Abstract
Radiotherapy is among the routine treatment options for malignant tumors. And it damages DNA and other cellular organelles in target cells by using ionizing radiation produced by various rays, killing the cells. In recent years, multiple studies have demonstrated that exosomes are mechanistically involved in regulating tumor formation, development, invasion and metastasis, and immune evasion. The latest research shows that radiation can affect the abundance and composition of exosomes as well as cell-to-cell communication. In the environment, exosome-carried miRNAs, circRNA, mRNA, and proteins are differentially expressed in cancer cells, while these molecules play a role in numerous biological processes, including the regulation of oncogene expression, mediation of signaling pathways in cancer cells, remodeling of tumor-related fibroblasts, regulation of cell radiosensitivity, and so forth. Therefore, elucidation of the mechanism underlying the role of exosomes in radiotherapy of malignant tumors is crucial for improving the efficacy of radiotherapy. This review will summarize the research advances in radiosensitivity of malignant tumors related to exosomes.
Keywords: Exosomes, Radiosensitivity, microRNA, Tumor
Introduction
The morbidity and mortality of cancer have been increasing rapidly during the past half century. In 2020, approximately 19.3 million new cancer cases and almost 10 million cancer deaths were reported worldwide [1]. At present, there has been a rapid progress in early diagnosis techniques for cancer (nano-flow detection technology [2] and circulating tumor DNA [3] ) as well as new therapeutic means (immunotherapy [4] and targeted therapy [5]. However, cancer remains a significant public issue threatening the human health. In recent years, biological effects of exosomes on cancer formation, development and prognosis have gradually been unveiled, while the application of exosomes as a biomarker for disease diagnosis and prognostic prediction or a drug delivery vehicle has rapidly been promoted [6–8]. In 1987, Johnstone et al. [9] observed an exocytotic pleomorphic vesicular body termed exosome during the study of maturation of sheep reticulocytes. In 1996, Raposo et al. [10] showed that exosomes play a role in immune system by acting as a vehicle for intercellular communication, thus entering people’s vision. Later on, Ekström et al. [11] revealed that genetic materials can be delivered via exosomes in certain cells in order to reciprocally regulate gene expression by protein and miRNA. This important finding suggests to us exosomes may be closely related to human physio-pathological state and potentially serve as a novel therapeutic target for diseases.
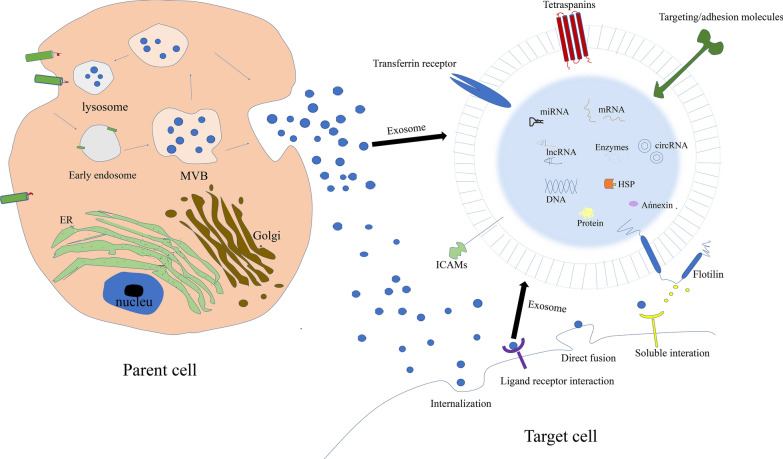
Exosomes are a class of bilayer-bound extracellular vesicles with a diameter of 30–100 nm, which contain various bioactive components such as protein, DNA, mRNA, miRNA, other non-coding RNA, lipid, and so forth. And they have been widely detected in body fluids, including cerebrospinal fluid, saliva, breast milk, blood and urine (among others) [12–15]. It is generally recognized that exosomes are derived from early endosomes formed by cell membrane invagination. Early endosomes bud inward and selectively encapsulate protein, nucleic acids, lipid, and others to form multivesicular bodies. While a part of multivesicular bodies fuse with intracellular lysosomes to degrade the contents, the others fuse with plasma membrane under the traction of intracellular molecular motor to secret exosomes for exerting biological function [16]. Upon release, exosomes act as a new vehicle for cell-to-cell communication to play an important role in various biological processes, including intercellular signal transduction, substance delivery, gene expression, and biochemical metabolism. Exosomes may directly bind to target cell receptor via their surface ligands to stimulate target cells, or function in horizontal transfer of bioactive components into target cells through fusing with target cells and endocytosis, thus affecting cell function (Fig. 1).
Fig. 1.
Exosomes biogenesis and release mechanism to recipient cells
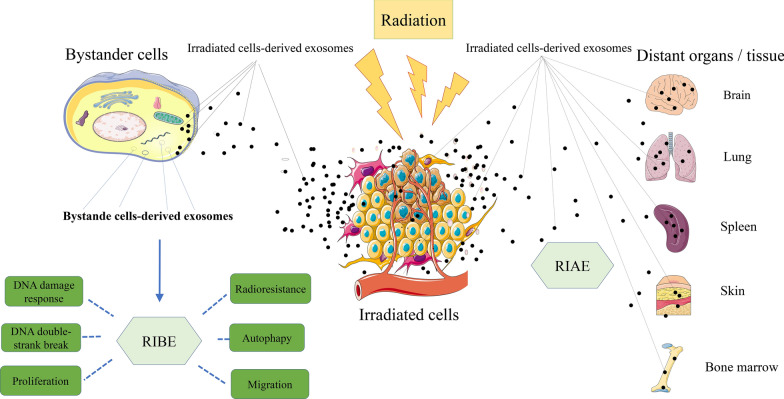
With the deepening research on exosome function, the mechanism underlying the role of exosomes in tumorigenesis has been further unveiled. The above achievement provides a theoretical basis for implementing cancer precision therapy and individualized therapy. It has been shown that while tumor cells secret much higher number of exosomes than normal cells, the level of exosomes is correlated with cancer prognosis [17]. Moreover, exosomes carry source cell-specific bioactive components including protein, mRNAs and miRNAs, which could indicate an alteration in genetics or signaling within cancer cells. Therefore, exosomes may have bright prospects in serving as a biomarker for early diagnosis and prognostic prediction of cancer [18, 19]. While good biocompatibility, low immunogenicity, liposolubility and nano-scale diameter allow exosomes to penetrate various biological barriers such as blood brain barrier, the bilayer membrane structure of exosomes can function as a natural protective barrier for protecting their contents from hydrolysis by the surrounding enzymes. Hence, exosomes could be used as a delivery vehicle in gene therapy or an ideal drug carrier [20–23]. Radiotherapy is a routine treatment option for malignant tumors. And it damages DNA and other cellular organelles in target cells by using ionizing radiation produced by various rays, killing the cells. However, while radiotherapy kills tumor cells, it can also cause damage to bystander cells and even distant tissues and organs, which is called radiation-induced bystander effects (RIBE) and radiation-induced abscopal effects (RIAE) (Fig. 2) [24, 25]. There is an increasing number of evidences that exosomes play a significant role in RIBE and RIAE, including activating the RIBE and inducing biological effects such as genomic instability, stress response, apoptosis and changes in cell proliferation after radiotherapy (Fig. 2) [26–28]. It has recently been shown that radiation leads to alterations in the secretory rate and components of released exosomes in both malignant tumor cells and normal cells [27] , which are essential for radioresistance of radiated cells as well as signal transmission between radiated cells and non-radiated cells. Not only that, exposure to radiation also increases the uptake of exosomes by cells (Fig. 2). Studies have found that radiation can elicit information communication between cancer cells and tumor microenvironment. Exosomes can be used as a means of intercellular communication. In the environment, exosome-carried miRNAs, circRNA, mRNA, and proteins are differentially expressed in cancer cells, while these molecules play a role in numerous biological processes, including the regulation of oncogene expression, mediation of signaling pathways in cancer cells, remodeling of tumor-related fibroblasts, regulation of cell radiosensitivity, and so forth [29–32]. Hence, elucidation of the mechanism underlying the role of exosomes in radioresponse is crucial for improving the efficacy of radiotherapy (Figs. 3, 4, Tables 1, 2).
Fig. 2.
Exosomes and exosomal contents participate in multiple RIBE, including DNA damage response, DNA double-strank break, cell proliferation and migration changes, promoted cell autophagy, and to tumor cell radioresistance changes. The contents of exosomes are packaged and protected to escape degradation, cross various physiological barriers, target unirradiated distant tissues and cells, and induce RIAE
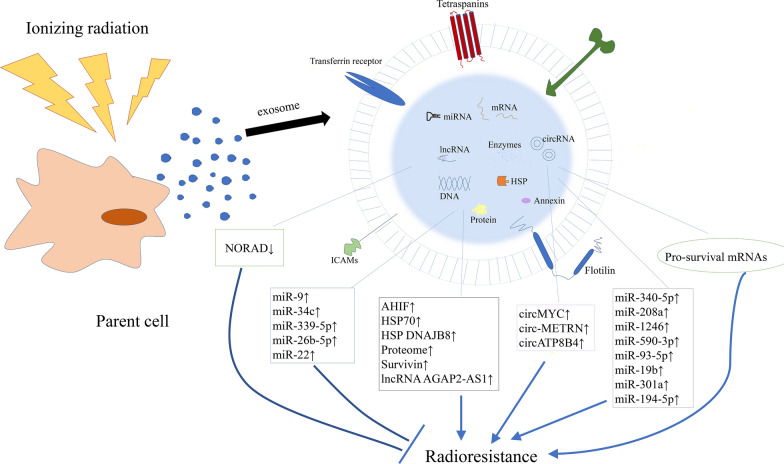
Fig. 3.
An overview of exosomes/exosomal cargo contents in the regulation of cancer radiosensitivity. Exosomes/exosomal cargo contents exert important function to regulate the radiosensitivity of cancer cells, via complicate interaction with multiple biological processes including DNA damage response, cell cycle and apoptosis, hypoxic tumor microenvironment, epithelial–mesenchymal transition, cancer stem cells and radiation-induced signaling pathways
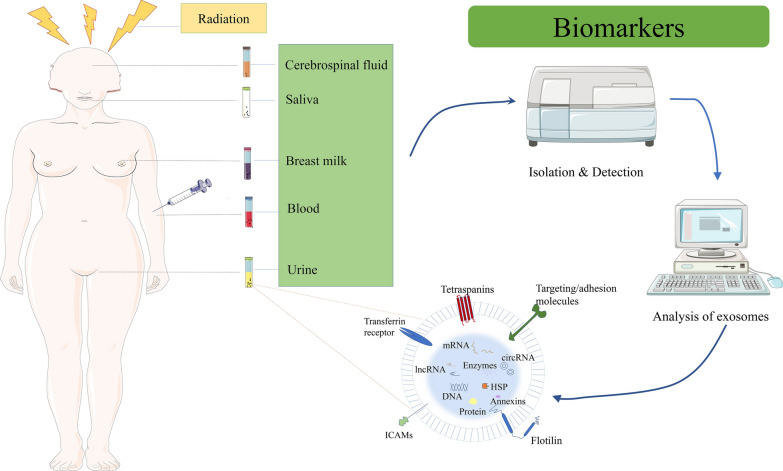
Fig. 4.
Exosomes can be isolated from body fluids, including cerebrospinal fluid, saliva, breast milk, blood, and urine (among others). Analysis of the contents of exosomes exposed to radiation, with altered levels of exosome content expression, which makes exosomes promising as a biomarker for early diagnosis and prognostic prediction of diseases
Table 1.
Roles of exosomes/exosomal cargo contents in regulating cancer radiosensitivity
| Exosomes/Exosomal contents | Cancer types | Mechanism | Function | References |
|---|---|---|---|---|
| Exosomes (↑) | HNSCC | Increase DNA double-strand break repair and promote proliferation | Promote radioresistance | [34] |
| Exosome miR-9 (↑) | HPV + HNSCC | Polarize macrophages into M1 phenotype via downregulating PPARγ | Promote radiosensitivity | [35] |
| Exosome miR-34c(↑) | NPC | Block the EMT process by directly targeting β-catenin | Promote radiosensitivity | [37] |
| Exosome circMYC (↑) | NPC | Sponge miR-20b-5p and let-7e-3p | Promote radioresistance | [38] |
| LMP1-positive exosomes (↑) | NPC | Stimulate p38 MAPK signaling through the exosomal transfer of LMP1 to recipient cells | Promote radioresistance | [30] |
| Exosome miR-340-5P(↑) | ESCC | Alleviate radiation-induced apoptosis and accelerate DNA damage repair by directly targeting KLF10 | Promote radioresistance | [29] |
| Exosome miR-339-5p(↑) | ESCC | Directly target Cdc25A | Promote radiosensitivity | [46] |
| Exosome NORAD(↓) | ESCC | Promote exosomal miR-199a-5p dispersion | Promote radiosensitivity | [47] |
| Exosome miR-208a(↑) | NSCLC | Target p21 with a corresponding activation of the AKT/mTOR pathway | Promote radioresistance | [48] |
| Exosome miR-1246(↑) | NSCLC | Target the DR5 | Promote radioresistance | [49] |
| Exosome miR-26b-5p(↑) | LUAD | Target ATF2 in DNA damage | Promote radiosensitivity | [53] |
| Exosome lncRNA AGAP2-AS1(↑) | NSCLC | Downregulate miR-296 and upregulate NOTCH2 | Promote radioresistance | [54] |
| Exosome HSP70(↑) | NSCLC | Synthesize hypoxia-related genes | Promote radioresistance | [55] |
| Exosomes(↑) | CRC | Promote colorectal cancer stem cell phenotype | Promote radioresistance | [61] |
| Exosome miR-590-3p(↑) | CRC | Regulate the CLCA4-dependent PI3K/Akt signaling pathway positively | Promote radioresistance | [62] |
| Exosome miR-93-5p(↑) | CRC | Downregulate FOXA1 and upregulate TGFB3 | Promote radioresistance | [63] |
| Exosome miR-19b(↑) | CRC | Target FBXWT to promote colorectal cancer stem cell stemness | Promote radioresistance | [64] |
| Exosome circ-0067835(↓) | CRC | Upregulate miR-296-5p and downregulate IGFIR | Promote radiosensitivity | [31] |
| Exosome AHIF (↑) | Glioblastoma | AHIF-mediated p53 downregulation and anti-apoptosis | Promote radioresistance | [77] |
| Exosome miR-301a (↑) | Glioblastoma | Directly target TCEAL7, TCEAL7 negatively regulate the Wnt/β-catenin pathway | Promote radioresistance | [78] |
| Exosome circ-METRN (↑) | Glioblastoma | MiR-4709-3p/GRB14/PDGFRα pathway | Promote radioresistance | [82] |
| Exosomes (↑) | Glioblastoma | Increase oncogenic miRNAs、mRNAs and pro-survival proteasome pathway;decrease levels of tumor-suppressive miRNAs and mRNAs | Promote radioresistance | [83] |
| Exosome circATP8B4(↑) | Glioblastoma | MiR766 sponge | Promote radioresistance | [84] |
| Exosomes (↑) | Neuroblastoma | Activate downstream dependent survival pathway | Promote radioresistance | [86] |
| Exosome miR-194-5p (↑) | Pancreatic cancer | Enhance DNA damage response in TRCs | Promote radioresistance | [87] |
| Exosomes (↑) | Prostate cancer | Induce autophagy | Promote radiosensitivity | [89, 90] |
| Exosome HSP DNAJB8(↑) | Renal cell carcinoma | Maintain RCC CSCs/CICs | Promote radioresistance | [92, 93] |
| Exosome survivin(↑) | cervical carcinoma | Bystander effect | Promote radioresistance | [95] |
| Exosome miR-22(↑) | cervical carcinoma | Upregulate apoptotic pathway | Promote radiosensitivity | [96] |
| Exosomes (↑) | Melanoma | Stimulate tumor cell death | Promote radiosensitivity | [97] |
| Exosomes (↑) | Breast cancer | Induce autophagy | Promote radiosensitivity | [89, 90] |
| Exosomes (↑) | Breast cancer | Paracrine and juxtacrine signaling | Promote radiosensitivity | [98] |
| Exosomes proteome (↑) | Breast cancer | Hypoxic microenvironments; upregulate pro-survival factors | Promote radioresistance | [99] |
| Exosomes (↑) | Breast cancer | Increase the activity of exosomal secretory pathway | Promote radioresistance | [79] |
HNSCC: Head and neck squamous cell cancer; NPC: Nasopharyngeal carcinoma; ESCC: Esophageal squamous carcinoma; NSCLC: Non—small cell lung cancer; LUAD: Lung adenocarcinoma; CRC: Colorectal cancer
Table 2.
Exosomes as diagnostic, prognostic or predictive biomarker in solid tumor
| Exosomes/Exosomal contents | Cancer types | Specimen origin | Biomarker function | References |
|---|---|---|---|---|
| Exosome circMYC | NPC | Serum | Differentiate radioresistant patients from patients with radiosensitive NPC | [38] |
| Exosome miR-339-5p | ESCC | Serum | Predict radiotherapeutic response | [46] |
| Exosome miR-96 | NSCLC | Plasma | Diagnostic and prognostic marker of radioresistant NSCLC | [50] |
| Exosome miR-378 | NSCLC | Serum | Predict radiotherapeutic response | [51] |
| Exosome miR-29a-3p miR-150-5p | NSCLC | Serum | Predict radiotherapeutic response; radiation-related | [52] |
| Exosome HSP70 | NSCLC | Plasma | Predict radiotherapeutic response | [55] |
| Exosome miR-663a | CRC | Plasma | Predict radiotherapeutic response | [67] |
| Exosome miR-574-3p | Glioblastoma | Serum | Predict radiotherapeutic response | [85] |
| Exosome miR-379-5p miR-654-3p | Prostate cancer | Serum | Predict radiotherapeutic response | [91] |
| Exosome proteome | Breast cancer | Body fluids | Differentiate radiation-resistant tumors | [99] |
Head and neck squamous cell carcinoma (HNSCC)
Over 95% of head and neck cancers are histopathologically classified as squamous epithelial cell carcinoma. At present, the tumor location in patients with HNSCC can be precisely detected using imaging technique, and better radiotherapy strategies have been developed. However, while not all patients with HNSCC exhibit the sensitivity to radiotherapy, a portion of the patients are resistant to the radiotherapy [33]. It has been shown that the exposure of HNSCC cells BHY and FaDu to γ-ray leads to an increased secretion of exosomes. Moreover, co-culture of BHY cells with the exosomes conferred the cells with radioresistance and promoted the proliferation of recipient cells within 6 h after administration with exosomes. In this case, the exosomes facilitated the radioresistance through promoting DNA double-strand break repair [34]. Meanwhile, studies have revealed that miR-9 enriched exosomes isolated from human papilloma virus (HPV) positive HNSCC patients can transform macrophages into M1 phenotype and facilitate the radiosensitivity of these patients [35]. Nasopharyngeal carcinoma (NPC) is a subtype of head and neck cancer frequently occurring in Southeast Asian countries and Southern China [36]. Wan et al. [37] reported that miR-34c-overexpressing exosomes inhibit malignant behaviors of NPC and restore its radioresistance. Exosomal miR-34c down-regulates epithelial-mesenchymal transformation in NPC by inhibiting β-catenin, enhancing the radiosensitivity. Luo et al. [38] collected and analyzed circulating exosomal circMYC samples from 210 NPC patients, and found that while circulating exosomal circMYC is highly expressed in the patients, the overexpression of circMYC promotes NPC cell proliferation as well as the radioresistance. It has been demonstrated that intercellular transport of LMP1 can occur via extracellular vesicles or exosomes, and exosome-mediated intercellular transport is closely related to the role of Epstein‐Barr virus(EBV) in carcinogenesis [39]. In addition, Zhang et al. [30] provided evidence that LMP1-containing exosomes derived from NPC cell line CNE1 can activate P38 MAPK signaling pathway, conferring radioresistance to recipient NPC cells. This finding confirms from a lateral perspective that a small portion of LMP1-expressing cells enhance the radioresistance of NPC cells presumably by affecting the infected host and regulating tumor microenvironment.
Esophageal cancer (EC)
EC is among malignant tumors seriously endangering people’s health and life. Radiotherapy has been a main treatment option for EC, particularly for cervical and upper thoracic EC with a relatively big surgical difficulty. Notably, EC patients vary greatly in the efficacy and prognosis of radiotherapy, suggesting a significant difference in sensitivity of esophageal cancer cells to radiotherapy among the individuals [40]. Hence, how to improve sensitivity of EC cells to radiotherapy has become a pivotal issue in the field of radiation oncology. Multiple studies have shown that exosomes derived from radiation exposed cells in the microenvironment facilitate the efficacy of radiotherapy possibly via the bystander effect and distal effect [41–44]. Hypoxia is a main reason to cause radioresistance. In this case, hypoxic tumor cells may release a certain type of exosome which are subsequently engulfed by the neighboring cells in the tumor environment, eliciting a series of biological changes [45]. In accordance with the above theory, Chen et al. [29] found that exosomes derived from hypoxic esophageal squamous cell carcinoma (ESCC) cells down-regulate the radiosensitivity of recipient cells through transporting miR-340-5p. Furthermore, miR-340-5p negatively regulates KLF10 and UVRAG, inhibiting ionizing radiation-induced apoptosis and facilitating DNA damage repair. Notably, Luo et al. [46] found that while miR-339-5p can be selectively secreted into blood via exosomes, the relatively high level of serum miR-339-5p is positively correlated with the radiosensitivity and survival rate. Moreover, exosome-derived miR-339-5p can mediate the radiosensitivity of ESCC by down-regulating Cdc25A and predict pathological response of locally advanced ESCC to pre-operative radiotherapy, indicating that the miR-339-5p may become a promising non-invasive biomarker. Besides, a high expression of lncRNA NORAD has been identified as an indicator of radioresistance for ESCC. While NORAD can be activated by radiation via enhanced enrichment of H3K4me2, highly expressed NORAD competitively binds to PUM1, decreasing the processing of pri-miR-199a1 and down-regulating the expression of miR-199a-5p. Knocking out NORAD in EC cells led to up-regulation of miR-199a-5p-targeted EEPD1 as well as down-regulation of ATR/Chk1 signaling in exosomes, thereby enhancing radiosensitivity of esophageal cancer cells [47].
Non-small cell lung cancer (NSCLC)
Radiotherapy or combined chemoradiotherapy is currently the main treatment option for patients with intermediate and advanced-stage NSCLC who lost the opportunity of radical surgery. Numerous studies have demonstrated that miRNA-containing exosomes act as a carrier for regulating radiosensitivity of tumor cells as well as a tool for monitoring the efficacy of radiotherapy. Tang et al. [48] reported that an alteration in serum miRNA expression pattern occurs in NSCLC patients after radiotherapy, including a significant increase in the level of serum miR-208a. Serum miR-208a-containing exosomes can be taken up by NSCLC cells, and miR-208a subsequently promotes the proliferation of the cancer cells and inhibits apoptosis through targeting p21 and AKT/mTOR pathway, thus affecting the radioresistance of cells. Yuan et al. [49] found that exosomes derived from irradiated tumor cells promote the radioresistance of recipient cells by transporting miR-1246. Zheng et al. [50] observed that the level of plasma miR-1246 and miR-96 is significantly increased in NSCLC patients compared with normal individuals, while the level of exosomal miR-96 in patients with radioresistant NSCLC is markedly higher than that in those with radiosensitive NSCLC. They, therefore, proposed that exosomal miR-96 could serve as a noninvasive diagnostic and prognostic biomarker for radioresistant NSCLC. In the meantime, Zhang et al. [51] showed that the level of serum exosomal miR-378 is markedly reduced in over 50% of NSCLC patients following radiotherapy, implying that miR-378 may act as an indicator for response of NSCLC patients to radiotherapy. It has also been demonstrated that among 752 exosomal miRNAs identified from patients with locally advanced NSCLC, the expression of miR29a-3p and miR150-5p is decreased with the increasing radiation dose [52]. Besides, exosomal miRNAs derived from tumor or stromal cells have been shown to play a role in the progression and therapy resistance of lung adenocarcinoma. Han et al. [53] proved that exosomal miR-26b-5p facilitates radiosensitivity of lung adenocarcinoma by regulating ATF2. Meanwhile, Zhang et al. [54] confirmed that exosomal lncRNA AGAP2-AS1 derived from M2 macrophages can enhance radioresistance of radiation-resistant NSCLC through down-regulating microRNA-296 and up-regulating NOTCH2. Ostheimer et al. [55] presented data showing that exosomal Hsp70 significantly enhances radioresistance of NSCLC.
Colorectal cancer (CRC)
CRC remains one of digestive tumors with the highest incidence. And radiotherapy is an important part of the comprehensive treatment of CRC. Previous studies have shown that the tumor microenvironment mediates the resistance to radiotherapy [56]. As an important component of the tumor environment, tumor-related fibroblasts are significantly involved in the resistance to radiotherapy [57–60]. Liu et al. [61] found that exosomes derived from tumor-related fibroblasts increase the stemness of CRC cells by activating TGF-β signaling pathway, enhancing radioresistance of the cancer cells. It has also been demonstrated that exosomal miR-590-3p derived from tumor-related fibroblasts enhances the radioresistance of CRC cells by regulating CLCA4-dependent PI3K/Akt signaling pathway in a positive feedback loop [62]. Likewise, Chen et al. [63] observed that tumor-related fibroblast derived exosomes for transporting miR-93-5p promote the proliferation and colony formation of tumor cells and inhibit apoptosis through down-regulating FOXA1 and up-regulating TGFB3, thereby enhancing the radioresistance of CRC cells. In the meantime, Sun et al. [64] proved that exosomal miR-19b derived from CRC cells inhibits the maintenance of CRC stem cells and enhances radiosensitivity of the cancer cells by activating Wnt/β-catenin signaling pathway via down-regulation of FBXW7. Besides, circRNA has been demonstrated to regulate radiosensitivity of CRC cells through exosome-mediated transport [65, 66]. Studies have revealed that serum exosomal circ_0067835 is remarkably upregulated in CRC patients following radiotherapy, while being transported via exosomes in the patients. Notably, a combined functional assay demonstrated that knocking out circ_0067835 regulates the expression of IGF1R by sponging miR-1236-3p, thus suppressing the progression of CRC and enhancing radiosensitivity of the cancer [31]. Moreover, Bjørnetrø et al. [67] showed that miR-663a is expressed in exosomes released from hypoxic CRC cells and provided evidence that exosomal miR-663a is related to radioresistance caused by hypoxic tumor environment.
Glioblastoma
Glioblastoma is the most common primary central nervous system malignant tumor [68]. While surgery is the main treatment option for glioblastoma, unsuccessful surgical resection often occurs mainly due to the fact that glioblastoma usually displays an invasive growth invading parenchyma [69, 70]. Hence, radiotherapy has become a main adjuvant therapy for glioblastoma patients [71]. However, radiation tolerance even radioresistance could significantly affect the treatment and prognosis of glioblastoma patients receiving radiotherapy because of the presence of individual variation and tumor heterogeneity [72–74]. It has been shown that radiation exposure can lead to a significant release of CTGF-containing exosomes, being involved in the tumor formation. Co-culture of CTGF-containing exosomes with unirradiated cells may cause the overexpression of CTGF in recipient cells. Moreover, radiation has been found to affect the abundance of exosomes; in particular, it may change the components of exosomes and facilitate the migratory phenotype during the uptake process [75]. Kucharzewska [76] found that exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Thereafter, Dai et al. [77] validated that AHIF is markedly up-regulated in cancerous tissue and radiation resistant glioblastoma, while AHIF promotes the progression and radioresistance of glioblastoma via exosomes. The above findings suggest that AHIF may serve as a biomarker for predicting the progression and radioresistance of glioblastoma, while exosomes could potentially become a therapeutic target for glioblastoma. Yue et al. [78] showed that exosomal miR-301a released from hypoxic glioblastoma cells activates Wnt/β-catenin signaling pathway through inhibiting the expression of tumor suppressor gene TCEAL7, ultimately facilitating the tumor radioresistance. Thus, exo-miR-301a/TCEAL7-signaling axis would most likely provide new ideas for overcoming radioresistance in glioblastoma patients. In the meantime, numerous studies have demonstrated that irradiation can not only promote the generation and transport of exosomes, but also facilitate the absorption of exosomes by other cells [79–81]. Wang et al. [82] provided the first demonstration that while circ-METRN is highly enriched in low-dose radiation-induced exosomes, circ-METRN in the exosomes exerts an effect on the progression and radioresistance of glioblastoma via miR-4709-3p/GRB14/PDGFRα pathway. Meanwhile, Mrowczynski et al. [83] observed that exosomes can increase the levels of carcinogenic miRNAs (miR-889), mRNAs and survival-promoting pathway proteins, while decreasing the levels of tumor suppressive miRNAs (miR-516 and miR-365) and mRNAs, thereby promoting the survival rate of cells exposed to irradiation. Besides, Zhao et al. [84] found that circATP8B4 in RR-U251 cell derived exosomes could be transported to glioma cells in which it sponges miR-766 to enhance radioresistance of the cells. Li et al. [85] demonstrated that radiotherapy leads to a marked decrease in the level of miR-574-3p in plasma exosomes, indicating that miR-574-3p could serve as an important biomarker for monitoring the efficacy of radiotherapy.
Other types of carcinomas
Neuroblastoma is characterized by insidious onset, high malignancy and extremely rapid progression. And radiotherapy is currently one of the breakthrough points in promoting the survival rate of neuroblastoma. Tortolici et al. [86] demonstrated that early release of the extracellular vesicles from irradiated neuroblastoma cells can induce the activation of the survival pathway, promoting the proliferation and invasion of recipient cells related to epithelial-mesenchymal transitions. Upon irradiation of these cells, EVs induce a cell cycle arrest at G2/M phase for DNA damage repair. The above process is an adaptive cellular response that is essential for the formation of a stronger phenotype of invasiveness and irradiation resistance.
Pancreatic cancer is a highly invasive malignancy. While radiotherapy plays a crucial role in the treatment of pancreatic cancer, it could lower the rate of local recurrence and increase the chance of surgical operation in locally advanced pancreatic cancer. Notably, pancreatic cancer cells are relatively insensitive to radiotherapy. Jiang et al. [87] reported that exosomes can facilitate the radioresistance through transporting proliferation-suppressing miRNAs. Strikingly, irradiation-induced dying tumor cells have been shown to regulate tumor cell repopulation. In this case, irradiated dying cells secret exosomes, induce a cell cycle arrest, and promote DNA damage repair, thus improving the survival capacity of ALDH1A1 + repopulated tumor cells.
Prostatic cancer is highly invasive, and radiotherapy is currently an effective approach for the treatment of prostatic cancer. Hurwitz et al. [88] comparatively analyzed prostatic cancer tissue specimens before and after radiotherapy and found that radiation exposure leads to a certain increase in the level of exosomal HSP72. Moreover, exosomes derived from prostatic cancer stem cells have been demonstrated to induce autophagy, while autophagy has been proved to regulate the sensitivity of tumor cells to radiotherapy [89, 90]. In addition, Yu et al. [91] showed that a number of miRNAs are detected in serum exosomes from prostatic cancer patients who underwent carbon ion radiotherapy, including miR-493-5p, miR-323a-3p, miR-411-5p, miR-494-3p, miR-379-5p, miR-654-3p, miR-409-3p, miR-543, and miR-200c-3p. Among these miRNAs, miR-379-5p and miR-654-3p could be used for predicting the sensitivity of tumor cells to carbon ion radiotherapy.
Renal cell carcinoma is among the most common malignancies in urinary system, and radiotherapy is an effective adjuvant therapy option for advanced renal cancer. It has been shown that exosomal Hsp DNAJB8, a Hsp40 family member, plays a certain role in maintaining radioresistance of CSCs/CICs in renal cell carcinoma [92, 93].
At present, radiotherapy is a main option for clinical treatment of intermediate and advanced cervical cancer. While a good curative effect could be achieved at the early stage of radiotherapy, the accumulating radiation dose at the later stage may lead to a decrease in sensitivity of cervical cancer cells to radiotherapy [94]. Proton beam therapy (PBT) is one type of radiotherapy, and exosomes have been recently reported to mediate PBT resistance. Proton-irradiated HeLa cervical cancer cells can secret exosomes containing a high level of survivin, facilitating tumor growth and resistance to the therapies [95]. It has been demonstrated that miR-22 enriched exosomes can enhance sensitivity of cervical cancer to radiotherapy in vitro possibly by promoting apoptosis. In this case, forced expression of miR-22 via gene transfection results in inhibited expression of MYCBP and a subsequent decrease in hTERT, thereby promoting radiosensitivity of cervical cancer cells [96].
Melanoma is a melanocyte derived highly malignant tumor with strong invasiveness, high metastasis rate and rapid progression. Clinically, radiotherapy is the important treatment option for surgically unresectable melanoma. Studies have shown that exosomes derived from mesenchymal stem cells can improve the efficacy of radiotherapy in melanoma via bystander effect and distal effect, thus preventing the metastasis and spread of melanoma cells [97].
Breast cancer is one of the most common carcinomas endangering women’s health, and radiotherapy has become the standard treatment for locally advanced breast cancer. It has been shown that crosstalk between stromal cells and breast cancer cells acts through paracrine and juxtacrine signals, and exosomal RNAs regulate the radioresistance through anti-viral STAT1/NOTCH3 pathway [98]. Meanwhile, studies have found that exosomes released from breast cancer stem cells can induce autophagy, while autophagy has been proved to regulate the sensitivity of cancer to radiotherapy [89, 90]. Thomas et al. [99] demonstrated that proteomic characteristics of tumor-related exosomes may reflect the oxygenation state of breast cancer cells, indicating that it can be used for identifying irradiation resistant tumor cells. Moreover, it has been reported that ionizing radiation increases the activity of exosome secretory pathway in breast cancer cell line MCF-7, probably resulting in radioresistance in the cancer cells [79].
Perspectives
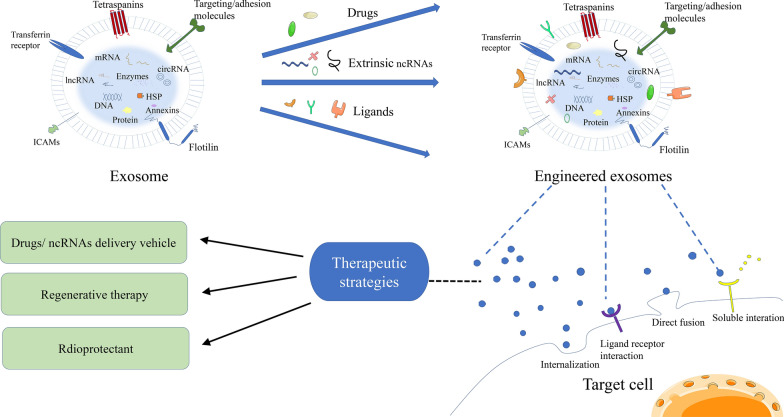
Radiation therapy remains an important part of cancer treatment, and most cancer patients receive radiotherapy during their illness. Yet, tolerance to radiotherapy or even radioresistance is the main reason for radiation therapy failure, and there is an urgent need for biomarkers that can predict the efficacy of radiation therapy to screen the superior treatment population and assist in rapid clinical adjustment of treatment plans through real-time monitoring. Numerous studies have shown that exosomes can mediate resistance to radiotherapy, and changes in the expression levels of their contents are closely related to treatment response. Conversely, radiation can also affect the production, secretion, composition and uptake of exosomes [100]. These solid research foundations enable exosomes promising as biomarkers for monitoring radiosensitivity (Fig. 3). In addition to being a liquid biopsy tool, exosomes themselves as natural nanoscale vesicles are also a research hotspot in the field of cancer formation, progression, migration, invasion, metastasis and treatment. A growing number of studies have confirmed that exosomes can promote tumor development, thus, inhibition of exosome biosynthesis, secretion and uptake [101] has anti-tumor potential [101]. Based on this scenario, the following measures could be taken: blocking the generation and release of exosomes as well as exosome-mediated intercellular communications, e.g. decreasing exosome production using small-molecule enzymes and protein inhibitors; blocking adhesion molecules on the surface of exosomes such as phosphatidylserine, intercellular adhesion molecule 1 and its receptor in order to effectively decrease the uptake of exosomes; or disturbing the downstream signaling pathway in recipient cells elicited by exosomes. All the above strategies would provide new directions for cancer therapy. In the meantime, exosomes can be used as delivery systems for therapeutic drugs such as drugs and various nucleic acids such as mRNA/miRNA and other non-coding RNA/interfering small RNA (siRNA), and are expected to become an important tool for malignant tumor treatment through precision drug delivery. Besides, nano-scale diameter, lipid bilayer structure, high biocompatibility, high stability and low immunogenicity of exosomes enable exosomes to persist for a longer time in blood circulation, thus promoting tissue-directed transport as well as uptake of encapsulated contents of exosomes by the recipient cells. In these cases, while exosomes can selectively penetrate tumor tissues by enhancing permeability and retention effect due to their nano-scale diameter, the presence of phospholipid bilayer structure in exosomes protects their contents from bioenzyme-mediated hydrolysis for maintaining the activities of various biomolecules. In addition, the high biocompatibility allows exosomes to evade immune monitoring and penetrate into the tissue. Exosome-based drug delivery is currently undergoing extensive clinical trials, with preliminary evidence of safety of delivery and tolerability in oncology patients. Moreover, exosomes have been shown to be pivotal in the prevention and treatment of radiation-induced tissue injury [102–104]. As the risk of radiation exposure increases, it can damage normal tissue and cause various side effects. Traditional radioprotectants have limitations, therefore, it is essential to develop new radioprotectors, just in time exosomes as radioprotective agents hold great promise. In particular, MSC-derived exosomes showed tremendous advantages in the treatment of refractory graft vs. host disease (Fig. 5) [105]. Together, exosomes could become an extremely promising research direction in tumor radiotherapy.
Fig. 5.
Clinical therapeutic strategies of exosomes in the field of cancer radiotherapy
To date, there have been content-rich research reports with novel entry points on exosomes at home and abroad. However, limited understanding of biogenesis, content sorting, subtype analysis, fluid transmission, target cell uptake, and content delivery of exosomes has greatly restricted accurate investigation on the role of exosomes in malignant tumor progression as well as individualized therapy [15]. The main challenges of exosome application in cancer therapy are as follows: how to effectively load exogenous ncRNAs or drugs into exosomes and further increase cell-specific delivery; how to prevent immune responses when utilizing non-autologous exosomes; how to prolong the half-life of modified exosomes in vivo, avoiding their rapid clearance; how to control the quality of exosomes administered to patients and the technical challenges associated with clinically graded production. Due to the complexity of exosome biology and these clinical challenges, it is critical to carefully establish standards for exosome quality and improve their effectiveness in vivo prior to their widespread use in clinical trials. Moreover, the mechanism underlying response of exosomes to ionizing radiation remains to be fully understood. Besides, the complete information of radiation-induced exosomes has yet to be fully unveiled. In short, there is still a long way to go for exosomes to achieve clinical translation, which requires the efforts of most researchers.
Conclusion
The clinical application of exosomes in the field of radiation therapy for malignant tumors is still in the initial stage. There are several challenges facing radiotherapy specialists in the coming years, including elucidation of the mechanism underlying radiation-induced formation of exosomes, a fully understanding of impacts of the exosomes on the microenvironment and cell signaling transduction, reducing the probability of radioresistance and metastasis of tumor cells, and promoting the efficacy of malignant tumor radiotherapy. We expect exosomes will be used in clinical application as soon as possible with the continuous progress of technology, so that more tumor patients can benefit from them.
Acknowledgements
Not applicable.
Abbreviations
- RIBE
Radiation-induced bystander effect
- RIAE
Radiation-induced abscopal effects
- AKT
Serine/threonine kinase 1
- ATF2
Activating transcription factor 2
- CRC
Colorectal cancer
- EBV
Epstein-Barr virus
- EC
Esophageal cancer
- EEPD1
Endonuclease/exonuclease/phosphatase family domain containing 1
- ESCC
Esophageal squamous cell carcinoma
- FBXW7
F-box and WD repeat domain containing 7
- FOXA1
Forkhead box A1
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human papilloma virus
- HSP72
Heat shock protein family A (Hsp70) member 1A
- IGF1R
Insulin like growth factor 1 receptor
- KLF10
Kruppel-like factor 10
- LMP1
Latent membrane protein 1
- MAPK
Mitogen-activated protein kinase
- NPC
Nasopharyngeal carcinoma
- NSCLC
Non-small cell lung cancer
- NOTCH2
Notch receptor 2
- PBT
Proton beam therapy
- PUM1
Pumilio RNA binding family member 1
- TCEAL7
Transcription elongation factor A like 7
- TGF-β
Transforming growth factor beta
- TGFB1
Transforming growth factor beta 3
- UVRAG
UV radiation resistance-associated gene
Author contributions
HHS, YJS and WDG conceived and designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81803036), China Postdoctoral Science Foundation (2021M700545), Natural science foundation of Jiangsu province (BK20180186), Scientific Research Project of Jiangsu Health Commission (H2019104), the Science and Technology Bureau Foundation Application Project of Changzhou (CJ20200117), Funding from Young Talent Development Plan of Changzhou Health Commission (CZQM2020010), Young Talent Science and Technology Project of Changzhou Municipal Health Commission (QN201906 and QN201804).
Availability of data and materials
The data supporting the conclusion of this review have been included within the article.
Declarations
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huihui Sun, Email: 20215235071@stu.suda.edu.cn.
Rui Sun, Email: rui198608@163.com.
Xing Song, Email: songxingjoy@163.com.
Wendong Gu, Email: guwendong1415@czfph.com.
Yingjie Shao, Email: shaoyingjie@suda.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Morales-Kastresana A, Welsh JA, Jones JC. Detection and Sorting of Extracellular Vesicles and Viruses Using nanoFACS. Curr Protoc Cytom. 2020;95(1):e81. doi: 10.1002/cpcy.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7(30):48832–48841. doi: 10.18632/oncotarget.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-Driven NSCLC: implication for optional immune targeted therapy for NSCLC Patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Aheget H, Tristan-Manzano M, Mazini L, Cortijo-Gutierrez M, Galindo-Moreno P, Herrera C, Martin F, Marchal JA, Benabdellah K. Exosome: a new player in translational nanomedicine. J Clin Med. 2020;9:8. doi: 10.3390/jcm9082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10(1):3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni J, Bucci J, Malouf D, Knox M, Graham P, Li Y. Exosomes in Cancer Radioresistance. Front Oncol. 2019;9:869. doi: 10.3389/fonc.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–9420. doi: 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 10.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 13.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med. 2018;379(10):958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 15.Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya T, Yashiro M, Hirakawa K, Kosaka T, Makino H, Akiyama H, Kunisaki C, et al. Exosomal miRNAs from Peritoneum Lavage Fluid as Potential Prognostic Biomarkers of Peritoneal Metastasis in Gastric Cancer. PLoS ONE. 2015;10(7):e0130472. doi: 10.1371/journal.pone.0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013;1:e201. doi: 10.7717/peerj.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang MK, Wong AS. Exosomes: Emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015;367(1):26–33. doi: 10.1016/j.canlet.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Morad G, Carman CV, Hagedorn EJ, Perlin JR, Zon LI, Mustafaoglu N, Park TE, Ingber DE, Daisy CC, Moses MA. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13(12):13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, Zhang M, Zheng L, Liang Q, Li H, Chen JT, Guo H, Yoshina S, Chen YZ, Zhao X, et al. Cysteine protease cathepsin B mediates radiation-induced bystander effects. Nature. 2017;547(7664):458–462. doi: 10.1038/nature23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Ma H, Lv C, Lan F, Wang Y, Deng Y. Exosomes and exosomal microRNA in non-targeted radiation bystander and abscopal effects in the central nervous system. Cancer Lett. 2021;499:73–84. doi: 10.1016/j.canlet.2020.10.049. [DOI] [PubMed] [Google Scholar]
- 27.Tuncay Cagatay S, Mayah A, Mancuso M, Giardullo P, Pazzaglia S, Saran A, Daniel A, Traynor D, Meade AD, Lyng F, et al. Phenotypic and functional characteristics of exosomes derived from irradiated mouse organs and their role in the mechanisms driving non-targeted effects. Int J Mol Sci. 2020;21:21. doi: 10.3390/ijms21218389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Abedi R, Tuncay Cagatay S, Mayah A, Brooks SA, Kadhim M. Ionising Radiation Promotes Invasive Potential of Breast Cancer Cells: The Role of Exosomes in the Process. Int J Mol Sci. 2021;22:21. doi: 10.3390/ijms222111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen F, Xu B, Li J, Yang X, Gu J, Yao X, Sun X. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. 2021;40(1):38. doi: 10.1186/s13046-021-01834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Yu X, Zhou Z, Li B, Peng J, Wu X, Luo X, Yang L. LMP1-positive extracellular vesicles promote radioresistance in nasopharyngeal carcinoma cells through P38 MAPK signaling. Cancer Med. 2019;8(13):6082–6094. doi: 10.1002/cam4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P, Sun Y, Yang Y, Chen Y, Liu H. Circ_0067835 Knockdown Enhances the Radiosensitivity of Colorectal Cancer by miR-296-5p/IGF1R Axis. Onco Targets Ther. 2021;14:491–502. doi: 10.2147/OTT.S281011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, Wang F, Li X, Liu Q, Li Y, et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology. 2019;70(1):241–258. doi: 10.1002/hep.30607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akervall J, Nandalur S, Zhang J, Qian CN, Goldstein N, Gyllerup P, Gardinger Y, Alm J, Lorenc K, Nilsson K, et al. A novel panel of biomarkers predicts radioresistance in patients with squamous cell carcinoma of the head and neck. Eur J Cancer. 2014;50(3):570–581. doi: 10.1016/j.ejca.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Mutschelknaus L, Peters C, Winkler K, Yentrapalli R, Heider T, Atkinson MJ, Moertl S. Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS ONE. 2016;11(3):e0152213. doi: 10.1371/journal.pone.0152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong F, Mao X, Zhang S, Xie H, Yan B, Wang B, Sun J, Wei L. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020;478:34–44. doi: 10.1016/j.canlet.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 36.Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, Soerjomataram I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. doi: 10.3322/caac.21384. [DOI] [PubMed] [Google Scholar]
- 37.Wan FZ, Chen KH, Sun YC, Chen XC, Liang RB, Chen L, Zhu XD. Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma. J Transl Med. 2020;18(1):12. doi: 10.1186/s12967-019-02203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, Ma J, Liu F, Guo J, Gui R. Diagnostic value of exosomal circMYC in radioresistant nasopharyngeal carcinoma. Head Neck. 2020;42(12):3702–3711. doi: 10.1002/hed.26441. [DOI] [PubMed] [Google Scholar]
- 39.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Liu J, Zhang W, Deng W, Yue J. Treatment of esophageal cancer with radiation therapy -a pan-Chinese survey of radiation oncologists. Oncotarget. 2017;8(21):34946–34953. doi: 10.18632/oncotarget.16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res. 2012;177(5):539–545. doi: 10.1667/RR2868.1. [DOI] [PubMed] [Google Scholar]
- 42.Al-Mayah A, Bright S, Chapman K, Irons S, Luo P, Carter D, Goodwin E, Kadhim M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat Res. 2015;772:38–45. doi: 10.1016/j.mrfmmm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng W, Hao Y, He C, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer. 2019;18(1):57. doi: 10.1186/s12943-019-0982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo A, Zhou X, Shi X, Zhao Y, Men Y, Chang X, Chen H, Ding F, Li Y, Su D, et al. Exosome-derived miR-339-5p mediates radiosensitivity by targeting Cdc25A in locally advanced esophageal squamous cell carcinoma. Oncogene. 2019;38(25):4990–5006. doi: 10.1038/s41388-019-0771-0. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Wang J, Ma Y, Li J, Sun X, Zhao X, Shi X, Hu Y, Qu F, Zhang X. Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. J Exp Clin Cancer Res. 2021;40(1):306. doi: 10.1186/s13046-021-02084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He Y, Chen G, Zhou Q, Wang W, Zhou X, et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res. 2016;35:7. doi: 10.1186/s13046-016-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan D, Xu J, Wang J, Pan Y, Fu J, Bai Y, Zhang J, Shao C. Extracellular miR-1246 promotes lung cancer cell proliferation and enhances radioresistance by directly targeting DR5. Oncotarget. 2016;7(22):32707–32722. doi: 10.18632/oncotarget.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Q, Ding H, Wang L, Yan Y, Wan Y, Yi Y, Tao L, Zhu C. Circulating Exosomal miR-96 as a Novel Biomarker for Radioresistant Non-Small-Cell Lung Cancer. J Oncol. 2021;2021:5893981. doi: 10.1155/2021/5893981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Xu H. Serum exosomal miR-378 upregulation is associated with poor prognosis in non-small-cell lung cancer patients. J Clin Lab Anal. 2020;34(6):e23237. doi: 10.1002/jcla.23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dinh TK, Fendler W, Chalubinska-Fendler J, Acharya SS, O'Leary C, Deraska PV, D'Andrea AD, Chowdhury D, Kozono D. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat Oncol. 2016;11:61. doi: 10.1186/s13014-016-0636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han F, Huang D, Huang X, Wang W, Yang S, Chen S. Exosomal microRNA-26b-5p down-regulates ATF2 to enhance radiosensitivity of lung adenocarcinoma cells. J Cell Mol Med. 2020;24(14):7730–7742. doi: 10.1111/jcmm.15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang F, Sang Y, Chen D, Wu X, Wang X, Yang W, Chen Y. M2 macrophage-derived exosomal long non-coding RNA AGAP2-AS1 enhances radiotherapy immunity in lung cancer by reducing microRNA-296 and elevating NOTCH2. Cell Death Dis. 2021;12(5):467. doi: 10.1038/s41419-021-03700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ostheimer C, Gunther S, Bache M, Vordermark D, Multhoff G. Dynamics of heat shock protein 70 serum levels as a predictor of clinical response in non-small-cell lung cancer and correlation with the hypoxia-related marker osteopontin. Front Immunol. 2017;8:1305. doi: 10.3389/fimmu.2017.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji X, Ji J, Shan F, Zhang Y, Chen Y, Lu X. Cancer-associated fibroblasts from NSCLC promote the radioresistance in lung cancer cell lines. Int J Clin Exp Med. 2015;8(5):7002–7008. [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai KK, Stuart J, Chuang YY, Little JB, Yuan ZM. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiat Res. 2009;172(3):306–313. doi: 10.1667/RR1764.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verset L, Tommelein J, Moles Lopez X, Decaestecker C, Boterberg T, De Vlieghere E, Salmon I, Mareel M, Bracke M, De Wever O, et al. Impact of neoadjuvant therapy on cancer-associated fibroblasts in rectal cancer. Radiother Oncol. 2015;116(3):449–454. doi: 10.1016/j.radonc.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y, Wang R, Qiao M, Xu Y, Guan W, Wang L. Cancer associated fibroblasts tailored tumor microenvironment of therapy resistance in gastrointestinal cancers. J Cell Physiol. 2018;233(9):6359–6369. doi: 10.1002/jcp.26433. [DOI] [PubMed] [Google Scholar]
- 61.Liu L, Zhang Z, Zhou L, Hu L, Yin C, Qing D, Huang S, Cai X, Chen Y. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp Cell Res. 2020;391(2):111956. doi: 10.1016/j.yexcr.2020.111956. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, Liu Y, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Liu J. Exosomal miR-590-3p derived from cancer-associated fibroblasts confers radioresistance in colorectal cancer. Mol Ther Nucleic Acids. 2021;24:113–126. doi: 10.1016/j.omtn.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Ge H, Liu Y. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 2020;39(1):65. doi: 10.1186/s13046-019-1507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun T, Yin YF, Jin HG, Liu HR, Tian WC. Exosomal microRNA-19b targets FBXW7 to promote colorectal cancer stem cell stemness and induce resistance to radiotherapy. Kaohsiung J Med Sci. 2021;89:e45. doi: 10.1002/kjm2.12449. [DOI] [PubMed] [Google Scholar]
- 65.Du S, Zhang P, Ren W, Yang F, Du C. Circ-ZNF609 accelerates the radioresistance of prostate cancer cells by promoting the glycolytic metabolism through miR-501-3p/HK2 Axis. Cancer Manag Res. 2020;12:7487–7499. doi: 10.2147/CMAR.S257441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin Y, Su Z, Sheng H, Li K, Yang B, Li S. Circ_0086720 knockdown strengthens the radiosensitivity of non-small cell lung cancer via mediating the miR-375/SPIN1 axis. Neoplasma. 2021;68(1):96–107. doi: 10.4149/neo_2020_200331N333. [DOI] [PubMed] [Google Scholar]
- 67.Tonje Bjørnetrø KRH, Sebastian M, Rampradeep S, Lars GL, Caroline J, Linda S, Nirujah ST, Kathrine RR, Anne HR. Abstract 4514: Low release of exosomal miR-663a from hypoxic tumor cells and poor tumor response to neoadjuvant radiotherapy in rectal cancer patients. Mol Cell Biol Genet. 2017;89:33. [Google Scholar]
- 68.Sun L, Joh DY, Al-Zaki A, Stangl M, Murty S, Davis JJ, Baumann BC, Alonso-Basanta M, Kaol GD, Tsourkas A, et al. Theranostic application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J Biomed Nanotechnol. 2016;12(2):347–356. doi: 10.1166/jbn.2016.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157(3):580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caruso C, Carcaterra M, Donato V. Role of radiotherapy for high grade gliomas management. J Neurosurg Sci. 2013;57(2):163–169. [PubMed] [Google Scholar]
- 72.Orzan F, De Bacco F, Crisafulli G, Pellegatta S, Mussolin B, Siravegna G, D'Ambrosio A, Comoglio PM, Finocchiaro G, Boccaccio C. Genetic evolution of glioblastoma stem-like cells from primary to recurrent tumor. Stem Cells. 2017;35(11):2218–2228. doi: 10.1002/stem.2703. [DOI] [PubMed] [Google Scholar]
- 73.Godlewski J, Ferrer-Luna R, Rooj AK, Mineo M, Ricklefs F, Takeda YS, Nowicki MO, Salinska E, Nakano I, Lee H, et al. MicroRNA signatures and molecular subtypes of glioblastoma: the role of extracellular transfer. Stem Cell Reports. 2017;8(6):1497–1505. doi: 10.1016/j.stemcr.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abou-El-Ardat K, Seifert M, Becker K, Eisenreich S, Lehmann M, Hackmann K, Rump A, Meijer G, Carvalho B, Temme A, et al. Comprehensive molecular characterization of multifocal glioblastoma proves its monoclonal origin and reveals novel insights into clonal evolution and heterogeneity of glioblastomas. Neuro Oncol. 2017;19(4):546–557. doi: 10.1093/neuonc/now231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arscott WT, Tandle AT, Zhao S, Shabason JE, Gordon IK, Schlaff CD, Zhang G, Tofilon PJ, Camphausen KA. Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl Oncol. 2013;6(6):638–648. doi: 10.1593/tlo.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai X, Liao K, Zhuang Z, Chen B, Zhou Z, Zhou S, Lin G, Zhang F, Lin Y, Miao Y, et al. AHIF promotes glioblastoma progression and radioresistance via exosomes. Int J Oncol. 2019;54(1):261–270. doi: 10.3892/ijo.2018.4621. [DOI] [PubMed] [Google Scholar]
- 78.Yue X, Lan F, Xia T. Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/beta-catenin signaling and promotes radiation resistance by targeting TCEAL7. Mol Ther. 2019;27(11):1939–1949. doi: 10.1016/j.ymthe.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jabbari N, Nawaz M, Rezaie J. Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. Int J Mol Sci. 2019;20:15. doi: 10.3390/ijms20153649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mutschelknaus L, Azimzadeh O, Heider T, Winkler K, Vetter M, Kell R, Tapio S, Merl-Pham J, Huber SM, Edalat L, et al. Radiation alters the cargo of exosomes released from squamous head and neck cancer cells to promote migration of recipient cells. Sci Rep. 2017;7(1):12423. doi: 10.1038/s41598-017-12403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hazawa M, Tomiyama K, Saotome-Nakamura A, Obara C, Yasuda T, Gotoh T, Tanaka I, Yakumaru H, Ishihara H, Tajima K. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem Biophys Res Commun. 2014;446(4):1165–1171. doi: 10.1016/j.bbrc.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Cao Q, Shi Y, Wu X, Mi Y, Liu K, Kan Q, Fan R, Liu Z, Zhang M. Identification of low-dose radiation-induced exosomal circ-METRN and miR-4709-3p/GRB14/PDGFRalpha pathway as a key regulatory mechanism in Glioblastoma progression and radioresistance: Functional validation and clinical theranostic significance. Int J Biol Sci. 2021;17(4):1061–1078. doi: 10.7150/ijbs.57168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mrowczynski OD, Madhankumar AB, Sundstrom JM, Zhao Y, Kawasawa YI, Slagle-Webb B, Mau C, Payne RA, Rizk EB, Zacharia BE, et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget. 2018;9(90):36083–36101. doi: 10.18632/oncotarget.26300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao M, Xu J, Zhong S, Liu Y, Xiao H, Geng L, Liu H. Expression profiles and potential functions of circular RNAs in extracellular vesicles isolated from radioresistant glioma cells. Oncol Rep. 2019;41(3):1893–1900. doi: 10.3892/or.2019.6972. [DOI] [PubMed] [Google Scholar]
- 85.Li Z, Ye L, Wang L, Quan R, Zhou Y, Li X. Identification of miRNA signatures in serum exosomes as a potential biomarker after radiotherapy treatment in glioma patients. Ann Diagn Pathol. 2020;44:151436. doi: 10.1016/j.anndiagpath.2019.151436. [DOI] [PubMed] [Google Scholar]
- 86.Tortolici F, Vumbaca S, Incocciati B, Dayal R, Aquilano K, Giovanetti A, Rufini S. Ionizing Radiation-Induced Extracellular Vesicle Release Promotes AKT-Associated Survival Response in SH-SY5Y Neuroblastoma Cells. Cells. 2021;10:1. doi: 10.3390/cells10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang MJ, Chen YY, Dai JJ, Gu DN, Mei Z, Liu FR, Huang Q, Tian L. Dying tumor cell-derived exosomal miR-194-5p potentiates survival and repopulation of tumor repopulating cells upon radiotherapy in pancreatic cancer. Mol Cancer. 2020;19(1):68. doi: 10.1186/s12943-020-01178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hurwitz MD, Kaur P, Nagaraja GM, Bausero MA, Manola J, Asea A. Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer. Radiother Oncol. 2010;95(3):350–358. doi: 10.1016/j.radonc.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar D, Gupta D, Shankar S, Srivastava RK. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget. 2015;6(5):3280–3291. doi: 10.18632/oncotarget.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li L, Liu WL, Su L, Lu ZC, He XS. The Role of Autophagy in Cancer Radiotherapy. Curr Mol Pharmacol. 2020;13(1):31–40. doi: 10.2174/1874467212666190809154518. [DOI] [PubMed] [Google Scholar]
- 91.Yu Q, Li P, Weng M, Wu S, Zhang Y, Chen X, Zhang Q, Shen G, Ding X, Fu S. Nano-vesicles are a potential tool to monitor therapeutic efficacy of carbon ion radiotherapy in prostate cancer. J Biomed Nanotechnol. 2018;14(1):168–178. doi: 10.1166/jbn.2018.2503. [DOI] [PubMed] [Google Scholar]
- 92.Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H, Morita R, et al. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012;72(11):2844–2854. doi: 10.1158/0008-5472.CAN-11-3062. [DOI] [PubMed] [Google Scholar]
- 93.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci U S A. 2015;112(19):E2497–2506. doi: 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhen X, Chen J, Zhong Z, Hrycushko B, Zhou L, Jiang S, Albuquerque K, Gu X. Deep convolutional neural network with transfer learning for rectum toxicity prediction in cervical cancer radiotherapy: a feasibility study. Phys Med Biol. 2017;62(21):8246–8263. doi: 10.1088/1361-6560/aa8d09. [DOI] [PubMed] [Google Scholar]
- 95.Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Konishi H, Hayashi M, Taniguchi K, Nakamura M, Kuranaga Y, Ito Y, Kondo Y, Sasaki H, Terai Y, Akao Y, et al. The therapeutic potential of exosomal miR-22 for cervical cancer radiotherapy. Cancer Biol Ther. 2020;21(12):1128–1135. doi: 10.1080/15384047.2020.1838031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Araujo FV, O'Valle F, Serrano-Saenz S, Anderson P, Andres E, Lopez-Penalver J, Tovar I, Nieto A, Santos A, Martin F, et al. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer. 2018;17(1):122. doi: 10.1186/s12943-018-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomas SN, Liao Z, Clark D, Chen Y, Samadani R, Mao L, Ann DK, Baulch JE, Shapiro P, Yang AJ. Exosomal proteome profiling: a potential multi-marker cellular phenotyping tool to characterize hypoxia-induced radiation resistance in breast cancer. Proteomes. 2013;1(2):87–108. doi: 10.3390/proteomes1020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian T, Liang R, Erel-Akbaba G, Saad L, Obeid PJ, Gao J, Chiocca EA, Weissleder R, Tannous BA. Immune Checkpoint Inhibition in GBM primed with radiation by engineered extracellular vesicles. ACS Nano. 2022;16(2):1940–1953. doi: 10.1021/acsnano.1c05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang T, Jian Z, Baskys A, Yang J, Li J, Guo H, Hei Y, Xian P, He Z, Li Z, et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials. 2020;257:120264. doi: 10.1016/j.biomaterials.2020.120264. [DOI] [PubMed] [Google Scholar]
- 103.Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF, Ricardo SD. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24(7):1290–1301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zuo R, Liu M, Wang Y, Li J, Wang W, Wu J, Sun C, Li B, Wang Z, Lan W, et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/beta-catenin signaling. Stem Cell Res Ther. 2019;10(1):30. doi: 10.1186/s13287-018-1121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusion of this review have been included within the article.