Abstract
Previously, we reported on the use of rifampin-loaded microspheres to effectively treat Mycobacterium tuberculosis-infected macrophages and mice. Using similar biocompatible polymeric excipients of lactide and glycolide copolymers, we have increased the rifampin loading of small microsphere formulations (1 to 10 μm) by fourfold. Improved formulations were evaluated individually and in combination with oral regimens of isoniazid for the treatment of Mycobacterium tuberculosis H37Rv-infected mice. Groups (10 mice per group) consisted of mice that received (i) oral dosages of isoniazid (25 to 0.19 mg/kg of body weight/day), (ii) two intraperitoneal injections of rifampin-loaded microspheres on days 0 and 7, (iii) a combination of small rifampin-loaded microspheres on days 0 and 7 and isoniazid orally for 25 days (12.5 to 0.39 mg/kg/day), (iv) placebo injections, and (v) no treatment. Treatment with rifampin-loaded microspheres alone resulted in significant reductions in the numbers of CFU in the lungs and spleens by day 26. A bioassay revealed that plasma rifampin levels from the microspheres exceeded the MICs by more than twofold throughout the 26-day experimental period. Susceptibility testing demonstrated continued sensitivity to rifampin during the treatment period. Whereas isoniazid alone significantly reduced the numbers of CFU for dosages ranging from 12.5 to 1.56 mg/kg, combination therapy with rifampin-loaded microspheres increased the effective range to 0.39 mg/kg. In many cases, complete elimination of CFU was obtained with the combination therapy, something not achieved with most of the single therapies. These results demonstrate the ability to use small microsphere formulations alone to achieve significant results in a murine tuberculosis model and also the ability to use them safely in combination with another antimycobacterial agent.
Tuberculosis is one of a number of diseases that have afflicted the human race for centuries. Even though a vaccine and numerous effective antimycobacterial agents are available for its treatment, several million people die from the disease each year (8). An important consideration in the treatment of tuberculosis is the fact that the etiological agent, Mycobacterium tuberculosis, has the ability to persist intracellularly in the host macrophage for long periods of time. Optimum therapy, therefore, must depend upon the intracellular delivery of antimycobacterial agents for prolonged periods. This becomes even more important when one considers the ability of M. tuberculosis to persist in a dormant state, thus giving rise to a large group of infected individuals who carry the organism in a subclinical state without having active disease (8). It has been estimated that about 0.3% of U.S. residents are infected and at risk of development of active disease (5). Worldwide, it is estimated that one-third of the population is infected with M. tuberculosis, which results in about 8 million new cases of tuberculosis annually (2).
Properly devised delivery techniques should theoretically circumvent these problems by positioning effective drugs within host macrophages, thus giving direct access to dormant organisms that presumably would be within macrophages or in the surrounding lymphatic area. In the case of a drug that is effective against actively multiplying mycobacteria, this would be advantageous because the drug would continually be available for prolonged periods at the site in the event the organism underwent any multiplication cycle. Microsphere technology has the capability of accomplishing these goals by achieving intracellular delivery of antimycobacterial drugs and allowing programmed controlled release over a prolonged period (24). As discussed in our previous publications, the microsphere formulations used in these studies are known to be biocompatible (26, 27, 28) and capable of degradation to lactic and glycolic acids by nonenzymatic reactions (24). This technology has been used for sustained delivery of various biological components, including antigens, steroids, peptides, proteins, and antibiotics (1, 3, 9, 10, 11, 12, 14, 15, 21, 23, 25).
In previous studies, we have developed microsphere formulations containing rifampin, one of the first-line drugs used to treat tuberculosis (4, 22). Those formulations consisted of two types, one developed for targeted delivery to host macrophages (1 to 10 μm in diameter; small microspheres) (4) and the other developed for systemic delivery (10 to 150 μm in diameter; large microspheres) (22). Formulations were developed in such a way as to provide sustained programmed release of the drugs in order to circumvent the multiple dosing required for conventional therapy and to provide a means of delivery of the drugs to the macrophages where mycobacteria reside during an infection. Although the small microsphere formulations were capable of significantly reducing the numbers of CFU in macrophages infected with M. tuberculosis (4), they were not able to achieve significant reductions in the numbers of CFU when used alone for the treatment of M. tuberculosis-infected mice (22). Increased loading was therefore something that we proposed would be accomplished in future studies.
Rifampin and isoniazid are both first-line drugs for use in the therapy of tuberculosis and are included in the list of recommended drug regimens for treatment of latent M. tuberculosis infection in adults (6). They have been used in combination for treatment of tuberculosis in clinical trials of human immunodeficiency virus-negative (13, 16) and human immunodeficiency virus-positive persons (30). As a part of this study, we also believed that it was important to evaluate the use of rifampin-loaded microspheres in a combined therapeutic regimen with oral dosages of isoniazid.
The objectives of this extended study were, therefore, to (i) increase the drug loading of the small microspheres, (ii) test their ability to treat an M. tuberculosis infection without the use of the larger microsphere formulations, and (iii) evaluate the safety of combined therapy with the rifampin-loaded small microspheres and an oral regimen of another antimycobacterial drug. In this case, we chose isoniazid.
MATERIALS AND METHODS
Preparation of microspheres for extended release of rifampin.
The process used to prepare the small microspheres (i.e., 1 to 10 μm in diameter) has previously been described in detail (4, 22). Briefly, excipient solution was prepared by dissolving poly(dl-lactide-co-glycolide) in methylene chloride. Rifampin was added to that solution, and a homogeneous solution was obtained by thorough mixing. The resulting mixture was then introduced into aqueous process medium consisting of polyvinyl alcohol (Air Products Inc., Allentown, Pa.). An emulsion consisting of appropriately sized microdroplets was formed with the aid of a Silverson emulsifier (Silverson Machines, East Longmeadow, Mass.). The emulsion was then transferred to 5.0 liters of water. The resulting microspheres were then concentrated by centrifugation and collected by lyophilization. In previous studies, we were able to accomplish only 1.4 to 1.8% (wt/wt) rifampin loading (4, 22). In order to increase the loading for these formulations, the initial theoretical dry concentration was increased from 5 to 10% (wt/wt). The rest of the process remained the same.
Each microsphere formulation was analyzed for drug content by spectrophotometric and high-pressure liquid chromatographic assays (4, 22). The size distribution for each lot of rifampin-loaded microspheres was determined with a Coulter LS Particle Size Analyzer (Coulter Electronics Inc., Hialeah, Flo.). Microsphere lots were sterilized with gamma radiation (2.5-Mrad dose) by Neutron Products Inc., Dickerson, Md. Following sterilization, microsphere formulations are stored desiccated at −20°C until use. The surface morphologies of microsphere formulations are examined by scanning electron microscopy (SEM). Matching placebo formulations were made for each drug-loaded preparation.
Drug content of rifampin-loaded microspheres.
The rifampin content of each lot of microspheres was determined by first extracting the rifampin from a known quantity of microspheres and quantifying the amount of drug spectrophotometrically (4). The concentration of rifampin contained in each sample was determined by measuring the absorbance on a spectrophotometer at a λ of 474 nm. A series of rifampin solutions of known concentrations in ethyl acetate were prepared, and their absorbances were measured in order to generate a standard curve. The rifampin concentrations in the microsphere and control samples were then obtained by linear regression, and the total amount of rifampin was calculated as (rifampin concentration [in micrograms per milliliter])(number of milligrams/1,000 μg)(sample volume [in milliliters])/(microsphere sample weight [in milligrams]).
Mycobacterial strains.
M. tuberculosis H37Rv (ATCC 27294, SRI-1345) was maintained on Middlebrook 7H10 agar slants containing 0.5% glycerol and 10% oleic acid-albumin-dextrose-citrate (OADC) (Difco). The MIC of rifampin for this strain is 0.06 to 0.25 μg/ml (31).
Mice.
Female CD-1 mice (weight, 14 to 16 g) were obtained from Charles River Laboratories and were maintained on a diet of Teklad sterilizable laboratory feed (Harlan) and water in a biosafety level III facility throughout the studies. All animal research programs and facilities at Southern Research Institute are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. Animals were euthanatized by CO2 asphyxiation, consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Approval for the studies was given by the institutional animal care and use committee at Southern Research Institute.
Infection and treatment of mice.
The mouse model described here is a nonlethal, short-term model (22) that was developed from previous studies (7, 17, 18, 19) in order to investigate antituberculous drugs. The inoculum size and time frame were therefore selected to ensure that death would not occur due to the large inoculum size and also that the drugs could be screened with a short turnaround time. Mice were inoculated via the lateral tail vein on day 0 with approximately 105 viable M. tuberculosis H37Rv organisms in a volume of 0.1 ml of 0.9% sterile sodium chloride solution. Drug treatments were initiated approximately 4 h postinoculation. Each treatment group contained 10 mice.
In order to develop a manageable experimental process, it was necessary to use two separate efficacy studies. For that reason, two different batches of microspheres were formulated; one contained 5.8% (wt/wt) rifampin (lot 1) and the other contained 5.0% (wt/wt) rifampin (lot 2). Considering that these were small laboratory-scale lots, the variation (16%) is reasonable. It should be noted that when similar formulations are developed on a larger scale, the variation can be reduced to about 5 to 10%. Microsphere formulations, including rifampin-loaded and placebo microsphere formulations, were injected intraperitoneally on days 0 and 7 by using 50- and 58-mg doses for the 5.8 and 5.0% (wt/wt) formulations, respectively, and 100- or 116-mg doses for the 5.8 and 5.0% (wt/wt) formulations, respectively (see Tables 1 and 2). This was done to keep the total rifampin content equal for each group. Therefore, members of each group received equivalent doses of rifampin, which were 2.9 and 5.8 mg per mouse. Assuming an average weight of 0.03 kg (22), this would be equivalent to approximately 193 and 387 mg/kg of body weight, respectively. Each injection was suspended in sterile saline by using a sterile tuberculin syringe with a 23-gauge needle.
TABLE 1.
Experimental protocol for combination therapy with rifampin loaded small microspheres and oral regimens of free isoniazid, experiment 1a
| Treatment | Dosage | Route | Schedule |
|---|---|---|---|
| Nontreated | NA | NA | NA |
| INH | 25 mg/kg | p.o. | Once daily, 0–25 days |
| INH | 12.5 mg/kg | p.o. | Once daily, 0–25 days |
| INH | 6.25 mg/kg | p.o. | Once daily, 0–25 days |
| INH | 3.125 mg/kg | p.o. | Once daily, 0–25 days |
| INH | 1.56 mg/kg | p.o. | Once daily, 0–25 days |
| Placebo (microspheres) | 100 mg | i.p. | Days 0 and 7 |
| 5.8% RIF | 50 mg | i.p. | Days 0 and 7 |
| 5.8% RIF | 50 mg | i.p. | Days 0 and 7 |
| INH | 12.5 mg/kg | p.o. | Once daily, 0–25 days |
| 5.8% RIF | 50 mg | i.p. | Days 0 and 7 |
| INH | 6.25 mg/kg | p.o. | Once daily, 0–25 days |
| 5.8% RIF | 50 mg | i.p. | Days 0 and 7 |
| INH | 3.125 mg/kg | p.o. | Once daily, 0–25 days |
| 5.8% RIF | 100 mg | i.p. | Days 0 and 7 |
| 5.8% RIF | 100 mg | i.p. | Days 0 and 7 |
| INH | 12.5 mg/kg | p.o. | Once daily, 0–25 days |
| 5.8% RIF | 100 mg | i.p. | Days 0 and 7 |
| INH | 6.25 mg/kg | p.o. | Once daily, 0–25 days |
| 5.8% RIF | 100 mg | i.p. | Days 0 and 7 |
| INH | 3.125 mg/kg | p.o. | Once daily, 0–25 days |
Rifampin-loaded microspheres (lot 1; 5.8% [wt/wt]) were injected intraperitoneally (i.p.), and isoniazid was given orally (p.o.), as described in Material and Methods. There were 10 mice in each treatment group. Abbreviations: RIF, rifampin-loaded microspheres; INH, isoniazid; NA, not applicable.
TABLE 2.
Experimental protocol for combination therapy with rifampin-loaded small microspheres and oral regimens of free isoniazid experiment 2a
| Treatment | Dosage | Route | Schedule | Number of mice |
|---|---|---|---|---|
| Nontreated | NA | NA | NA | 10 |
| INH | 1.56 mg/kg | p.o. | Once daily, 0–25 days | 10 |
| INH | 0.78 mg/kg | p.o. | Once daily, 0–25 days | 10 |
| INH | 0.39 mg/kg | p.o. | Once daily, 0–25 days | 10 |
| INH | 0.19 mg/kg | p.o. | Once daily, 0–25 days | 10 |
| Placebo (microspheres) | 100 mg | i.p. | Days 0 and 7 | 5 |
| Placebo (microspheres) | 116 mg | i.p. | Days 0 and 7 | 5 |
| 5.0% RIF microspheres | 58 mg | i.p. | Days 0 and 7 | 10 |
| 5.0% RIF microspheres | 58 mg | i.p. | Days 0 and 7 | 10 |
| INH | 1.56 mg/kg | p.o. | Once daily, 0–25 days | |
| 5.0% RIF microspheres | 58 mg | i.p. | Days 0 and 7 | 10 |
| INH | 0.78 mg/kg | p.o. | Once daily, 0–25 days | |
| 5.0% RIF microspheres | 58 mg | i.p. | Days 0 and 7 | 10 |
| INH | 0.39 mg/kg | p.o. | Once daily, 0–25 days | |
| 5.0% RIF microspheres | 116 mg | i.p. | Days 0 and 7 | 10 |
| 5.0% RIF microspheres | 116 mg | i.p. | Days 0 and 7 | 10 |
| INH | 1.56 mg/kg | p.o. | Once daily, 0–25 days | |
| 5.0% RIF microspheres | 116 mg | i.p. | Days 0 and 7 | 10 |
| INH | 0.78 mg/kg | p.o. | Once daily, 0–25 days | |
| 5.0% RIF microspheres | 116 mg | i.p. | Days 0 and 7 | 10 |
| INH | 0.39 mg/kg | p.o. | Once daily, 0–25 days |
Rifampin-loaded microspheres (lot 2; 5.0% [wt/wt]) were injected intraperitoneally (i.p.), and isoniazid was given peroral (p.o.) as described in Material and Methods. Abbreviations: RIF, rifampin-loaded microspheres; INH, isoniazid; NA, not applicable.
For mice receiving isoniazid, each was dosed by individual weight by a gavage technique (atraumatic stainless steel oral dosing needle attached to a sterile 1-ml syringe) with a volume of 0.1 ml/10 g of body weight. Isoniazid (Sigma, St. Louis, Mo.) was dissolved in sterile water prior to oral administration. The oral gavage of isoniazid was performed daily from day 0 to day 25 (see Tables 1 and 2). All mice were weighed daily and were observed for clinical signs of toxicity. On day 26, mice were anesthetized with ketamine-xylazine for aseptic blood collection with a sterile tuberculin syringe and an heparinized needle to withdraw volumes of 0.5 to 1.0 ml of blood from the heart. The mice were immediately euthanatized with CO2. The organs were removed and frozen individually in sterile Tekmar bags, thawed, hand homogenized with a Bayer roller, diluted with sterile saline containing 0.05% Tween 80, and plated onto OADC-supplemented 7H11 Middlebrook Mycobacterium solid agar. The colonies were enumerated after 14 to 21 days of incubation in a 37°C, 5% CO2 incubator.
Combination therapy with rifampin-loaded microspheres and oral regimen of isoniazid.
The mice were treated with a combination of small rifampin-loaded microspheres and various oral regimens of isoniazid by the procedures described above for individual therapies. The experimental protocol for the combined therapy is also given in Tables 1 and 2.
In vivo release characteristics of microspheres.
During the efficacy studies, the levels of rifampin in mouse plasma were determined for each formulation for mice receiving only the rifampin-loaded microsphere therapy. At 7, 14, and 21 days, three mice per group were anesthetized with ketamine-xylazine and blood was obtained by retro-orbital bleeding. At 26 days, all 10 mice were anesthetized with ketamine-xylazine and exsanguinated by cardiac puncture. Plasma was assayed for rifampin by using Staphylococcus aureus ATCC 29213 in the bioassay described previously (4).
RESULTS
Microspheres.
In previous studies, we were able to achieve 1.4 to 1.8% (wt/wt) loading. In order to increase the loading for these formulations, the initial theoretical dry concentration was increased from 5 to 10% (wt/wt). The rest of the process remained the same. Two lots were produced for this study, lot 1 (5.8%; wt/wt) and lot 2 (5.0%; wt/wt). Each lot was produced in order to obtain a diameter of 1 to 10 μm. The size distributions of both lots of rifampin-loaded microspheres demonstrated a Gaussian curve similar to those for previous formulations (4, 22). The means ± standard deviations for particle size distributions were 3.98 ± 2.74 and 4.2 ± 3.2 μm for lots 1 and 2, respectively. The 90th percentiles for the microspheres were 7.99 and 4.2 μm for lots 1 and 2, respectively. These data mean that 90% of the microspheres in lots 1 and 2 were ≤7.99 and ≤4.2 μm, respectively. Examination by SEM revealed no evidence of cracks, holes, or major defects in the outer films of the formulations.
Results of isoniazid oral therapy.
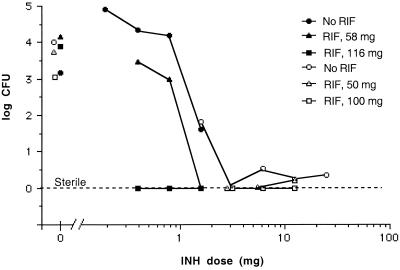
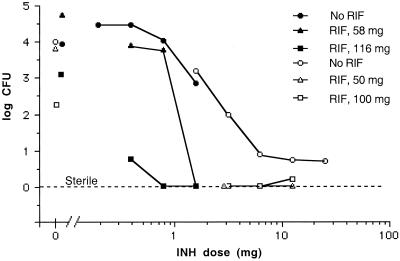
In experiment 1 (Table 1), the oral regimen for isoniazid consisted of the following dosages: 25, 12.5, 6.25, 3.125, and 1.56 mg/kg (Fig. 1 and 2). In experiment 2 (Table 2) dosages were 1.56, 0.78, 0.39, and 0.19 mg/kg (Fig. 1 and 2). Analysis of the data reveals that the effective range for significant reductions (P < 0.05) of the numbers of CFU in the lungs and spleens was from 25 to 1.56 mg of isoniazid/kg (Fig. 1 and 2). Dosages below 1.56 mg of isoniazid/kg did not result in significant reductions in the numbers of CFU (Fig. 1 and 2). It should be noted that although the higher concentrations of oral isoniazid (i.e., 25 to 1.56 mg/kg) were able to lower the numbers of CFU by more than 3 logs, with the exception of the 3.125-mg/kg dose, none of the doses were able to completely eliminate all CFU (Fig. 1 and 2). For isoniazid dosages of 0.39 and 0.19 mg/kg (Fig. 1 and 2), a significant increase in the numbers of CFU was observed. This was due to the stress induced by the extra handling (i.e., daily dosing) of these treated groups compared to that required for the mice in the nontreated group, which were not handled on a daily basis.
FIG. 1.
Synergistic interaction of rifamipin-loaded small microspheres (RIF) with orally administered isoniazid (INH) on mycobacterial CFU in the lungs of mice infected with M. tuberculosis H37Rv. The mice were infected and the tissues were processed for determination of the numbers of CFU as described in Materials and Methods. Results from experiment 1 (Table 1) are given as open symbols, and results from experiment 2 (Table 2) are given as closed symbols. Each point represents the mean for 10 mice, unless otherwise discussed in the text.
FIG. 2.
Synergistic interaction of rifamipin-loaded small microspheres (RIF) with orally administered isoniazid (INH) on mycobacterial CFU in the spleens of mice infected with M. tuberculosis H37Rv. The mice were infected and the tissues were processed for determination of the numbers of CFU as described in Materials and Methods. Results from experiment 1 (Table 1) are given as open symbols, and results from experiment 2 (Table 2) are given as closed symbols. Each point represents the mean for 10 mice, unless otherwise discussed in the text.
Results of microsphere therapy.
The results of the microsphere therapy revealed that treatment with 50- or 58-mg rifampin-loaded microspheres (given as two separate intraperitoneal injections on days 0 and 7) did not result in significant reductions in the numbers of CFU in the lungs or spleens at the end of 26 days (Fig. 1 and 2). The same treatment with 100-mg rifampin-loaded microspheres did result in significant reductions in the numbers of CFU in the lungs and spleens (P < 0.01) by the end of the 26 days (Fig. 1 and 2). Likewise, treatment with 116-mg rifampin-loaded microspheres also resulted in significant reductions in the numbers of CFU in the spleens (P < 0.01; Fig. 2) but not in the lungs (Fig. 1). Unfortunately, in the latter case, only four samples were worthy of processing due to contamination. It is noteworthy that these results are an improvement over those from our previous experiments, in which we were not able to show significant reductions in the numbers of CFU with small microspheres containing 1.8% (wt/wt) rifampin (22). This improvement has been achieved by a threefold increase in loading.
In experiment 1, 10 mice were injected with placebo microspheres with the 100-mg dosage. In experiment 2, two groups of five mice each were injected with placebo dosages of 100 and 116 mg. None of the placebo-treated groups showed any significant decrease in the numbers of CFU at the end of the experimental period in either the lungs or the spleens (data not shown).
Results of combined therapy.
With the therapeutic range used in experiment 1 for isoniazid (Table 1), significant reductions in the numbers of CFU in the lungs and the spleens (P < 0.0002) were observed for all dosages (Fig. 1 and 2). Thus, it was difficult to show significant differences in the reduction in the numbers of CFU with the combined microsphere therapies compared with that achieved with the oral regimen of isoniazid alone (Fig. 1 and 2). The one exception was the isoniazid dose of 3.125 mg/kg in the spleens (Fig. 2), for which it was possible to show a statistically significant difference between the combined therapy with the microspheres and the oral isoniazid therapy. Both the 50-mg and 100-mg doses of microspheres (Fig. 2) resulted in significant improvements in the reduction in the numbers of CFU compared with that achieved by therapy with only the oral isoniazid at 3.125 mg/kg (P = 0.0021 for both the 50- and 100-mg doses). For the oral isoniazid therapy (3.125 mg/kg), 1.96 log10 CFU was observed, whereas when combined with the 50- or 100-mg microsphere therapy, complete elimination of CFU was obtained (Fig. 2).
With the combination of microspheres plus the higher range of oral isoniazid therapy (Figure 1), complete elimination of CFU from the lungs was observed for two of three combinations with the 50-mg microsphere dose (Fig. 1) and three of three combinations with the 100-mg microsphere dose (Fig. 1). In the spleens, five of the six combination therapies with the 50- and 100-mg microsphere dosages (Fig. 2) resulted in the complete elimination of CFU, something not achieved with any of the dosages of isoniazid alone (Fig. 2). The single exception to the complete elimination of CFU with the combination therapy resulted from 50 CFU being observed on only 1 of 10 culture plates (which contained 100-mg microspheres plus 12.5 mg of isoniazid per kg; Fig. 2). All nine other plates were void of CFU.
In experiment 2 (Table 2), the therapeutic dose range for isoniazid was decreased to 1.56 to 0.19 mg/kg in order to evaluate less effective dosages (Fig. 1 and 2). Among the doses in this dose range, only the dose of 1.56 mg/kg was able to produce a significant reduction (P = 0.0029) in the numbers of CFU in the spleens (Fig. 2) and in the case of the lower dosages (i.e., 0.39 and 0.19 mg/kg), a significant increase in the numbers of CFU was observed (Fig. 2). As discussed above, this was due to the stress induced by the extra handling.
The 5.0% (wt/wt) rifampin-loaded microsphere formulation (lot 2) was able to significantly reduce the numbers of CFU in the spleens (P = 0.0089) but not in the lungs when given as the 116-mg dose (Fig. 2 and 1, respectively). Lack of a significant reduction in the numbers of CFU may best be attributed to the availability of only four samples for CFU determination. All other culture plates in that set were contaminated and could not be used.
The lower microsphere dose (58 mg; Fig. 1) was able to significantly reduce the numbers of CFU in the lungs (P = 0.0001) when combined with the isoniazid dose of 1.56 mg/kg, whereas the isoniazid dose alone (P = 0.07) was unable to do so (Fig. 1). In fact, use of the 58-mg microsphere dose with the oral 1.56-mg/kg isoniazid regimen resulted in complete elimination of the CFU in both the lungs and the spleens (Fig. 1 and 2). Likewise, combination therapy with the larger 116-mg dose of microspheres resulted in complete elimination of the CFU in the lungs and spleens for isoniazid dosages of 1.56, 0.78, and 0.39 mg/kg (Fig. 1 and 2) with the exception of the combination of microspheres and isoniazid at 0.39 mg/kg in the spleens (Fig. 2). In that particular set, only two of eight culture plates contained colonies, which were present at 900 and 1,050 CFU, respectively. The other six plates were void of CFU.
At the end of each experiment, M. tuberculosis was isolated from mice treated with the rifampin-loaded microspheres and retested for susceptibility to rifampin. The MIC remained the same, 0.06 to 0.25 μg/ml.
Deposition of microspheres following therapy.
As the result of our previous study involving macrophages, we know that the small microspheres (i.e., 1 to 10 μm in diameter) are readily phagocytosed by host macrophages (4). Because this has been well documented, we did not attempt to monitor the precise distribution of the microspheres in the treated mice in this experiment. Postmortem observations did reveal that microspheres had formed aggregates which had adhered to various sites within the abdomen (e.g., intestines, spleen, stomach, liver, and mesentery). Noteworthy is the fact that the presence of the microspheres did not cause any adhesions of internal structures and the microspheres could easily be removed by means of forceps. We believe that this indicates that the microspheres were likely trafficked through the mouse by means of macrophages, similar to what one would observe with intraperitoneal injection of any particles of this size. As discussed below, the aggregate formation is a likely explanation for the different release patterns observed for the low versus the high dosages. As discussed in the Discussion section, we realize that intraperitoneal injection is not the best route for demonstration of the optimum use of the small microspheres; better routes would be the intravenous or the intranasal-intratracheal route. We used the intraperitoneal route in this study to be consistent with the model that we had used previously (22).
In vivo release of microsphere formulations.
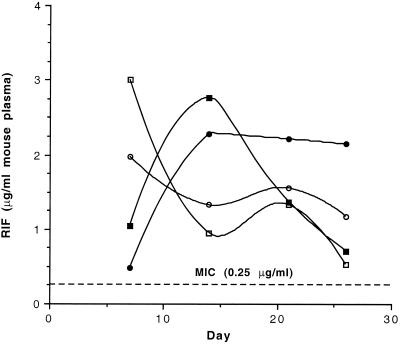
Plasma rifampin levels were determined in three randomly selected mice from the groups treated with the rifampin-loaded microspheres at days 7, 14, and 21 and in all 10 mice after the conclusion of the experiment at 26 days. A similar protocol was conducted with the nontreated groups to provide appropriate negative controls. Examination of the results from the first experiment, in which the 5.8% (wt/wt) rifampin-loaded microspheres were used (Fig. 3), indicates that plasma rifampin levels were 2- to 12-fold and 3- to 11-fold above the upper MIC throughout the experimental period for the 50- and 100-mg dosages, respectively. In the second experiment with the 5.0% (wt/wt) rifampin-loaded microspheres, plasma rifampin levels were five- to eight-fold and two- to nine-fold above the upper MIC throughout the experimental period for the 58- and 116-mg dosages, respectively (Fig. 3). Thus, considering the results of both experiments, overall coverage was two- to nine-fold above the upper MIC of 0.25 μg/ml.
FIG. 3.
Release characteristics of small rifampin-loaded microspheres: lot 1 (5.8%; wt/wt) in experiment 1 and lot 2 (5.0%; wt/wt) in experiment 2. Mice were randomly chosen from experimental group 1, which received the 58-mg (○) and 116-mg (•) doses or experimental group 2, which received the 50-mg (▫) and 100-mg (▪) doses. At days 7, 14, and 21, blood was obtained from three mice by retro-orbital bleeding. At the conclusion of the experiment (day 26), all 10 mice in each of the experimental groups were exsanguinated by cardiac puncture. Plasma was assayed by using S. aureus, as described in Materials and Methods. Each point represents the mean for either 3 (days 7, 14, and 21) or 10 (day 26) assayed samples. The upper MIC for M. tuberculosis H37Rv is given as a dotted line (0.25 μg/ml) and corresponds to the upper value of the MIC range discussed in Materials and Methods (0.06 to 0.25 μg/ml).
There were differences in the release patterns of the two dosages of rifampin-loaded microspheres. At 7 days, the levels of release from the small dosages (i.e., 50 and 58 mg) were three- and fourfold greater than those from the larger dosages of 100 and 116 mg, respectively (Fig. 3). By 14 days, however, those levels had reversed (Fig. 3). The release patterns of the small dosages in both experiments are very similar, as are the release patterns of the large dosages (Fig. 3). This can be explained by the fact that immediately following injection, the microspheres loaded with the larger dosages tended to cluster more than those loaded with the smaller dosages, thus producing less surface area for optimum diffusion. This would result in decreased levels of release initially, followed by increased levels of release throughout the rest of the experiment as the microspheres dissolved.
Comparison with previous studies.
Table 3 presents a summary of the data obtained in this study and compares those data with some data obtained from our previous study with a small microsphere formulation (22). We have been able to increase the loading from 1.4 to 5.8% (wt/wt), a fourfold increase. As a result, the total amount of rifampin that can be delivered to the mice over a 26-day period has increased more than eightfold, from 47 to 387 mg/kg (Table 3). With our previous small formulation, the rifampin level was never above the MIC, whereas with the new formulation the drug level remains above the MIC throughout the 26-day experimental period (Table 3). In addition, peak drug levels were increased from 0.2 μg/ml to a maximum of 3.0 μg/ml with the new formulation (Table 3). That represents a 15-fold increase in peak drug levels.
TABLE 3.
Comparison of previously reported small microsphere formulation with new formulations described in this report
| Microsphere | Microsphere dose (mg) | Total amt of microsphere (mg) | Total amt of RIFa delivered (mg) | Total amt of RIF delivered by body weight (mg/kg)b | No. of days the RIF concn was above the MICc | Peak level (μg) throughout 26 days |
|---|---|---|---|---|---|---|
| Previous small microspheresd | 50e | 100 | 1.4 | 47 | 0 | 0.2 |
| New batches of small microspheres | ||||||
| Low dose | 50 and 58 | 100 and 116 | 5.8 | 193 | 26 | 2.0–3.0 |
| High dose | 100 and 116 | 200 and 232 | 11.6 | 387 | 26 | 2.3–2.8 |
RIF, rifampin.
Amount is calculated assuming an average weight of 0.03 kg/mouse.
MIC, 0.25 μg/ml.
Results were reported previously (22). This formulation contained 1.4% (wt/wt) rifampin.
Each dose was administered twice, once at day 0 and once at day 7.
DISCUSSION
Previously, we reported on the initial development of microsphere technology for use in the delivery of the potent antimycobacterial drug rifampin. Our initial report described the use of small microspheres (1 to 10 μm in diameter) for the targeting of rifampin to macrophages and their ability to significantly reduce the level of intracellular replication of M. tuberculosis H37Rv (4). A subsequent report described the successful use of both small (1 to 10 μm in diameter) and large (10 to 150 μm in diameter) microspheres for the treatment of mice infected with M. tuberculosis H37Rv (22). In an effort to demonstrate further application of this technology, we report on the combined use of microspheres with an oral regimen of isoniazid for the treatment of mice infected with M. tuberculosis H37Rv.
As stated in the introduction, the objectives of this extended study were to (i) increase the drug loading of the small microspheres, (ii) test their ability to treat an M. tuberculosis infection without the use of the larger microsphere formulations, and (iii) evaluate the safety of combined therapy with the rifampin-loaded small microspheres and an oral regimen of another antimycobacterial drug.
In our two previous studies, the maximum rifampin loadings achieved with the small microspheres were 1.4 and 1.8% (wt/wt) (4, 22). For the present study, we have been able to increase the loading to 5.0 and 5.8% (wt/wt) rifampin, a three- to fourfold increase. Previously, the small microspheres had not been able to achieve significant reductions of viable mycobacteria in mice infected with M. tuberculosis H37Rv when used alone (22). As reported here, the small microspheres with the increased loading were able to significantly reduce the numbers of CFU in M. tuberculosis H37Rv-infected mice without assistance from the larger microsphere formulations. The new formulation was able to deliver an eightfold increased concentration of rifampin and achieve drug levels above the MIC throughout the 26-day experimental period. In addition, the new formulation was able to produce a peak level in blood that was 15-fold above that achieved with the previous formulation. These are significant refinements that further support the hypothesis that appropriate microsphere formulations can be used to effectively improve tuberculosis therapy.
Because treatment of tuberculosis uses multiple drug therapies, it was important to evaluate the safety of the use of the rifampin-loaded microspheres with another antimycobacterial agent. For the present study, we chose isoniazid because it is a frequently used first-line drug for the treatment of tuberculosis. A therapeutic regimen with the small rifampin-loaded microspheres in combination with oral regimens of isoniazid was able to safely increase the effective therapeutic range of isoniazid by some twofold (from 1.56 down to 0.39 mg/kg). In most cases, the combination therapy reduced the numbers of CFU in the lungs and spleens to nondetectable levels, something not achieved with the oral regimen of isoniazid alone. The results were achieved by using a much reduced dosing schedule for rifampin. Instead of dosing the mice daily for 26 days, as would be the case with an oral dose (22), we were able to dose the mice only twice during that time period and achieve significant improvement of the isoniazid oral therapy. These findings are encouraging and demonstrate that microsphere technology can be safely used in combination with another antimycobacterial agent.
In order to assess the potential value of these findings, it is important to examine the amount of rifampin delivered to the mice and the period of sustained release obtained with these formulations. Considering the amount of rifampin delivered in the two formulations and assuming an average weight of 0.03 kg, each mouse received an equivalent of 193 and 387 mg of rifampin/kg for the two dosages, respectively. If those values are divided by the length of the experiment (i.e., 26 days, assuming equivalent releases on each day), then each mouse received approximately 7.4 mg of rifampin/kg for the lower dose and 14.9 mg of rifampin/kg for the higher dose. This is actually a conservative estimate, because bioassay results indicated that the levels in plasma were still approximately twofold above the upper MIC at the end of the experiment. It is likely that therapeutic levels would have persisted for at least up to 30 days, something that is generally observed with this size of microsphere (unpublished data).
In the mouse model used in the present study, it is necessary to administer ≥5.0 mg of rifampin/kg/day orally in order to achieve a significant reduction in the numbers of M. tuberculosis H37Rv CFU by 26 days; dosages of 2.5, 1.25, and 0.42 mg/kg are not sufficient (22). We have used this mouse model for several years to consistently produce similar results. It is also meaningful to compare the reduction of the numbers of CFU achieved with the oral rifampin dosing observed in our previous study (22) and those achieved with the microspheres in the present study. With oral regimens of 5 and 10 mg/kg, the log CFU reductions were 0.42 and 1.70, respectively (22). With the small microsphere treatment in this study, the larger dosages of small microspheres (i.e., 100 and 116 mg) were able to achieve 0.93 log reductions in the lungs and 1.72 and 0.84 log reductions in the spleens. As discussed previously, this is within the range of the highest clinically tolerated dosages in humans, disregarding differences in metabolism (22). It is noteworthy that the small microsphere formulations were administered only twice, while the oral rifampin regimen was administered daily throughout the experimental period (22).
Intraperitoneal administration of soluble test compounds is routinely used for efficacy experiments with mice (22). Although intravenous injection would be a better route for delivery of the small microsphere formulations, our attempts to do so have not been successful due to such problems as low diluent volumes, small vein size, and small needle size (22). These will always be problems in studies with mice. We are conducting studies with rats and nonhuman primates to evaluate intravenous administration of the small microsphere formulations. With that information, we should be able to rationally deduce the maximum potential for microsphere delivery in humans and the upper ranges of dosing that might be used in clinical trials. Another potential route of administration for the small microspheres would be the intranasal route (e.g., aerosolization). Those studies are being considered for future evaluation of microsphere technology for the treatment of tuberculosis.
As discussed in our previous publication, liposomes can be used to deliver biological agents such as antimicrobics (22). Some liposome products are approved the Food and Drug Administration and are marketed for the treatment of microbial infections. Previously, we compared the poly(lactide-co-glycolide) microspheres with liposomes for the delivery of biological agents (22). For that reason, a lengthy discussion will not be presented here. However, it is important to realize some of the major differences between the two technologies. The poly(lactide-co-glycolide) microspheres allow greater flexibility with respect to the timed release of encapsulated material and are more stable than liposomes (22). While biological degradation of poly(lactide-co-glycolide) microspheres is well understood (26, 27), biological degradation of liposomes is not. In addition, microspheres can be formulated to hold a larger amount of drug for longer sustained release than liposomes, on a weight basis comparison (20, 29). With the addition of the results presented in this report, it seems apparent that microsphere technology can offer an alternative if not a better solution for improving antimycobacterial therapy.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grant AI38185 (principal investigator, W. W. Barrow).
Technical assistance for the animal model work by Gloria Triggs, Shixiong Li, and Anthony King is greatly appreciated.
REFERENCES
- 1.Abazinge M, Jackson T, Yang Q, Owusu-Ababio G. In vitro and in vivo characterization of biodegradable enoxacin microspheres. Eur J Pharm Biopharm. 2000;49:191–194. doi: 10.1016/s0939-6411(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 2.Arachi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 3.Bahk J Y, Hyun J S, Lee J Y, Kim J, Cho Y H, Lee J H, Park J S, Kim M O. Concentration of ofloxacin in canine prostate tissue and prostate fluid after intraprostatic injection of biodegradable sustained-releasing microspheres containing ofloxacin. J Urol. 2000;163:1560–1564. [PubMed] [Google Scholar]
- 4.Barrow E L W, Winchester G A, Staas J K, Quenelle D C, Barrow W W. Use of microsphere technology for sustained and targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob Agents Chemother. 1998;42:2682–2689. doi: 10.1128/aac.42.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. CDC revises HIV infection estimates. HIV/AIDS Prevent. 1996;st:2. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Morb Mortal Wkly Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 7.Chadwick M, Nicholson G, Gaya H. Brief report: combination chemotherapy with ciprofloxacin for infection with Mycobacterium tuberculosis in mouse models. Am J Med. 1989;87:35S–36S. doi: 10.1016/0002-9343(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 8.Collins F M. Mycobacterial pathogenesis: a historical perspective. Frontiers Biosci. 1998;3:123–132. doi: 10.2741/a285. [DOI] [PubMed] [Google Scholar]
- 9.Cowsar D R, Tice T R, Gilley R M, English J P. Poly(lactide-co-glycolide) microcapsules for controlled release of steroids. Methods Enzymol. 1985;112:101–116. doi: 10.1016/s0076-6879(85)12010-0. [DOI] [PubMed] [Google Scholar]
- 10.Eldridge J H, Staas J K, Meulbroek J A, Tice T R, Gilley R M. Biodegradable and biocompatible poly(dl-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991;59:2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elices M J, Goldstein I J. Glycosyltransferase activities of Ehrlich ascites tumor cells: detection, isolation, and characterization using oligosaccharide-synsorb beads. Arch Biochem Biophys. 1987;254:329–341. doi: 10.1016/0003-9861(87)90109-3. [DOI] [PubMed] [Google Scholar]
- 12.Fallon M T, Shafer W, Jacob E. Use of cefazolin microspheres to treat localized methicillin-resistant Staphylococcus aureus infections in rats. J Surg Res. 1999;86:97–102. doi: 10.1006/jsre.1999.5686. [DOI] [PubMed] [Google Scholar]
- 13.Madras T R C Hong Kong Chest Service; British Medical Research Council. A double-blind placebo controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis. 1992;145:36–41. doi: 10.1164/ajrccm/145.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Hora M S, Rana R K, Nunberg J H, Tice T R, Gilley R M, Hudson M E. Release of human serum albumin from poly(lactide-co-glycolide) microspheres. Pharm Res. 1990;7:1190–1194. doi: 10.1023/a:1015948829632. [DOI] [PubMed] [Google Scholar]
- 15.Jacob E, Setterstrom J A, Bach D E, Heath J R, McNiesh L M, Cierny I G. Evaluation of biodegradable ampicillin anhydrate microcapsules for local treatment of experimental staphylococcal osteomyelitis. Clin Orthopaed Rel Res. 1991;267:237–244. [PubMed] [Google Scholar]
- 16.Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom. Thorax. 1998;53:536–548. [PMC free article] [PubMed] [Google Scholar]
- 17.Khor M, Lowrie D B, Coates A R M, Mitchison D A. Recombinant interferon-gamma and chemotherapy with isoniazid and rifampicin in experimental murine tuberculosis. Br J Exp Pathol. 1986;67:587–596. [PMC free article] [PubMed] [Google Scholar]
- 18.Kradolfer F, Schnell R. Incidence of resistant pulmonary tuberculosis in relation to initial bacterial load. Chemotherapy (Basel) 1970;15:242–249. doi: 10.1159/000220688. [DOI] [PubMed] [Google Scholar]
- 19.Kradolfer F, Schnell R. The combination of rifampicin and other antituberculous agents in chronic murine tuberculosis. Chemotherapy (Basel) 1971;16:173–182. doi: 10.1159/000220725. [DOI] [PubMed] [Google Scholar]
- 20.Orozco L C, Quintana F O, Beltrán R M, deMoreno I, Wasserman M, Rodriguez G. The use of rifampicin and isoniazid entrapped in liposomes for the treatment of murine tuberculosis. Tubercle. 1986;67:91–97. doi: 10.1016/0041-3879(86)90002-4. [DOI] [PubMed] [Google Scholar]
- 21.Prior S, Gamazo C, Irache J M, Merkle H P, Gander B. Gentamicin encapsulation in PLA/PLGA microspheres in view of treating Brucella infections. Int J Pharm. 2000;196:115–125. doi: 10.1016/s0378-5173(99)00448-2. [DOI] [PubMed] [Google Scholar]
- 22.Quenelle D C, Staas J K, Winchester G A, Barrow E L W, Barrow W W. Efficacy of microencapsulated rifampin in Mycobacterium tuberculosis-infected mice. Antimicrob Agents Chemother. 1999;43:1144–1151. doi: 10.1128/aac.43.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redding T W, Schally A V, Tice T R, Meyers W E. Long-acting delivery systems for peptides: inhibition of rat prostate tumors by controlled release of [d-Trp6]luteinizing hormone-releasing hormone from injectable microcapsules. Proc Natl Acad Sci USA. 1984;81:5845–5848. doi: 10.1073/pnas.81.18.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tice T R, Cowsar D R. Biodegradable controlled-release parenteral systems. J Pharm Technol. 1984;8:26–35. [Google Scholar]
- 25.Ustariz-Peyret C, Coudane J, Vert M, Kaltsatos V, Boisrame B. Cephradin-plaga microspheres for sustained delivery to cattle. J Microencapsul. 1999;16:181–194. doi: 10.1080/026520499289167. [DOI] [PubMed] [Google Scholar]
- 26.Visscher G E, Robison R L, Argentieri G I. Tissue response to biodegradable injectable microcapsules. J Biomaterials Appl. 1987;2:118–131. doi: 10.1177/088532828700200103. [DOI] [PubMed] [Google Scholar]
- 27.Visscher G E, Robison R L, Maulding H V, Fong J W, Pearson J E, Argentieri G I. Biodegradation of and tissue reaction to 50:50 poly(dl-lactide-co-glycolide) microcapsules. J Biomed Materials Res. 1985;19:349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- 28.Visscher G E, Robison R L, Maulding H V, Fong J W, Pearson J E, Argentieri G I. Biodegradation of and tissue reaction to microcapsules. J Biomed Materials Res. 1986;20:667–676. doi: 10.1002/jbm.820200510. [DOI] [PubMed] [Google Scholar]
- 29.Vladimirsky M A, Ladigina G A. Antibacterial activity of liposome-entrapped streptomycin in mice infected with Mycobacterium tuberculosis. Biomedicine. 1982;36:375–377. [PubMed] [Google Scholar]
- 30.Whalen C C, Johnson J L, Okwera A, Hom D L, Huebner R, Mugyenyi P, Mugerwa R D, Ellner J J. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 31.Wright E L, Quenelle D C, Suling W J, Barrow W W. Use of Mono Mac 6 human monocytic cell line and J774 murine macrophage cell line in parallel antimycobacterial drug studies. Antimicrob Agents Chemother. 1996;40:2206–2208. doi: 10.1128/aac.40.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]