Abstract
Gatifloxacin (8-methoxy, 7-piperazinyl-3′-methyl) at the MIC selected mutant strains that possessed gyrA mutations at a low frequency (3.7 × 10−9) from wild-type strain Streptococcus pneumoniae IID553. AM-1147 (8-methoxy, 7-piperazinyl-3′-H) at the MIC or higher concentrations selected no mutant strains. On the other hand, the respective 8-H counterparts of these two compounds, AM-1121 (8-H, 7-piperazinyl-3′-methyl) and ciprofloxacin (8-H, 7-piperazinyl-3′-H), at one and two times the MIC selected mutant strains that possessed parC mutations at a high frequency (>2.4 × 10−6). The MIC of AM-1147 increased for the gyrA mutant strains but not for the parC mutant strains compared with that for the wild-type strain. These results suggest that fluoroquinolones that harbor 8-methoxy groups select mutant strains less frequently and prefer DNA gyrase, as distinct from their 8-H counterparts. The in vitro activities of gatifloxacin and AM-1147 are twofold higher against the wild-type strain, eight- and twofold higher against the first-step parC and gyrA mutant strains, respectively, and two- to eightfold higher against the second-step gyrA and parC double mutant strains than those of their 8-H counterparts. These results indicate that the 8-methoxy group contributes to enhancement of antibacterial activity against target-altered mutant strains as well as the wild-type strain. It is hypothesized that the 8-methoxy group of gatifloxacin increases the level of target inhibition, especially against DNA gyrase, so that it is nearly the same as that for topoisomerase IV inhibition in the bacterial cell, leading to potent antibacterial activity and a low level of resistance selectivity.
Streptococcus pneumoniae is one of the most important pathogens and is responsible for community-acquired pneumonia, acute otitis media, and meningitis. Recently, the worldwide prevalence of penicillin-resistant S. pneumoniae has become a serious problem in clinical settings. Therefore, antibiotics that possess potent activity against penicillin-resistant as well as penicillin-susceptible S. pneumoniae are urgently needed.
Some of the recently developed fluoroquinolones have improved activities against respiratory pathogens, including S. pneumoniae, and are expected to be useful as chemotherapeutic agents for the treatment of patients infected with such pathogens (1, 6, 11, 12, 20, 21, 33, 34). Recent clinical assessments of the susceptibility of S. pneumoniae to antibacterial agents have indicated that most clinical isolates continue to retain their quinolone susceptibility (9, 15, 31). Nevertheless, an outbreak of quinolone-resistant S. pneumoniae has recently been reported (K. Weiss, C. Restieri, M. Laverdiere, R. J. Davidson, A. McGeer, J. De Azavedo, and D. E. Low, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., Abstr. 824, p. 110, 1999). In conjunction with the increasing clinical use of fluoroquinolone for the treatment of respiratory infections, the increasing prevalence of quinolone resistance is anticipated in S. pneumoniae, as recently occurred in methicillin-resistant Staphylococcus aureus. Therefore, it is important to try to prevent the acquisition of quinolone resistance in S. pneumoniae.
In number of studies on the in vitro selection of quinolone-resistant strains of S. pneumoniae, investigators have reported observing different frequencies of resistance selectivity among fluoroquinolones (4, 8). It has been suggested that gatifloxacin and clinafloxacin possess potent antipneumococcal activities and select mutant strains less frequently than other fluoroquinolones because of their inhibition of DNA gyrase and topoisomerase IV (TopoIV), which occur at nearly the same levels in bacterial cells (dual-targeting property) (8, 23). On the other hand, it has been reported that the ease of resistance selectivity in S. pneumoniae correlated with the susceptibilities of the agents to the bacterial NorA-type efflux system (4).
Gatifloxacin harbors a characteristic methoxy group at the 8 position of the quinolone ring. The contributions of the methoxy groups of certain fluoroquinolones, including gatifloxacin, to antibacterial activity and/or resistance selectivity have been investigated in some bacteria. The methoxy group has been shown to correlate with the prevention of emergence of the mutant strains and/or potent in vitro activity against Escherichia coli (17, 38), S. aureus (13, 14, 39), and mycobacteria (5, 29, 37).
In the study described in this report, we investigated the contribution of the 8-methoxy group of gatifloxacin to resistance selectivity, target preference, and the antibacterial activity against S. pneumoniae.
(This study was presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, California, 26 to 29 September 1999.)
MATERIALS AND METHODS
Fluoroquinolones.
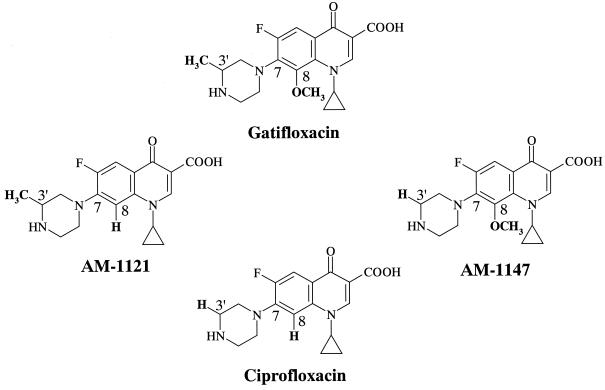
Figure 1 shows the fluoroquinolones used in the present study. Gatifloxacin (8-methoxy, 7-piperazinyl-3′-methyl) and the structurally related compounds ciprofloxacin (8-H, 7-piperazinyl-3′-H), AM-1121 (8-H, 7-piperazinyl-3′-methyl), and AM-1147 (8-methoxy, 7-piperazinyl-3′-H) were synthesized at Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan. These fluoroquinolones were used for the selection of the mutant strains and susceptibility testing.
FIG. 1.
Chemical structures of gatifloxacin (8-methoxy, 7-piperazinyl-3′-methyl) and its structurally related compounds ciprofloxacin (8-H, 7-piperazinyl-3′-H), AM-1121 (8-H, 7-piperazinyl-3′-methyl), and AM-1147 (8-methoxy, 7-piperazinyl-3′-H).
Bacterial strains.
Quinolone-susceptible, wild-type S. pneumoniae IID553 (a type strain collected at the Institute of Medical Science, University of Tokyo) was provided through the Japanese Society for Bacteriology. Overnight cultures of IID553 on Mueller-Hinton agar plates containing 5% defibrinated horse blood were suspended in saline. The mutant strains were selected by plating the bacterial suspension (approximately 109 CFU) on Mueller-Hinton agar plates containing 5% defibrinated horse blood with gatifloxacin, ciprofloxacin, AM-1121, and AM-1147 at 1, 2, 4, 8, and 16 times the MICs. The selection plates were incubated aerobically at 37°C for at least 48 h before being scored for the number of bacterial colonies. The incidence of the appearance of resistant strains was calculated as the ratio of the number of colonies that emerged to the number of bacteria inoculated (in CFU).
Four types of first-step mutant strains possessing a deduced alteration in either the GyrA subunit (S81F or S81Y) or the ParC subunit (S79Y or D83N) have been obtained from wild-type strain IID553 by selection with various fluoroquinolones (8). The second-step mutant strains have continuously been selected from the first-step gyrA and parC mutant strains (9). The results of the second-step mutation showed that all of the second-step mutant strains possessed deduced alterations in both ParC and GyrA subunits, as described in Table 3. In order to investigate the antibacterial activities of gatifloxacin and its related compounds, these first- and second-step mutant strains were used for MIC determinations.
TABLE 3.
Activities of gatifloxacin and its related compounds against target-altered mutant strains
| Straina (amino acid substitutions) | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Gatifloxacin | AM-1121 | AM-1147 | Ciprofloxacin | |
| Wild-type strain IID553 (ParC, none; GyrA, none) | 0.39 | 0.78 | 0.39 | 0.78 |
| First-step mutant strains | ||||
| NF9884 (ParC, S79Y; GyrA, none) | 0.39 | 3.13 | 0.39 | 3.13 |
| LF9853 (ParC, D83N; GyrA, none) | 0.39 | 3.13 | 0.39 | 3.13 |
| SF9863 (ParC, none; GyrA, S81F) | 0.78 | 1.56 | 0.78 | 1.56 |
| GF9821 (ParC, none; GyrA, S81Y) | 0.78 | 1.56 | 0.78 | 1.56 |
| Second-step mutant strains | ||||
| NG9951 (ParC, S79Y; GyrA, S81F) | 6.25 | 25 | 6.25 | 25 |
| NG9952 (ParC, S79Y; GyrA, E85K) | 6.25 | 25 | 12.5 | 25 |
| ST9941 (ParC, S79F; GyrA, S81F) | 6.25 | 25 | 6.25 | 25 |
| SN9981 (ParC, D83Y; GyrA, S81F) | 3.13 | 25 | 3.13 | 12.5 |
Mutations of QRDRs of the parC, parE, gyrA, and gyrB genes.
To amplify gene fragments, including the quinolone resistance-determining region (QRDRs) of the gyrA, gyrB, parC, and parE genes, which correspond to the QRDRs of the E. coli gyrA and gyrB genes (35, 36), each pair of primers, the sequences of which were the same as those reported by Pan et al. (24), was synthesized. The gene fragments were amplified, using the genomic DNAs of S. pneumoniae strains as templates, by 25 PCR cycles on a Perkin-Elmer thermal cycler with recombinant Taq DNA polymerase (Takara Shuzo Co., Ltd., Shiga, Japan). The PCR conditions were as follows: 30 s at 94°C for denaturation, 30 s at 55°C for annealing, and 2 min at 72°C for primer extension. The PCR-amplified gene fragments were sequenced with 5′-biotinylated primers (5′-AAATCTGCTCGTATTACAGGGGATG-3′, nucleotide positions 187 to 211 of the gyrA gene [3]; 5′-CAGGGAAACTAGCAGACTGTTCTTC-3′, nucleotide positions 1238 to 1262 of the gyrB gene [25]; 5′-GACAAGAGCTACCGTAAGTCGGCCAAG-3′, nucleotide positions 166 to 192 of the parC gene [25]; 5′-CAGCCCAATCTAAGAATCCTGCTAAG-3′, nucleotide positions 1253 to 1278 of the parE gene [25]) by direct cycle sequencing. The samples were subjected to electrophoresis in a 5% polyacrylamide gel containing 8 M urea at 45 W for 2.5 h. Thereafter, the DNA on the gel was transferred to a nylon membrane sheet (Boehringer Mannheim GmbH, Mannheim, Germany). The dried nylon membrane was then treated by use of a Phototope 6K detection kit (New England Biolabs Inc., Mass.), and the bands were visualized by exposing the membrane to X-ray film.
MIC determination.
The MICs of each fluoroquinolone for the resistant strains were determined. The MIC was defined as the lowest concentration of an antibacterial agent that inhibited visible growth of the cells on Mueller-Hinton agar plates with 5% defibrinated horse blood after 18 to 20 h of incubation at 37°C (12).
RESULTS AND DISCUSSION
Resistance selection.
Gatifloxacin at the MIC selected mutant strains at a low frequency (3.7 × 10−9). AM-1147 at the MIC or higher concentrations selected no mutant strains. On the other hand, ciprofloxacin and AM-1121 at one and two times their MICs selected mutant strains at high frequencies (>2.4 × 10−6) (Table 1). These results indicate that the compounds harboring an 8-methoxy group selected mutant strains less frequently than their 8-H counterparts did. For some fluoroquinolones, the presence of an 8-methoxy group has been shown to correlate with the prevention of the emergence of mutant strains of E. coli (38), mycobacteria (5), and S. aureus (13). The potent bactericidal activities of the agents have been interpreted as factors that influence resistance selectivity in these bacteria. On the other hand, it has been reported that some fluoroquinolones selected mutant strains of S. pneumoniae or S. aureus less frequently, since those agents seemed to inhibit DNA gyrase and TopoIV at nearly the same levels in bacterial cells (dual-targeting property) (8, 23). Therefore, the dual-targeting property of the agents that harbor an 8-methoxy group might be one of the causes of the low frequency of resistance selectivity in S. pneumoniae.
TABLE 1.
Frequencies of appearance of mutant strains by selection with gatifloxacin and its related compounds
| Selecting quinolone | Substitutions at the following positiona:
|
Frequency at the following multiple of the MICb:
|
|||||
|---|---|---|---|---|---|---|---|
| 8 | 3′ | 1 | 2 | 4 | 8 | 16 | |
| Gatifloxacin | OCH3 | CH3 | 3.7 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 |
| AM-1121 | H | CH3 | >2.4 × 10−6 | >2.4 × 10−6 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 |
| AM-1147 | OCH3 | H | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 |
| Ciprofloxacin | H | H | >2.4 × 10−6 | >2.4 × 10−6 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 |
8, position C-8 of the quinolone ring; 3′, position 3′ of the C-7 piperazinyl moiety.
The reproducibility of the frequency was confirmed by repeated experiments.
It has been reported that the ease of resistance selectivity in S. pneumoniae correlates with the susceptibilities of the agents to the NorA-type efflux system (4). We have tested the effects of reserpine (10 μg/ml), which is an inhibitor of the NorA-type streptococcal PmrA efflux system (10), on the activities of gatifloxacin and the related compounds. No effects of reserpine on the MICs of these quinolones for wild-type and mutant strains were observed (H. Fukuda, unpublished data). These results suggest that the activities of these compounds are little influenced by the intrinsic PmrA and/or other reserpine-sensitive efflux systems.
As described previously (18), to investigate the differences in the magnitude of the effects of reserpine between the compounds in detail, studies of the activities against S. pneumoniae strains that possess an activated reserpine-sensitive efflux system will be necessary. Unfortunately, we have no efflux system-activated S. pneumoniae strains. However, we have an S. aureus norA strain and have determined the staphylococcal NorA efflux system susceptibilities of some fluoroquinolones, including gatifloxacin and ciprofloxacin, as the ratio of the MIC for the S. aureus norA strain to the MIC for its parent strain (MIC ratio) (7). On the other hand, we have also investigated resistance selectivity in S. pneumoniae (8). The NorA efflux system-susceptible quinolones norfloxacin and ciprofloxacin selected mutant strains of S. pneumoniae at a high frequency. However, the NorA efflux system-resistant quinolone sparfloxacin selected mutant strains of S. pneumoniae at a high frequency. We have also investigated the antibacterial activities of AM-1121 and AM-1147 against the S. aureus norA strain and its parent strain. The NorA efflux system susceptibilities of AM-1147 and AM-1121 were almost the same as that of ciprofloxacin (MIC ratios, 32 to 64). It is not clear, on the basis of these results, whether the ease of resistance selectivity in S. pneumoniae correlates with the susceptibilities of the agents to the NorA-type efflux system.
Target preference.
We and other workers have already obtained first-step mutant strains of S. pneumoniae by selection with various fluoroquinolones (8, 11, 16, 19, 23, 24, 26, 28, 30). Trovafloxacin, levofloxacin, ciprofloxacin, and norfloxacin select parC mutant strains, whereas gatifloxacin, sparfloxacin, clinafloxacin, and moxifloxacin select gyrA mutant strains. These genetic studies suggest that the former and the latter groups of fluoroquinolones show preferences for TopoIV and DNA gyrase, respectively. On the other hand, studies with purified pneumococcal DNA gyrase and TopoIV have shown that TopoIV is more sensitive to sparfloxacin and clinafloxacin according to a simple comparison of the 50% inhibitory concentrations (IC50s) (22, 27). The IC50s of these quinolones for the target enzymes were determined with different in vitro assay systems, the conditions of which were also different from the conditions in bacterial cells. Therefore, target preference cannot necessarily be determined by simple comparison of the IC50s in vitro. Further studies on the correlation between the IC50 ratio (IC50 for TopoIV/IC50 for DNA gyrase) and target preference in bacterial cells will be needed.
We therefore investigated the mutations of the QRDRs in the gyrA and parC genes of the first-step mutant strains selected with gatifloxacin and its related compounds. Gatifloxacin and ciprofloxacin naturally selected the gyrA and the parC mutant strains, respectively (Table 2). AM-1121 selected the parC mutant strains (Table 2). These results suggest that gatifloxacin prefers DNA gyrase and that AM-1121 and ciprofloxacin prefer TopoIV in the wild-type strain.
TABLE 2.
Mutations in QRDRs of the gyrA and parC genes of representative mutant strains
| Selecting quinolone | Strain | Mutationa
|
|
|---|---|---|---|
| gyrA | parC | ||
| Gatifloxacin | GF9821 | S81(TCC)→Y(TAC) | None |
| GF9822 | S81(TCC)→F(TTC) | None | |
| AM-1121 | 219841 | None | D83(GAT)→N(AAT) |
| 219842 | None | S79(TCT)→Y(TAT) | |
| Ciprofloxacin | CF9841 | None | S79(TCT)→Y(TAT) |
| CF9842 | None | D83(GAT)→N(AAT) | |
No mutant strains were obtained by selection with the MIC or higher concentrations of AM-1147 (Table 1). However, the MIC of AM-1147 increased for the first-step gyrA mutant strains but not for the first-step parC mutant strains compared with that for the wild-type strain (Table 3). These results suggest that AM-1147 prefers DNA gyrase. In S. aureus, we reported that the 8-methoxy groups of gatifloxacin and AM-1147 contribute to enhancement of DNA gyrase inhibition rather than TopoIV inhibition (M. Takei, H. Fukuda, Y. Oomori, and M. Hosaka, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 758, p. 80, 2000). Moreover, Pestova et al. (28) suggest that the 8-methoxy quinolone moxifloxacin prefer DNA gyrase in S. pneumoniae. Therefore, the 8-methoxy groups of the fluoroquinolones might contribute to the target preference for DNA gyrase by enhancing DNA gyrase inhibition in the wild-type S. pneumoniae strain. Some quinolones with 8-halogen groups, such as clinafloxacin (8-chlorine) and sparfloxacin (8-fluorine), also prefer DNA gyrase (23, 26). Therefore, substituents other than the methoxy group at the C-8 position might be correlated with the target preference.
Antibacterial activity.
We previously obtained second-step mutant strains from first-step gyrA and parC mutant strains with gatifloxacin, trovafloxacin, levofloxacin, ciprofloxacin, and sparfloxacin (9). All of the fluoroquinolones selected the gyrA and the parC mutant strains from the first-step parC and gyrA mutants, respectively. The results of studies of the second-step mutations showed that all of the second-step mutant strains possessed both parC and gyrA mutations (9).
To investigate the contribution of the 8-methoxy group to the antibacterial activity, we studied the activities of gatifloxacin and its related compounds against various types of the target-altered first- and second-step mutant strains as well as the wild-type strain.
Against the wild-type strain, the activities of the 8-methoxy quinolones gatifloxacin and AM-1147 were two-fold higher than those of their respective 8-H counterparts, AM-1121 and ciprofloxacin (Table 3). Moreover, the activities of these 8-methoxy quinolones were eight-and twofold higher against the first-step parC and gyrA mutant strains, respectively, and two- to eightfold higher against the second-step gyrA and parC mutant strains with double mutations (Table 3). These results indicate that the 8-methoxy group contributes to enhancement of antibacterial activity against target-altered mutant strains as well as the wild-type strain.
The results of studies of strains with second-step mutations suggest that the fluoroquinolones prefer DNA gyrase and TopoIV in the first-step parC and gyrA mutant strains of S. pneumoniae, respectively (9). On the basis of the assumed target preferences of the fluoroquinolones, 8-methoxy quinolones seem to prefer DNA gyrase in the wild-type strains and the parC mutant strains, and their inhibition of DNA gyrase might be greatly correlated with their activities against the wild-type and parC mutant strains, whereas 8-methoxy quinolones seem to prefer TopoIV in the gyrA mutant strains, and their inhibition of TopoIV might be greatly correlated with their activities against the gyrA mutant strains.
The MICs of the 8-H quinolones ciprofloxacin and AM-1121 increased not only for the first-step parC mutant strains but also for the gyrA mutant strains compared with those for the wild-type strain, although the increase in the MIC for the gyrA mutant strains was less than that for the parC mutant strains (Table 3). This slight crossover effect has previously been reported in S. pneumoniae (32) and S. aureus (13). These results suggest that the preferential target (TopoIV) inhibition of 8-H quinolones is correlated with the activity against the wild-type strain and also that the secondary target (DNA gyrase) inhibition might be slightly involved in the activity.
Concluding comments.
It is hypothesized that the 8-methoxy group of gatifloxacin increases the level of target inhibition, especially against DNA gyrase, so that it is nearly the same as that for TopoIV inhibition in the bacterial cell, leading to potent antibacterial activity and a low level of resistance selectivity in S. pneumoniae, although further enzyme analysis will be necessary to validate this hypothesis.
Alovero et al. (2) suggested that some C-7 substituents of fluoroquinolones affect not only antibacterial activity but also target preference in S. pneumoniae. However, the piperazinyl 3′-methyl group of gatifloxacin at the C-7 position did not contribute to enhancement of the antibacterial activities or the target preferences of the compounds used in the present study. Further study of the contribution of C-8 and C-7 substituents to target preference, resistance selectivity, and antibacterial activity will provide important information for the development of quinolones that possess potent antibacterial activity and low levels of resistance selectivity.
REFERENCES
- 1.Akasaka T, Kurosaka S, Uchida Y, Tanaka M, Sato K, Hayakawa I. Antibacterial activities and inhibitory effects of sitafloxacin (DU-6859a) and its optical isomers against type II topoisomerases. Antimicrob Agents Chemother. 1998;42:1284–1287. doi: 10.1128/aac.42.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alovero F L, Pan X-S, Morris J E, Manzo R H, Fisher L M. Engineering the specificity of antibacterial fluoroquinolones: benzenesulfonamide modifications at C-7 of ciprofloxacin change its primary target in Streptococcus pneumoniae from topoisomerase IV to gyrase. Antimicrob Agents Chemother. 2000;44:320–325. doi: 10.1128/aac.44.2.320-325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balas D, Fernández-Moreira E, De La Campa A G. Molecular characterization of the gene encoding the DNA gyrase A subunit of Streptococcus pneumoniae. J Bacteriol. 1998;180:2854–2861. doi: 10.1128/jb.180.11.2854-2861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer R, Pestova E, Millichap J J, Stosor V, Noskin G A, Peterson L R. A convenient assay for estimating the possible involvement of efflux of fluoroquinolones by Streptococcus pneumoniae and Staphylococcus aureus: evidence for diminished moxifloxacin, sparfloxacin, and trovafloxacin efflux. Antimicrob Agents Chemother. 2000;44:798–801. doi: 10.1128/aac.44.3.798-801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Xu C, Zhao X, Domagala J, Drlica K. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on growth, survival, and resistance. Antimicrob Agents Chemother. 1998;42:2978–2984. doi: 10.1128/aac.42.11.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu K P, Lafredo S C, Foleno B, Isaacson D M, Barrett J F, Tobia A J, Rosenthale M E. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother. 1992;36:860–866. doi: 10.1128/aac.36.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda H, Hori S, Hiramatsu K. Antibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:1917–1922. doi: 10.1128/aac.42.8.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410–412. doi: 10.1128/aac.43.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda H. Genetic study of the mechanisms of action of fluoroquinolones in Streptococcus pneumoniae. Jpn J Chemother. 2000;48:243–250. [Google Scholar]
- 10.Gill M N, Brenwald N P, Wise R. Identification of efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:187–189. doi: 10.1128/aac.43.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gootz T D, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer R J. Activity of the new fluoroquinolone trovafloxacin (CP-99, 219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. doi: 10.1128/aac.40.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosaka M, Yasue T, Fukuda H, Tomizawa H, Aoyama H, Hirai K. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1992;36:2108–2117. doi: 10.1128/aac.36.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ince D, Hooper D C. Mechanisms and frequency of resistance to premafloxacin in Staphylococcus aureus: novel mutations suggest novel drug-target interactions. Antimicrob Agents Chemother. 2000;44:3344–3350. doi: 10.1128/aac.44.12.3344-3350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Matsumoto M, Nishino T. Improved bactericidal activity of Q-35 against quinolone-resistant staphylococci. Antimicrob Agents Chemother. 1995;39:1522–1525. doi: 10.1128/aac.39.7.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones M E, Sahm D F, Martin N, Scheuring S, Heisig P, Thornsberry C, Köhrer K, Schmitz F-J. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during the 1997–1998 respiratory season. Antimicrob Agents Chemother. 2000;44:462–466. doi: 10.1128/aac.44.2.462-466.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junior C, Keller V, Kitzis M-D, Moreau N J, Gutmann L. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob Agents Chemother. 1996;40:2760–2764. doi: 10.1128/aac.40.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu T, Zhao X, Drlica K. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activities by the C-8-methoxy group. Antimicrob Agents Chemother. 1999;43:2969–2974. doi: 10.1128/aac.43.12.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markham P N. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob Agents Chemother. 1999;43:988–989. doi: 10.1128/aac.43.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz R, De la Campa A G. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of quinolones and cooperates with DNA gyrase A subunit in forming resistant phenotype. Antimicrob Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Minami A, Nakata K, Kurobe N, Kouno K, Sakaguchi Y, Kashimoto S, Yoshida H, Kojima T, Ohue T, Fujimoto K, Nakamura M, Hashimoto M, Shimizu M. In vitro and in vivo antibacterial activities of AT-4140, a new broad-spectrum quinolone. Antimicrob Agents Chemother. 1989;33:1167–1173. doi: 10.1128/aac.33.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neu H C, Novelli A, Chin N-X. Comparative in vitro activity of a new quinolone, AM-1091. Antimicrob Agents Chemother. 1989;33:1036–1041. doi: 10.1128/aac.33.7.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onodera Y, Uchida Y, Tanaka M, Sato K. Dual inhibitory activity of sitafloxacin (DU-6859a) against DNA gyrase and topoisomerase IV of Streptococcus pneumoniae. J Antimicrob Chemother. 1999;44:533–536. doi: 10.1093/jac/44.4.533. [DOI] [PubMed] [Google Scholar]
- 23.Pan X-S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan X-S, Ambler J, Mehter S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X-S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan X-S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan X-S, Fisher L M. Streptococcus pneumoniae DNA gyrase and topoisomerase IV: overexpression, purification, and differential inhibition by fluoroquinolones. Antimicrob Agents Chemother. 1999;43:1129–1136. doi: 10.1128/aac.43.5.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pestova E, Millichap J J, Noskin G A, Peterson L R. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J Antimicrob Chemother. 2000;45:583–590. doi: 10.1093/jac/45.5.583. [DOI] [PubMed] [Google Scholar]
- 29.Renau T E, Gage J W, Dever J A, Roland G E, Joannides E T, Shapiro M A, Sanchez J P, Gracheck S J, Domagala J M, Jacobs M R, Reynolds R C. Structure-activity relationships of quinolone agents against mycobacteria: effect of structural modifications at the 8 position. Antimicrob Agents Chemother. 1996;40:2363–2368. doi: 10.1128/aac.40.10.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tankovic J, Perichon B, Duval J, Courvalin P. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob Agents Chemother. 1996;40:2502–2510. doi: 10.1128/aac.40.11.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsurumaki Y, Manda H, Takei M, Hosaka M. In vitro antimicrobial activity of gatifloxacin against 873 clinical isolates from respiratory tract, urinary tract and surgical infections during 1997–1998 in Japan. J Antimicrob Chemother. 2000;45:685–689. doi: 10.1093/jac/45.5.685. [DOI] [PubMed] [Google Scholar]
- 32.Varon E, Janoir C, Kitzis M-D, Gutmann L. ParC and GyrA may be interchangeable initial targets of some fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:302–306. doi: 10.1128/aac.43.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakebe H, Imada T, Yoneda H, Mukai F, Ohguro K, Ohmori K, Tamaoka H, Yabuuchi Y. Evaluation of OPC-17116 against important pathogens that cause respiratory tract infections. Antimicrob Agents Chemother. 1994;38:2340–2345. doi: 10.1128/aac.38.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida H, Bogaki M, Nakamura M, Yamanaka L M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991;35:1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao B Y, Pine R, Domagala J, Drlica K. Fluoroquinolone action against clinical isolates of Mycobacterium tuberculosis: effects of a C-8 methoxyl group on survival in liquid media and in human macrophages. Antimicrob Agents Chemother. 1999;43:661–666. doi: 10.1128/aac.43.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Xu C, Domagala J, Drlica K. DNA topoisomerase target of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc Natl Acad Sci USA. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Wang J-Y, Xu C, Dong Y, Zhou J, Domagala J, Drlica K. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob Agents Chemother. 1998;42:956–958. doi: 10.1128/aac.42.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]