Abstract
Objective
To investigate relationships of education and intracranial volume (ICV) (factors related to cognitive and brain reserve, respectively) with cognitive trajectories and mortality in individuals with biomarker-defined Alzheimer disease (AD).
Methods
We selected 1,298 β-amyloid–positive memory clinic patients with subjective cognitive decline (SCD, n = 142), mild cognitive impairment (MCI, n = 274), or AD dementia (n = 882) from the Amsterdam Dementia Cohort. All participants underwent baseline MRI and neuropsychological assessment, and 68% received cognitive follow-up (median 2.3 years, interquartile range 2.4). Mortality data were collected from the Central Public Administration. In the total sample and stratified by disease stage (i.e., SCD/MCI vs dementia), we examined education and ICV as predictors of baseline and longitudinal cognitive performance on 5 cognitive domains (memory, attention, executive, language, and visuospatial functions; linear mixed models) and time to death (Cox proportional hazard models). Analyses were adjusted for age, sex, whole brain gray matter atrophy, and MRI field strength.
Results
Education and ICV showed consistent positive associations with baseline cognition across disease stages. Longitudinally, we observed a relationship between higher education and faster cognitive decline among patients with dementia on global cognition, memory, executive function, and language (range β = −0.06 to −0.13; all p < 0.05). Furthermore, in the total sample, both higher education and larger ICV were related to lower mortality risk (hazard ratio 0.84 and 0.82, respectively; p < 0.05).
Discussion
In this β-amyloid–positive memory clinic sample, both cognitive and brain reserve were positively associated with baseline cognition, whereas only education was related to longitudinal cognition (i.e., accelerated decline among more highly educated patients with dementia). Higher education and ICV both moderately attenuated overall mortality risk in AD.
Alzheimer disease (AD) is associated with cognitive deterioration and increased mortality risk.1-3 However, even among patients with AD with comparable biomarker-defined neurodegeneration, clinical trajectories are not uniform. To explain this heterogeneity, the concept of reserve has been proposed. Cognitive reserve (CR) represents the brain's adaptive response to pathologic changes, which is generally higher among persons with higher education.4,5 Brain reserve (BR), in contrast, constitutes a more passive form of reserve, which shows a well-established link with MRI-based intracranial volume (ICV).4,6
In a previous cross-sectional study of individuals across the AD spectrum, we demonstrated positive associations of education and ICV with cognitive performance after adjusting for gray matter (GM) atrophy, providing support for the notion of reserve.7 There is limited research, however, that illustrates how CR and BR factors affect longitudinal cognition and mortality in AD. Therefore, our aim was to investigate these relationships among prospectively followed β-amyloid (Aβ)+ memory clinic patients with subjective cognitive decline (SCD), mild cognitive impairment (MCI), or AD dementia.
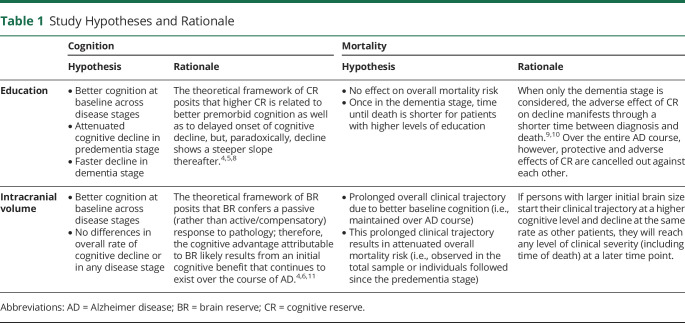
According to existing theoretical frameworks,4 we hypothesized that apart from better cognition at baseline, higher education would relate to attenuated cognitive decline in individuals in the predementia stage and faster decline among patients with dementia.8 Consequently, we expected this CR factor to be adversely associated with mortality risk in the dementia stage.9,10 Regarding ICV, we hypothesized that besides positive associations with baseline cognitive performance, this BR factor would show no relationships with longitudinal cognition.11 We expected this pattern to result in a prolonged clinical trajectory and thus a lower overall mortality risk (see detailed hypotheses and rationale in Table 1).
Table 1.
Study Hypotheses and Rationale
Methods
Participants
We selected 1,298 Aβ+ participants from the Amsterdam Dementia Cohort, which consists of patients who visited the Alzheimer Center Amsterdam of the Amsterdam UMC between 2000 and 2019 and gave informed consent to use their medical data for scientific purposes.12,13 As part of standardized dementia screening, participants received MRI, neuropsychological assessment, physical and neurologic examination, and an informant-based interview on family and medical history and interference in activities of daily living. They also underwent a lumbar puncture or PET scan to determine Aβ status. This diagnostic workup is provided to all participants from the Amsterdam Dementia Cohort and a high rate provide consent to use these data for research purposes.13 The clinical diagnosis of MCI or AD dementia was established by consensus in a multidisciplinary meeting following conventional published criteria.14,15 Individuals with cognitive complaints without detectable impairment on neuropsychological tests were classified as SCD.16
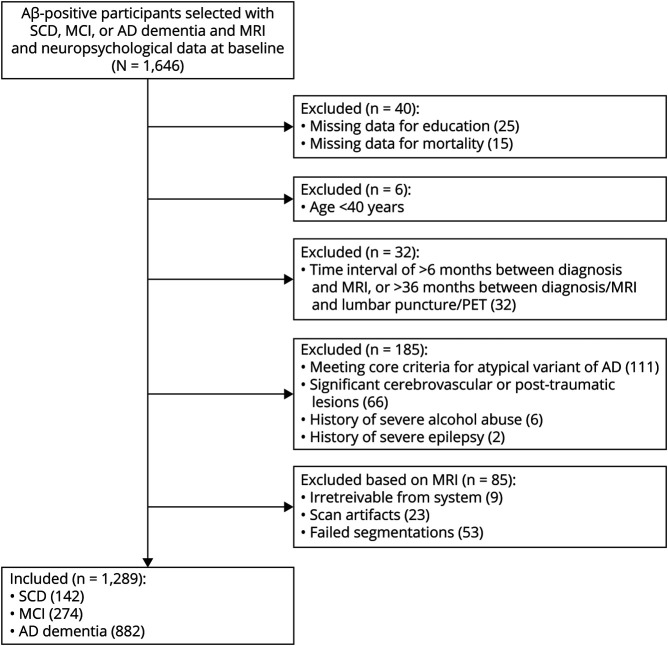
Participants were selected for this study if they had Aβ+ biomarkers (i.e., [drift-corrected] Aβ42 < 813 pg/L in CSF in 79% of cases, or a positive visual rating of a [18F]flutemetamol, [11C]Pittsburgh compound B, [18F]florbetaben, or [18F]florbetapir PET scan in the remaining part of the sample),17,18 a diagnosis of SCD, MCI, or AD dementia, and availability of MRI and neuropsychological data at baseline. Most of the participants (68%) also had neuropsychological follow-up data available, with a median follow-up duration of 2.3 years (interquartile range 2.4). We had access to mortality data for all individuals (details are described in more detail in the Mortality section) and the median follow-up duration was 5.5 years (interquartile range 4.9). Exclusion criteria were (1) missing data for education, ICV, or mortality; (2) age <40 years; (3) meeting core clinical criteria for an atypical variant of AD19-21; (4) significant cerebrovascular or posttraumatic lesions; (5) history of severe epilepsy; (6) history of severe substance abuse; (7) a time interval of >6 months between baseline diagnosis and MRI; (8) a time interval of >36 months between baseline diagnosis or MRI and lumbar puncture or PET (we accepted this large time interval by virtue of a relatively slow progression of Aβ biomarkers over time). Our final sample (n = 1,298) consisted of 142 SCD, 274 MCI, and 882 AD dementia cases (Figure 1). Among a total number of participants in the current study, 564 (43%) were also included in our previous cross-sectional study.7
Figure 1. Flowchart of the Sample Selection.
Aβ = β-amyloid; AD = Alzheimer disease; MCI = mild cognitive impairment; SCD = subjective cognitive decline.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained for participation in the Amsterdam Dementia Cohort and study procedures were approved by the institutional review board of the Amsterdam UMC.
MRI Acquisition and Preprocessing
We acquired T1-weighted images from 9 different MRI scanners according to standardized acquisition protocols (as described elsewhere).22 3T MRI scans were performed in the majority of cases (71%) and the remaining individuals received 1.5T or 1T scans (29%). In accordance with previous work, we included field strength as a covariate in our statistical models (i.e., 3T vs <3T).23 We used statistical parametric mapping 12 software to segment T1-weighted images into GM, white matter (WM), and CSF and carried out visual checks to ensure data quality. ICV was quantified as the sum of GM, WM, and CSF. We calculated whole-brain GM atrophy as the GM/ICV ratio, with lower values reflecting greater atrophy.
Education and ICV
Education (a factor related to CR) was measured with a qualitative Dutch 7-item scale, ranging from “1 = primary school not completed” to “7 = university degree” (see eTable 1, links.lww.com/WNL/B852).24 Similar to our previous research, this ordinal variable was binarized based on a median split, dividing our sample into groups with lower education scores (1–5, n = 776) and higher education scores (6–7, n = 522).7 For ICV (a BR factor), we used a mean split in our sample (1,487.6 cm3) to create groups with lower (n = 662) and higher ICV (n = 636). We chose to binarize both reserve factors as a harmonization approach that would facilitate comparability between observed effects of education (i.e., an ordinal variable) and ICV (i.e., a continuous measure). These binarized measures were included in the statistical analyses.
Neuropsychological Assessment
Participants completed a standard neuropsychological test battery at baseline and follow-up visits. We assessed global cognition with the Mini-Mental State Examination (MMSE)25 and performance across 5 cognitive domains: memory (Rey Auditory Verbal Learning Test immediate and delayed recall; total recall on condition A of the Visual Association Test), attention (Trail Making Test [TMT] part A; Digit Span Forward; Stroop test form I and II), executive function (Digit Span Backward; Frontal Assessment Battery; TMT part B; Stroop test form III; Letter Fluency), language (Category Fluency Test [animal naming]; short version of the Boston Naming Test; naming condition of the Visual Association Test), and visuospatial ability (Dot Counting; Number Location; Fragmented Letters).7 All test scores were converted into z scores based on the mean and SD of the total sample. The TMT and Stroop tests were subsequently inverted to ensure that lower scores reflected worse performance. Composite scores were calculated by averaging the standardized tests across domains only if data were available on at least 2 tests within each domain.
There was variability between tests in data availability, including the number of participants per cognitive domain (range n = 941 to n = 1,290) and the total number of observations per test (range n = 1,688 to n = 3,777). In addition, interindividual differences existed in the total number of follow-up measurements (eTable 2, links.lww.com/WNL/B852), as well as the length of the time intervals between them. We did not conduct multiple imputation, as the linear mixed models described in our Statistical Analysis are capable of handling these types of variability.26,27
Mortality
We collected information on mortality from the Central Public Administration (i.e., dead/alive and date of death28). Data from this register were collected for all participants in our sample on April 1, 2020. Follow-up duration was defined as the time between the date of diagnosis and the date of death or April 1, 2020 (if still alive).
Data Availability
Derived data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.
Statistical Analysis
Statistical analyses were carried out in SPSS 26.0 and R v4.0. We conducted t tests, Mann-Whitney U tests, and χ2 (for continuous, ordinal, and nominal variables, respectively) to compare individuals in the predementia stage (i.e., SCD and MCI) vs participants with dementia on baseline characteristics. In the total sample, we determined the correlation between education and ICV, as well as their associations with whole-brain GM atrophy and global cognition.
To evaluate relationships of education and ICV with cognition, we performed linear mixed models with time (years), education, or ICV, and their interaction term (i.e., education/ICV × time), as predictors of performance on each cognitive domain. All models contained random intercepts and slopes per participant and were corrected for age, sex, whole brain GM atrophy, and MRI field strength. First, we carried out univariate analyses for education and ICV separately and examined their relationships with both cognition at baseline (i.e., simple main effects) and cognitive changes (i.e., interactions with time). Next, we combined education and ICV as predictors into multivariate models to evaluate their independent effects on cognition. We repeated these models after stratification by disease stage (i.e., predementia vs dementia). Results were considered disease stage–specific when the effect sizes of education and ICV among participants in the predementia stage fell outside the 95% CI of these effect sizes in the dementia group and vice versa.29
To examine associations between education and ICV with mortality, we applied Cox proportional hazard models in which these factors predicted time (years) from baseline diagnosis to death. We used lower education and lower ICV as reference categories, such that the reported hazard ratios (HRs) reflected how mortality risk was affected by higher reserve. Similar to the linear mixed models, we first ran 2 univariate models and then combined education and ICV into a multivariate model. In addition, the models were repeated after stratification by disease stage. Again, all analyses were corrected for age, sex, whole brain GM atrophy, and MRI field strength. Statistical significance was set at p < 0.05. We applied a correction for multiple comparisons using the Benjamini-Hochberg procedure with a false discovery rate Q value of 5% for the linear mixed models and report both corrected and uncorrected levels of significance.
In sensitivity analyses, we further stratified the predementia sample into SCD and MCI, repeating all the analyses to check whether clinical stage had an effect on the results. Similarly, we reran all models adjusting for whole brain atrophy operationalized as GM residualized by ICV (instead of the GM/ICV ratio used in the main analyses). To investigate a potential confounding effect of sex on the relationship between ICV and education with mortality, we assessed the interaction term between sex and ICV/education in additional Cox models in the total sample.
Results
Clinical Characteristics
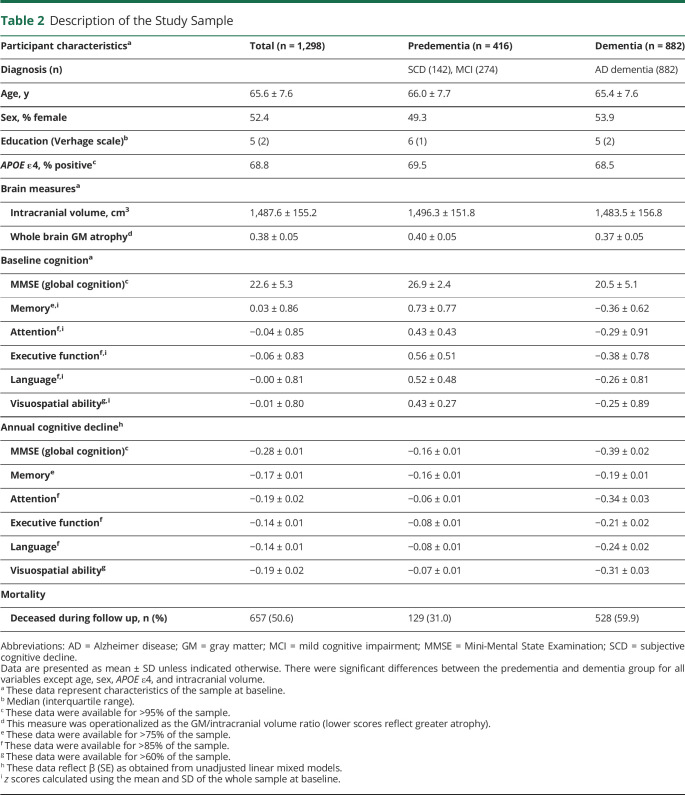
Table 2 and eTable 3 (links.lww.com/WNL/B852) provide a description of the study sample at baseline. Comparisons between disease stages indicated that participants in the predementia stage (i.e., MCI and SCD) were more highly educated than persons with dementia (which has also been reported in previous studies).30,31 As expected, whole brain GM atrophy, CSF biomarkers, and cognitive performance were more abnormal in the dementia group. Of those individuals who entered the sample in predementia stages, 31% died during follow-up, compared to 60% of persons diagnosed with dementia at baseline.
Table 2.
Description of the Study Sample
In the total sample, there was an association between education and ICV, such that 67% of individuals with lower ICV also had lower education (χ2 = 32.4; p < 0.001). This percentage was even higher (73%) in the dementia group, whereas participants in the predementia stage demonstrated a more even distribution of education levels across ICV groups (53% of individuals with lower ICV also had lower education). Moreover, participants in the higher education or higher ICV group showed higher MMSE scores (education: t = 10.0; p < 0.001; ICV: t = 4.2; p < 0.001). Whole brain GM atrophy was more pronounced in participants with lower compared to higher education (t = 2.2; p < 0.05) and in individuals with higher compared to lower ICV (t = −8.2; p < 0.001; note that this is an inherent consequence of our methodologic decision to calculate whole brain GM atrophy as the GM/ICV ratio).
Relationships of Education and ICV With Cognition
Total Sample
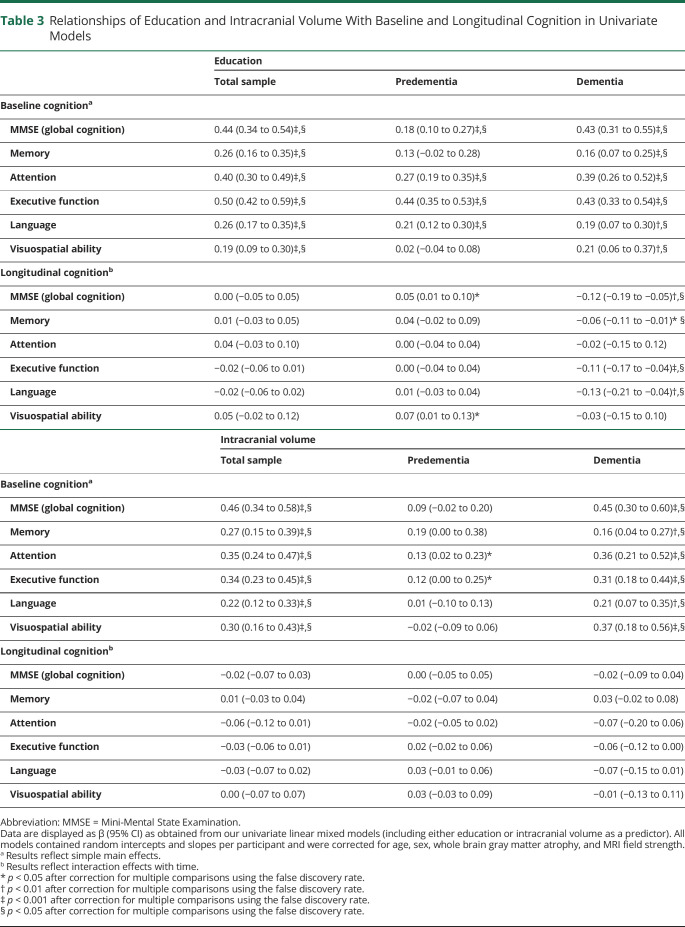
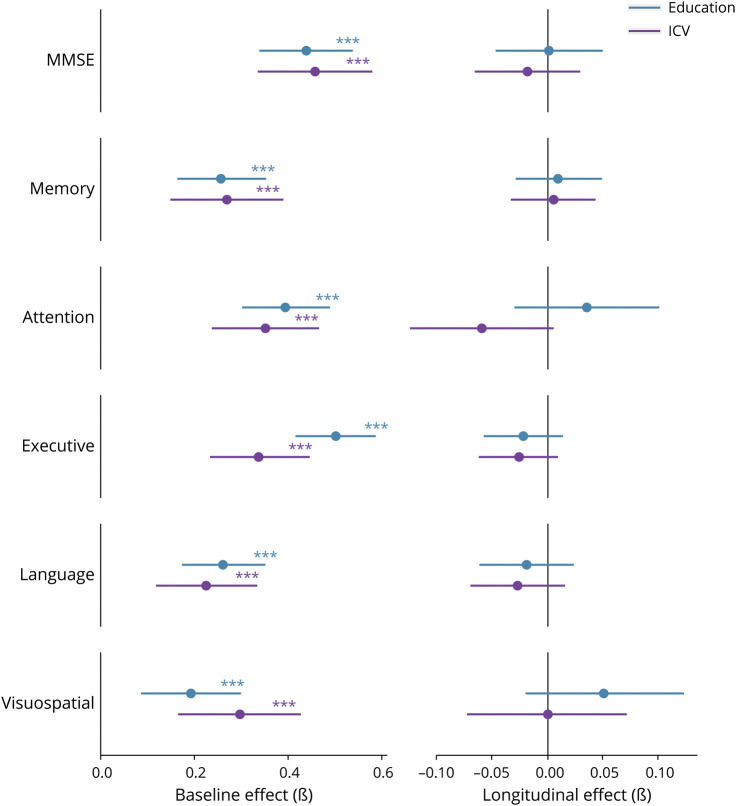
Univariate linear mixed models showed that higher education was associated with better cognitive performance on all cognitive domains at baseline (range β = 0.19–0.50; all p < 0.001; Table 3), and the same pattern was found for ICV (range β = 0.22–0.46; all p < 0.001). Both reserve factors were unrelated to longitudinal cognition in the total sample (Figure 2). Similar findings were observed in multivariate models, which included education and ICV simultaneously (eTable 4, links.lww.com/WNL/B852). When using residualized atrophy, results remained essentially the same for education; for ICV, associations with baseline cognition were attenuated compared to the analyses that included the GM/ICV ratio to adjust for atrophy (eFigure 1).
Table 3.
Relationships of Education and Intracranial Volume With Baseline and Longitudinal Cognition in Univariate Models
Figure 2. Relationships of Education and Intracranial Volume With Baseline and Longitudinal Cognition in the Total Sample.
Results are based on univariate linear mixed models and reflect effects that were estimated after correction for all covariates. *p < 0.05, **p < 0.01, ***p < 0.001. ICV = intracranial volume; MMSE = Mini-Mental State Examination.
Stratified by Disease Stage
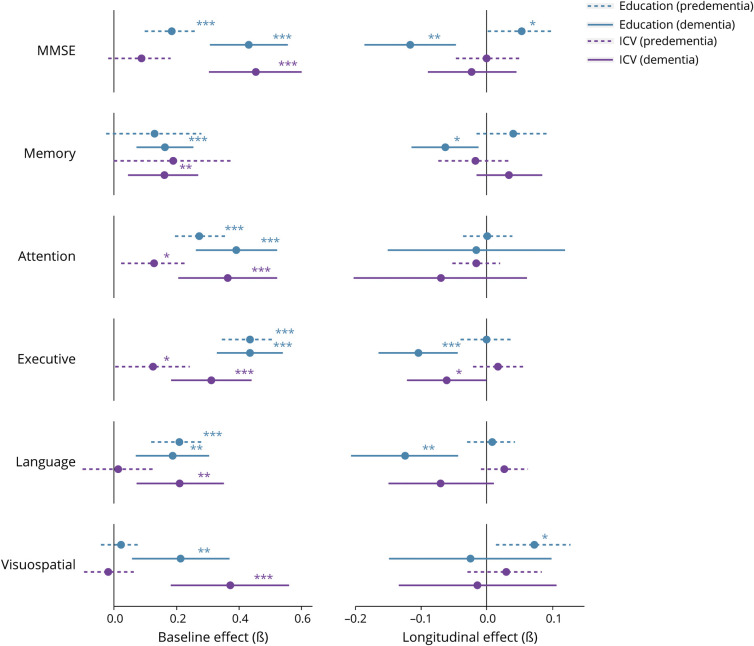
Similar to results reported in the total sample, univariate models demonstrated that in the predementia group, education was positively related to baseline cognition on most cognitive domains (range β = 0.18–0.44; all p < 0.001; Table 3). Again, we generally found no relationships between education and longitudinal cognition (except for MMSE and visuospatial ability, for which we observed slower cognitive decline with higher education, β = 0.05–0.07; p < 0.05). Whereas higher ICV was associated with better cognition in the total sample, this pattern largely disappeared in the predementia group. Moreover, ICV among participants in the predementia stage was unrelated to longitudinal cognition.
In the dementia group, most positive associations of education with baseline cognition that were observed in the total sample remained significant (range β = 0.16–0.43; all p < 0.01; Table 3). In addition, a pronounced disease stage–specific negative relationship with longitudinal cognition emerged: patients with dementia with higher education showed faster cognitive decline on the majority of domains (i.e., MMSE, memory, executive function, and language; range β = −0.06 to −0.13; all p < 0.05), which is in contrast to our findings observed in the predementia group. Regarding ICV, patients with dementia demonstrated positive relationships with baseline cognition that were comparable to results in the total sample (range β = 0.16–0.45; all p < 0.01). The same was true for longitudinal cognition (with the exception of executive functioning, which showed faster decline among patients with dementia with higher ICV, β = −0.06; p < 0.05; Figure 3). Further stratifying the predementia group into SCD and MCI groups revealed that associations of education and ICV with cognition were comparable in these 2 diagnostic groups (eFigure 2). Nearly all stratified findings from our univariate models were replicated in multivariate models, which simultaneously included education and ICV to assess their independent effects (eTable 4, links.lww.com/WNL/B852).
Figure 3. Relationships of Education and Intracranial Volume With Baseline and Longitudinal Cognition After Stratification by Disease Stage.
Results are based on univariate linear mixed models and reflect effects that were estimated after correction for all covariates. *p < 0.05, **p < 0.01, ***p < 0.001. ICV = intracranial volume; MMSE = Mini-Mental State Examination.
Relationships of Education and ICV With Mortality
Total Sample
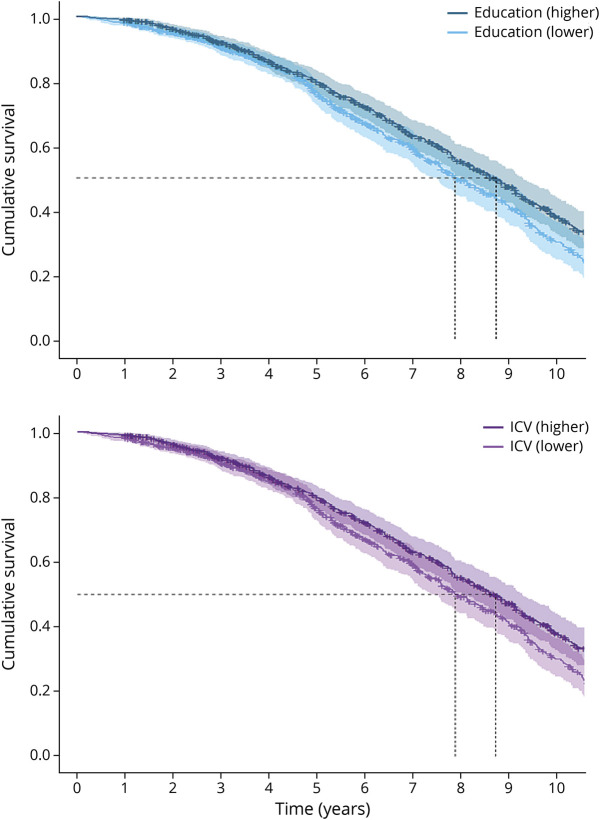
Univariate Cox proportional hazard models indicated that higher education (HR 0.84, 95% CI 0.72–0.99; p < 0.05) and higher ICV (HR 0.82, 95% CI 0.67–0.99; p < 0.05; Figure 4) were both related to lower mortality risk. In multivariate models, these effects fell above the significance threshold, although effect sizes remained in the same order of magnitude (education: HR 0.86, 95% CI 0.73–1.01; ICV: HR 0.83, 95% CI 0.68–1.00; both p = 0.06). Similarly, when using the residualized atrophy measure, neither education (HR 0.86, 95% CI 0.73–1.00; p = 0.06) nor ICV (HR 0.92, 95% CI 0.76–1.11; p = 0.40) was significantly associated with mortality (eFigure 3, links.lww.com/WNL/B852). Sex did not moderate the associations between education (pinteraction = 0.889) or ICV (pinteraction = 0.721) and mortality (eFigure 4).
Figure 4. Survival of Participants in Higher and Lower Education and Intracranial Volume Groups.
Results are based on univariate Cox proportional hazard models and reflect survival curves that were estimated after correction for all covariates. ICV = intracranial volume.
Stratified by Disease Stage
There were no significant associations of education (predementia: HR 0.76, 95% CI 0.53–1.09; p = 0.14; dementia: HR 0.95, 95% CI 0.79–1.13; p = 0.54) and ICV (predementia: HR 1.10, 95% CI 0.71–1.69; p = 0.67; dementia: HR 0.85, 95% CI 0.69–1.06; p = 0.15) with mortality risk after stratification, although some findings were in the same direction and similar in magnitude as effects reported in the total sample. Further stratifying the predementia group into SCD and MCI groups revealed that associations of education and ICV with mortality were comparable in these 2 diagnostic groups (eFigure 5). Stratified results were comparable between univariate and multivariate models (eTable 5, links.lww.com/WNL/B852).
Discussion
In this study, we investigated relationships of education and ICV (factors related to CR vs BR, respectively) with cognitive trajectories and mortality in memory clinic patients with biomarker-defined AD. We found that both participants with higher education and higher ICV showed better baseline neuropsychological test performance across cognitive domains. Furthermore, while ICV was generally unrelated to longitudinal cognition, education was associated with the rate of cognitive decline. This effect was disease stage–specific, such that higher education related to faster cognitive decline among patients with dementia, whereas this relationship was absent (or, in the case of MMSE and visuospatial ability, in the opposite direction) in the predementia group. Finally, in the total sample, we demonstrated that higher education and larger ICV were both associated with a lower mortality risk. Our study thus suggests that although education and ICV differentially affect the cognitive trajectory of AD, both reserve factors are protective against mortality.
The reported associations between education and cognition support a previously postulated theoretical framework of CR.4,8 CR may exert an “active response” (e.g., increased functional connectivity or network reorganization) to pathologic changes, such that cognition is initially protected against impairment. However, when pathology surpasses a certain threshold, this protective mechanism is no longer sufficient and consequently, cognitive performance decreases at an accelerated rate.8 Evidence for this manifestation of CR is supported by extensive literature showing that higher educational attainment is associated with an accelerated decline in cognitive performance in the dementia stage.32-40 Furthermore, in a sample of 312 individuals with incident dementia at follow-up, the accelerated decline in memory and executive function (but not in other domains) in more highly educated individuals was already present in the years prior to diagnosis.35 However, the relationship between education and decline rates in earlier (predementia) stages is largely debatable. While several studies have reported no association between education level and change in cognition over time,31,39,41-43 others reported attenuated decline in global cognition, as measured with MMSE44,46 or a composite score.47 These latter results, however, are generally associated with small effect sizes, are not transferable to other domains,45 and vary across cohorts.45,46 The initial protective effect that education confers is thought to occur through a delayed onset of decline, i.e., offsetting the deleterious effect of neuropathologic changes and therefore the emergence of clinical symptoms, rather than through differential rates of decline before onset.36,37,40 Our results are in line with the literature, as we comprehensively demonstrated the stage-specific effects of education on cognitive decline among an AD biomarker-defined sample. We speculate that the weak empirical support for the protective effect of education in the predementia stage could be due to a timing issue. That is, if participants had been included in the Amsterdam Dementia Cohort earlier (i.e., prior to any subtle cognitive changes that motivated their memory clinic visit), we might have been better able to capture the initial phase of CR, when cognition is still optimally protected despite accumulating pathology. The observed relationship between ICV and (baseline) cognition is in accordance with the concept of BR.4,6,11 BR has been described as a passive model, in which persons with more neurobiological capital (e.g., brain size, neuronal count) can undergo greater neurodegeneration before it results in (detectable) cognitive impairment. Theoretically, their clinical trajectory is characterized by a higher premorbid cognitive level, with no distinct differences in the rate of cognitive decline. The relative absence of a relationship between ICV and longitudinal cognition in the current study supports this idea. In our sample, associations between ICV and baseline cognitive performance were mainly driven by the dementia group. We speculate that (subtle) effects among participants in the predementia stage less often reached significance because this group was considerably smaller (n = 416) compared to the number of individuals with dementia (n = 882).
With respect to education and mortality, results differed from our hypotheses. Based on a theoretical framework of CR, we expected education to be unrelated to mortality risk in the total sample (i.e., before stratification by disease stage), whereas our results indicated a modest protective effect. This suggests that the overall duration between the first memory clinic visit and death was longer for individuals with higher education. Comparable findings have been reported in a previous study.49 Our results disappeared after stratification, which may be explained by a reduction in sample size and thus statistical power. Alternatively, it is possible that disease stage itself was a confounding factor in the relationship between education and mortality. In our sample, participants with higher education were more often in predementia stages of AD, which may directly explain why mortality rates were lower in this (clinically less advanced) group. However, it is important to note that this unequal distribution of educational levels across disease stages might actually be reserve-related, as described in the next section.
Our hypothesis that mortality risk would be increased with higher education in the dementia stage was also not confirmed. This seems somewhat counterintuitive given the observed accelerated cognitive decline among participants with dementia. However, the procedure by which diagnostic status was determined in the Amsterdam Dementia Cohort provides a reasonable explanation. In multidisciplinary meetings of the Alzheimer Center Amsterdam, dementia diagnoses were established based on all available clinical and demographic information, including education. Because premorbid cognitive function is expected to differ according to a patient's educational level, the same neuropsychological test profile could have been sufficient to provide an AD dementia diagnosis to a highly educated individual, whereas it may have resulted in a predementia diagnosis (e.g., MCI) in a lower-educated person. Hence, participants in the higher education group were assigned to the dementia group at clinically less advanced levels, which likely prolonged their overall duration of this disease stage. This, in turn, could have undermined the hypothesized compressing effect of education on the interval between dementia onset and death.
Regarding ICV, we confirmed our hypothesis that higher ICV was associated with lower mortality risk in the total sample. In agreement with our findings for cognition, we argue that BR is related to a higher cognitive level that is maintained across the AD spectrum, which results in a longer clinical trajectory and thereby a lower overall mortality risk. To our knowledge, no previous literature exists on the relationship between ICV and mortality. Our study thus takes an important step towards a better understanding of the manifestation of BR at the final endpoint of AD.
Strengths of this study include the large sample size (n = 1,298), availability of AD biomarkers in all participants, the assessment of different disease stages across the AD spectrum, and availability of longitudinal neuropsychological data on multiple cognitive domains over a reasonably extended follow-up period.
There are also several limitations. First, the Amsterdam Dementia Cohort comprises relatively highly educated participants. In terms of generalizability and the evaluation of CR across the full range, it would have been desirable if a larger proportion of our sample had consisted of individuals with lower education. Moreover, education was unequally distributed across disease stages, such that the predementia group was more highly educated than the dementia group. While this adds complexity to the interpretation of our findings, this pattern has been observed in previous studies30,31 and may in fact be a direct consequence of CR. According to a theoretical framework, individuals with higher CR have a prolonged predementia stage of AD, which may in some cases lead to the dementia stage never being reached during life.46,50 This creates an unavoidable selection bias in which highly educated Aβ+ individuals will more often enter a research sample in predementia stages as opposed to the dementia stage of AD.
Second, as this study was memory clinic–based, our results cannot be directly translated to the general AD population. Participants became part of the Amsterdam Dementia Cohort in different disease stages, and there was no information available to estimate the actual onset of their symptoms. In case of the mortality results, for example, we cannot adjudicate whether individuals with higher education or ICV actually live longer with AD or simply get diagnosed sooner (potentially due to individual differences in health care behavior). Similarly, a number of potential confounding factors related to socioeconomic status (e.g., a healthier lifestyle or better access to health care) that are known to relate to our reserve factors and outcomes were not accounted for in our analyses as they were not available in this dataset. Either way, our results have clinical relevance as they contribute to an improved prognostic accuracy for patients with AD once they enter the health care system.
Third, most participants had a large time interval between the last cognitive assessment (follow-up duration: median 2.3 years) and the moment at which mortality information (median 5.5 years) was obtained. Hence, although we measured longitudinal cognition across a major part of the AD spectrum, the final stages of each individual's cognitive trajectory were often not captured. Whereas cognitive impairment inevitably becomes too severe to allow neuropsychological assessment near the endpoint of AD, this is presumably not the only reason for loss to follow-up (especially among participants who were in predementia stages of AD at baseline). Nonetheless, a sample selection bias (which is inevitable in longitudinal studies of aging and dementia) is likely present as only 68% of participants had longitudinal cognitive assessments available on at least 1 cognitive domain and, as expected, the group of participants lost to follow-up included a larger proportion of individuals in dementia stages (eTable 6, links.lww.com/WNL/B852).
Fourth, our mortality data did not include information on the cause of death, which implies that among the deceased participants, not all necessarily died as a clinical consequence of AD. Similar to the intention-to-treat principle in clinical trials,51 however, one could argue that it is clinically most meaningful to take into account all-cause mortality in statistical analyses, which would justify our approach.
Fifth, we chose to dichotomize the 2 reserve measures for harmonization and interpretability purposes, as well as to account for the ordinal and skewed nature of the education variable available in our dataset. Nonetheless, when an ordinal or continuous measure is transformed into a 2-category variable (i.e., higher vs lower), more subtle within-category variability between individuals is lost, leading to a decrease in precision. This might have reduced the statistical power to detect effects of education and ICV on cognition. Nonetheless, we argue that in this particular case, the potential costs of dichotomization do not outweigh the benefits of increased interpretability and harmonization (i.e., between our variables of interest and between the current study and previous work in which education and ICV were also dichotomized).7
Finally, the results varied slightly according to the method used to operationalize atrophy (i.e., whole brain GM volume residualized for ICV vs the GM/ICV ratio). This might be due to the differential associations of each atrophy measure with ICV (i.e., the residualized variable is orthogonal to ICV, whereas the ratio variable is negatively correlated with ICV). However, the relationship between ICV as a measure of brain reserve with mortality was not confounded by sex.
We demonstrated that education and ICV are related to individual differences in cognitive trajectories and mortality in AD. Our results could enhance prognostic accuracy in a clinical setting and provide novel insights to refine theoretical frameworks of CR and BR. Future research in community-dwelling cohorts could complement this work by capturing Aβ+ individuals in prediagnostic disease stages to enable the assessment of the earliest effects of reserve.
Acknowledgment
Research of the Alzheimer Center Amsterdam is part of the neurodegeneration research program of the Neuroscience Campus Amsterdam. The Alzheimer Center Amsterdam is supported by Alzheimer Nederland and Stichting Amsterdam UMC Fonds. The clinical database structure was developed with funding from Stichting Dioraphte. F.B. is supported by the NIHR biomedical research center at UCLH.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- BR
brain reserve
- CR
cognitive reserve
- GM
gray matter
- HR
hazard ratio
- ICV
intracranial volume
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- SCD
subjective cognitive decline
- TMT
Trail Making Test
- WM
white matter
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62(5):779-784. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti O, Solerte SB, Cantoni F. Life expectancy in Alzheimer's disease (AD). Arch Gerontol Geriatr. 2009;49(suppl 1):237-243. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. White paper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenaza-Urquijo EM, Wirth M, Chételat G. Cognitive reserve and lifestyle: moving towards preclinical Alzheimer's disease. Front Aging Neurosci. 2015;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Loenhoud AC, Groot C, Vogel JW, van der Flier WM, Ossenkoppele R. Is intracranial volume a suitable proxy for brain reserve? Alzheimers Res Ther. 2018;10(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groot C, van Loenhoud AC, Barkhof F, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90(2):e149–e156. [DOI] [PubMed] [Google Scholar]
- 8.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer’s disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37(5):590-595. [DOI] [PubMed] [Google Scholar]
- 10.Contador I, Stern Y, Bermejo-Pareja F, Sanchez-Ferro A, Benito-Leon J. Is educational attainment associated with increased risk of mortality in people with dementia? A population-based study. Curr Alzheimer Res. 2017;14(5):571-576. [DOI] [PubMed] [Google Scholar]
- 11.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448-460. [PubMed] [Google Scholar]
- 12.van der Flier WM, Pijnenburg YA, Prins N, et al. . Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimers Dis. 2014;41(1):313-327. [DOI] [PubMed] [Google Scholar]
- 13.van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62(3):1091-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wilde A, Reimand J, Teunissen CE, et al. . Discordant amyloid-β PET and CSF biomarkers and its clinical consequences. Alzheimers Res Ther. 2019;11(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ossenkoppele R, Prins ND, Pijnenburg YA, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9(4):414-421. [DOI] [PubMed] [Google Scholar]
- 19.Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Brain. 2015;138(Pt 9):2732-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. . Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crutch SJ, Schott JM, Rabinovici GD, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13(8):870-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodius-Meester HFM, Benedictus MR, Wattjes MP, et al. . MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front Aging Neurosci. 2017;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Loenhoud AC, van der Flier WM, Wink AM, et al. . Cognitive reserve and clinical progression in Alzheimer disease: a paradoxical relationship. Neurology. 2019;93(4):e334–e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhage F. Intelligentie en Leeftijd: Onderzoek bij Nederlanders van Twaalf tot Zevenenzeventig Jaar [Intelligence and Age: Research Study in Dutch Individuals Age Twelve to Seventy-Seven]. Van Gorcum; 1964. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 26.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16(20):2349-2380. [DOI] [PubMed] [Google Scholar]
- 27.Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66(9):1022-1028. [DOI] [PubMed] [Google Scholar]
- 28.Government of the Netherlands. Central public administration. government.nl/topics/public-administration/central-public-administration
- 29.Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Loenhoud AC, Wink AM, Groot C, et al. . A neuroimaging approach to capture cognitive reserve: application to Alzheimer's disease. Hum Brain Mapp. 2017;38(9):4703-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadar D, Piccinin AM, Hofer SM, Johansson B, Muniz-Terrera G. Education, occupational class, and cognitive decline in preclinical dementia. GeroPsych. 2016;29:5-15. [Google Scholar]
- 32.Wilson RS, Li Y, Aggarwal NT, et al. Education and the course of cognitive decline in Alzheimer disease. Neurology. 2004;63(7):1198-1202. [DOI] [PubMed] [Google Scholar]
- 33.Amieva H, Jacqmin-Gadda H, Orgogozo JM, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128(pt 5):1093-1101. [DOI] [PubMed] [Google Scholar]
- 34.Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer's patients. J Int Neuropsychol Soc. 2006;12(1):147-152. [DOI] [PubMed] [Google Scholar]
- 35.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(3):308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69(17):1657-1664. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Boyle P, Wilson RS, et al. A random change point model for cognitive decline in Alzheimer's disease and mild cognitive impairment. Neuroepidemiology. 2012;39(2):73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verlinden VJA, van der Geest JN, de Bruijn RFAG, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement. 2016;12(2):144-153. [DOI] [PubMed] [Google Scholar]
- 39.Soldan A, Pettigrew C, Cai Q, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer's disease. Neurobiol Aging. 2017;60:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clouston SAP, Smith DM, Mukherjee S, et al. Education and cognitive decline: an integrative analysis of global longitudinal studies of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):e151–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucker-Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age-associated declines in reasoning and processing speed. Develop Psychol. 2009;45(2):431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimäki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70(2):296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G, Larson EB, Shofer JB, et al. Cognitive trajectory changes over 20 years before dementia diagnosis: a large cohort study. J Am Geriatr Soc. 2017;65(12):2627-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritsch T, McClendon MJ, Smyth KA, Ogrocki PK. Effects of educational attainment and occupational status on cognitive and functional decline in persons with Alzheimer-type dementia. Int Psychogeriatr. 2002;14(4):347-363. [DOI] [PubMed] [Google Scholar]
- 45.Lipnicki DM, Crawford JD, Dutta R, et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 2017;14(3):e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robitaille A, van den Hout A, Machado RJM, et al. Transitions across cognitive states and death among older adults in relation to education: a multistate survival model using data from six longitudinal studies. Alzheimers Dement. 2018;14(4):462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology. 2015;29(4):649-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cadar D, Robitaille A, Clouston S, Hofer SM, Piccinin AM, Muniz-Terrera G. An international evaluation of cognitive reserve and memory changes in early old age in 10 European countries. Neuroepidemiology. 2017;48(1-2):9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geerlings MI, Deeg DJ, Schmand B, Lindeboom J, Jonker C. Increased risk of mortality in Alzheimer's disease patients with higher education? A replication study. Neurology. 1997;49(3):798-802. [DOI] [PubMed] [Google Scholar]
- 50.EClipSE Collaborative Members, Brayne C, Ince PG, et al. . Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(pt 8):2210-2216. [DOI] [PubMed] [Google Scholar]
- 51.McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med. 2017;18(6):1075-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.