Abstract
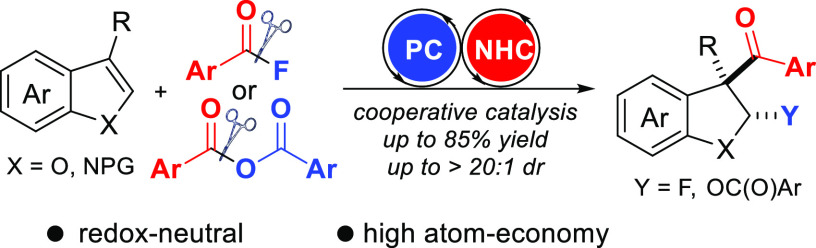
The 2,3-dihydrobenzofuran scaffold is widely found in natural products and biologically active compounds. Herein, dearomatizing 2,3-fluoroaroylation of benzofurans with aroyl fluorides as bifunctional reagents to access 2,3-difunctionalized dihydrobenzofurans is reported. The reaction that occurs by cooperative NHC/photoredox catalysis provides 3-aroyl-2-fluoro-2,3-dihydrobenzofurans with moderate to good yield and high diastereoselectivity. Cascades proceed via radical/radical cross-coupling of a benzofuran radical cation generated in the photoredox catalysis cycle with a neutral ketyl radical formed through the NHC catalysis cycle. The redox-neutral transformation exhibits broad substrate scope and high functional group compatibility. With anhydrides as bifunctional reagents, dearomatizing aroyloxyacylation of benzofurans is achieved and the strategy can also be applied to N-acylated indoles to afford 3-aroyl-2-fluoro-dihydroindoles.
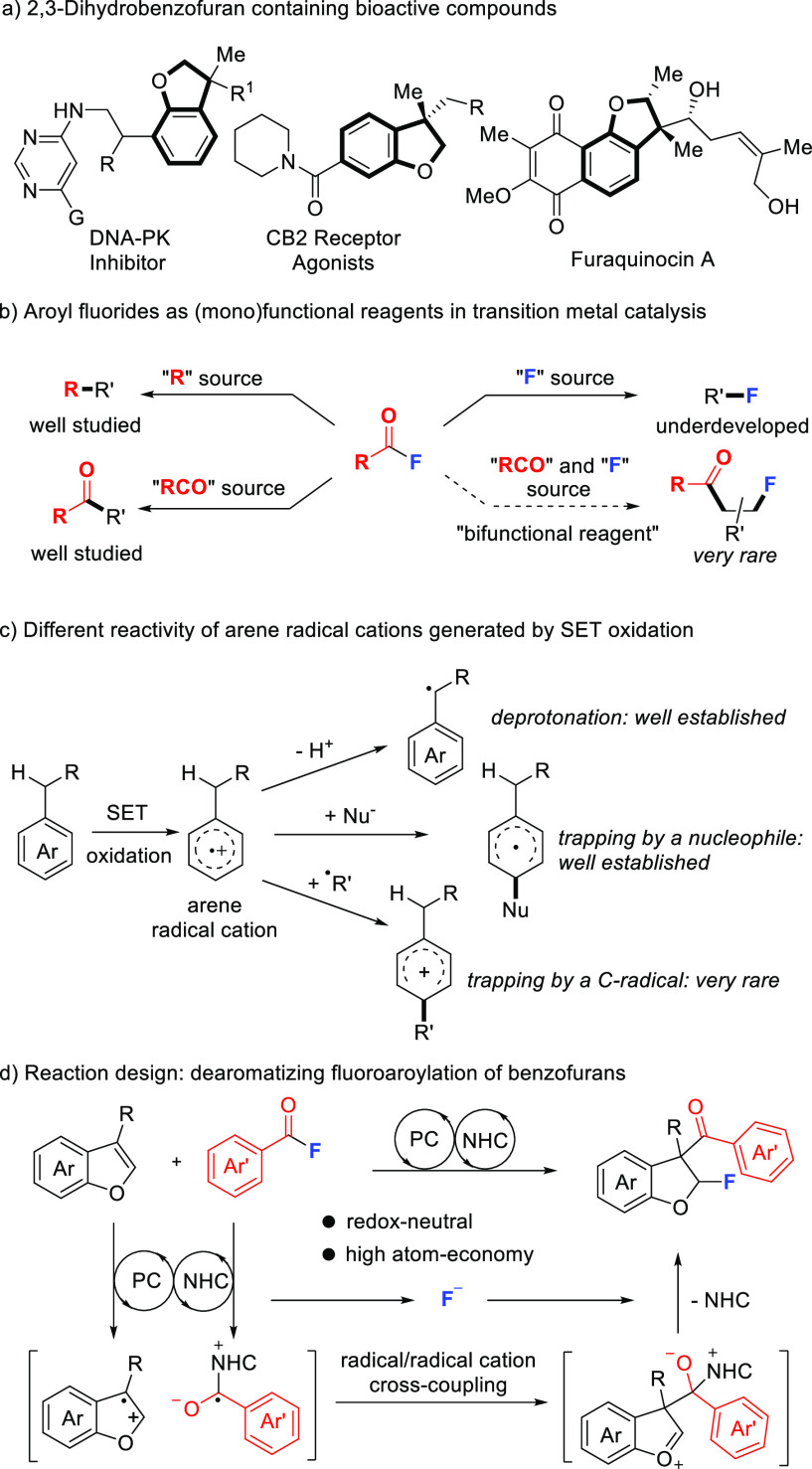
2,3-Dihydrobenzofurans are core motifs that appear in biologically active compounds (Scheme 1a).1,2 For example, DNA-PK inhibitors, CB2 receptor agonists, and furaquinocin A contain a functionalized 2,3-dihydrobenzofuran scaffold.1−6 Therefore, it is important to develop methods to access such compounds.7 Along these lines, benzofuran dearomatization is a straightforward route to 2,3-dihydrobenzofurans.8 Known dearomatization strategies involve cycloaddition,9−14 direct hydrogenation,15,16 cyclopropanation,17 radical cyclization,18−20 and radical addition21 among other reactions.22−27 Halofunctionalizations have been used for benzofuran dearomatization.28−30 However, a multistep operation for prior installation of substituents bearing nucleophilic moieties is generally required in these halofunctionalizations.
Scheme 1. Functionalized 2,3-Dihydrobenzofurans: Occurrence and Novel Synthetic Approach Using Aroyl Fluorides As Reagents via Redox Processes.
The incorporation of fluorine atoms into organic compounds generally improves their metabolic stability and bioavailability.31,32 In this context, the development of methods for construction of the C–F bond is of significance. In the past, aroyl fluorides have attracted considerable attention in chemistry.33−35 They are valuable alternatives for the other aroyl halides or anhydrides due to their higher stability.36 Accordingly, aroyl fluorides have been used as acylation reagents in ionic transformations.37−39 Moreover, transition-metal catalyzed cross-coupling of aroyl fluorides has also been developed (Scheme 1b).33−35,40−44 In general, aroyl fluorides mainly serve as “RCO” or “R” sources but reports on their use as fluorination reagents remain rare.45−48 Notably, reactions where the aroyl fluoride acts as a bifunctional reagent49 with the aroyl moiety and also the fluoride being incorporated into the product are very rare.48
Photoredox catalysis50−68 has been used for arene functionalization. Along with reductive processes, established reactivity is the single electron transfer (SET) oxidation of the arene to give an arene radical cation that can be deprotonated at the α-position of an alkyl substituent to give a benzylic radical (Scheme 1c).69−76 Alternatively, the arene radical cation can be trapped by a nucleophile to give a cyclohexadienyl radical.77−81 However, the trapping of an arene radical cation with a C-radical to generate a cyclohexadienyl-type cation has rarely been reported.82−84 This is challenging since both the arene radical cation and the C-radical are reactive intermediates and should be present in low concentrations. Successful examples use photoinduced82,83 or electrochemical84 electron transfer to generate a C-radical and an arene radical cation in close proximity that allows for efficient coupling.
Herein, we present dearomatizing 2,3-fluoroaroylation of benzofurans with aroyl fluorides as bifunctional reagents for both C–C and C–F bond formation via cooperative NHC/photoredox catalysis (Scheme 1d).85−97 Reaction of an aroyl fluoride with an NHC catalyst will lead to an aroyl azolium ion that can be SET reduced by a photocatalyst (PC) to generate a ketyl radical.91−96 Oxidation of benzofuran by the PC will give its radical cation that should cross-couple with the ketyl radical to give an oxocarbenium ion. Trapping by the F-anion and NHC fragmentation should lead to the fluoroaroylation product. Since the F-anion is a poor nucleophile and is present in low concentration only, we assumed that the radical cation/radical cross-coupling to be faster than trapping by the F-anion. Moreover, potential deprotonation of the benzofuran radical cation (for R = primary or secondary alkyl) has to be suppressed.
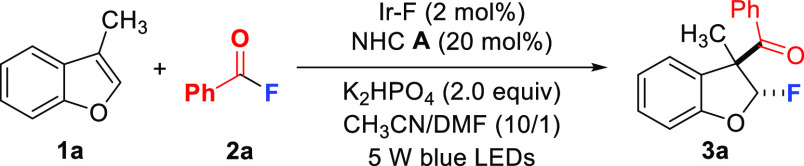
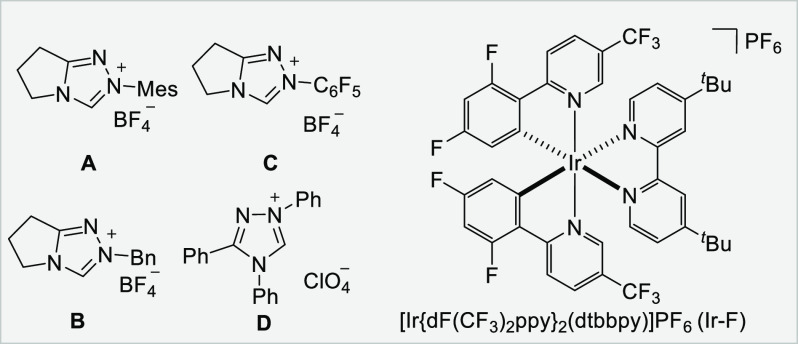
Initial experiments were conducted with 3-methylbenzofuran (1a) and benzoyl fluoride (2a). Optimization revealed that this reaction is best conducted in a 10:1 mixture of CH3CN/DMF, with K2HPO4 as base in the presence of Ir–F as the photocatalyst and the NHC catalyst A upon irradiation with a 5 W blue LED (λmax = 450 nm) at room temperature for 24 h to provide the racemic dihydrobenzofuran 3a in 70% isolated yield with high (15:1) trans-diastereoselectivity (Table 1, entry 1).98 The relative configuration of the major isomer of 3a was assigned in analogy to compound 6 where an X-ray structure was obtained (see below). With Ru(bpy)3(PF6)2 or 9-mesityl-10-methylacridinium in place of Ir–F, 3a was not formed (entries 2 and 3). In CH3CN or in a mixture of CH3CN/acetone, the yield of 3a decreased (entries 4 and 5) and Cs2CO3 in place of K2HPO4 led to a reduced yield (entry 6). NHC catalyst screening showed the best result with the triazolium salt A, and no conversion or only a trace amount of 3a was observed with precatalysts B–D (entries 7–9). Control experiments demonstrated the necessity of visible light irradiation, the photocatalyst, and also the NHC catalyst (entries 10–12).
Table 1. Reaction Optimizationa.
| entry | variation from the standard condition | yield of 3a (%)b |
|---|---|---|
| 1 | none | 73 (70)c |
| 2 | Ru(bpy)3(PF6)2 instead of Ir–F | ND |
| 3 | 9-Mesityl-10-methylacridinium instead of Ir–F | ND |
| 4 | CH3CN instead of CH3CN/DMF | 50 |
| 5 | CH3CN/Acetone instead of CH3CN/DMF | 61 |
| 6 | Cs2CO3 instead of K2HPO4 | 40 |
| 7 | NHC B instead of NHC A | 2 |
| 8 | NHC C instead of NHC A | 35 |
| 9 | NHC D instead of NHC A | ND |
| 10 | no light irradiation | ND |
| 11 | no NHC catalyst | ND |
| 12 | no photocatalyst | ND |
Reaction conditions: 1a (0.1 mmol), 2a (0.4 mmol), NHC A (20 mol %), Ir–F (2 mol %), K2HPO4 (2.0 equiv), and CH3CN/DMF (1 mL/0.1 mL) under irradiation with 5 W blue LEDs for 24 h, 15:1 d.r.
Yields were determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
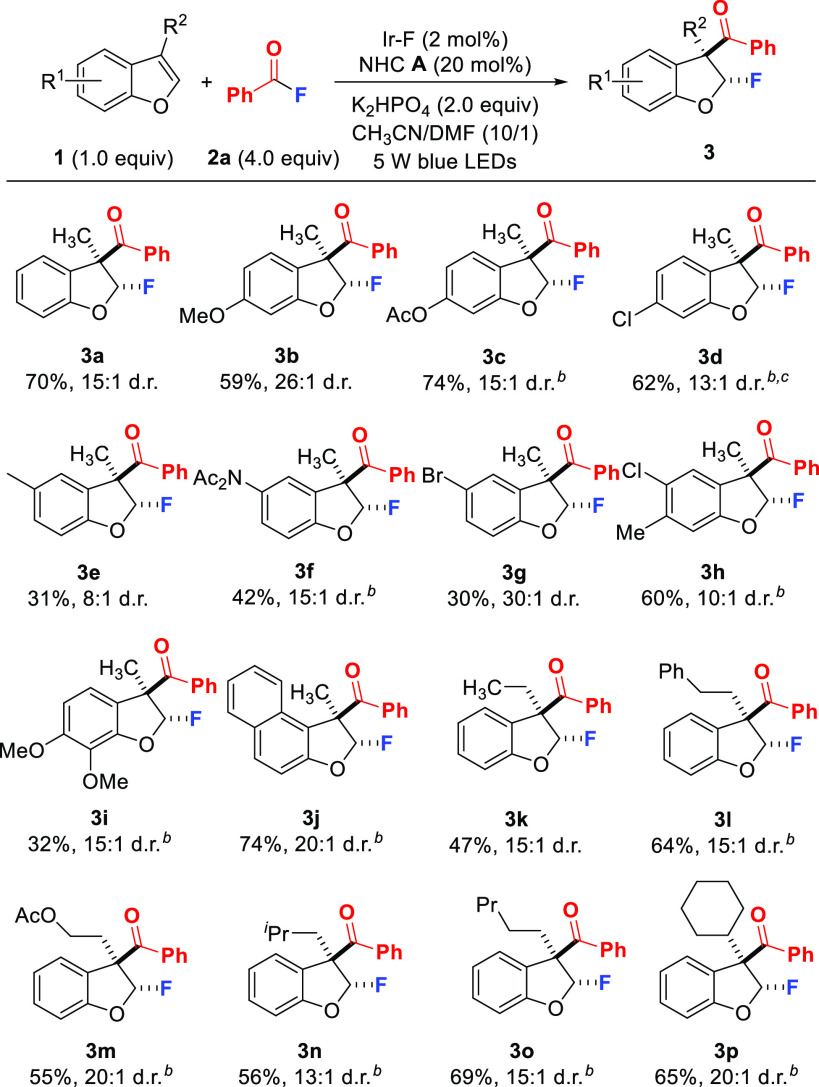
With the optimized conditions identified, the generality of the protocol by first varying the benzofuran was explored (Scheme 2). We found that various R1-substituents on the aromatic ring were tolerated and the dihydrobenzofurans 3b–3j were obtained with high trans-selectivity (8:1 to 30:1). Electronic effects at the C6-position are not pronounced and benzofurans bearing electron-donating (1b, 1c) as well as halo substituents (1d) worked well, affording 3b–d in 59–74% yields. However, lower yields were noted for C5-substituted systems. The methyl- and amide-substituted benzofurans 1e and 1f underwent dearomatizing fluoroaroylation in moderate yields with high diastereoselectivity (31–42%, 8:1–15:1 d.r.). For 1e, a complex mixture resulted and 3e was obtained in 31% yield. In contrast, benzofuran 1f reacted cleanly, but conversion was low and starting material was recovered. A low efficiency was also found for the 5-bromo derivative 1g (30%), where unreacted starting material was recovered. The disubstituted 1h and 1i engaged in the dearomatization to provide 3h and 3i in 60% and 32% yield. For 1i, the reaction was clean and the low yield is a result of a low conversion with recovery of 1i. Prolonging reaction time did not increase the conversion. The reason for the low conversion of 1f, 1g, and 1i is not understood. Back-electron transfer after benzofuran oxidation might play a role.99
Scheme 2. Substrate Scope: Variation of the Benzofuran.
Reactions conducted on a 0.1 mmol scale for 24 h.
Using 2 × 45 W blue LEDs.
Reactions conducted for 72 h.
1-Methylnaphtho[2,1-b]furan 1j was converted to 3j with a high yield and high diastereoselectivity (74%, 20:1 d.r.). The influence of the R2-substituent was investigated, and the ethyl 1k, phenethyl 1l, acetoxyethyl 1m, isobutyl 1n, pentyl 1o, and cyclohexyl 1p derivatives all worked rather well to deliver 3k–3p with moderate to good yields and high diastereoselectivity (47–69%, 13:1–20:1 d.r.).
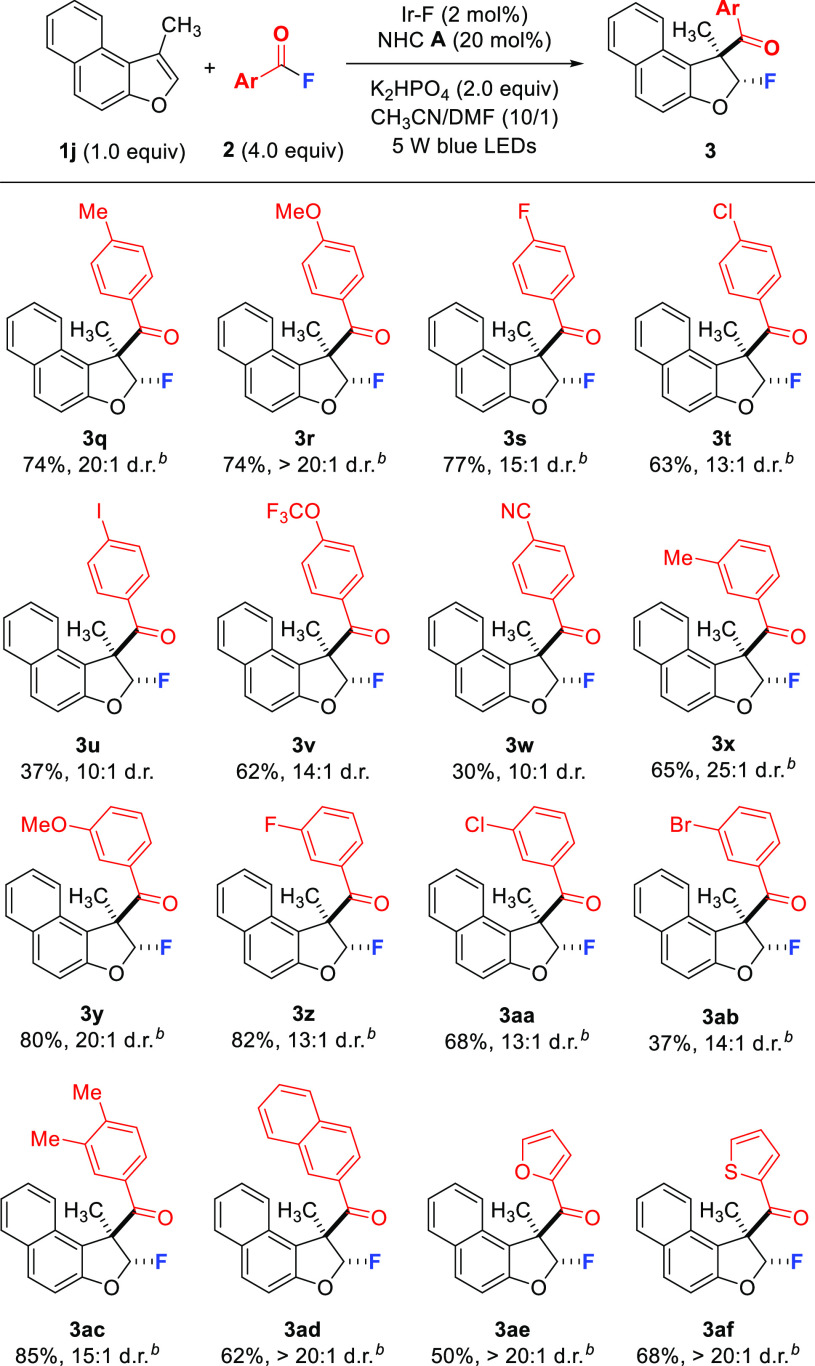
Next, we studied the scope with respect to the aroyl fluoride by using 1j as the reaction partner (Scheme 3). Fluorides bearing electron-donating (e.g., Me, MeO) or electron-withdrawing (e.g., F, Cl, I, OCF3, CN) substituents at the para-position of the phenyl ring are tolerated, and 3q–3w were isolated in 30–77% yields with high diastereoselectivity. Moreover, aroyl fluorides with methyl, methoxy, fluoro, chloro, and bromo substituents at the meta-position of the phenyl ring engaged in the reaction to provide 3x–3ab in 37–82% yields (13:1–25:1 d.r.). Hence, no clear trend regarding electronic effects could be deduced. The reaction of the 3,4-dimethylated benzoyl fluoride proceeded efficiently to give 3ac (85%), and 2-naphthoyl fluoride was also compatible providing 3ad in 62% yield and excellent diastereoselectivity (>20:1). Pleasingly, heteroaroyl fluorides such as the 2-furyl and 2-thienyl derivatives worked well to afford 3ae and 3af (50% and 68%, >20:1 d.r.).
Scheme 3. Substrate Scope: Varying the Aroyl Fluoride.
Reactions conducted on a 0.1 mmol scale for 24 h.
Using 2 × 45 W blue LEDs
In order to explore the potential of the methodology, dearomatizing indole 2,3-difunctionalization was tested (Scheme 4a). Pleasingly, N-acetyl and N-benzoyl substituted indoles 4a and 4b reacted under slightly modified conditions using 4CzlPN as the PC (5 mol %) with 2a to give the fluorodihydroindoles 5a and 5b in moderate yield and excellent diastereoselectivity. 5-Methyl and 5-methoxy N-acetyl-indole also reacted well, but the corresponding fluorinated indoles were unstable and could not be isolated. The 5-bromo and 6-fluoro N-acetyl-indole reacted with very low efficiency to the corresponding unstable products that were not isolable. Moreover, a larger scale reaction of 1j with 2a was conducted without compromising the yield (Scheme 4b). Next, various follow-up transformations were carried out to document the value of the products. Treatment of 3j with hydroxylamine hydrochloride afforded the oxime 6 in 57% yield along with 10% of the cyclized 2-isoxazoline 7 (Scheme 4c). The relative configuration in 6 was assigned by X-ray structure analysis. We found that 6 fully cyclizes to 7 in the presence of p-TsOH (4 equiv) (Scheme 4c). Reduction of 3j with NaBH4 provided quantitatively the alcohol 8 as a 1:1 diastereoisomeric mixture (Scheme 4d).
Scheme 4. Indole Dearomatization and Synthetic Applications.
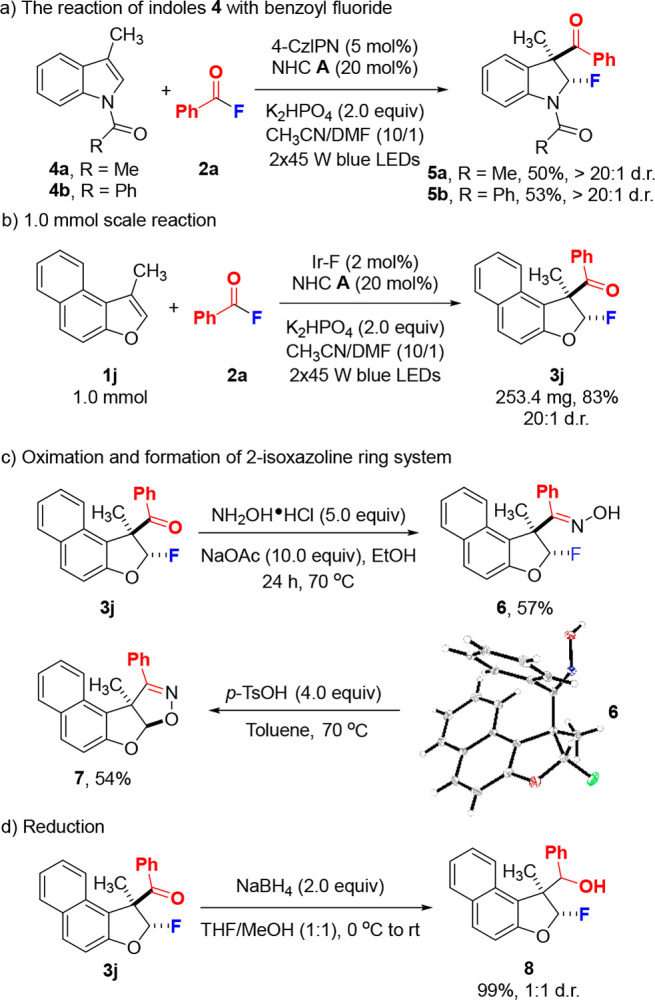
5 (0.1 mmol), 2a (0.4 mmol), NHC A (20 mol %), 4-CzlPN (5 mol %), K2HPO4 (2.0 equiv), and CH3CN/DMF (1 mL/0.1 mL) under irradiation with 2 × 45 W blue LEDs for 24 h.
1j (1.0 mmol), 2a (4.0 mmol), NHC A (20 mol %), Ir–F (2 mol %), K2HPO4 (2.0 equiv), and CH3CN/DMF (10.0 mL/1.0 mL) under irradiation with 2 × 45 W blue LEDs for 24 h.
Hydroxylamine hydrochloride (5.0 equiv), NaOAc (10.0 equiv), EtOH, 70 °C; p-TsOH (4.0 equiv), Toluene, 70 °C.
NaBH4 (2.0 equiv), MeOH/THF (1:1), 0 °C.
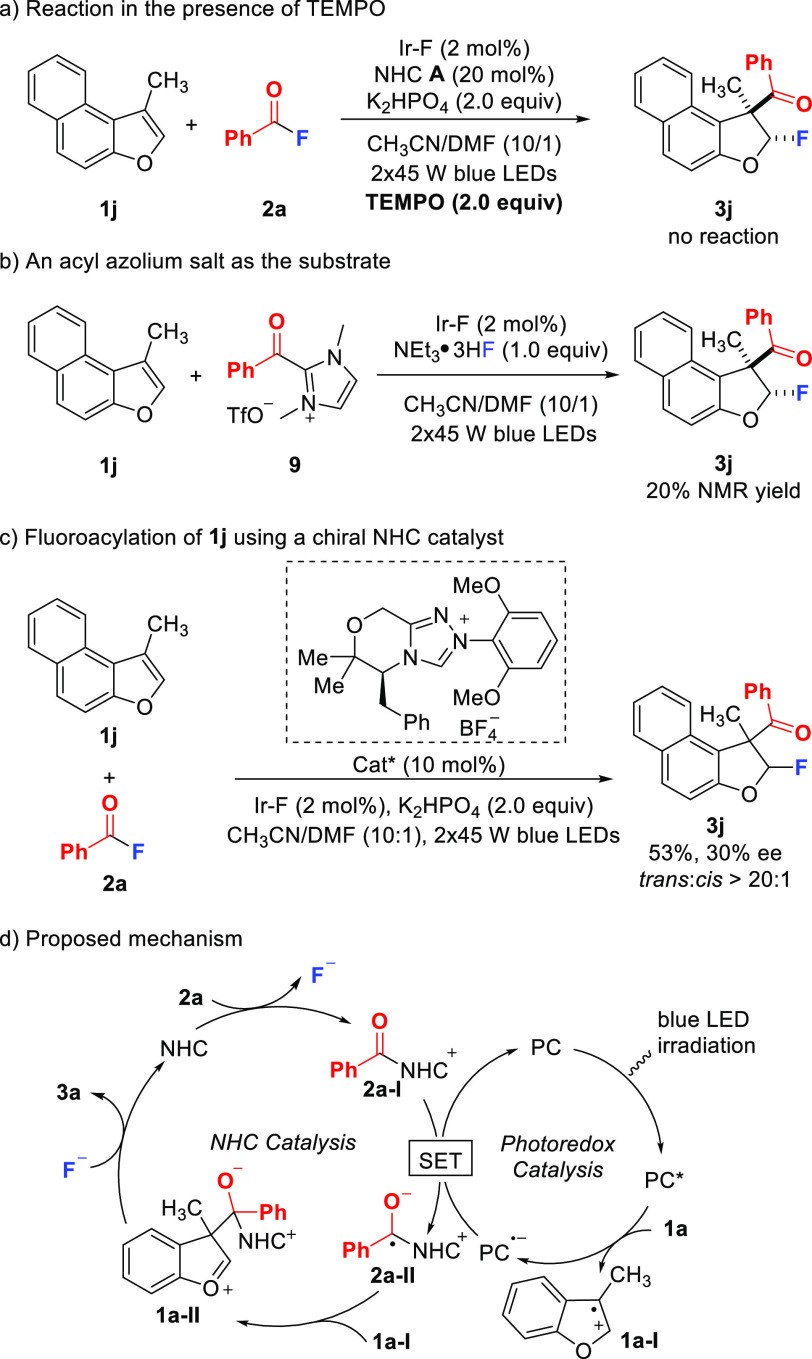
To elucidate the mechanism, control experiments were performed (Scheme 5). When adding a stoichiometric amount of TEMPO, complete suppression of the dearomatization was noted and benzofuran was recovered (Scheme 5a). Reaction of the acylazolium ion 9 and benzofuran 1j in the presence of NEt3·3HF (1 equiv) without the NHC and K2HPO4 afforded 3j (Scheme 5b). This result demonstrated that acyl azoliums of type 2a-I are competent intermediates. In addition, Stern–Volmer quenching experiments revealed that benzofuran 1j could efficiently quench the excited photocatalyst while acylazolium ion 9 did not quench the excited state of the PC (see Supporting Information). Importantly, running the reaction of 1j with 2a with a chiral NHC provided 3j in 53% yield with 30% ee and complete diastereoselectivity (Scheme 5c). This experiment indicated that the radical C–C coupling as the enantiodetermining step may occur prior to C–F bond formation. Although another reaction pathway involving C–F bond formation prior to C–C coupling might be possible and cannot be totally ruled out, the fact that moderate enantioselectivity was observed when chiral NHC catalyst was used in the reaction argues in favor of a preferential occurrence of radical cross-coupling. On basis of these experiments and previous reports,91−96,100 a mechanism is proposed in Scheme 5d. Upon visible light irradiation, benzofuran 1a (E1/2= 1.28 V vs SCE) is oxidized to its radical cation 1a-1 by photoexcited Ir(III)*. On the other hand, the reaction of 2a with the NHC gives the acyl azolium ion 2a-I (E1/2= −1.29 V vs SCE),91 which is reduced by Ir(II) (E1/2(P/P•–) = −1.37 V vs SCE) to regenerate Ir(III) with the formation of the persistent ketyl radical 2a-II.91−96,100 Subsequent cross-coupling of 2a-II with the radical cation 1a-I leads to the oxocarbenium ion 1a-II. Diastereoselective trapping of this carbenium ion by the F-anion trans to the bulky alcoholate moiety and NHC fragmentation provide 3a closing the NHC catalysis cycle. Alternatively, NHC fragmentation can occur prior the trapping of the oxocarbenium ion by the F-anion.
Scheme 5. Mechanistic Studies.
Finally, symmetrical anhydrides were tested as bifunctional reagents for the dearomatizing aroyloxyacylation of benzofurans (Scheme 6). Under slightly modified conditions (CH3CN in place of CH3CN/DMF), the symmetric anhydrides 10a–10c were converted with moderate to good yield and excellent diastereoselectivity to 11a–11c (46–64%, > 20:1 d.r.). The relative configuration of 11c was assigned by X-ray structure analysis. Repeating the reaction of 1j with 10a using a chiral NHC afforded 11a in 39% ee indicating that a similar mechanism as with the aroyl fluorides is operative.
Scheme 6. Dearomatizing Benzofuran Functionalization with Anhydrides.
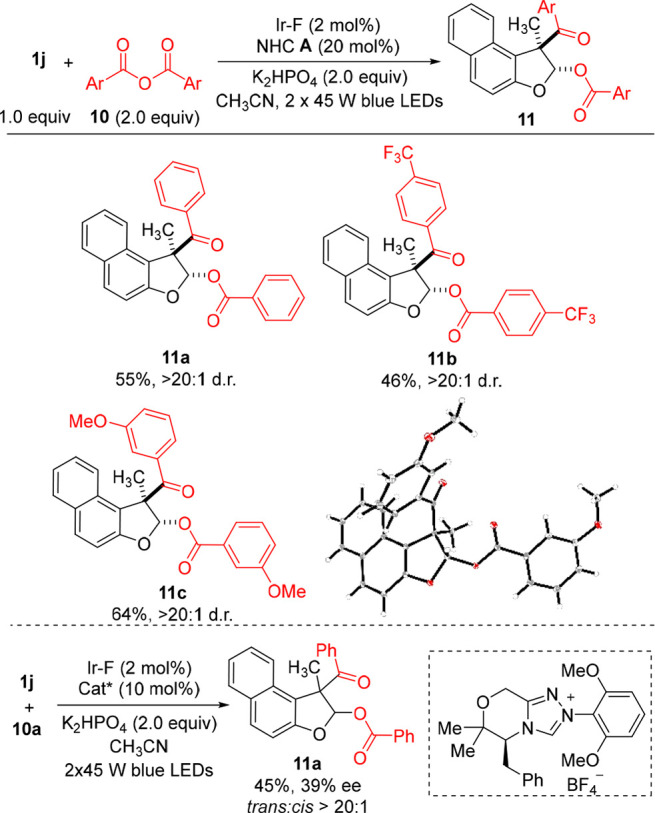
1j (0.1 mmol), 10 (0.2 mmol), NHC A (20 mol %), Ir–F (2 mol %), K2HPO4 (2.0 equiv), and CH3CN/DMF (1.0 mL/0.1 mL) under irradiation with 2 × 45 W blue LEDs for 24 h.
In summary, fluoroaroylation of benzofurans via cooperative NHC and photoredox catalysis has been achieved. Aroyl fluorides were shown to react as bifunctional reagents to incorporate both the aroyl moiety and also the fluoride into the product. The mild photocatalytic protocol shows a broad scope and high functional group tolerance. Of note, N-acyl indoles were found to be eligible substrates for the 2,3-difunctionalizing dearomatization. The synthetic value was further demonstrated by follow up transformations. Along with aroyl fluorides, anhydrides also serve as bifunctional reagents for benzofuran dearomatization. Mechanistic studies reveal that these transformations proceed via a rare radical/radical cation cross-coupling reaction as a key step.
Acknowledgments
We thank the Alexander von Humboldt Foundation (postdoctoral fellowship to X.Y.) and European Research Council ERC (advanced grant agreement No. 692640) for supporting this work. We also thank Matthew James Milner for conducting the CV studies.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c01735.
Experimental details and characterization data; NMR spectrum of new compounds; X-ray data (PDF)
Accession Codes
CCDC 2150837 and 2150839 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
∥ X.Y. and Q.-Y.M. contributed equally.
The authors declare no competing financial interest.
This paper published ASAP on March 22, 2022 with an error in Scheme 1. The scheme was replaced and the revised manuscript reposted on March 29, 2022.
Supplementary Material
References
- Nevagi R. J.; Dighe S. N.; Dighe S. N. Biological and Medicinal Significance of Benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. 10.1016/j.ejmech.2014.10.085. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Huang X.; Giuseppone N.; Rao P. B.; Bella M.; Reddy M. V.; Snyder S. A. Construction of the Complete Aromatic Core of Diazonamide A by a Novel Hetero Pinacol Macrocyclization Cascade Reaction. Angew. Chem., Int. Ed. 2001, 40, 4705–4709. . [DOI] [PubMed] [Google Scholar]
- Diaz P. S.; Phatak S.; Xu J.; Fronczek F. R.; Astruc-Diaz F.; Thompson C. M.; Cavasotto C. N.; Naguib M. 2,3-Dihydro-1-Benzofuran Derivatives as a Series of Potent Selective Cannabinoid Receptor 2 Agonists: Design, Synthesis, and Binding Mode Prediction through Ligand-Steered Modeling. ChemMedChem. 2009, 4, 1615–1629. 10.1002/cmdc.200900226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L. N.; Vo D. D.; Nakhai A.; Andersson C. D.; Elofsson M. Diversity-Oriented Synthesis of Libraries Based on Benzofuran and 2,3-Dihydrobenzofuran Scaffolds. ACS Comb. Sci. 2017, 19, 370–376. 10.1021/acscombsci.7b00014. [DOI] [PubMed] [Google Scholar]
- Zhang Z.-M.; Xu B.; Qian Y.; Wu L.; Wu Y.; Zhou L.; Liu Y.; Zhang J. Palladium-Catalyzed Enantioselective Reductive Heck Reactions: Convenient Access to 3,3-Disubstituted 2,3-Dihydrobenzofuran. Angew. Chem., Int. Ed. 2018, 57, 10373–10377. 10.1002/anie.201806372. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Pitchakuntla M.; Jia Y. Synthetic Approaches to Natural Products Containing 2,3-Dihydrobenzofuran Skeleton. Nat. Prod. Rep. 2019, 36, 666–690. 10.1039/C8NP00072G. [DOI] [PubMed] [Google Scholar]
- Laurita T.; D’Orsi R.; Chiummiento L.; Funicello M.; Lupattelli P. Recent Advances in Synthetic Strategies to 2,3-Dihydrobenzofurans. Synthesis 2020, 52, 1451–1477. 10.1055/s-0039-1690820. [DOI] [Google Scholar]
- Nair S. R.; Baire B. Recent Dearomatization Strategies of Benzofurans and Benzothiophenes. Asian J. Org.Chem. 2021, 10, 932–948. 10.1002/ajoc.202100025. [DOI] [Google Scholar]
- Suneja A.; Schneider C. Phosphoric Acid Catalyzed [4 + 1]-Cycloannulation Reaction of ortho-Quinone Methides and Diazoketones: Catalytic, Enantioselective Access toward cis-2,3-Dihydrobenzofurans. Org. Lett. 2018, 20, 7576–7580. 10.1021/acs.orglett.8b03311. [DOI] [PubMed] [Google Scholar]
- Yang Q.-Q.; Xiao W.-J. Catalytic Asymmetric Synthesis of Chiral Dihydrobenzofurans through a Formal [4 + 1] Annulation Reaction of Sulfur Ylides and In Situ Generated ortho-Quinone Methides. Eur. J. Org. Chem. 2017, 2017, 233–236. 10.1002/ejoc.201601186. [DOI] [Google Scholar]
- Cheng Q.; Zhang H.-J.; Yue W.-J.; You S.-L. Palladium-Catalyzed Highly Stereoselective Dearomative [3 + 2] Cycloaddition of Nitrobenzofurans. Chem. 2017, 3, 428–436. 10.1016/j.chempr.2017.06.015. [DOI] [Google Scholar]
- Wu X.; Xue L.; Li D.; Jia S.; Ao J.; Deng J.; Yan H. Organocatalytic Intramolecular [4 + 2] Cycloaddition between In Situ Generated Vinylidene ortho-Quinone Methidesand Benzofurans. Angew. Chem., Int. Ed. 2017, 56, 13722–13726. 10.1002/anie.201707523. [DOI] [PubMed] [Google Scholar]
- Liang X.-S.; Li R.-D.; Wang X.-C. Copper-Catalyzed Asymmetric Annulation Reactions of Carbenes with 2-Iminyl- or 2-Acyl-Substituted Phenols: Convenient Access to Enantioenriched 2,3-Dihydrobenzofurans. Angew. Chem., Int. Ed. 2019, 58, 13885–13889. 10.1002/anie.201907943. [DOI] [PubMed] [Google Scholar]
- Soldi C.; Lamb K. N.; Squitieri R. A.; González-López M.; Di Maso M. J. D.; Shaw J. T. Enantioselective Intramolecular C–H Insertion Reactions of Donor–Donor Metal Carbenoids. J. Am. Chem. Soc. 2014, 136, 15142–15145. 10.1021/ja508586t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.-G. Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res. 2007, 40, 1357–1366. 10.1021/ar700094b. [DOI] [PubMed] [Google Scholar]
- Ortega N.; Urban S.; Beiring B.; Glorius F. Ruthenium NHC Catalyzed Highly Asymmetric Hydrogenation of Benzofurans. Angew. Chem., Int. Ed. 2012, 51, 1710–1713. 10.1002/anie.201107811. [DOI] [PubMed] [Google Scholar]
- Vargas D. A.; Khade R. L.; Zhang Y.; Fasan R. Biocatalytic Strategy for Highly Diastereo- and Enantioselective Synthesis of 2,3-Dihydrobenzofuran-Based Tricyclic Scaffolds. Angew. Chem., Int. Ed. 2019, 58, 10148–10152. 10.1002/anie.201903455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-M.; Procter D. J. Dearomatizing Radical Cyclizations and Cyclization Cascades Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. J. Am. Chem. Soc. 2017, 139, 1661–1667. 10.1021/jacs.6b12077. [DOI] [PubMed] [Google Scholar]
- Kyei A. S.; Tchabanenko K.; Baldwin J. E.; Adlington R. M. Radical Dearomatising Spirocyclisations onto the C-2 Position of Benzofuran And Indole. Tetrahedron Lett. 2004, 45, 8931. 10.1016/j.tetlet.2004.09.148. [DOI] [Google Scholar]
- Huang H.-M.; McDouall J. J. W.; Procter D. J. SmI2-Catalysed Cyclization Cascades by Radical Relay. Nat. Catal. 2019, 2, 211–218. 10.1038/s41929-018-0219-x. [DOI] [Google Scholar]
- Li Y.; Studer A. Transition-Metal-Free Trifluoromethylaminoxylation of Alkenes. Angew. Chem., Int. Ed. 2012, 51, 8221–8224. 10.1002/anie.201202623. [DOI] [PubMed] [Google Scholar]
- Wang M.; Liu X.; Zhou L.; Zhu J.; Sun X. Fluorination of 2-Substituted Benzo[B]Furans with Selectfluor. Org. Biomol. Chem. 2015, 13, 3190–3193. 10.1039/C4OB02691H. [DOI] [PubMed] [Google Scholar]
- Oderinde M. S.; Mao E.; Ramirez A.; Pawluczyk J.; Jorge C.; Cornelius L. A. M.; Kempson J.; Vetrichelvan M.; Pitchai M.; Gupta A.; Gupta A. K.; Meanwell N. A.; Mathur A.; Dhar T. G. M. Synthesis of Cyclobutane-Fused Tetracyclic Scaffolds Via Visible-Light Photocatalysis for Building Molecular Complexity. J. Am. Chem. Soc. 2020, 142, 3094–3103. 10.1021/jacs.9b12129. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-Z.; Zhao Q.-R.; Zhang X.; You S.-L. Asymmetric Dearomatization of Indole Derivatives with N-Hydroxycarbamates Enabled by Photoredox Catalysis. Angew. Chem., Int. Ed. 2019, 58, 18069–18074. 10.1002/anie.201911144. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-Z.; Zhou K.; Zhu M.; Li L.-A.-C.; Zhang X.; You S.-L. Visible-Light Promoted Intermolecular Oxidative Dearomatization of β-Naphthols with N-Hydroxycarbamates. Chem.—Eur. J. 2018, 24, 12519–12523. 10.1002/chem.201803149. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-Z.; Huang X.-L.; Zhuang W.-H.; Zhao Q.-R.; Zhang X.; Mei T.-S.; You S.-L. Intermolecular Dearomatization of Naphthalene Derivatives by Photoredox-Catalyzed 1,2-Hydroalkylation. Angew. Chem., Int. Ed. 2020, 59, 18062–18067. 10.1002/anie.202008358. [DOI] [PubMed] [Google Scholar]
- Huang X.-L.; Cheng Y.-Z.; Zhang X.; You S.-L. Photoredox-Catalyzed Intermolecular Hydroalkylative Dearomatization of Electron-Deficient Indole Derivatives. Org. Lett. 2020, 22, 9699–9705. 10.1021/acs.orglett.0c03759. [DOI] [PubMed] [Google Scholar]
- Liang X.-W.; Zheng C.; You S.-L. Dearomatization through Halofunctionalization Reactions. Chem.—Eur. J. 2016, 22, 11918–11933. 10.1002/chem.201600885. [DOI] [PubMed] [Google Scholar]
- Liang X.-W.; Zheng C.; You S.-L. Catalytic Asymmetric Chlorinative Dearomatization Reaction of Benzofurans. Adv. Synth. Catal. 2016, 358, 2066–2071. 10.1002/adsc.201501184. [DOI] [Google Scholar]
- Liang X.-W.; Chen X.; Zhang Z.; You S.-L. Catalytic asymmetric brominative dearomatization reaction of benzofurans. Chin. Chem. Lett. 2018, 29, 1212–1214. 10.1016/j.cclet.2018.01.039. [DOI] [Google Scholar]
- Muller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- Ogiwara Y.; Sakai N. Acyl Fluorides in Late-Transition-Metal Catalysis. Angew. Chem., Int. Ed. 2020, 59, 574–594. 10.1002/anie.201902805. [DOI] [PubMed] [Google Scholar]
- Blanchard N.; Bizet V. Acid Fluorides in Transition-Metal Catalysis: A Good Balance between Stability and Reactivity. Angew. Chem., Int. Ed. 2019, 58, 6814–6817. 10.1002/anie.201900591. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Szostak M. Redox-Neutral Decarbonylative Cross-Couplings Coming of Age. ChemSusChem 2019, 12, 2983–2987. 10.1002/cssc.201900408. [DOI] [PubMed] [Google Scholar]
- O’Hagan D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]
- Carpino L. A.; Beyermann M.; Wenschuh H.; Bienert M. Peptide Synthesis via Amino Acid Halides. Acc. Chem. Res. 1996, 29, 268–274. 10.1021/ar950023w. [DOI] [Google Scholar]
- Birrell J. A.; Desrosiers J. N.; Jacobsen E. N. Enantioselective Acylation of Silyl Ketene Acetals through Fluoride Anion-Binding Catalysis. J. Am. Chem. Soc. 2011, 133, 13872–13875. 10.1021/ja205602j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C. S.; Förster P. M.; Carreira E. M. Facile Formation of N-Acyl-oxazolidinone Derivatives Using Acid Fluorides Org. Lett. 2010, 12, 4102–4105. 10.1021/ol1016977. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Rovis T. A Unique Catalyst Effects the Rapid Room-Temperature Cross-Coupling of Organozinc Reagents with Carboxylic Acid Fluorides, Chlorides, Anhydrides, and Thioesters. J. Am. Chem. Soc. 2004, 126, 15964–15965. 10.1021/ja044113k. [DOI] [PubMed] [Google Scholar]
- Keaveney S. T.; Schoenebeck F. Palladium-Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides. Angew. Chem., Int. Ed. 2018, 57, 4073–4077. 10.1002/anie.201800644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapit C. A.; Bour J. R.; Brigham C. E.; Sanford M. S. Base-free Nickel-Catalysed Decarbonylative Suzuki-Miyaura Coupling of Acid Fluorides. Nature 2018, 563, 100–104. 10.1038/s41586-018-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C. D.-T.; Zivkovic F. G.; Schoenebeck F. Synthesis of N-CF3 Alkynamides and Derivatives Enabled by Ni-Catalyzed Alkynylation of N-CF3 Carbamoyl Fluorides. J. Am. Chem. Soc. 2021, 143, 13029–13033. 10.1021/jacs.1c07780. [DOI] [PubMed] [Google Scholar]
- Ogiwara Y.; Hosaka S.; Sakai N. Benzoyl Fluorides as Fluorination Reagents: Reconstruction of Acyl Fluorides via Reversible Acyl C–F Bond Cleavage/Formation in Palladium Catalysis. Organometallics 2020, 39, 856–861. 10.1021/acs.organomet.0c00028. [DOI] [Google Scholar]
- Kalow J. A.; Doyle A. G. Enantioselective Ring Opening of Epoxides by Fluoride Anion Promoted by a Cooperative Dual- Catalyst System. J. Am. Chem. Soc. 2010, 132, 3268–3269. 10.1021/ja100161d. [DOI] [PubMed] [Google Scholar]
- Kalow J. A.; Doyle A. G. Mechanistic Investigations of Cooperative Catalysis in the Enantioselective Fluorination of Epoxides. J. Am. Chem. Soc. 2011, 133, 16001–16012. 10.1021/ja207256s. [DOI] [PubMed] [Google Scholar]
- Kalow J. A.; Doyle A. G. Enantioselective Fluoride Ring Opening of Aziridines Enabled by Cooperative Lewis Acid Catalysis. Tetrahedron 2013, 69, 5702–5709. 10.1016/j.tet.2013.01.062. [DOI] [Google Scholar]
- Fujimoto H.; Kodama T.; Yamanaka M.; Tobisu M. Phosphine-Catalyzed Intermolecular Acylfluorination of Alkynes via a P(V) Intermediate. J. Am. Chem. Soc. 2020, 142, 17323–17328. 10.1021/jacs.0c08928. [DOI] [PubMed] [Google Scholar]; In a side reaction, bifunctional reactivity of an aroyl fluoride was observed; see:Ryan S. J.; Schimler S. D.; Bland D. C.; Sanford M. S. Acyl Azolium Fluorides for Room Temperature Nucleophilic Aromatic Fluorination of Chloro- and Nitroarenes. Org. Lett. 2015, 17, 1866–1869. 10.1021/acs.orglett.5b00538. [DOI] [PubMed] [Google Scholar]
- Huang H.-M.; Bellotti P.; Ma J.; Dalton T.; Glorius F. Bifunctional Reagents in Organic Synthesis. Nature Rev. Chem. 2021, 5, 301–321. 10.1038/s41570-021-00266-5. [DOI] [PubMed] [Google Scholar]
- Xuan J.; Xiao W.-J. Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2012, 51, 6828–6838. 10.1002/anie.201200223. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanam J. M. R.; Stephenson C. R. J. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113. 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]
- Gentry E. C.; Knowles R. R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 2016, 49, 1546–1556. 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubi K. L.; Blum T. R.; Yoon T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T.; Akita M. Fine Design of Photoredox Systems for Catalytic Fluoromethylation of Carbon–Carbon Multiple Bonds. Acc. Chem. Res. 2016, 49, 1937–1945. 10.1021/acs.accounts.6b00268. [DOI] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Xie J.; Jin H.; Hashmi A. S. K. The Recent Achievements of Redox-Neutral Radical C–C Cross-Coupling Enabled by Visible-Light. Chem. Soc. Rev. 2017, 46, 5193–5203. 10.1039/C7CS00339K. [DOI] [PubMed] [Google Scholar]
- Silvi M.; Melchiorre P. Enhancing the Potential of Enantioselective Organocatalysis with Light. Nature 2018, 554, 41–49. 10.1038/nature25175. [DOI] [PubMed] [Google Scholar]
- Cannalire R.; Pelliccia S.; Sancineto L.; Novellino E.; Tron G. C.; Giustiniano M. Visible Light Photocatalysis in the Late-Stage Functionalization of Pharmaceutically Relevant Compounds. Chem. Soc. Rev. 2021, 50, 766–897. 10.1039/D0CS00493F. [DOI] [PubMed] [Google Scholar]
- Bell J. D.; Murphy J. A. Recent Advances in Visible Light-Activated Radical Coupling Reactions Triggered by (I) Ruthenium, (II) Iridium And (III) Organic Photoredox Agents. Chem. Soc. Rev. 2021, 50, 9540–9685. 10.1039/D1CS00311A. [DOI] [PubMed] [Google Scholar]
- Yu X.-Y.; Chen J.-R.; Xiao W.-J. Visible Light-Driven Radical-Mediated C-C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. 10.1021/acs.chemrev.0c00030. [DOI] [PubMed] [Google Scholar]
- Melchiorre P. Introduction: Photochemical Catalytic Processes. Chem. Rev. 2022, 122, 1483–1484. 10.1021/acs.chemrev.1c00993. [DOI] [PubMed] [Google Scholar]
- Candish L.; Collins K. D.; Cook G. C.; Douglas J. J.; Gómez-Suárez A.; Jolit A.; Keess S. Photocatalysis in the Life Science Industry. Chem. Rev. 2022, 122, 2907–2980. 10.1021/acs.chemrev.1c00416. [DOI] [PubMed] [Google Scholar]
- Tay N. E. S.; Lehnherr D.; Rovis T. Photons or Electrons? A Critical Comparison of Electrochemistry and Photoredox Catalysis for Organic Synthesis. Chem. Rev. 2022, 122, 2487–2649. 10.1021/acs.chemrev.1c00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. Y.; Perry I. B.; Bissonnette N. B.; Buksh B. F.; Edwards G. A.; Frye L. I.; Garry O. L.; Lavagnino M. N.; Li B. X.; Liang Y.; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. 10.1021/acs.chemrev.1c00383. [DOI] [PubMed] [Google Scholar]
- Murray P. R. D.; Cox J. H.; Chiappini N. D.; Roos C. B.; McLoughlin E. A.; Hejna B. G.; Nguyen S. T.; Ripberger H. H.; Ganley J. M.; Tsui E.; Shin N. Y.; Koronkiewicz B.; Qiu G.; Knowles R. R. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017–2291. 10.1021/acs.chemrev.1c00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitre S. P.; Overman L. E. Strategic Use of Visible-Light Photoredox Catalysis in Natural Product Synthesis. Chem. Rev. 2022, 122, 1717–1751. 10.1021/acs.chemrev.1c00247. [DOI] [PubMed] [Google Scholar]
- Bortolato T.; Cuadros S.; Simionato G.; Dell’Amico L. The Advent and Development of Organophotoredox Catalysis. Chem. Commun. 2022, 58, 1263–1283. 10.1039/D1CC05850A. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Wang F.; McCann S. D.; Wang D.; Chen P.; Stahl S. S.; Liu G. Enantioselective Cyanation of Benzylic C-H Bonds via Copper-Catalyzed Radical Relay. Science 2016, 353, 1014–1018. 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J.; DeGlopper K. S.; Yoon T. P. Site-Selective Alkoxylation of Benzylic C-H Bonds by Photoredox Catalysis. Angew. Chem., Int. Ed. 2020, 59, 197–202. 10.1002/anie.201910602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betori R. C.; May C. M.; Scheidt K. A. Combined Photoredox/Enzymatic C-H Benzylic Hydroxylations. Angew. Chem., Int. Ed. 2019, 58, 16490–16494. 10.1002/anie.201909426. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xu W.; Wang W.; Liu T.; Xie J.; Zhu C. Late-Stage Trifluoromethylthiolation of Benzylic C–H Bonds. Nat. Commun. 2019, 10, 4867. 10.1038/s41467-019-12844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Zhang L.-K.; Buevich A. V.; Li G.; Tang H.; Vachal P.; Colletti S. L.; Shi Z.-C. Chemoselective Peptide Modification via Photocatalytic Tryptophan β-Position Conjugation. J. Am. Chem. Soc. 2018, 140, 6797–6800. 10.1021/jacs.8b03973. [DOI] [PubMed] [Google Scholar]
- Kim W.; Koo J.; Lee H. G. Benzylic C(sp3)–C(sp2) Cross-Coupling of Indoles Enabled by Oxidative Radical Generation and Nickel Catalysis. Chem. Sci. 2021, 12, 4119–4125. 10.1039/D0SC06666D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Hu X. Nickel Catalysis Enables Convergent Paired Electrolysis for Direct Arylation of Benzylic C–H Bonds. Chem. Sci. 2020, 11, 10786–10791. 10.1039/D0SC01445A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel T.; Muñiz K. Cooperative Iodine and Photoredox Catalysis for Direct Oxidative Lactonization of Carboxylic Acids. Chem. Commun. 2019, 55, 933–936. 10.1039/C8CC08594C. [DOI] [PubMed] [Google Scholar]
- McManus J. B.; Nicewicz D. A. Direct C–H Cyanation of Arenes via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 2880–2883. 10.1021/jacs.6b12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay N. E. S.; Nicewicz D. A. Cation Radical Accelerated Nucleophilic Aromatic Substitution via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 16100–16104. 10.1021/jacs.7b10076. [DOI] [PubMed] [Google Scholar]
- Chen W.; Huang Z.; Tay N. E. S.; Giglio B.; Wang M.; Wang H.; Wu Z.; Nicewicz D. A.; Li Z. Direct Arene C–H Fluorination with 18F– via Organic Photoredox Catalysis. Science 2019, 364, 1170–1174. 10.1126/science.aav7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlili A.; Lakhdar S. Acridinium Salts and Cyanoarenes as Powerful Photocatalysts: Opportunities in Organic Synthesis. Angew. Chem., Int. Ed. 2021, 60, 19526–19549. 10.1002/anie.202102262. [DOI] [PubMed] [Google Scholar]
- Tay N. E. S.; Chen W.; Levens A.; Pistritto V. A.; Huang Z.; Wu Z.; Li Z.; Nicewicz D. A. 19F- and 18F-Arene Deoxyfluorination via Organic Photoredox-Catalysed Polarity-Reversed Nucleophilic Aromatic Substitution. Nat. Catal. 2020, 3, 734–742. 10.1038/s41929-020-0495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. J.; Kanigan T. Electron Transfer Reactions between Bis(4-methoxyphenyl)methyl Cations and Triplet 1-Methoxynaphthalene. J. Am. Chem. Soc. 1990, 112, 1271–1273. 10.1021/ja00159a072. [DOI] [Google Scholar]
- Saeva F. D.; Breslin D. T.; Luss H. R. Intramolecular Photoinduced Rearrangements via Electron-Transfer-Induced, Concerted Bond Cleavage and Cation Radical/Radical Coupling. J. Am. Chem. Soc. 1991, 113, 5333–5337. 10.1021/ja00014a028. [DOI] [Google Scholar]
- Liu K.; Tang S.; Huang P.; Lei A. External oxidant-free electrooxidative [3 + 2] annulation between phenol and indole derivatives. Nat. Commun. 2017, 8, 775. 10.1038/s41467-017-00873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.-Z.; Zeng R.; Han B.; Li J.-L. Single-Electron Transfer Reactions Enabled by N-Heterocyclic Carbene Organocatalysis. Chem.—Eur. J. 2021, 27, 3238–3250. 10.1002/chem.202004059. [DOI] [PubMed] [Google Scholar]
- Ishii T.; Nagao K.; Ohmiya H. Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci. 2020, 11, 5630–5636. 10.1039/D0SC01538E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRocco D. A.; Rovis T. Catalytic Asymmetric α-Acylation of Tertiary Amines Mediated by a Dual Catalysis Mode: N-Heterocyclic Carbene and Photoredox Catalysis. J. Am. Chem. Soc. 2012, 134, 8094–8097. 10.1021/ja3030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.; Hu W.; Dong X.; Li X.; Sun J. N-Heterocyclic Carbene Catalyzed γ-Dihalomethylenation of Enals by Single-Electron Transfer. Angew. Chem., Int. Ed. 2016, 55, 15783–15786. 10.1002/anie.201608371. [DOI] [PubMed] [Google Scholar]
- Dai L.; Xia Z.-H.; Gao Y.-Y.; Gao Z.-H.; Ye S. Visible-Light-Driven N-Heterocyclic Carbene Catalyzed γ- and ϵ-Alkylation with Alkyl Radicals. Angew. Chem., Int. Ed. 2019, 58, 18124–18130. 10.1002/anie.201909017. [DOI] [PubMed] [Google Scholar]
- Xia Z.-H.; Dai L.; Gao Z.-H.; Ye S. N-Heterocyclic carbene/photo-cocatalyzed oxidative Smiles rearrangement: synthesis of aryl salicylates from O-aryl salicylaldehydes. Chem. Commun. 2020, 56, 1525–1528. 10.1039/C9CC09272B. [DOI] [PubMed] [Google Scholar]
- Bay A. V.; Fitzpatrick K. P.; Betori R. C.; Scheidt K. A. Combined Photoredox and Carbene Catalysis for the Synthesis of Ketones from Carboxylic Acids. Angew. Chem., Int. Ed. 2020, 59, 9143–9148. 10.1002/anie.202001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.; Döben N.; Studer A. Cooperative NHC and Photoredox Catalysis for the Synthesis of β-Trifluoromethylated Alkyl Aryl Ketones. Angew. Chem., Int. Ed. 2020, 59, 19956–19960. 10.1002/anie.202008040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.; Lezius L.; Studer A. Benzylic C–H Acylation by Cooperative NHC and Photoredox Catalysis. Nat. Commun. 2021, 12, 2068. 10.1038/s41467-021-22292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.; Studer A. Direct α-Acylation of Alkenes via N-Heterocyclic Carbene, Sulfinate and Photoredox Cooperative Triple Catalysis. J. Am. Chem. Soc. 2021, 143, 4903–4909. 10.1021/jacs.1c01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; Daniliuc C. G.; Studer A. Cooperative NHC/Photoredox Catalyzed Ring-Opening of Aryl Cyclopropanes to 1-Aroyloxylated-3-Acylated Alkanes. Angew. Chem., Int. Ed. 2021, 60, 25252–25257. 10.1002/anie.202110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Fitzpatrick K. P.; Scheidt K. A. Combined Photoredox and Carbene Catalysis for the Synthesis of g-Aryloxy Ketones. Adv. Synth. Catal. 2022, 364, 518–524. 10.1002/adsc.202101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See also:; Ishii T.; Kakeno Y.; Nagao K.; Ohmiya H. N-Heterocyclic Carbene-Catalyzed Decarboxylative Alkylation of Aldehydes. J. Am. Chem. Soc. 2019, 141, 3854–3858. 10.1021/jacs.9b00880. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information for more details. The relative configuration was assigned in analogy based on a derivative; see X-ray structure of compound 6 in Scheme 4.
- Zou C.; Miers J. B.; Ballew R. M.; Dlott D. D.; Schuster G. B. Electron transfer and back electron transfer in photoexcited ion pairs: forward and back directions have different maximum rates. J. Am. Chem. Soc. 1991, 113, 7823–7825. 10.1021/ja00020a088. [DOI] [Google Scholar]
- Leifert D.; Studer A. The Persistent Radical Effect in Organic Synthesis. Angew. Chem., Int. Ed. 2020, 59, 74–108. 10.1002/anie.201903726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.