Abstract
(R)-9-[4-Hydroxy-2-(hydroxymethy)butyl]guanine (H2G) is a potent and selective inhibitor of herpesvirus replication. It is a nucleoside analog, and its triphosphate derivative (H2G-TP) is a competitive inhibitor of herpesvirus DNA polymerases. In this study, the antiviral activities of H2G and acyclovir (ACV) and the development of viral resistance to these agents were compared in varicella-zoster virus (VZV)-infected cells. In plaque reduction assays, the 50% effective concentration of H2G for VZV was 60- to 400-fold lower than that of ACV, depending on the virus strain and the cell line tested. The enhanced efficacy of H2G against VZV can be accounted for in part by the fact that the intaracellular H2G-TP level (>170 pmol/106 cells) is higher than the intracellular ACV-TP level (<1 pmol/106 cells). In addition, H2G-TP has extended half-lives of 3.9 and 8.6 h in VZV-infected MRC-5 and MeWo cells, respectively. To assess the emergence of H2G-resistant VZV in vitro, VZV was passaged in the presence of increasing concentrations of H2G. Earlier in the passage, when the concentration of H2G was relatively low, the predominant variant had the (A)76 deletion in the viral thymidine kinase (TK) gene. This mutant was identical to an ACV-resistant mutant generated in parallel experiments. However, higher concentrations of H2G appeared to favor a novel mutant, which had deletions of two consecutive nucleotides at positions 805 and 806 of the TK gene. All of these changes introduced frameshift mutations in the TK gene resulting in the expression of truncated polypeptides. H2G-resistant viruses were cross-resistant to ACV, and vice versa.
Varicella-zoster virus (VZV), a member of the herpesvirus family, is the etiologic agent of two distinct diseases, varicella (chicken pox) and shingles (herpes zoster). Chicken pox, caused by primary infection of the host, is a benign disease in healthy children. However, the reactivation of the virus, usually associated with aging and immunosuppression, produces herpes zoster, which is characterized by localized rash and pain. Some herpes zoster patients develop the serious neurologic complication of postherpetic neuralgia (PHN), which is a debilitating and severe chronic pain that can last for months. Three antiviral drugs (acyclovir [ACV], valacyclovir, and famciclovir) are indicated for the treatment of zoster, but their effectiveness in preventing or treating PHN remains unsatisfactory. Clearly, there exists a significant need for an antiviral agent with superior activity against VZV that may lead to improved efficacy in controlling zoster and its consequences over that of current therapies.
(R)-9-[4-Hydroxy-2-(hydroxymethy)butyl]guanine (H2G; USAN, omaciclovir) is a nucleoside analog with in vitro inhibitory activity against VZV, herpes simplex virus types 1 and 2 (HSV-1 and -2), Epstein-Barr virus, and human herpesvirus 6 (1, 4, 5, 15). It is more active against VZV than the currently approved agents, ACV and penciclovir (PCV) (1, 5, 15). H2G is also efficacious in simian varicella virus-infected monkeys, currently the best model for predicting efficacy against VZV-related disease in humans (23). H2G is selectively phosphorylated within VZV-infected cells by the viral thymidine kinase (TK) to a monophosphorylated derivative, H2G-MP (3). Subsequent phosphorylation of H2G-MP is thought to be carried out by cellular enzymes producing H2G-triphosphate (H2G-TP), which is an effective inhibitor of VZV DNA polymerase (2, 15). The mode of action of H2G is thus similar to those of ACV and PCV, which require conversion to their triphosphates for potent anti-VZV activity. A prodrug of H2G enhancing its oral bioavailability has given promising results in a phase II clinical trial in zoster patients and is being developed by Medivir AB (Huddinge, Sweden) (unpublished data).
In this study, we compared the anti-VZV activity of H2G with that of ACV in both MeWo and MRC-5 cells. We found that H2G was much more effective than ACV in inhibiting the growth of VZV. The 50% effective concentration (EC50) of H2G for VZV was markedly lower in infected MeWo cells than in infected MRC-5 cells. We also describe the first report of the isolation and characterization of VZV mutants generated by serial passage of the virus in increasing concentrations of H2G. Some of the H2G-resistant mutants had TK mutations identical to those found in ACV-resistant VZV generated in parallel experiments, while other H2G-resistant mutants had TK mutations not reported before.
MATERIALS AND METHODS
Antiviral compounds and radiochemicals.
H2G and ACV were synthesized at Abbott Laboratories. [8-3H]H2G (7.4 Ci/mmol) was prepared by tritiation of unlabeled H2G by Moravek Biochemicals (Brea, Calif.) and had a radiochemical purity of 99.9%. [8-3H]ACV (30 Ci/mmol) was also obtained from Moravek Biochemicals, and its radiochemical purity was 99.8%.
Cells and viruses.
Human melanoma cells (MeWo) were grown in minimum essential medium supplemented with 2 mM glutamine, 1% nonessential amino acids, antibiotics, and 10% fetal bovine serum (12). MRC-5 and Vero cells were obtained from the American Type Culture Collection and propagated according to recommended conditions. VZV-32 was isolated from a child with chicken pox (12). Both MeWo cells and VZV-32 were kindly supplied by Charles Grose (University of Iowa). VZ11 and VZ30 were gifts of Medivir AB. They were isolated from patients at the Swedish Institute for Disease Control. Two other VZV strains (Molly and Emily) were isolated from patients and kindly supplied by Jeffrey Cohen (National Institutes of Health [NIH]). HSV-1(F) and HSV-1(F)Δ305 were kindly supplied by Bernard Roizman (University of Chicago) (10, 21). HSV-2(G) was obtained from the American Type Culture Collection.
Determination of antiviral activity in vitro.
Plaque reduction assays with VZV were performed with MeWo or MRC-5 cells in 25-cm2 culture flasks by modification of a method described previously (12). Briefly, subconfluent monolayers of MeWo (5 × 106) or MRC-5 (1.5 × 106) cells were infected with approximately 100 PFU of cell-associated VZV from virus stocks prepared in the respective cell lines. After adsorption for 2 h at 37°C, 5 ml of media containing different concentrations of H2G or ACV in duplicate were added to the infected cells. The infected MeWo and MRC-5 cells were incubated in a CO2 incubator at 34°C for 4 to 5 and 6 days, respectively, fixed, and stained with crystal violet. Plaque reduction assays with HSV-1 and HSV-2 were performed with infected Vero cells as described previously (16). The EC50 is the concentration of drug that gives 50% inhibition of plaque formation.
Determination of cellular toxicity in MeWo and Vero cells.
Cytotoxicity was determined by a protocol modified from a previously published method (20). Subconfluent MeWo or Vero cells were incubated in 96-well plates with increasing concentrations of H2G or ACV in ½-log increments in triplicate. MeWo cells were incubated with the drug for 4 days at 34°C, and Vero cells were incubated with the drug for 2 days at 37°C, which were the conditions used for the plaque reduction assays on the respective cell lines. Cell controls were incubated with no drug. A stock solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma), at a concentration of 3 mg/ml in phosphate-buffered saline (PBS) for MeWo cells or 2 mg/ml for Vero cells, was added to all wells at 25 μl per well. Plates were further incubated for 3 to 4 h, and each well was then treated with 50 μl of a solution containing 20% sodium dodecyl sulfate (SDS) and 0.02 N HCl. After an overnight incubation, optical density was measured by reading the plates at wavelengths of 570 and 650 nm on a Bio-Tek microtiter plate reader.
Analyses of intracellular H2G and ACV metabolites.
To determine the accumulation of H2G and ACV metabolites in VZV-infected MeWo cells, cells grown on 6-well plates were either mock infected or infected with VZV-32 at an input ratio of 1:0.3 (ratio of uninfected cells to VZV-infected MeWo cells exhibiting 60 to 70% cytopathic effect [CPE]) and allowed to adsorb for 2 h at 37°C. The infected cells were washed and incubated at 34°C in fresh medium for another 2 h. The medium was then replaced with medium containing 5 μM [3H]H2G or [3H]ACV, and the plates were incubated further at 34°C. Additional plates, containing no radioactive medium, were used for cell counts. At each time point, the medium was removed and the cells were washed twice with PBS before they were trypsinized and washed in PBS again. The trypsinized cells were centrifuged and extracted with phosphate-buffered methanol for at least 2 h at −20°C. After extraction, the soluble extract was collected and the cell debris was solubilized in Laemmli sample buffer (62.5 mM Tris-HCl [pH 6.8], 25% glycerol, 2% SDS, and 0.01% bromophenol blue). A portion of the extract and cell debris was counted in a scintillation counter to monitor the efficiency of extraction. Extraction efficiency was usually more than 95%. After the removal of the methanol by speed vacuuming, the extracts were lyophilized to dryness and stored at −80°C until analyzed. To investigate the stability of phosphorylated H2G during processing, a fresh infected-cell extract was aliquoted into different samples. The amount of phosphorylated H2G in aliquots without processing was compared with that in aliquots that had been processed by speed vacuuming, lyophilization, and reconstitution. To investigate the stability of phosphorylated H2G during storage, a fresh infected-cell extract was aliquoted into different samples and processed as described above. The amount of phosphorylated H2G in aliquots without storage was compared with that in aliquots that had been stored at −80°C for 1, 2, 5, 12, or 15 days. These control experiments showed that there was no apparent loss of phosphorylated H2G species during the processing and storage of lyophilized extracts for as long as 15 days at −80°C. The lyophilized samples were usually analyzed after less than a week of storage at −80°C.
To determine the intracellular half-life of H2G-TP in VZV-infected MeWo or MRC-5 cells, infection was carried out as described above with inoculum prepared from viral stocks of VZV-32-infected MeWo or MRC-5 cells. At 4 h postinfection, the infected cells were labeled for 20 h with medium containing 5 μM [3H]H2G. The medium was then removed, and the cells were washed twice with 4 ml of prewarmed PBS. The infected cells were subsequently incubated with fresh medium. The medium was changed every hour to minimize the reanabolism of nucleosides which might have been released from the infected cells into the medium. Intracellular metabolites were extracted from infected cultures at 2-h intervals with phosphate-buffered methanol as described above.
To compare the metabolism of H2G in cells infected with wild-type VZV with that in cells infected with the purified VZV mutant viruses, MeWo cells grown on 6-well plates were first inoculated with 5 × 104 PFU of cell-associated VZV and then labeled for 20 h and harvested as described above.
To analyze the extracts by high-pressure liquid chromatography (HPLC), the lyophilized sample was resuspended in column buffer A (5 mM tetrabutylammonium chloride [TBACl] –112.5 μM triethylamine [TEA] [pH 7.4]) with 1 mM GTP as an internal standard. Samples were analyzed by gradient HPLC using a Phenomenex Lichrospher 5 RP-18e column (125 by 4.0 mm) at 1 ml/min. Elution was achieved from a gradient of 100% buffer A to 80% buffer A–20% buffer B (5 mM TBACl–112.5 μM TEA–50% acetonitrile [ACN] [pH 7.4]) over 10 min, continuing to 100% buffer B over a further 25 to 35 min. This was followed by a 35-min wash alternating between 100% distilled H2O and 100% ACN and reequilibration with 100% buffer A prior to the next injection.
The radioactivity of the eluent and the UV absorbance of the internal standard were analyzed by continuous on-line radiochemical detection using a Packard Radiomatic Flow Scintillation Analyzer and mixing with Packard Ultima Flo M scintillation fluid. Results were expressed as picomoles of nucleotide per 106 cells.
Retention times of H2G and ACV derivatives in the extracts were identified by HPLC peak comparison to the di- and triphosphate compounds synthesized enzymatically from H2G-MP or ACV-MP (Moravek Biochemicals) using a previously described method (15). The retention times and masses of authentic H2G and ACV derivatives were determined using a combination of reverse-phase ion-pair HPLC and atmospheric pressure chemical ionization (APCI) tandem mass spectrometry. For the H2G derivatives, retention times and masses were determined to be 15.3 min for H2G-MP with the [M + 1] ion at m/z 333; 20.9 min for H2G-DP with the [M − 1] ion at m/z 412 and the [M + TBA − 1] ion at m/z 654; and 24.6 min for H2G-TP with the [M + TBA − 1] ion at m/z 734. For the ACV derivatives, retention times and masses were 21.6 min for ACV-DP with the [M − 1] and [M + TBA − 1] ions at m/z 385 and 626, respectively, and 25.0 min for ACV-TP with the [M − 1] ion at m/z 465 and the [M + TBA − 1] ion at m/z 707.
Generation of VZV resistant to H2G and ACV by in vitro passage.
Parallel cultures of MeWo cells were infected with 6,500 PFU of cell-associated VZV-32 for 2 h and subsequently incubated in the presence of H2G or ACV, each at a concentration twice the EC50 (0.03 μM H2G and 13 μM ACV). Viral replication was monitored by the observation of CPE in the infected cultures. On the 4th day postinfection, when the CPE exceeded 50 to 60% of the infected cells, the infected cells were trypsinized, aliquoted, and stored in liquid nitrogen. The passaged virus was amplified once at the same drug concentration by infecting fresh cells with one aliquot of the infected cells. The amplified virus was harvested as above for further drug selection or determination of viral DNA sequences. To continue the selection, the virus was serially passaged and amplified in concentrations twice the previous drug concentrations. Selection was carried out for a total of 11 passages for H2G and 6 passages for ACV. Viral stocks of selected passages were titered on MeWo cells, and their susceptibilities to H2G and ACV were determined using a plaque reduction assay on MeWo cells as described above. To obtain purified drug-resistant VZV mutants, individual viruses were plaque purified at least twice in the absence of drug from passage 6 (P6) of ACV selection and P11 of H2G selection, according to a previously published method (12).
DNA sequence analyses.
To examine the VZV TK coding regions from viruses in selected passages of the selection, DNA from VZV-infected cells was first extracted with a QIAamp Blood Kit (Qiagen) according to the manufacturer's instructions. The entire VZV TK coding sequence was PCR amplified from DNA extracted from infected cells using the forward primer 1 (5′ CCG TCT AGA CAA GAC GCG TTT GTC TAC A 3′) and the reverse primer 2 (5′ GCA CTC GAG ACA GGC TTG GCG GCT TT 3′). All PCRs were performed for 30 cycles under the following conditions: melting at 94°C for 30 s, annealing at 55°C for 1 min, and extension at 72°C for 2 min. The resulting PCR product was ligated into the TA vector pCR 2.1-TOPO (Invitrogen), and the ligation product was used to transform competent TOP10F′ cells. Plasmid DNA from the resulting white colonies was isolated and purified using a Wizard Plus Miniprep Kit (Promega) or a QIAprep Spin Miniprep Kit (Qiagen). VZV TK genes from multiple clones were sequenced by the dideoxy-chain termination method in an automated DNA sequencer using the forward primers 3 (5′ CAC CAC TTT GCA ATA ACA CC 3′) and 4 (5′ GGG ACC AAC TTG GTA GTT TGT ACC G 3′) and the reverse primers 5 (5′ AAC ACG TAC ACG CGA GTA TGA CAA 3′) and 6 (5′ ATA ACC CAG GAA GCG CCG CTG GGG 3′).
DNA from wild-type VZV- as well as mutant VZV-infected cells was extracted as described above to analyze the sequences of both the TK and DNA polymerase genes. The TK gene was PCR amplified from the DNA extract and sequenced using the primers described above. The DNA polymerase gene was PCR amplified into two different products due to its large size (approximately 3.6 kb). One-half of the polymerase gene was PCR amplified to a product (Pol PCR1) of about 2 kb by using the forward primer pol #8 (5′ GCT AGT GGA CCG AAT ACA CG 3′) and the reverse primer pol #1 (5′ GAG ACT GTG GTG CCA TCC ATT G 3′). The other half of the polymerase gene was amplified to a product (Pol PCR2) of about 2.4 kb using the forward primer pol #16 (5′ CAA ATC AGA GTC CGT GCT ACG AGC 3′) and the reverse primer pol #7 (5′ CAA TAC GAC CAC CGG ATC G 3′). Both PCRs were performed for 30 cycles under the following conditions: melting at 94°C for 30 s, annealing at 55°C for 1 min, and extension at 72°C for 3 min. Pol PCR1 was sequenced by the dideoxy-chain termination method in an automated DNA sequencer using the following primers: pol #1 (described above), pol #2 (reverse primer; 5′ CAT AAG GGA TGC GTT CTC G 3′), pol #3 (forward primer; 5′ CAT AAA CCG TCG CTT GGC TC 3′), pol #4 (reverse primer; 5′ CTC GCC GAT TTT AGC TAT CC 3′), pol #5 (forward primer; 5′ ATG GAG ATA CGG ATT CTG TG 3′), pol #6 (forward primer; 5′ CCT TTA CAG TTG GAG GAA AAC G 3′), pol #7 (reverse primer; 5′ CAA TAC GAC CAC CGG ATC G 3′), and pol #8 (described above). Pol PCR2 was sequenced in the same way using the following primers: pol #7 (described above), pol #8 (described above), pol #9 (reverse primer; 5′ CGT TGA TCT TTA CCT TGC TTC G 3′), pol #10 (forward primer; 5′ CAT CTG GAG GAT CTT GTA ATC C 3′), pol #11 (reverse primer; 5′ GAC TTG CCG GTC GAA CTC G 3′), pol #12 (forward primer; 5′ GCA TCT CCA GAA AGC TTT CG 3′), pol #13 (reverse primer; 5′ CCA AGC GGT CGT GTT GCA GTT GC 3′), pol #14 (forward primer; 5′ GAT GTG CCC ATG GAA GAA CG 3′), pol #15-1 (reverse primer; 5′ TTC TCT GTT ACT ACC GCG C 3′), pol #15-2 (reverse primer; 5′ CCG TTC TGA TCG CCA TTT-3′), and pol #16 (described above).
RESULTS
Antiviral activity of H2G against herpesviruses in vitro.
Previous studies from several laboratories have shown that H2G has potent activity against VZV in vitro (1, 5, 15). These assessments have usually been done using plaque reduction assays on human embryonic fibroblasts such as MRC-5 cells. Human melanoma (MeWo) cells, an excellent cell line in which to propagate VZV, have seldom been used to assay for the potency of antiviral compounds. It has been reported that the in vitro antiviral activity of a drug varies with the cell line tested (17). To determine if the inhibitory activity of H2G in VZV-infected MeWo cells was different from that in MRC-5 cells, the potencies of H2G in the two cell lines were compared in plaque reduction assays.
Table 1 summarizes the susceptibilities of a laboratory VZV strain (VZV-32) and several clinical VZV isolates (Molly, Emily, VZ11, and VZ30) to H2G and ACV in MRC-5 and MeWo cells. The EC50s of ACV and H2G in infected MRC-5 cells, similar to those published previously, were approximately 40 and 0.7 μM, respectively (15). However, the antiviral activities of both compounds for VZV were much higher in infected MeWo cells than in infected MRC-5 cells, with EC50s ranging from 0.015 to 0.048 μM for H2G and from 6.4 to 16 μM for ACV. The relative potency improvements observed for H2G versus ACV varied from approximately 60-fold in MRC-5 cells to more than 300-fold in MeWo cells.
TABLE 1.
Antiviral activities of H2G and ACV against different human herpesviruses
| Cell line | Virus and strain | EC50 (μM)b
|
|
|---|---|---|---|
| ACV | H2G | ||
| MRC-5 | VZV | ||
| VZV-32 | 37.3 ± 5.2 | 0.72 ± 0.1 | |
| Molly | 41.6 ± 8.5 | 0.62 ± 0.2 | |
| MeWo | VZV | ||
| VZV-32 | 6.4 ± 1.9 | 0.015 ± 0.004 | |
| Molly | 14.6 ± 4.9 | 0.048 ± 0.023 | |
| Emily | 16.0 ± 2.4 | 0.047 ± 0.004 | |
| VZ11 | 9.5 ± 3.0 | 0.035 ± 0.022 | |
| VZ30 | 7.0 ± 2.6 | 0.016 ± 0.003 | |
| Vero | HSV-1 | ||
| (F) (WTa) | 0.04 ± 0.016 | 0.08 ± 0.013 | |
| (F)Δ305 (TK−) | 22 ± 7.6 | 29 ± 17.4 | |
| HSV-2(G) (WT) | 0.34 ± 0.079 | 1.7 ± 0.1 | |
WT, wild type.
Determined by plaque reduction assays on VZV-, HSV-1-, or HSV-2-infected cells. Values are means ± standard errors from at least two separate experiments.
H2G was also evaluated against HSV-1 and HSV-2 in infected Vero cells (Table 1). H2G showed a potency similar to that of ACV against HSV-1, but less potency against HSV-2. Previous studies done on TK-deficient VZV strains indicated that a functional viral TK was necessary in order to achieve the potent antiviral effect seen with H2G in vitro (1). In this study, we tested a TK-deficient HSV-1 mutant, HSV-1(F)Δ305, which has a genetically engineered deletion in the viral TK gene (21). As expected, HSV-1(F)Δ305 had a much higher EC50 than its wild-type parent virus, HSV-1(F).
The cytotoxic effects of H2G and ACV were examined using uptake of the tetrazolium dye MTT. The 50% cytotoxic concentrations (CC50s) of H2G and ACV were more than 1,000 μM in MeWo cells, while the CC50 of H2G was more than 500 μM in Vero cells. All of these concentrations were the highest drug concentrations tested in the respective cells.
Accumulations of H2G-TP and ACV-TP in VZV-infected MeWo cells.
H2G-TP is an effective inhibitor of VZV DNA polymerase (2). To establish the basis for the superior activity of H2G over ACV in inhibiting the replication of VZV in infected MeWo cells, the accumulations of H2G-TP and ACV-TP were determined. VZV-infected MeWo cells were labeled with [3H]H2G or [3H]ACV at 4 h postinfection, and the amount of H2G or ACV metabolites was determined at different time points postinfection by HPLC. HPLC analyses of the extracts found that the proportions of H2G-MP, -DP, and -TP at 20 h after labeling were approximately 1:3:40 in VZV-infected MeWo cells, whereas the proportions of ACV metabolites were not determined, as explained below.
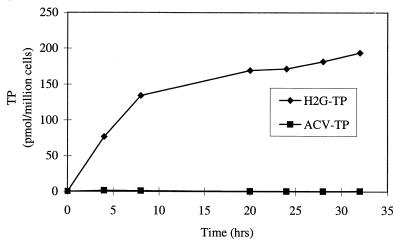
Figure 1 shows results of a representative experiment detecting the accumulations of H2G-TP and ACV-TP in VZV-infected MeWo cells. The accumulation of H2G-TP increased steadily during the first 8 h of labeling and slowly leveled off after 20 h of labeling, reaching a level of approximately 170 pmol/106 cells. Furthermore, even after 32 h of labeling, the amount of H2G-TP remained very high. In contrast to the high level of H2G-TP, the accumulation of ACV metabolites was very low at all time points, with the ACV-TP level usually less than 1 pmol/106 cells. Our limit of detection for ACV-TP was estimated to be 0.1 pmol. The low level of ACV-TP in VZV-infected cells was also reported by other studies (9, 15). However, when [3H]ACV was used to label HSV-2-infected MRC-5 cells in a control experiment, the ACV metabolites accumulated to substantial levels (data not shown).
FIG. 1.
Accumulation of H2G-TP and ACV-TP in VZV-infected MeWo cells. MeWo cells were infected with VZV and labeled with medium containing 5 μM [3H]H2G or [3H]ACV at 4 h postinfection. At the indicated time points postlabeling, infected cells were extracted and the samples were assayed by HPLC as described in Materials and Methods.
Intracellular stability of H2G-TP in VZV-infected MeWo and MRC-5 cells.
To determine if the high accumulation of H2G-TP is related to the intracellular half-life of H2G-TP, the half-lives of H2G-TP in VZV-infected MeWo and MRC-5 cells were determined. In these experiments, MeWo or MRC-5 cells were infected with the same input ratio of respective VZV-infected cells and then labeled with [3H]H2G for 20 h such that the initial triphosphate level was high enough to allow a determination (Fig. 1). After the labeling, the cells were washed, overlaid with fresh medium, and harvested at different time points after the removal of the medium containing [3H]H2G. The medium was changed on an hourly basis throughout the remainder of the experiment to reduce the reanabolism of the compound diffused out from the infected cells.
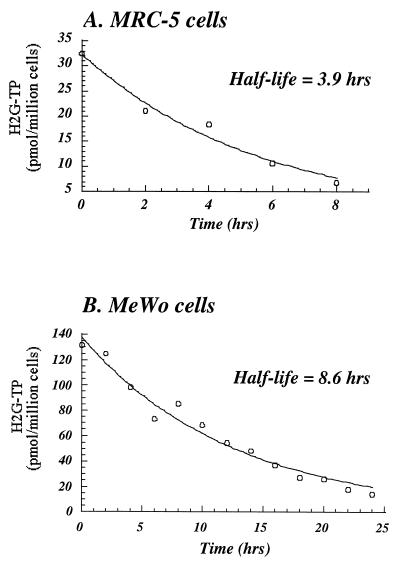
Figure 2A and B depict representative experiments examining the stability profiles of H2G-TP in infected MRC-5 and MeWo cells, respectively. The length of the experiments allowed the decay of H2G-TP to 2 half-lives (8 h) in MRC-5 cells (Fig. 2A) and 3 half-lives (24 h) in MeWo cells (Fig. 2B). To determine the half-lives of H2G-TP, the amount of H2G-TP was plotted against time using a first-order decay formula. In agreement with the results of a previously published study, the half-life of H2G-TP in infected MRC-5 cells was about 4 h (15). H2G-TP was more stable in infected MeWo cells, with a half-life of approximately 8.6 h. We were unable to determine the half-life of ACV-TP because of its low accumulation (Fig. 1).
FIG. 2.
Half-lives of H2G-TP in VZV-infected MRC-5 cells (A) and MeWo cells (B). Cells were infected with VZV and labeled with medium containing 5 μM [3H]H2G at 4 h postinfection. At 20 h postlabeling, the infected cells were washed and then incubated with fresh medium. At the indicated time points after the removal of the labeled medium, infected cells were extracted and the samples were assayed by HPLC as described in Materials and Methods. The half-lives of H2G-TP were calculated by a first-order decay formula.
In vitro selection of VZV resistant to H2G or ACV.
The mode of action of H2G is believed to be similar to that of ACV. The drug target can be identified by analyzing the gene in which mutations confer a resistance phenotype. To compare the mutations conferring resistance to H2G with those conferring resistance to ACV, we generated VZV variants resistant to H2G or ACV by passaging and amplifying wild-type VZV-32 in MeWo cells in the presence of increasing concentrations of each drug (Tables 2 and 3). VZV was initially grown and amplified in the presence of 13 μM ACV or 0.03 μM H2G (P1), which represented twice the EC50 of the respective drug. During subsequent passages, the concentrations of ACV and H2G were again increased to twice the previous drug selection concentrations. Six passages of ACV selection were carried out because sequencing data as described in detail below indicated that all of the 10 clones isolated from virus in P4 and P6 had mutations (Table 4). For the H2G selection, 11 passages of VZV were done, because even after all these passages, a small population of VZV still had wild-type TK sequences (Table 4).
TABLE 2.
In vitro selection and phenotypic susceptibility of VZV passaged in ACV
| Passage | ACV selection concn (μM) | EC50 (μM)a
|
|
|---|---|---|---|
| ACV | H2G | ||
| VZV-32 (WT)b | NAc | 6.4 ± 1.9 | 0.015 ± 0.004 |
| P1 | 13 | NDd | ND |
| P2 | 26 | 5.5 ± 2.6 | 0.024 ± 0.017 |
| P3 | 52 | 72 ± 5.9 | 5.1 ± 0.1 |
| P4 | 104 | 124 ± 26.2 | 118 ± 24.0 |
| P5 | 208 | ND | ND |
| P6 | 416 | 227 ± 51.6 | 207 ± 32.2 |
Determined by plaque reduction assays on VZV-infected MeWo cells. Values are means ± standard errors from at least two separate experiments.
WT, wild type.
NA, not applicable.
ND, not determined.
TABLE 3.
In vitro selection and phenotypic susceptibility of VZV passaged in H2G
| Passage | H2G selection concn (μM) | EC50 (μM)a
|
|
|---|---|---|---|
| ACV | H2G | ||
| VZV-32 (WT)b | NAc | 6.4 ± 1.9 | 0.015 ± 0.004 |
| P1 | 0.03 | NDd | ND |
| P2 | 0.06 | ND | ND |
| P3 | 0.12 | 9.2 ± 0.9 | 0.061 ± 0.016 |
| P4 | 0.24 | 59 ± 14.1 | 4.5 ± 0.7 |
| P5 | 0.48 | 128 ± 14.4 | 95 ± 8.7 |
| P6 | 0.96 | ND | ND |
| P7 | 1.92 | 206 ± 39.7 | 169 ± 4.9 |
| P8 | 3.84 | ND | ND |
| P9 | 7.68 | 137 ± 35.1 | 146 ± 52.3 |
| P10 | 15.36 | ND | ND |
| P11 | 30.72 | 182 ± 28.4 | 112 ± 28.6 |
Determined by plaque reduction assays on VZV-infected MeWo cells. Values are means ± standard errors from at least two separate experiments.
WT, wild type.
NA, not applicable.
ND, not determined.
TABLE 4.
Frequency of mutations in TK gene from VZV passaged in H2G or ACVa
| Selection drug | Viral passage | Frequency of TK gene mutation | TK mutationb |
|---|---|---|---|
| ACV | P3 | 9/26 | None |
| 17/26 | Deletion of A(76)b | ||
| P4 | 10/10 | Deletion of A(76) | |
| P6 | 10/10 | Deletion of A(76) | |
| H2G | P5 | 3/10 | None |
| 4/10 | Deletion of A(76) | ||
| 2/10 | Deletion of GA(805, 806) | ||
| 1/10 | Deletion of A(76), GA(805, 806) | ||
| P7 | 4/20 | None | |
| 4/20 | Deletion of A(76) | ||
| 11/20 | Deletion of GA(805, 806) | ||
| 1/20 | Deletion of A(76), GA(805, 806) | ||
| P9 | 3/9 | None | |
| 2/9 | Deletion of A(76) | ||
| 4/9 | Deletion of GA(805, 806) | ||
| P11 | 2/10 | None | |
| 2/10 | Deletion of A(76) | ||
| 5/10 | Deletion of GA(805, 806) | ||
| 1/10 | Deletion of A(76), GA(805, 806) |
The TK sequences of the clones were compared with that of wild-type VZV-32.
Number(s) in parentheses indicates nucleotide position.
Phenotypic evaluation of VZV passaged in H2G and ACV.
To monitor the appearance of drug-resistant virus, four viral passages (P2, P3, P4, and P6) from ACV selection and six viral passages (P3, P4, P5, P7, P9, and P11) from H2G selection were examined to determine their phenotypic susceptibilities to the selection drug and levels of cross-resistance to the other nucleoside analog (Tables 2 and 3). These passages were chosen to reflect the gradual generation of drug-resistant virus during the selection. As expected, viruses at the earlier passages, such as P2 in the ACV selection and P3 in the H2G selection, had drug susceptibilities similar to that of the wild-type virus. The susceptibility of the passaged virus decreased starting from P3 in the ACV selection and P4 in the H2G selection. The EC50 reached a maximum in P6 of ACV selection (Table 2). In H2G selection, the EC50 gradually reached a plateau by P7 (Table 3). As judged by their EC50s, mutant viruses selected in the presence of ACV were cross-resistant to H2G, and vice versa.
DNA sequence analyses of the TK coding region from selected passages.
The VZV TK gene is 1,023 nucleotides in length and encodes a polypeptide of 341 amino acids. VZV mutants that are resistant to nucleoside analogs usually harbor mutations in the TK gene (7, 26). Moreover, the resistance of a TK-deficient mutant to H2G as described above [HSV-1(F)Δ305] (Table 1) and by another study (1) strongly demonstrated the requirement for a functional TK in order for H2G to exert its antiviral effect. To determine whether the drug-resistant mutants generated in this study harbor mutations in the coding region of TK, the TK gene was PCR amplified from the viral DNA prepared from cells infected with wild-type VZV-32 or from a number of viral passages in the presence of drug. The amplified TK gene was then cloned into the TA vector pCR2.1-TOPO. TK sequences from individual bacterial colonies transformed with the plasmid were then sequenced. Using this protocol, the DNA sequence of the TK gene from wild-type VZV-32, completely matched the published TK sequence of VZV (Dumas strain), except for the nucleotide 863 change (C→T) that has been identified in most VZV strains sequenced to date (8). Sequences of the individual clones from drug selection were then compared with that of wild-type VZV-32. Table 4 lists the frequencies and positions of TK mutations of VZV at selected passages.
In the ACV selection, the deletion of the nucleotide A at position 76 emerged in P3 and was found in 17 of 26 clones. This mutation was present in all clones in P4 as well as in P6. This deletion created a frameshift mutation that introduced a stop codon at amino acid position 38, producing a highly truncated polypeptide. As in the H2G selection, three kinds of mutants were identified by P5. First of all, a mutant with a deletion of the nucleotide A at position 76, which was also found in the ACV selection, was present at a frequency of 4 out of 10 clones. A novel mutant with deletions of two consecutive nucleotides at positions 805 and 806 was observed in 2 out of 10 clones. A third mutant, which had deletions of both A(76) and GA(805, 806), was present in 1 of 10 clones. As the selection continued with higher drug concentrations, the relative percentage of the A(76) mutant decreased while that of the GA(805, 806) mutant increased, and the amount of the double mutant [A(76) GA(805, 806)] remained at a constant low level. Interestingly, in all the H2G passages for which sequencing was performed, 20 to 30% of VZV had the wild-type TK sequence.
Characterization of plaque-purified VZV mutants.
To confirm that the mutants carrying the TK mutations were truly resistant to ACV and/or H2G, individual viruses were plaque purified from the last passages of selection (P6 of ACV selection and P11 of H2G selection) and characterized phenotypically and genotypically. Two viral mutants with the A(76) deletion and one mutant with the GA(805, 806) deletion in the TK gene were isolated from the ACV and H2G selection. The sequences of the DNA polymerase genes in all of these purified mutant viruses and the wild-type virus (VZV-32) were determined and compared with the sequence (Dumas strain) published by Davison and Scott (8) to ensure that there was no mutation in the polymerase genes.
To study whether the metabolism of H2G in cells infected with these purified mutant viruses was different from that in cells infected with the wild-type virus, the intracellular H2G-TP concentration in infected cells labeled with [3H]H2G was determined. After 20 h of labeling, the level of H2G-TP in cells infected with all the mutant viruses was similar to that in the mock-infected cells (<1 pmol/106 cells), while that in cells infected with the wild-type virus was similar to the level (time = 20 h) shown in Fig. 1 (about 150 pmol/106 cells).
The susceptibilities of these purified mutant viruses to both drugs were also determined (Table 5). Their EC50s clearly showed that they were resistant to the drug they were selected against and also cross-resistant to the other nucleoside analog. The A(76) mutant, which was truncated close to the N terminus of TK, was more resistant to both H2G and ACV than the GA(805, 806) mutant, which was truncated close to the C terminus.
TABLE 5.
Antiviral activities of H2G and ACV against plaque-purified VZV mutants
| Plaque-purified virus | Mutation | EC50 (μM)a
|
|
|---|---|---|---|
| ACV | H2G | ||
| VZV-32 (WT)b | None | 7.5 ± 1.3 | 0.018 ± 0.006 |
| ACV-P6-1 | Deletion of A(76)c | 513 ± 116.5 | 413 ± 81.9 |
| ACV-P6-3 | Deletion of A(76) | 465 ± 21.2 | 485 ± 49.5 |
| H2G-P11-13 | Deletion of GA(805, 806) | 127 ± 12.9 | 156 ± 37.5 |
Determined by plaque reduction assays on VZV-infected MeWo cells. Values are means ± standard errors from at least two separate experiments.
WT, wild type.
Number(s) within parentheses indicates nucleotide position(s).
DISCUSSION
H2G is more active than ACV against VZV in vitro. Although ACV-TP is a more potent inhibitor of VZV DNA polymerase than H2G-TP (2), the results presented in this report and by Lowe et al, (15) suggest that H2G-TP in VZV-infected cells reaches sufficiently high levels to yield a more effective inhibition of this important viral enzyme. These results underscore the variety of parameters that contribute to the enhanced antiviral efficacy observed with H2G relative to ACV in VZV-infected cells, e.g., a higher relative rate of H2G phosphorylation by the VZV TK (3, 15), higher intracellular H2G-TP concentrations (approximately 170 pmol/106 cells [Fig. 1]), and the extended intracellular half-life of H2G-TP (8.6 h [Fig. 2B]).
VZV can be readily propagated in vitro in only a very limited number of cell lines. Human embryonic fibroblasts such as MRC-5 cells are usually used in plaque reduction assays of VZV to determine the activities of different antiviral agents. MeWo cells were used in this study because of their excellent ability to support the growth of VZV (12). This unusual cell line was derived from human melanoma tumors. The melanoma cell is the neoplastic counterpart of the melanocyte, which is derived embryologically from the neural crest. This origin of derivation may have biological importance because of the preference of alphaherpesviruses, such as VZV, for growth in neuronal cells.
The antiviral activity of H2G was higher in VZV-infected MeWo cells than in VZV-infected MRC-5 cells (Table 1). Results from two of our experiments may provide some of the reasons for its superior activity in MeWo cells. A high level of H2G-TP was found to accumulate in infected MeWo cells even at 32 h postlabeling (i.e., 36 h postinfection) (Fig. 1). In a previous study, the accumulation of H2G-TP in infected MRC-5 cells was reported to reach a maximum concentration at about 20 h postinfection, and the amount of H2G-TP declined after this time point (15). The high and continuous accumulation of H2G-TP in infected MeWo cells might result from the extended intracellular half-life of H2G-TP in MeWo cells (8.6 h), which was considerably longer than that in MRC-5 cells (3.9 h) (Fig. 2).
The ACV-resistant VZV variant generated by in vitro selection in this study, namely, A(76), is identical to the clinical mutants V8811-4 and V8811-13 isolated from an AIDS patient (26). A VZV mutant with the same mutation was also found in the H2G selection (Table 4). This suggests that the nucleotide A at position 76 of the VZV TK gene is a mutation hot spot that confers resistance to nucleoside analogs such as ACV and H2G. This mutation hot spot is located within a stretch of 5 A's encoding part of the ATP binding site of TK as described by Talarico et al. (26). Incidentally, TK mutation hot spots in ACV-resistant HSV-1 and HSV-2 variants were mapped to homopolymer nucleotide stretches of G's and C's present in the TK coding sequence (13, 22). Homopolymer nucleotide stretches have been found to be very susceptible to frameshift mutations because these sequences, presumably, provide sites for misaligned but complementary base pairing or the viral DNA polymerase is more prone to slip or stutter at these sequences (6, 18, 24, 27). The deletion in the A(76) mutant created a frameshift in the open reading frame of TK and produced a highly truncated polypeptide. A clinical isolate harboring this mutation was severely impaired in its TK activity (26).
In the H2G selection, a novel TK mutant, GA(805, 806), was detected (Table 4). This mutation was never reported in ACV-resistant VZV variants generated in vitro or isolated from patients. The deletion of the two nucleotides changed the open reading frame of TK after the Gly-268 codon and introduced a stop codon at amino acid position 333, producing a truncated peptide with a C terminus different from that of the wild-type TK. Previous studies identified at least two ACV-resistant VZV mutants isolated from AIDS patients that had mutations at the C terminus of TK. The first mutant, 11H, had an insertion of 2 nucleotides at positions 889 and 890, changing codon 298 to a stop codon (7). The second mutant had a C-to-T substitution at position 907 of the TK gene, changing the glutamine codon into a stop codon at position 303 of TK (11). The loss or alteration of TK activity in these mutants indicated that the C terminus of VZV TK is very important to its activity. Indeed, the crystal structure of TK encoded by another member of the herpesvirus family, HSV-1, showed that a mutation at the C terminus could disrupt the three-dimensional structure of the whole enzyme active site (14). In spite of the lack of information about the three-dimensional structure of VZV TK, the mutations mapped to its C terminus by our study and other studies (7, 11, 25) identify regions that are crucial to the activity of this VZV enzyme.
Two kinds of evidence showed that the A(76) and GA(805, 806) mutants were deficient in TK activity. First, we have shown that the metabolism of H2G in cells infected with either the A(76) or the GA(805, 806) mutant was impaired. The great difference in the intracellular H2G-TP concentration between cells infected with the wild-type virus and cells infected with these mutant viruses indicated that these TK mutations altered the activity of TK. Second, the deficiency in TK activity in TK mutants harboring a mutation identical to that in the A(76) mutant or mutations at the C terminus of TK like that in the GA(805, 806) mutant has been demonstrated in biochemical (TK) assays (7, 11, 26).
Apparently, the GA(805, 806) mutant was more predominant than the A(76) mutant when the selection proceeded to higher concentrations of H2G (Table 4). It is not clear why the selection appeared to favor the growth of a mutant virus for which the EC50 was lower than it was for the A(76) mutant (Table 5), although the drug selection concentrations were still well below the EC50 for the GA(805, 806) mutant (Table 3). It is possible that the A(76) mutant appeared early in the selection process, because this deletion was easily generated in the mutation hot spot area, as discussed above. As the selection went on, the GA(805, 806) mutant prevailed because this particular mutant might be more stable than the A(76) mutant in the increasing concentrations of H2G.
In contrast to the total absence of VZV with the wild-type TK sequence by P4 in the ACV selection, a low percentage of VZV had the wild-type TK sequence in the H2G selection even by P11 (Table 4). We could not rule out the possibility that these viruses might have mutations in an area outside the TK coding sequence, e.g., the DNA polymerase gene. ACV-resistant VZV with mutations in the DNA polymerase gene has been described (19). Interestingly, Boivin et al. (7) also reported the isolation of an ACV-resistant VZV from an AIDS patient that did not have a mutation in the TK gene.
In conclusion, the in vitro selection and characterization of H2G-resistant VZV mutants provide some important information about the development of viral resistance to H2G. First, as in ACV mutants, a single mutation in the TK gene is sufficient to confer resistance to H2G. Second, during the course of selection with H2G in vitro, H2G selected the A(76) mutant, which was also found in the ACV selection. However, higher H2G selection concentrations favored the selection of the novel mutant GA(805, 806). All these mutations introduced frameshift mutations in the TK gene, resulting in the expression of truncated polypeptides. Third, H2G-resistant VZV is cross-resistant to ACV, and vice versa. The results presented in this study are important in the prediction of the mutation profile of VZV isolated from patients who develop resistance to H2G.
ACKNOWLEDGMENTS
We thank Charles Grose for MeWo cells and VZV-32, Medivir AB for VZV11 and VZV30, Jeffrey Cohen for VZV strains (Molly and Emily), and Bernard Roizman for HSV-1(F) and HSV-1(F)Δ305. We are grateful to Mike Tang for the mass spectroscopy studies and to Tim Middleton for critical reading of the manuscript.
REFERENCES
- 1.Abele G, Cox S, Bergman S, Lindborg B, Vissgarden A, Karlstrom A, Harmenberg J, Wahren B. Antiviral activity against VZV and HSV type 1 and type 2 of the (+) and (−) enantiomers of (R,S)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine, in comparison to other closely related acyclic nucleosides. Antivir Chem Chemother. 1991;2:163–169. [Google Scholar]
- 2.Abele G, Eriksson B, Harmenberg J, Wahren B. Inhibition of varicella-zoster virus-induced DNA polymerase by a new guanosine analog, 9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine triphosphate. Antimicrob Agents Chemother. 1988;32:1137–1142. doi: 10.1128/aac.32.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abele G, Karlstrom A, Harmenberg J, Shigeta S, Larsson A, Lindborg B, Wahren B. Inhibiting effect of (R,S)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine on varicella-zoster virus replication in cell culture. Antimicrob Agents Chemother. 1987;31:76–80. doi: 10.1128/aac.31.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akesson-Johansson A, Harmenberg J, Wahren B, Linde A. Inhibition of human herpesvirus 6 replication by 9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine (2HM-HBG) and other antiviral compounds. Antimicrob Agents Chemother. 1990;34:2417–2419. doi: 10.1128/aac.34.12.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrei G, Snoeck R, Reymen D, Liesnard C, Goubau P, Desmyter J, De Clercq E. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur J Clin Microbiol Infect Dis. 1995;14:318–328. doi: 10.1007/BF02116525. [DOI] [PubMed] [Google Scholar]
- 6.Baumann B, Potash M J, Kohler G. Consequences of frame-shift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985;4:351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin G, Edelman C K, Pedneault L, Talarico C L, Biron K K, Balfour H H., Jr Phenotypic and genotypic characterization of acyclovir-resistant varicella-zoster viruses isolated from persons with AIDS. J Infect Dis. 1994;170:68–75. doi: 10.1093/infdis/170.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 9.Earnshaw D L, Bacon T H, Darlison S J, Edmonds K, Perkins R M, Vere Hodge R A. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob Agents Chemother. 1992;36:2747–2757. doi: 10.1128/aac.36.12.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejercito P, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 11.Fillet A, Dumont B, Caumes E, Visse B, Agut H, Bricaire F, Huraux J. Acyclovir-resistant varicella-zoster virus: phenotypic and genetic characterization. J Med Virol. 1998;55:250–254. [PubMed] [Google Scholar]
- 12.Grose C, Brunell P A. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32°C. Infect Immun. 1978;19:199–203. doi: 10.1128/iai.19.1.199-203.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kit S, Sheppard M, Ichimura H, Nusinoff-Lehrman S, Ellis M N, Fyfe J A, Otsuka H. Nucleotide sequence changes in thymidine kinase gene of herpes simplex type 2 clones from an isolate of a patient treated with acyclovir. Antimicrob Agents Chemother. 1987;31:1483–1490. doi: 10.1128/aac.31.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kussmann-Gerber S, Kuonen O, Folkers G, Pilger B D, Scapozza L. Drug resistance of herpes simplex virus type 1: structural consideration at the molecular level of the thymidine kinase. Eur J Biochem. 1998;255:472–481. doi: 10.1046/j.1432-1327.1998.2550472.x. [DOI] [PubMed] [Google Scholar]
- 15.Lowe D M, Alderton W K, Ellis M R, Parmar V, Miller W H, Roberts G B, Fyfe J A, Gaillard R, Ertl P, Snowden W, Littler E. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng T I, Talarico C, Burnette T C, Biron K, Roizman B. Partial substitution of the functions of the herpes simplex virus 1 UL13 gene by the human cytomegalovirus UL97 gene. Virology. 1996;225:347–358. doi: 10.1006/viro.1996.0609. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien J J, Campoli-Richards D M. Acyclovir: an updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989;37:233–309. doi: 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]
- 18.Owen J E, Schultz D W, Taylor A, Smith G R. Nucleotide sequence of the lysozyme gene of bacteriophage T4. Analysis of mutations involving repeated sequences. J Mol Biol. 1983;165:229–248. doi: 10.1016/s0022-2836(83)80255-1. [DOI] [PubMed] [Google Scholar]
- 19.Pahwa S, Biron K, Lim W, Swenson P, Kaplan M H, Sadick N, Pahwa R. Continuous varicella-zoster infection associated with acyclovir resistance in a child with AIDS. JAMA. 1988;260:2879–2882. [PubMed] [Google Scholar]
- 20.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 21.Post L E, Mackem S, Roizman B. Regulation of α genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with α gene promoters. Cell. 1981;24:555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 22.Sasadeusz J J, Tufaro F, Safrin S, Schubert K, Hubinette M M, Cheung P K, Sacks S L. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J Virol. 1997;71:3872–3878. doi: 10.1128/jvi.71.5.3872-3878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soike K F, Bohm R, Huang J, Oberg B. Efficacy of (−)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine in African green monkeys infected with simian varicella virus. Antimicrob Agents Chemother. 1993;37:1370–1372. doi: 10.1128/aac.37.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streisinger G, Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985;109:633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Susutani T, Lacey S F, Powell K L, Purifoy D J M, Honess R W. Random mutagenesis of the thymidine kinase gene of varicella-zoster virus. J Virol. 1992;66:2118–2124. doi: 10.1128/jvi.66.4.2118-2124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talarico C L, Phelps W C, Biron K K. Analysis of the thymidine kinase genes from acyclovir-resistant mutants of varicella-zoster virus isolated from patients with AIDS. J Virol. 1993;67:1024–1033. doi: 10.1128/jvi.67.2.1024-1033.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson J B, Hayday A, Courtneidge S, Fried M. A frameshift at a mutational hotspot in the polyoma virus early region generates two new proteins that define T-antigen functional domains. Cell. 1986;44:477–487. doi: 10.1016/0092-8674(86)90469-1. [DOI] [PubMed] [Google Scholar]