Abstract
Background
Low levels of high-density lipoprotein cholesterol (HDL-C) and diabetes are common in patients undergoing peritoneal dialysis (PD). The aim of this study was to investigate the association between the coexistence of diabetes with a low level of HDL-C and the first episode of peritoneal dialysis-related peritonitis (PDRP) in patients with PD.
Methods
We retrospectively investigated patients with PD from January 1, 2003, to May 31, 2020, in four PD centers. Patients with PD were divided into four groups: no comorbidities, low HDL-C only, diabetes only, and diabetes plus low HDL-C. The clinical and laboratory baseline data of the four groups were collected and compared. The association between diabetes coexisting with low HDL-C levels and the first episode of PDRP was analyzed by multivariate Cox regression analysis.
Results
A total of 1013 patients with PD were included in our study. The mean age was 49.94 ± 14.32 years, and 597 (58.99%) patients were males. A total of 301 (29.7%) patients had their first episodes of PDRP, and low HDL-C levels coexisted with diabetes in 72 patients with PD. After adjusting for confounding factors, a low level of HDL-C coexisting with diabetes was significantly associated with the first episode of PDRP in our study (hazard ratio: 2.81, 95% CI 1.32 ~ 4.73, p = 0.005). The associations among HDL-C, diabetes and PDRP were consistent in the following subgroups: sex, age, and pre-existing CVD (all P interaction > 0.05).
Conclusions
Patients with both diabetes and low HDL-C levels were at higher risk for PDRP in patients with PD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-022-00832-x.
Keywords: Peritoneal dialysis, High-density lipoprotein cholesterol, Diabetes, Peritonitis, Infection
Background
Peritoneal dialysis (PD) is an important renal replacement therapy. Peritoneal dialysis-related peritonitis (PDRP) is associated with mortality and technical failure in patients with PD [1, 2]. Diabetic nephropathy has risen in recent years in China [3]. Patients with diabetes are at increased risk for bacterial infection [4] Whether diabetes is associated with PDRP in patients with PD is controversial. In some previous studies, diabetes was identified as an independent risk factor for PDRP in patients with PD [5, 6]. In contrast, diabetes was not identified as an independent risk factor for PDRP in other previous reports [7] Dyslipidemia is common in patients with diabetes. It has been reported that a low level of high-density lipoprotein cholesterol (HDL-C) is a risk factor for infection in patients with diabetes [8].
Low levels of HDL-C are a manifestation of dyslipidemia in patients with PD and have been associated with mortality and cardiovascular disease (CVD) in patients with PD in many reports [9, 10]. HDL-C protects patients from serious infection, and low levels of HDL-C are also a risk factor for adverse outcomes in sepsis [11]. It has been reported that 50% of people with type 2 diabetes have low HDL-C concentrations [12]. Since diabetes was not necessarily an independent risk factor for PDRP, we presumed that diabetes coexisting with low levels of HDL-C might be associated with PDRP in patients with PD. In this study, we investigated the association diabetes coexisting low HDL-C levels with the first episode of PDRP in patients with PD.
Methods
Patients
Patients were recruited from four peritoneal dialysis centers in three provinces in China in this retrospective multiple-center study. Our study included adult patients aged ≥ 18 with PD recruited from January 1, 2003, to May 31, 2020. These patients received continuous ambulatory peritoneal dialysis (CAPD) with a standard glucose solution. Patients were excluded if they were on PD for < 3 months or had no lipid testing.
Data collection and clinical definitions
The demographic and clinical characteristics, including age, sex, weight, height, blood pressure, history of smoking and alcohol use, pre-existing CVD, pre-existing stroke, residual urinary volume, use of statins, and laboratory test results, were recorded at baseline by at least two trained nurses. Laboratory characteristics included routine blood tests, biochemical tests, kidney and liver function tests, and lipid levels. These records were rechecked by at least two trained doctors.
Diabetes was defined as follows: (1) fasting plasma glucose ≥ 7.0 mmol/L, (2) 2 h plasma glucose ≥ 11.1 mmol/L during an oral glucose tolerance test (OGTT),o (3) glycated hemoglobin (HbA1c) ≥ 6.5%, (4) diabetes symptoms plus random plasma glucose ≥ 11.1 mmol/L, or (5) the use of glucose-lowering drugs. Diabetes symptoms are polydipsia, polyuria, polyphagia and unexplained weight loss. If patients had no symptoms of diabetes and only once had hyperglycemia, criteria 1 to 3 were confirmed by repeated testing [13]. A low level of HDL-C was defined as < 1.0 mmol/L according to Chinese guidelines on the prevention and treatment of dyslipidemia in adults [14].
A diagnosis of the first episode of PDRP was made if the patient had at least two of the following criteria according to the 2017 ISPD guidelines [15]: (1) abdominal pain with or without cloudy peritoneal dialysis effluent and with or without fever, (2) total leukocyte count of the dialysis effluent ≥ 100 × 106 cells/L, with more than 50% polymorphonuclear cells in the differential count, and (3) positive Gram staining or culture of peritoneal dialysis effluent.
Outcomes and follow-up
The outcome of our study was the first episode of PDRP. Patients with PD routinely returned to each center and were tested every 3 months in each center. If patients did not return, they received telephone interviews. September 1, 2020, was the final follow-up date in this study. Patients without PDRP were followed up until death or PD cessation. The time at which patients received hemodialysis, kidney transplantation, transferred care to another dialysis center, or were lost to follow-up were also recorded.
Statistical analysis
Quantitative data are presented as the mean ± standard deviation (SD) or median (interquartile range [IQR]) after testing for normality. Nominal data are described as percentages. Baseline patient characteristics were compared for each group by chi-squared, one-way ANOVA, or Kruskal–Wallis tests. Univariate Cox regression analysis was used for the preliminary exploration of variables to estimate hazard ratios (HRs) with 95% confidence intervals (95% CIs) for the first episode of PDRP.
Survival curves and the time to peritonitis were calculated using the Kaplan–Meier method. Multivariate Cox regression analysis was conducted to examine the association between diabetes coexisting with low HDL-C levels and the first episode of PDRP using the following models: Model 1, unadjusted; Model 2, Model 1 plus demographic and clinical characteristics; and Model 3, Model 2 plus laboratory variables and medications. Since other outcomes, such as death, renal transplantation, transferred to hemodialysis and transfer to other centre, may influence standard regression test results for peritonitis, further analyses were done taking the competing risk of these outcomes into consideration. Competing-risks regression analysis was performed and sub-hazard ratios (SHR) was presented using Fine and Gray's competing risk regression analysis. Subgroups of sex, age, and history of pre-existing CVD were also analyzed. A p value across groups and the interactions between sex, age, and history of pre-existing CVD and PDRP were examined. The results are presented as HRs and 95% confidence intervals (95% CI). P values were two-sided, and P < 0.05 was considered to be statistically significant. All statistical analyses were performed with SPSS statistical software (version 21.0; Chicago, IL, USA), R (http://www.R-project.org), EmpowerStats software (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA), and Stata software (version 16; StataCorp, USA).
Results
Clinical baseline data of enrolled patients
A total of 1307 patients were included in our study; they were recruited from four peritoneal dialysis centers from January 1, 2003, to May 31, 2020. The patients with diabetes in our study had type 2 diabetes. None of the patients were diagnosed with type 1 diabetes. A total of 145 patients were excluded due to no available lipid level results. Twenty-two patients were excluded because they were younger than 18 years. A total of 127 patients were excluded because the duration of follow-up was < 3 months. The remaining 1013 patients were analyzed in our study (Fig. 1).
Fig. 1.
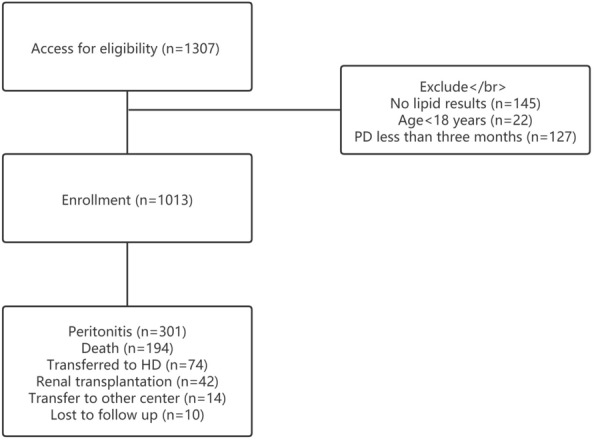
Process of patients inclusion
Patients were divided into four groups according to their HDL-C levels and the presence of diabetes: Group 0 (no comorbidity), Group 1 (low HDL-C only), Group 2 (DM only), and Group 3 (both DM and low HDL-C). A total of 472 patients (46.6%) were assigned to Group 0, 374 patients (36.9%) were assigned to Group 1, 95 patients (9.4%) were assigned to Group 2, and 72 patients (7.1%) were assigned to Group 3. A total of 167 patients (Group 2 plus Group 3, 16.5%) had diabetes. A total of 446 patients (Group 1 plus Group 3, 44.0%) had low HDL levels. The baseline demographics, clinical and laboratory characteristics, and medications are summarized in Table 1. The mean age was 49.94 ± 14.32 years old, and 597 patients (58.99%) were male. A total of 193 patients (19.05%) had a history of current smoking, 71 (7.01%) had a history of current alcohol consumption, and 39 (3.85%) had pre-existing stroke. A total of 104 patients (10.27%) had pre-existing CVD, and 145 (14.31%) received statin therapy prior to PD. There were no differences in serum calcium or 24 h urine volume among the four groups. Age and BMI were higher in the DM plus low HDL-C level group. The incidence of pre-existing stroke and pre-existing CVD was also higher in the DM plus low HDL-C level group. WBC and triglyceride levels were higher in the DM plus low HDL-C level group. Serum potassium was lower in the DM plus low HDL-C level group.
Table 1.
Baseline demographic characteristics, medications, and laboratory parameters
| Variablesa | Total (n = 1013) | No comorbidity (n = 472) | Low HDL-C (n = 374) | DM (n = 95) | DM plus low HDL-C (n = 72) | P-value |
|---|---|---|---|---|---|---|
| Age (years)b | 49.94 ± 14.32 (n = 1013) | 47.89 ± 14.04 | 47.98 ± 14.20 | 59.67 ± 9.93 | 60.74 ± 11.56 | < 0.001 |
| Male [n (%)] | 597 (59.0%, n = 1013) | 241(51.1%) | 267 (71.4%) | 51 (53.7%) | 38 (53.5%) | < 0.001 |
| Body mass index (kg/m2)b | 22.81 ± 3.23 (n = 994) | 22.04 ± 2.98 | 23.34 ± 3.36 | 23.76 ± 3.14 | 23.91 ± 3.10 | < 0.001 |
| Residual urine volume (mL)c | 800.00 (400.00–1245.00, n = 619) | 750.00 (400.00–1200.00) | 810.00 (450.00–1338.75) | 700.00 (487.50–1200.00) | 800.00 (325.00–1045.00) | 0.245 |
| Current smoking [n (%)] | 193 (19.1%, n = 1013) | 72 (15.3%) | 89 (23.8%) | 19 (20.0%) | 13 (18.1%) | 0.019 |
| Current alcohol consumption [n (%)] | 71 (7.0%, n = 1013) | 21 (4.5%) | 39 (10.4%) | 7 (7.4%) | 4 (5.6%) | 0.002 |
| Pre-existing stroke [n (%)] | 39 (3.9%, n = 1013) | 6 (1.3%) | 15 (4.0%) | 9 (9.5%) | 9 (12.5%) | < 0.001 |
| Pre-existing CVD [n (%)] | 104 (10.3%, n = 1013) | 21 (4.5%) | 24 (6.4%) | 30 (31.6%) | 29 (40.3%) | < 0.001 |
| WBC (109/L)b | 6.37 ± 2.30 (n = 1013) | 5.95 ± 2.10 | 6.53 ± 2.53 | 6.80 ± 2.09 | 7.57 ± 2.04 | < 0.001 |
| Hemoglobin (g/L)b | 97.07 ± 23.36 (n = 1009) | 97.83 ± 24.26 | 93.05 ± 22.67 | 105.35 ± 22.32 | 101.85 ± 17.59 | < 0.001 |
| Serum albumin (g/L)b | 34.49 ± 5.65 (n = 1008) | 34.83 ± 5.38 | 35.06 ± 5.97 | 31.81 ± 4.72 | 32.80 ± 5.66 | < 0.001 |
| AST (U/L)c | 17.00(13.00–22.00, n = 918) | 17.00 (13.00–22.00) | 16.00 (12.00–22.00) | 19.00 (15.50–24.50) | 18.50 (14.00–24.25) | < 0.001 |
| ALT (U/L)c | 13.00 (9.00–20.00, n = 918) | 13.00 (9.00–20.00) | 14.00 (8.00–20.00) | 15.00 (11.00–21.00) | 12.00 (8.00–16.25) | 0.024 |
| Cholesterol (mmol/L)b | 4.42 ± 1.31 (n = 1009) | 4.63 ± 1.25 | 3.92 ± 1.09 | 5.00 ± 1.41 | 4.90 ± 1.73 | < 0.001 |
| Triglyceride (mmol/L)c | 1.30 (0.94–1.88, n = 1009) | 1.13 (0.82–1.51) | 1.53 (1.11–2.17) | 1.20 (0.91–1.79) | 1.92 (1.24–2.79) | < 0.001 |
| Low-density lipoprotein cholesterol (mmol/L)b | 2.67 ± 0.97 (n = 1009) | 2.76 ± 0.98 | 2.47 ± 0.87 | 2.91 ± 1.17 | 2.74 ± 0.95 | < 0.001 |
| Serum calcium (mmol/L)b | 2.12 ± 0.28 (n = 1013) | 2.13 ± 0.28 | 2.09 ± 0.31 | 2.11 ± 0.23 | 2.16 ± 0.24 | 0.077 |
| Serum phosphorus (mmol/L)b | 1.66 ± 0.67 (n = 1013) | 1.65 ± 0.59 | 1.77 ± 0.70 | 1.50 ± 0.48 | 1.43 ± 1.04 | < 0.001 |
| Serum potassium (mmol/L)b | 4.11 ± 0.85 (n = 1013) | 4.12 ± 0.83 | 4.16 ± 0.81 | 4.12 ± 0.96 | 3.82 ± 0.97 | 0.019 |
| IPTH (pg/mL)c | 31.40 (12.25–93.56, n = 552) | 32.45 (12.05–106.45) | 32.90 (14.50–98.00) | 31.40 (14.20–97.35) | 17.10 (5.98–63.10) | 0.073 |
| Statins [n (%)] | 145 (14.3%, n = 1013) | 56 (11.9%) | 39 (10.4%) | 33 (34.7%) | 17 (23.6%) | < 0.001 |
| Peritonitis [n (%)] | 301 (29.7%, n = 1013) | 153 (32.4%) | 87 (23.3%) | 31 (32.6%) | 30 (41.7%) | 0.002 |
Statistically significant results are indicated in bold
HDL-C high-density lipoprotein cholesterol, DM diabetes mellitus, WBC white blood cell, CVD cardiovascular disease, ALT alanine aminotransferase, AST aspartate aminotransferase, iPTH intact parathyroid hormone
aData are expressed as number (%) unless otherwise indicated
bData are expressed as mean ± standard deviation
cData are expressed as median (interquar the range)
Risk factors for the first episode of PDRP in patients with PD
As shown in Table 2, after univariate Cox regression, diabetes, pre-existing stroke, pre-existing CVD, statins, hemoglobin, serum albumin, HDL-C, low HDL-C group, and diabetes plus low HDL-C group were associated with the first episode of PDRP in patients with PD.
Table 2.
Risk factor associated with the first episode of peritonitis
| Variables | HR (95% CI) | P-value |
|---|---|---|
| Age (decades) | 1.0 (0.9, 1.1) | 0.991 |
| Gender | ||
| Male | 1.0 | |
| Female | 1.1 (0.9, 1.4) | 0.417 |
| BMI (kg/m2) | 1.0 (1.0, 1.0) | 0.953 |
| Residual urine volume (mL) | 1.0 (1.0, 1.0) | 0.189 |
| Current smoking | 1.3 (1.0, 1.8) | 0.066 |
| Current alcohol consumption | 1.2 (0.8, 2.0) | 0.338 |
| DM | 1.8 (1.4, 2.4) | < 0.001 |
| Pre-existing stroke | 2.0 (1.2, 3.3) | 0.009 |
| Pre-existing CVD | 1.8 (1.3, 2.5) | < 0.001 |
| Statins | 1.9 (1.4, 2.6) | < 0.001 |
| WBC (109/L) | 1.1 (1.0, 1.1) | 0.130 |
| Hemoglobin (g/dL) | 1.0 (0.9, 1.0) | 0.023 |
| Serum albumin (g/dL) | 0.7 (0.6, 0.9) | 0.007 |
| AST (U/L) | 1.0 (1.0, 1.0) | 0.495 |
| ALT (U/L) | 1.0 (1.0, 1.0) | 0.578 |
| Cholesterol (mmol/L) | 0.9 (0.9, 1.0) | 0.253 |
| Triglyceride (mmol/L) | 0.9 (0.8, 1.1) | 0.301 |
| High-density lipoprotein cholesterol (mmol/L) | 1.3 (1.1, 1.6) | 0.002 |
| Low-density lipoprotein cholesterol (mmol/L) | 0.9 (0.8, 1.0) | 0.180 |
| Serum calcium (mmol/L) | 0.9 (0.6, 1.4) | 0.747 |
| Serum phosphorus (mmol/L) | 0.8 (0.7, 1.0) | 0.081 |
| IPTH (pg/mL) | 1.2 (1.0, 1.4) | 0.017 |
| Serum potassium (mmol/L) | 1.1 (1.0, 1.3) | 0.088 |
| GROUP | ||
| No comorbidity | 1.0 | |
| Low HDL-C | 0.8 (0.6, 1.0) | 0.037 |
| DM | 1.4 (1.0, 2.1) | 0.072 |
| DM plus LOW HDL-C | 1.9 (1.3, 2.8) | 0.002 |
Statistically significant results are indicated in bold
HR hazard ratio, CI confidence interval, WBC white blood cell, CVD cardiovascular disease, ALT alanine aminotransferase, AST aspartate aminotransferase, iPTH intact parathyroid hormone, HDL-C high-density lipoprotein cholesterol, DM diabetes mellitus
Observational period and outcome
The overall follow-up period was 403,213 patient-months, with a median period of 32.0 (4.0–211.0) months per patient. At the end of the study, 194 (19.15%) patients had died, 74 (7.31%) patients were transferred to hemodialysis, 42 (4.15%) patients received renal transplantation, 14 (1.38%) patients were transferred to other centers, and 10 (0.99%) patients were lost to follow-up. A total of 301 (29.7%) patients had their first episode of PDRP, and the incidences of a first episode of PDRP in Groups 0, 1, 2 and 3 were 32.4%, 23.3%, 32.6% and 41.7%, respectively.
Associations between low HDL-C levels and diabetes and the first episode of PDRP in patients with PD
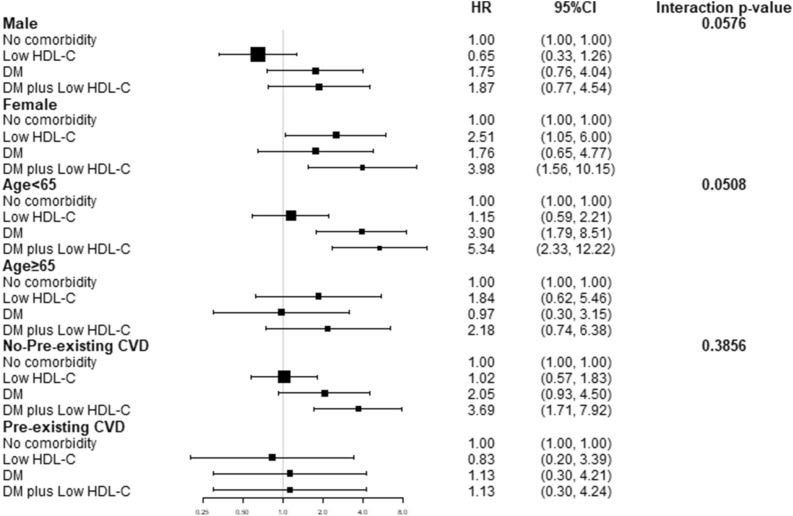
In the survival analyses, the overall peritonitis-free survival of patients in the DM plus low HDL group declined significantly faster than that in the other groups (p < 0.0001, Fig. 2). We validated the Cox regression using Stata 16, and the global test p value was 0.5005. The associations between low HDL-C levels and diabetes and PDRP are presented in Table 3. After adjusting for sex, age, BMI, current smoking, pre-existing CVD, pre-existing stroke, statins, and laboratory tests (Table 3), compared to Group 0, Groups 1, 2, and 3 had a 0.787 (95% CI 0.63 ~ 1.83), 1.83 (95% CI 0.98 ~ 3.41), and 2.81 (95% CI 1.32 ~ 4.73) higher risk for PDRP, respectively (using Model 3). Diabetes plus a «low level of HDL was significantly associated with a higher risk (HR = 2.81, 95% CI 1.32 ~ 4.73, p = 0.005) for the first episode of PDRP. In the competing risk model analysis, DM plus Low HDL-C (Group3) were significant for the first episode of peritonitis (sHR 1.99, 95% CI 1.07 ~ 3.73, p = 0.031), death (p = 0.001) and renal transplantation (p = 0.001), but they were not significantly different for transferred to hemodialysis (p = 0.187), or transfer to other centre (p = 0.873). An addional figure file shows the CIF curve [see Additional file 1]. The subgroups of sex, age, and pre-existing CVD are shown in Fig. 3. The p values for the interactions were > 0.05 for subgroups by sex (p = 0.0576), age (p = 0.0508) and pre-existing CVD (p = 0.3856).
Fig. 2.
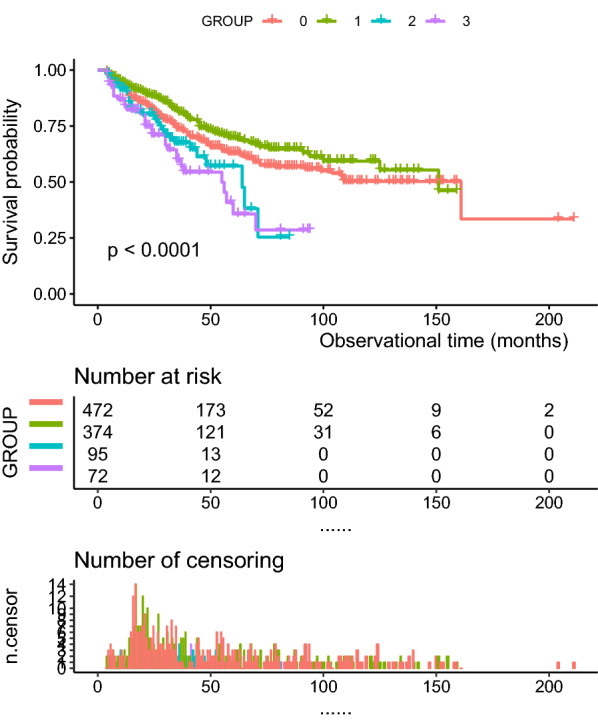
Kaplan–Meier curve of overall peritonitis-free survival
Table 3.
Association among DM and Low HDL-C and the first episode of peritonitis
| Model 1a | Model 2b | P-value | Model 3c | P-value | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | HR (95% CI) | |||
| No comorbidity | 1.0 (ref.) | 1.0 | 1.0 | |||
| Low HDL-C | 0.76 (0.58, 0.98) | 0.037 | 0.74 (0.56, 0.97) | 0.032 | 1.07 (0.63, 1.83) | 0.787 |
| DM | 1.43 (0.97, 2.11) | 0.072 | 1.32 (0.86, 2.01) | 0.202 | 1.83 (0.98, 3.41) | 0.057 |
| DM plus low HDL-C | 1.86 (1.25, 2.76) | 0.002 | 1.69 (1.09, 2.62) | 0.020 | 2.81 (1.32, 4.73) | 0.005 |
Statistically significant results are indicated in bold
HR hazard ratio, CI confidence interval, HDL-C high-density lipoprotein cholesterol, DM diabetes mellitus
aUnadjusted
bModel 1 plus age, sex, body mass index, current smoking, pre-existing stroke, pre-existing cardiovascular disease
cModel 2 plus hemoglobin, serum albumin, cholesterol, triglyceride, low-density lipoprotein, intact parathyroid hormone, statins and serum potassium
Fig. 3.
Subgroup analysis of gender, age and pre-existing cardiovascular disease
Discussion
The rate of diabetes in patients with PD was 16.5% in our study. This result is similar to previous literature reports. The cause of ESRD was diabetic nephropathy in 16.4% of patients in China [16]. We found that 44.0% patients had low HDL serum levels in our study. A total of 7.1% of patients with diabetes also had low HDL-C levels in our study. We found that comparing to no comorbidities, diabetes and concurrent low HDL-C levels were more associated with the first episode of PDRP in patients with PD than either diabetes or low HDL levels alone in our study.
It has been reported that the PDRP rate is higher in DM patients than in non-DM patients [17]. Diabetes was indicated as a risk factor for PDRP in previous reports [18, 19]. Diabetes alters the immunity of peritoneal defenses, such as leukocyte adherence, chemotaxis, and phagocytosis. Diabetes also interferes with the migration of phagocytic cells into the peritoneum and suppresses the phagocytic activity of resident peritoneal macrophages [5]. Not all studies support this conclusion. Some studies found that diabetes was not an independent risk factor for PDRP [20, 21]. Hyperglycemia was reported to be a predictor of the risk for tunneled catheters and existing infections but not for peritoneal infections [22]. Low HDL-C levels were observed in diabetes patients. A lack of apo AI and apo AII and increased clearance of HDL are the main reasons for low HDL-C levels in diabetes [23]. HDL plays an important role in fighting infection in many ways. HDL binds and neutralizes gram-negative bacterial lipopolysaccharide (LPS) and gram-positive bacterial lipoteichoic acid (LTA). HDL inhibits the expression of adhesion molecules that is induced by proinflammatory cytokines, such as V-CAM-1, ICAM-1, and E-selectin, after inflammation. HDL may also prevent monocyte activation and recruitment. As a result, the inflammatory response decreases after sepsis [11]. HDL limits oxidation by decreasing ROS production and inhibiting LDL oxidation. Low HDL levels lead to a decrease in antioxidation, which exacerbates damage from infection [24]. Low HDL levels are a risk factor for foot infection in diabetic foot osteomyelitis [25]. Low HDL levels were also associated with parasitic disease and Mycobacterium tuberculosis infection in patients with diabetes [26, 27]. Low HDL levels were associated with periodontal infection in patients with diabetes [28]. All these reports demonstrate that diabetes coexisting with low HDL-C levels is associated with infection. HDL-C binds to pathogenic microorganisms and reduces inflammatory damage in diabetes. PDRP is a typical bacterial infection in patients with PD. Diabetes plus low HDL-C levels increased the risk for PDRP in patients with PD in our study. The K-M curves confirmed this result. It is therefore important to maintain normal serum HDL levels in patients with diabetes with PD.
PD patients usually show increased levels of triglycerides (TGs), cholesterol (CHOL), and low-density lipoprotein cholesterol (LDL-C) and decreased levels of HDL-C [29]. Since disorders of HDL-C are associated with severe infection and exaggerate the systemic inflammatory response [30–32], we analyzed the association between HDL-C levels and PDRP in patients with PD. We found that low HDL-C levels were not independently associated with PDRP in PD patients in our study. The reason might be that dyslipidemia is a complicated process in patients with PD. Disorders of TG, CHOL and LDL-C also participate in the pathological process of PDRP. Low HDL-C levels alone were not enough to be an independent risk factor for PDRP in our study.
In previous studies, dyslipidemia and poor glycemic control were reported to be risk factors for ESRD and mortality in young patients and women [33–35]. HDL-C was inversely associated with the left ventricular mass index in patients with PD [36]. Subgroups of age, sex, and history of cardiovascular disease were analyzed in our study. We found that the association between DM plus low HDL-C levels and PDRP was not affected by age, sex, or history of cardiovascular disease after adjusting for age, sex, body mass index, current smoking, pre-existing stroke, pre-existing CVD, statins and laboratory tests except for the subgroup variable. We confirmed that DM plus low HDL levels is an independent risk factor for PDRP in patients with PD.
Our study has several limitations. First, although the study was a multicenter study with more than 1000 patients, the patients enrolled in our study had type 2 diabetes. We did not analyze patients with type 1 diabetes. Second, our study only looked at the association between DM plus a low level of HDL-C and PDRP. We could not determine the causality relationship between DM plus low HDL-C and PDRP. Third, the TG, CHOL, and LDL-C levels were associated with HDL-C levels. Thus, we should detect the detailed relationships among TGs, CHOL, LDL-C, and HDL-C in patients with PD and evaluate the effects of the interactions between HDL-C and other lipids on PDRP.
Conclusions
This study showed that comparing to no comorbidities, diabetes and concurrent low HDL-C levels were more associated with the first episode of PDRP in patients with PD than either diabetes or low HDL levels alone in our study. It is important to maintain normal levels of HDL-C in patients with diabetes with PD to avoid PDRP.
Supplementary Information
Additional file 1: Cumlative incidence function curve of peritonitis.
Acknowledgements
We thank all the doctors and nurses at the peritoneal dialysis centers. We thank all patients with PD who participated and provided data for the study.
Abbreviations
- ALT
Alanine amiotransferase
- ANOVA
Analysis of variance
- AST
Aspartate transaminase
- CHOL
Cholesterol
- CI
Confidence intervals
- CVD
Cardiovascular disease
- DM
Diabetes mellitus
- ESRD
End-stage renal disease
- HbA1c
Glycosylated hemoglobin, type A1c
- HDL
High-density lipoprotein
- HDL-C
High-density lipoprotein-cholesterol
- HR
Hazard ratio
- IQR
Interquartile range
- K-M
Kaplan–Meier
- LDL
Low-density lipoprotein
- LDL-C
Low-density lipoprotein-cholesterol
- OGTT
Oral glucose tolerance test
- PD
Peritoneal dialysis
- PDRP
Peritoneal dialysis-related peritonitis
- SD
Standard deviation
- TG
Triglyceride
- WBC
White blood cell
Author contributions
Y-JH and RZ designed of the entire study. XZ summarized and analyzed the data. NS provided guidance on this study. X-MT, L-WT, S-JS, XYW, Y-QW, X-RF and QZ were responsible for data acquisition. All authors read and approved the final manuscript.
Funding
This study was supported by the Dongguan Science and Technology Major Project, 201950715046061.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines detailed in the Declaration of Helsinki, and all procedures involving patients were approved by The Sixth Affiliated Hospital of Sun Yat-sen University (No. 2021ZSLYEC-177). Written informed consent was not required for this article because we retrospectively collected medical records available in the hospital, also approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-Sen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui Zhang and Xing Zhang contributed equally to this work
Contributor Information
Ning Su, Email: suning5@mail.sysu.edu.cn.
Yajuan Huang, Email: huangyj27@mail.sysu.edu.cn.
References
- 1.Cho Y, Johnson DW. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis. 2014;64:278–289. doi: 10.1053/j.ajkd.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Perl J, Fuller DS, Bieber BA, Boudville N, Kanjanabuch T, Ito Y, Nessim SJ, Piraino BM, Pisoni RL, Robinson BM, Schaubel DE, Schreiber MJ, Teitelbaum I, Woodrow G, Zhao J, Johnson DW. Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS) Am J Kidney Dis. 2020;76:42–53. doi: 10.1053/j.ajkd.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in chronic kidney disease in china. N Engl J Med. 2016;375:905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int. 2005;25:374–379. doi: 10.1177/089686080502500413. [DOI] [PubMed] [Google Scholar]
- 6.Nishina M, Yanagi H, Kakuta T, Endoh M, Fukagawa M, Takagi A. A 10-year retrospective cohort study on the risk factors for peritoneal dialysis-related peritonitis: a single-center study at Tokai University Hospital. Clin Exp Nephrol. 2014;18:649–654. doi: 10.1007/s10157-013-0872-y. [DOI] [PubMed] [Google Scholar]
- 7.Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Z, Shi J, Dorhoi A, Zhang J, Soodeen-Lalloo AK, Tan W, Yin H, Sha W, Li W, Zheng R, Liu Z, Yang H, Qin L, Wang J, Huang X, Wu C, Kaufmann SHE, Feng Y. Hemostasis and lipoprotein indices signify exacerbated lung injury in TB with diabetes comorbidity. Chest. 2018;153:1187–1200. doi: 10.1016/j.chest.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Lin T, Xia X, Yu J, Qiu Y, Yi C, Lin J, Mao H, Yang X, Huang F. The predictive study of the relation between elevated low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and mortality in peritoneal dialysis. Lipids Health Dis. 2020;19:51. doi: 10.1186/s12944-020-01240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan X, Yang M, Zhou R, Wei X, Chen Y, Chen Q. Triglyceride to high-density lipoprotein cholesterol ratio is associated with increased mortality in older patients on peritoneal dialysis. Lipids Health Dis. 2019;18:199. doi: 10.1186/s12944-019-1147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golucci A, Marson FAL, Ribeiro AF, Nogueira RJN. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition. 2018;55–56:7–14. doi: 10.1016/j.nut.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Xiang AS, Kingwell BA. Rethinking good cholesterol: a clinicians' guide to understanding HDL. Lancet Diabetes Endocrinol. 2019;7:575–582. doi: 10.1016/S2213-8587(19)30003-8. [DOI] [PubMed] [Google Scholar]
- 13.A American Diabetes Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joint Committee for Developing Chinese guidelines on, P. & Treatment of Dyslipidemia in, A. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007; 35: 390–419. [PubMed]
- 15.Szeto CC, Li PK, Johnson DW, Bernardini J, Dong J, Figueiredo AE, Ito Y, Kazancioglu R, Moraes T, Van Esch S, Brown EA. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37:141–154. doi: 10.3747/pdi.2016.00120. [DOI] [PubMed] [Google Scholar]
- 16.Liu ZH. Nephrology in china. Nat Rev Nephrol. 2013;9:523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 17.Ozener C, Arikan H, Karayaylali I, Utas C, Bozfakioglu S, Akpolat T, Ataman R, Ersoy F, Camsari T, Yavuz M, Akcicek F, Yilmaz ME. The impact of diabetes mellitus on peritoneal dialysis: the Turkey Multicenter Clinic Study. Ren Fail. 2014;36:149–153. doi: 10.3109/0886022X.2013.843275. [DOI] [PubMed] [Google Scholar]
- 18.Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol. 2009;4:1195–1200. doi: 10.2215/CJN.00910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai CC, Lee JJ, Liu TP, Ko WC, Wu CJ, Pan CF, Cheng SP. Effects of age and diabetes mellitus on clinical outcomes in patients with peritoneal dialysis-related peritonitis. Surg Infect. 2013;14:540–546. doi: 10.1089/sur.2012.195. [DOI] [PubMed] [Google Scholar]
- 20.Tian Y, Xie X, Xiang S, Yang X, Lin J, Zhang X, Shou Z, Chen J. Risk factors and outcomes of early-onset peritonitis in chinese peritoneal dialysis patients. Kidney Blood Press Res. 2017;42:1266–1276. doi: 10.1159/000485930. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Shi Y, Tao M, Jiang X, Wang Y, Zang X, Fang L, Jiang W, Du L, Jin D, Zhuang S, Liu N. Analysis of risk factors and outcome in peritoneal dialysis patients with early-onset peritonitis: a multicentre, retrospective cohort study. BMJ Open. 2020;10:e029949. doi: 10.1136/bmjopen-2019-029949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Carmona A, Perez-Fontan M, Lopez-Muniz A, Ferreiro-Hermida T, Garcia-Falcon T. Correlation between glycemic control and the incidence of peritoneal and catheter tunnel and exit-site infections in diabetic patients undergoing peritoneal dialysis. Perit Dial Int. 2014;34:618–626. doi: 10.3747/PDI.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantinou C, Karavia EA, Xepapadaki E, Petropoulou PI, Papakosta E, Karavyraki M, Zvintzou E, Theodoropoulos V, Filou S, Hatziri A, Kalogeropoulou C, Panayiotakopoulos G, Kypreos KE. Advances in high-density lipoprotein physiology: surprises, overturns, and promises. Am J Physiol Endocrinol Metab. 2016;310:E1–E14. doi: 10.1152/ajpendo.00429.2015. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Couret D, Tran-Dinh A, Duranteau J, Montravers P, Schwendeman A, Meilhac O. High-density lipoproteins during sepsis: from bench to bedside. Crit Care. 2020;24:134. doi: 10.1186/s13054-020-02860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie X, Gao L, Wang L, Wang J. Atherogenic index of plasma: a potential biomarker for clinical diagnosis of diabetic foot osteomyelitis. Surg Infect. 2020;21:9–14. doi: 10.1089/sur.2019.020. [DOI] [PubMed] [Google Scholar]
- 26.Segura-Cerda CA, Lopez-Romero W, Flores-Valdez MA. Changes in host response to mycobacterium tuberculosis infection associated with type 2 diabetes: beyond hyperglycemia. Front Cell Infect Microbiol. 2019;9:342. doi: 10.3389/fcimb.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakovac Tisdall A, Crowley VEF, Crook MA. Undetectable high-density lipoprotein cholesterol in acute malaria. J Clin Lipidol. 2018;12:822–825. doi: 10.1016/j.jacl.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Ylostalo P, Anttila S, Rajala U, Paivansalo M, Keinanen-Kiukaanniemi S, Sakki T, Knuuttila M. Periodontal infection and subclinical atherosclerosis: the role of high-density lipoprotein as a modifying factor. J Clin Periodontol. 2010;37:617–624. doi: 10.1111/j.1600-051X.2010.01572.x. [DOI] [PubMed] [Google Scholar]
- 29.Kwan BC, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007;18:1246–1261. doi: 10.1681/ASN.2006091006. [DOI] [PubMed] [Google Scholar]
- 30.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc Res. 2014;103:372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 31.Cirstea M, Walley KR, Russell JA, Brunham LR, Genga KR, Boyd JH. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care. 2017;38:289–294. doi: 10.1016/j.jcrc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Bermudes ACG, de Carvalho WB, Zamberlan P, Muramoto G, Maranhao RC, Delgado AF. Changes in lipid metabolism in pediatric patients with severe sepsis and septic shock. Nutrition. 2018;47:104–109. doi: 10.1016/j.nut.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22:1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler A, Casula A, Steenkamp R, Fogarty D, Wilkie M, Tomlinson L, Nitsch D, Roderick P, Tomson CR. Association between glycemia and mortality in diabetic individuals on renal replacement therapy in the U.K. Diabetes Care. 2014;37:1304–1311. doi: 10.2337/dc13-0553. [DOI] [PubMed] [Google Scholar]
- 35.Elley CR, Robinson T, Moyes SA, Kenealy T, Collins J, Robinson E, Orr-Walker B, Drury PL. Derivation and validation of a renal risk score for people with type 2 diabetes. Diabetes Care. 2013;36:3113–3120. doi: 10.2337/dc13-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikee R, Hamasaki Y, Oka M, Maesato K, Mano T, Moriya H, Ohtake T, Kobayashi S. High-density lipoprotein cholesterol and left ventricular mass index in peritoneal dialysis. Perit Dial Int. 2008;28:611–616. doi: 10.1177/089686080802800611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Cumlative incidence function curve of peritonitis.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.