation. Concomitant administration of aryl hydrocarbon receptor ligands (AhR) and cerulein in mice caused increased PF and IL-22 expression in T-cells. Importantly, IL-22 treatment with cerulein phenocopied the effects of AhR activation. Finally, in vitro, IL-22 treatment caused increased expression of collagen 1 and fibronectin in PMFs [140]. Overall, components of tobacco may exacerbate pancreatitis and promote a transition towards CP and PF.
Finally, metabolic conditions, including obesity and diabetes, are important risk factors for CP and PDAC and contribute biochemically to PMF activation. Obesity is associated with increased pancreatic inflammation, and, in murine PDAC models, is associated with increased IL1β-mediated PMF activation [60]. Importantly, inhibition of local renin-angiotensin signaling suppressed the effects of obesity on PMF activation in vivo. These findings are supported by two observations. First, ACE inhibitors, angiotensin receptor blockers, and knockout of angiotensin-II receptor I (AT2R1) each suppressed the formation of PF and overexpression of TGF-β1 resulting from spontaneous CP in Wistar rats and cerulein-treatment in mice [141–143]. Second, PMFs express significant levels of AT2R1 [112, 144]. In this context, AT2R1 transactivates receptor tyrosine kinases through non-canonical G-protein-coupled receptor signaling, resulting in activation of MAPK signaling downstream [112, 145]. Despite these findings, it remains unclear if obesity specifically acts to promote PMF activation through angiotensin as it was not directly assessed. Alternatively, one study demonstrated that obese mice have deficient vitamin A content in the pancreas. While not directly addressed, this finding would suggest that obesity promotes PMF activation through decreased retinol-mediated, suppressive signaling [146]. More directly, treatment of PMFs with VLDL increased their collagen synthesis, fibronectin expression, and proliferation [137]. Similarly, oleic acid augmented PMF proliferation and expression of TNF-α and fibronectin [147]. Further investigation is needed to understand the mechanism by which VLDL and fatty acids activate PMFs and the extent to which this mechanism plays a role in vivo.
Finally, high glucose and insulin exposure activates PMFs. Culture in high glucose concentrations drastically increased PMF proliferation and expression of TGF-β1, CTGF, and α-SMA [148–150]. Importantly suppression of angiotensin signaling abrogated the effects of glucose on PMF activation [148]. Consistent with the known mechanism of angiotensin signaling in PMFs, high glucose induced ERK and p38 activation [149, 150]. Inhibition of p38 and ERK signaling also suppressed the effects on the PMF activation. Finally, it should be noted that insulin also activates ERK signaling in PMFs, but the concomitant treatment of high glucose concentrations with insulin has an additive effect, suggesting that insulin resistance may be a key to the promotion of PF [149].
Conclusions
In conclusion, PMFs are activated by a diverse array of signals that encompass both specific signaling molecules as well as features of the CP and PDAC microenvironment. Despite the diversity in the molecules that can promote PMF activation, the downstream signaling of these factors converges on a small number of central intracellular signaling pathways crucial to promoting the activated PMF phenotype. The convergence of these diverse signals on a discrete number of signaling pathways provides an excellent opportunity for therapeutic targeting. However, the efficacy of such therapeutic mechanisms remains controversial in PDAC and mostly untested in CP. Finally, analysis of the risk factors for CP and PDAC in relation to PMF activation demonstrates a strong correlation between clinical features and molecular mechanisms, indicating that the highlighted pathways play critical roles in the development of PF.
Acknowledgements
The authors/work, in parts, were supported by the NIH grants (F30 CA225117, R21CA223429, R21 AA026428, R44 CA235991, P01 CA217798, Great Plains IDeA-CTR, R01 CA183459, R01 CA210637, and R01 CA228524), and the University of Nebraska Collaboration Initiative/System Science Seed Grant (20063).
Footnotes
Compliance with ethical standards
Conflict of interest SKB is one of the co-founders of the Sanguine Diagnostics and Therapeutics, Inc. The other authors declare no competing interests.
References
- 1.Beyer G, Habtezion A, Werner J, et al. Chronic pancreatitis. Lancet. 2020;396:499–512. [DOI] [PubMed] [Google Scholar]
- 2.Levy P, Dominguez-Munoz E, Imrie C, et al. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United Eur Gastroenterol J. 2014;2:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011;106:2192–9. [DOI] [PubMed] [Google Scholar]
- 5.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology. 2014;14:490–6. [DOI] [PubMed] [Google Scholar]
- 6.Machicado JD, Dudekula A, Tang G, et al. Period prevalence of chronic pancreatitis diagnosis from 2001–2013 in the commercially insured population of the United States. Pancreatology. 2019;19:813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capurso G, Archibugi L, Pasquali P, et al. Prevalence of chronic pancreatitis: results of a primary care physician-based population study. Dig Liver Dis. 2017;49:535–9. [DOI] [PubMed] [Google Scholar]
- 8.Wang LW, Li ZS, Li SD, et al. Prevalence and clinical features of chronic pancreatitis in China: a retrospective multicenter analysis over 10 years. Pancreas. 2009;38:248–54. [DOI] [PubMed] [Google Scholar]
- 9.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol. 1984;15:677–83. [DOI] [PubMed] [Google Scholar]
- 10.Pitchumoni CS, Glasser M, Saran RM, et al. Pancreatic fibrosis in chronic alcoholics and nonalcoholics without clinical pancreatitis. Am J Gastroenterol. 1984;79:382–8. [PubMed] [Google Scholar]
- 11.van Geenen EJ, Smits MM, Schreuder TC, et al. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol. 2011;106:1161–6 (quiz 1167). [DOI] [PubMed] [Google Scholar]
- 12.Levy P, Milan C, Pignon JP, et al. Mortality factors associated with chronic pancreatitis. Unidimensional and multidimensional analysis of a medical-surgical series of 240 patients. Gastroenterology. 1989;96:1165–72. [PubMed] [Google Scholar]
- 13.Fasanella KE, Davis B, Lyons J, et al. Pain in chronic pancreatitis and pancreatic cancer. Gastroenterol Clin N Am. 2007;36:335ix-ix364. [DOI] [PubMed] [Google Scholar]
- 14.DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–5. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19:7276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. [DOI] [PubMed] [Google Scholar]
- 18.SEER Cancer Statisitics Review, 1975–2017. In: Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Rhul J, Tatalovish Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (Eds.) The National Cancer Institute, https://seer.cancer.gov/csr/1975_2017/, 2020. [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 20.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 21.Hall BR, Cannon A, Atri P, et al. Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget. 2018;9:19396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masamune A, Hamada S, Kikuta K, et al. The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand J Gastroenterol. 2013;48:602–9. [DOI] [PubMed] [Google Scholar]
- 25.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon A, Thompson C, Hall BR, et al. Desmoplasia in pancreatic ductal adenocarcinoma: insight into pathological function and therapeutic potential. Genes Cancer. 2018;9:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–32. [DOI] [PubMed] [Google Scholar]
- 28.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen TW, Aardal NP, Bronner MP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–34. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marrache F, Pendyala S, Bhagat G, et al. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut. 2008;57:1113–20. [DOI] [PubMed] [Google Scholar]
- 34.Sparmann G, Kruse ML, Hofmeister-Mielke N, et al. Bone marrow-derived pancreatic stellate cells in rats. Cell Res. 2010;20:288–98. [DOI] [PubMed] [Google Scholar]
- 35.Akita S, Kubota K, Kobayashi A, et al. Role of bone marrow cells in the development of pancreatic fibrosis in a rat model of pancreatitis induced by a choline-deficient/ethionine-supplemented diet. Biochem Biophys Res Commun. 2012;420:743–9. [DOI] [PubMed] [Google Scholar]
- 36.Ino K, Masuya M, Tawara I, et al. Monocytes infiltrate the pancreas via the MCP-1/CCR2 pathway and differentiate into stellate cells. PLoS ONE. 2014;9:e84889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarlett CJ, Colvin EK, Pinese M, et al. Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction. PLoS ONE. 2011;6:e26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogelmann R, Ruf D, Wagner M, et al. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol. 2001;280:G164–72. [DOI] [PubMed] [Google Scholar]
- 39.Kruse ML, Hildebrand PB, Timke C, et al. TGFbeta1 autocrine growth control in isolated pancreatic fibroblastoid cells/stellate cells in vitro. Regul Pept. 2000;90:47–52. [DOI] [PubMed] [Google Scholar]
- 40.Luttenberger T, Schmid-Kotsas A, Menke A, et al. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47–55. [DOI] [PubMed] [Google Scholar]
- 41.Schneider E, Schmid-Kotsas A, Zhao J, et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2001;281:C532–43. [DOI] [PubMed] [Google Scholar]
- 42.Yoo BM, Yeo M, Oh TY, et al. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30:e71–9. [DOI] [PubMed] [Google Scholar]
- 43.Nagashio Y, Ueno H, Imamura M, et al. Inhibition of transforming growth factor beta decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice. Lab Invest. 2004;84:1610–8. [DOI] [PubMed] [Google Scholar]
- 44.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–21. [DOI] [PubMed] [Google Scholar]
- 45.Lohr M, Schmidt C, Ringel J, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–5. [PubMed] [Google Scholar]
- 46.Phillips PA, Wu MJ, Kumar RK, et al. Cell migration: a novel aspect of pancreatic stellate cell biology. Gut. 2003;52:677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarroll JA, Phillips PA, Kumar RK, et al. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol. 2004;67:1215–25. [DOI] [PubMed] [Google Scholar]
- 48.Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A. 2014;111:E3091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao R, Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin alpha5beta1 as a novel CCN2 receptor. Gastroenterology. 2005;129:1019–30. [DOI] [PubMed] [Google Scholar]
- 53.Karger A, Fitzner B, Brock P, et al. Molecular insights into connective tissue growth factor action in rat pancreatic stellate cells. Cell Signal. 2008;20:1865–72. [DOI] [PubMed] [Google Scholar]
- 54.di Mola FF, Friess H, Martignoni ME, et al. Connective tissue growth factor is a regulator for fibrosis in human chronic pancreatitis. Ann Surg. 1999;230:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wenger C, Ellenrieder V, Alber B, et al. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073–80. [DOI] [PubMed] [Google Scholar]
- 57.Schmid-Kotsas A, Gross HJ, Menke A, et al. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol. 1999;155:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sparmann G, Glass A, Brock P, et al. Inhibition of lymphocyte apoptosis by pancreatic stellate cells: impact of interleukin-15. Am J Physiol Gastrointest Liver Physiol. 2005;289:G842–51. [DOI] [PubMed] [Google Scholar]
- 60.Incio J, Liu H, Suboj P, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marzoq AJ, Giese N, Hoheisel JD, et al. Proteome variations in pancreatic stellate cells upon stimulation with proinflammatory factors. J Biol Chem. 2013;288:32517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das S, Shapiro B, Vucic EA, et al. Tumor cell-derived IL1beta promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 2020;80:1088–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aoki H, Ohnishi H, Hama K, et al. Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to proinflammatory cytokines. Am J Physiol Cell Physiol. 2007;292:C259–68. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida S, Ujiki M, Ding XZ, et al. Pancreatic stellate cells (PSCs) express cyclooxygenase-2 (COX-2) and pancreatic cancer stimulates COX-2 in PSCs. Mol Cancer. 2005;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun L, Chen K, Jiang Z, et al. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol Rep. 2018;39:2243–51. [DOI] [PubMed] [Google Scholar]
- 67.Charo C, Holla V, Arumugam T, et al. Prostaglandin E2 regulates pancreatic stellate cell activity via the EP4 receptor. Pancreas. 2013;42:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pomianowska E, Sandnes D, Grzyb K, et al. Inhibitory effects of prostaglandin E2 on collagen synthesis and cell proliferation in human stellate cells from pancreatic head adenocarcinoma. BMC Cancer. 2014;14:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Philip B, Roland CL, Daniluk J, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H, Chen J, Peng L, et al. Transgenic expression of cyclooxygenase-2 in pancreatic acinar cells induces chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2019;316:G179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masamune A, Kikuta K, Watanabe T, et al. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol. 2008;43:352–62. [DOI] [PubMed] [Google Scholar]
- 72.Masamune A, Kikuta K, Watanabe T, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–9. [DOI] [PubMed] [Google Scholar]
- 73.Kunzli BM, Nuhn P, Enjyoji K, et al. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology. 2008;134:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller-Ocuin JL, Liang X, Boone BA, et al. DNA released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. Oncoimmunology. 2019;8:e1605822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masamune A, Kikuta K, Watanabe T, et al. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–17. [DOI] [PubMed] [Google Scholar]
- 76.Erkan M, Reiser-Erkan C, Michalski CW, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eguchi D, Ikenaga N, Ohuchida K, et al. Hypoxia enhances the interaction between pancreatic stellate cells and cancer cells via increased secretion of connective tissue growth factor. J Surg Res. 2013;181:225–33. [DOI] [PubMed] [Google Scholar]
- 78.Sada M, Ohuchida K, Horioka K, et al. Hypoxic stellate cells of pancreatic cancer stroma regulate extracellular matrix fiber organization and cancer cell motility. Cancer Lett. 2016;372:210–8. [DOI] [PubMed] [Google Scholar]
- 79.Jesnowski R, Furst D, Ringel J, et al. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest. 2005;85:1276–91. [DOI] [PubMed] [Google Scholar]
- 80.Lachowski D, Cortes E, Pink D, et al. Substrate rigidity controls activation and durotaxis in pancreatic stellate cells. Sci Rep. 2017;7:2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal. 2008;1:pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klingberg F, Chow ML, Koehler A, et al. Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J Cell Biol. 2014;207:283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wipff PJ, Rifkin DB, Meister JJ, et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarper M, Cortes E, Lieberthal TJ, et al. ATRA modulates mechanical activation of TGF-beta by pancreatic stellate cells. Sci Rep. 2016;6:27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calvo F, Ege N, Grande-Garcia A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laklai H, Miroshnikova YA, Pickup MW, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elosegui-Artola A, Andreu I, Beedle AEM, et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397 e14–1410 e14. [DOI] [PubMed] [Google Scholar]
- 88.Hu C, Yang J, Su HY, et al. Yes-associated protein 1 plays major roles in pancreatic stellate cell activation and fibroinflammatory responses. Front Physiol. 2019;10:1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao Y, Zhang H, Ma Q, et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation. Cancer Lett. 2019;462:51–60. [DOI] [PubMed] [Google Scholar]
- 90.Morvaridi S, Dhall D, Greene MI, et al. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci Rep. 2015;5:16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fels B, Nielsen N, Schwab A. Role of TRPC1 channels in pressure-mediated activation of murine pancreatic stellate cells. Eur Biophys J. 2016;45:657–70. [DOI] [PubMed] [Google Scholar]
- 92.Storck H, Hild B, Schimmelpfennig S, et al. Ion channels in control of pancreatic stellate cell migration. Oncotarget. 2017;8:769–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuntze A, Goetsch O, Fels B, et al. Protonation of piezo1 impairs cell-matrix interactions of pancreatic stellate cells. Front Physiol. 2020;11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ottonello S, Scita G, Mantovani G, et al. Retinol bound to cellular retinol-binding protein is a substrate for cytosolic retinoic acid synthesis. J Biol Chem. 1993;268:27133–42. [PubMed] [Google Scholar]
- 95.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–24. [DOI] [PubMed] [Google Scholar]
- 96.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–54. [PubMed] [Google Scholar]
- 97.McCarroll JA, Phillips PA, Santucci N, et al. Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao W, Jiang W, Shen J, et al. Retinoic acid ameliorates pancreatic fibrosis and inhibits the activation of pancreatic stellate cells in mice with experimental chronic pancreatitis via suppressing the Wnt/beta-catenin signaling pathway. PLoS ONE. 2015;10:e0141462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jaster R, Hilgendorf I, Fitzner B, et al. Regulation of pancreatic stellate cell function in vitro: biological and molecular effects of all-trans retinoic acid. Biochem Pharmacol. 2003;66:633–41. [DOI] [PubMed] [Google Scholar]
- 100.Kim N, Yoo W, Lee J, et al. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut. 2009;58:1382–90. [DOI] [PubMed] [Google Scholar]
- 101.Chronopoulos A, Robinson B, Sarper M, et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun. 2016;7:12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baumert JT, Sparmann G, Emmrich J, et al. Inhibitory effects of interferons on pancreatic stellate cell activation. World J Gastroenterol. 2006;12:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fitzner B, Brock P, Nechutova H, et al. Inhibitory effects of interferon-gamma on activation of rat pancreatic stellate cells are mediated by STAT1 and involve down-regulation of CTGF expression. Cell Signal. 2007;19:782–90. [DOI] [PubMed] [Google Scholar]
- 104.Rateitschak K, Karger A, Fitzner B, et al. Mathematical modelling of interferon-gamma signalling in pancreatic stellate cells reflects and predicts the dynamics of STAT1 pathway activity. Cell Signal. 2010;22:97–105. [DOI] [PubMed] [Google Scholar]
- 105.Shen J, Gao J, Chen C, et al. Antifibrotic role of chemokine CXCL9 in experimental chronic pancreatitis induced by trinitrobenzene sulfonic acid in rats. Cytokine. 2013;64:382–94. [DOI] [PubMed] [Google Scholar]
- 106.Jaster R, Sparmann G, Emmrich J, et al. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yan Z, Ohuchida K, Fei S, et al. Inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis. J Exp Clin Cancer Res. 2019;38:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohnishi H, Miyata T, Yasuda H, et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem. 2004;279:8873–8. [DOI] [PubMed] [Google Scholar]
- 109.Xu XF, Liu F, Xin JQ, et al. Respective roles of the mitogen-activated protein kinase (MAPK) family members in pancreatic stellate cell activation induced by transforming growth factor-beta1 (TGF-beta1). Biochem Biophys Res Commun. 2018;501:365–73. [DOI] [PubMed] [Google Scholar]
- 110.Yoshida S, Yokota T, Ujiki M, et al. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Biophys Res Commun. 2004;323:1241–5. [DOI] [PubMed] [Google Scholar]
- 111.Jaster R, Brock P, Sparmann G, et al. Inhibition of pancreatic stellate cell activation by the hydroxymethylglutaryl coenzyme A reductase inhibitor lovastatin. Biochem Pharmacol. 2003;65:1295–303. [DOI] [PubMed] [Google Scholar]
- 112.Hama K, Ohnishi H, Yasuda H, et al. Angiotensin II stimulates DNA synthesis of rat pancreatic stellate cells by activating ERK through EGF receptor transactivation. Biochem Biophys Res Commun. 2004;315:905–11. [DOI] [PubMed] [Google Scholar]
- 113.Masamune A, Satoh M, Kikuta K, et al. Inhibition of p38 mitogen-activated protein kinase blocks activation of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2003;304:8–14. [DOI] [PubMed] [Google Scholar]
- 114.Masamune A, Kikuta K, Suzuki N, et al. A c-Jun NH2-terminal kinase inhibitor SP600125 (anthra[1,9-cd]pyrazole-6 (2H)-one) blocks activation of pancreatic stellate cells. J Pharmacol Exp Ther. 2004;310:520–7. [DOI] [PubMed] [Google Scholar]
- 115.Masamune A, Kikuta K, Satoh M, et al. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312:651–8. [DOI] [PubMed] [Google Scholar]
- 116.Kikuta K, Masamune A, Satoh M, et al. Hydrogen peroxide activates activator protein-1 and mitogen-activated protein kinases in pancreatic stellate cells. Mol Cell Biochem. 2006;291:11–20. [DOI] [PubMed] [Google Scholar]
- 117.Kikuta K, Masamune A, Satoh M, et al. 4-hydroxy-2, 3-nonenal activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. World J Gastroenterol. 2004;10:2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.An W, Zhu JW, Jiang F, et al. Fibromodulin is upregulated by oxidative stress through the MAPK/AP-1 pathway to promote pancreatic stellate cell activation. Pancreatology. 2020;20:278–87. [DOI] [PubMed] [Google Scholar]
- 119.Masamune A, Kikuta K, Satoh M, et al. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol. 2003;140:1292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whatcott CJ, Ng S, Barrett MT, et al. Inhibition of ROCK1 kinase modulates both tumor cells and stromal fibroblasts in pancreatic cancer. PLoS ONE. 2017;12:e0183871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masamune A, Watanabe T, Kikuta K, et al. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G99–108. [DOI] [PubMed] [Google Scholar]
- 122.Asaumi H, Watanabe S, Taguchi M, et al. Externally applied pressure activates pancreatic stellate cells through the generation of intracellular reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2007;293:G972–8. [DOI] [PubMed] [Google Scholar]
- 123.Xia D, Halder B, Godoy C, et al. NADPH oxidase 1 mediates caerulein-induced pancreatic fibrosis in chronic pancreatitis. Free Radic Biol Med. 2020;147:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ryu GR, Lee E, Chun HJ, et al. Oxidative stress plays a role in high glucose-induced activation of pancreatic stellate cells. Biochem Biophys Res Commun. 2013;439:258–63. [DOI] [PubMed] [Google Scholar]
- 125.Ohnishi N, Miyata T, Ohnishi H, et al. Activin A is an autocrine activator of rat pancreatic stellate cells: potential therapeutic role of follistatin for pancreatic fibrosis. Gut. 2003;52:1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aoki H, Ohnishi H, Hama K, et al. Autocrine loop between TGF-beta1 and IL-1β eta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2006;290:C1100–8. [DOI] [PubMed] [Google Scholar]
- 127.Aoki H, Ohnishi H, Hama K, et al. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem. 2006;99:221–8. [DOI] [PubMed] [Google Scholar]
- 128.He J, Sun X, Qian KQ, et al. Protection of cerulein-induced pancreatic fibrosis by pancreas-specific expression of Smad7. Biochim Biophys Acta. 2009;1792:56–60. [DOI] [PubMed] [Google Scholar]
- 129.Kuang C, Xiao Y, Liu X, et al. In vivo disruption of TGF-beta signaling by Smad7 leads to premalignant ductal lesions in the pancreas. Proc Natl Acad Sci USA. 2006;103:1858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao X, Cao Y, Staloch DA, et al. Bone morphogenetic protein signaling protects against cerulein-induced pancreatic fibrosis. PLoS ONE. 2014;9:e89114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao X, Cao Y, Yang W, et al. BMP2 inhibits TGF-beta-induced pancreatic stellate cell activation and extracellular matrix formation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Staloch D, Gao X, Liu K, et al. Gremlin is a key pro-fibrogenic factor in chronic pancreatitis. J Mol Med (Berl). 2015;93:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis+ Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–94. [DOI] [PubMed] [Google Scholar]
- 134.Masamune A, Kikuta K, Satoh M, et al. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302:36–42. [DOI] [PubMed] [Google Scholar]
- 135.Hu R, Wang YL, Edderkaoui M, et al. Ethanol augments PDGF-induced NADPH oxidase activity and proliferation in rat pancreatic stellate cells. Pancreatology. 2007;7:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCarroll JA, Phillips PA, Park S, et al. Pancreatic stellate cell activation by ethanol and acetaldehyde: is it mediated by the mitogen-activated protein kinase signaling pathway+ Pancreas. 2003;27:150–60. [DOI] [PubMed] [Google Scholar]
- 137.Siech M, Zhou Z, Zhou S, et al. Stimulation of stellate cells by injured acinar cells: a model of acute pancreatitis induced by alcohol and fat (VLDL). Am J Physiol Gastrointest Liver Physiol. 2009;297:G1163–71. [DOI] [PubMed] [Google Scholar]
- 138.Vonlaufen A, Phillips PA, Xu Z, et al. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut. 2011;60:238–46. [DOI] [PubMed] [Google Scholar]
- 139.Li Z, Zhang X, Jin T, et al. Nicotine promotes activation of human pancreatic stellate cells through inducing autophagy via alpha7nAChR-mediated JAK2/STAT3 signaling pathway. Life Sci. 2020;243:117301. [DOI] [PubMed] [Google Scholar]
- 140.Xue J, Zhao Q, Sharma V, et al. Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis. Gastroenterology. 2016;151:1206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuno A, Yamada T, Masuda K, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–9. [DOI] [PubMed] [Google Scholar]
- 142.Yamada T, Kuno A, Masuda K, et al. Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther. 2003;307:17–23. [DOI] [PubMed] [Google Scholar]
- 143.Nagashio Y, Asaumi H, Watanabe S, et al. Angiotensin II type 1 receptor interaction is an important regulator for the development of pancreatic fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G170–7. [DOI] [PubMed] [Google Scholar]
- 144.Liu WB, Wang XP, Wu K, et al. Effects of angiotensin II receptor antagonist, Losartan on the apoptosis, proliferation and migration of the human pancreatic stellate cells. World J Gastroenterol. 2005;11:6489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reinehr R, Zoller S, Klonowski-Stumpe H, et al. Effects of angiotensin II on rat pancreatic stellate cells. Pancreas. 2004;28:129–37. [DOI] [PubMed] [Google Scholar]
- 146.Trasino SE, Tang XH, Jessurun J, et al. Obesity leads to tissue, but not serum vitamin A deficiency. Sci Rep. 2015;5:15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ben-Harosh Y, Anosov M, Salem H, et al. Pancreatic stellate cell activation is regulated by fatty acids and ER stress. Exp Cell Res. 2017;359:76–85. [DOI] [PubMed] [Google Scholar]
- 148.Ko SH, Hong OK, Kim JW, et al. High glucose increases extracellular matrix production in pancreatic stellate cells by activating the renin-angiotensin system. J Cell Biochem. 2006;98:343–55. [DOI] [PubMed] [Google Scholar]
- 149.Hong OK, Lee SH, Rhee M, et al. Hyperglycemia and hyperinsulinemia have additive effects on activation and proliferation of pancreatic stellate cells: possible explanation of islet-specific fibrosis in type 2 diabetes mellitus. J Cell Biochem. 2007;101:665–75. [DOI] [PubMed] [Google Scholar]
- 150.Nomiyama Y, Tashiro M, Yamaguchi T, et al. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas. 2007;34:364–72. [DOI] [PubMed] [Google Scholar]