Abstract
The incidence of ciprofloxacin resistance in Streptococcus pneumoniae is low but steadily increasing, which raises concerns regarding the clinical impact of potential cross-resistance with newer fluoroquinolones. To investigate this problem, we utilized an in vitro pharmacodynamic model and compared the activities of gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin to that of ciprofloxacin against two laboratory-derived, ciprofloxacin-resistant derivatives of S. pneumoniae (strains R919 and R921). Ciprofloxacin resistance in these strains involved the activity of a multidrug efflux pump and possibly, for R919, a mutation resulting in an amino acid substitution in GyrA. Gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin achieved 99.9% killing of both R919 and R921 in ≤28 h. With respect to levofloxacin, significant regrowth of both mutants was observed at 48 h (P < 0.05). For gatifloxacin, grepafloxacin, moxifloxacin, and trovafloxacin, regrowth was minimal at 48 h, with each maintaining 99.9% killing against both mutants. No killing of either R919 or R921 was observed with exposure to ciprofloxacin. During model experiments, resistance to gatifloxacin, grepafloxacin, moxifloxacin, and trovafloxacin did not develop but the MICs of ciprofloxacin and levofloxacin increased 1 to 2 dilutions for both R919 and R921. Although specific area under the concentration-time curve from 0 to 24 h (AUC0–24)/MIC and maximum concentration of drug in serum (Cmax)/MIC ratios have not been defined for the fluoroquinolones with respect to gram-positive organisms, our study revealed that significant regrowth and/or resistance was associated with AUC0–24/MIC ratios of ≤31.7 and Cmax/MIC ratios of ≤3.1. It is evident that the newer fluoroquinolones tested possess improved activity against S. pneumoniae, including strains for which ciprofloxacin MICs were elevated.
Streptococcus pneumoniae is a significant cause of morbidity and mortality and is one of the primary pathogens implicated in community-acquired pneumonia (2, 3, 5, 17, 36). Newer fluoroquinolones, such as levofloxacin, trovafloxacin, and clinafloxacin, have been shown to possess greater activity than older agents of this class against gram-positive organisms, including S. pneumoniae (3, 6, 8, 13, 19, 23, 34). The newer fluoroquinolones thus are potential alternatives for therapy of infections caused by multidrug-resistant pneumococci. With the increasing use of these drugs, however, there is growing concern regarding the development of quinolone resistance among gram-positive bacteria (10, 21).
Animal models, in vitro pharmacodynamic models, and limited human data have demonstrated that the primary pharmacodynamic parameters which most closely correlate with therapeutic efficacy of fluoroquinolones are area under the concentration-time curve from 0 to 24 h (AUC0–24)/MIC and maximum concentration of drug in serum (Cmax)/MIC ratios (1, 16, 28, 37). However, there are limited data correlating pharmacodynamic parameters with the development of resistance (39).
Ciprofloxacin-resistant strains of S. pneumoniae have been reported, and most troubling is the possibility of cross-resistance to the newer fluoroquinolones (11, 21, 26, 27, 32). These compounds have improved activity against topoisomerase IV, which is thought to be the primary target for most fluoroquinolones in gram-positive organisms such as S. pneumoniae and Staphylococcus aureus. In vitro data on the susceptibility of penicillin-susceptible and -resistant pneumococci to fluoroquinolones have demonstrated that both ciprofloxacin and ofloxacin MICs tend to cluster around their susceptibility breakpoints of 2 and 4 μg/ml, respectively, whereas the MICs of the newer quinolones are much lower: levofloxacin, 0.5 to 2 μg/ml; gatifloxacin, 0.25 to 0.5 μg/ml; moxifloxacin, 0.015 to 0.06 μg/ml; and trovafloxacin, 0.06 to 0.5 μg/ml (13, 22, 34, 41).
The purpose of the present study was to evaluate pharmacodynamic relationships such as AUC/MIC and Cmax/MIC by comparing the activities of several newer fluoroquinolones with that of ciprofloxacin against ciprofloxacin-resistant S. pneumoniae using an in vitro pharmacodynamic model. Additionally, we characterized the impact of ciprofloxacin resistance in S. pneumoniae on fluoroquinolone pharmacodynamics in relationship to killing and the emergence of higher-level resistance.
MATERIALS AND METHODS
Bacterial strains.
The parent isolate, S. pneumoniae 79, was a penicillin- and erythromycin-resistant clinical isolate (MICs of 2 and 12 μg/ml, respectively). Additional study strains consisted of two laboratory-derived, ciprofloxacin-resistant mutants of strain 79. These mutants were produced from the parent strain by serial passage in the presence of ciprofloxacin (21). Briefly, strain 79 was streaked onto agar plates containing increasing multiples of the ciprofloxacin MIC. After several passages, two mutants (R919 and R921) for which ciprofloxacin MICs were apparently increased were recovered and used in further studies.
Antimicrobial agents.
Gatifloxacin was supplied by Bristol-Myers Squibb, New Brunswick, N.J.; grepafloxacin was supplied by Glaxo Wellcome, Research Triangle Park, N.C.; ciprofloxacin and moxifloxacin were supplied by Bayer Corporation, West Haven, Conn.; and trovafloxacin was supplied by Pfizer Inc., Groton, Conn. Levofloxacin for injection was commercially purchased from Ortho-McNeil, Raritan, N.J. One lot number of each drug was used throughout the study.
Susceptibility testing.
MICs were determined by broth microdilution using Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with calcium (25 mg/ml), magnesium (12.5 mg/ml), and 5% lysed horse blood (Rockland, Inc., Gilbertsville, Pa.) (SMHB-LHB) according to NCCLS guidelines (31). MICs also were determined in Todd-Hewitt broth supplemented with 0.5% yeast extract (Difco Laboratories) (THB-Y). In addition, trovafloxacin MICs were determined in the presence of albumin (4 g/dl) to evaluate the effect of protein binding on the activity of the drug. The possible presence of an efflux mechanism of resistance was investigated by determining the MICs of several common substrates of multidrug efflux pumps (ethidium bromide, acriflavine, benzalkonium chloride, cetrimide, and tetraphenylphosphonium bromide) and by evaluating the effect of reserpine (10 μg/ml) on selected MICs (7, 24, 30, 42). MIC plates were incubated in candle jars at 37°C for 24 h in the presence of approximately 3% CO2.
Sequence determination of the QRDRs of gyrA, gyrB, parC, and parE.
The quinolone resistance-determining regions (QRDRs) of gyrA, gyrB, parC, and parE were amplified from bacterial strains using PCR. Primers and conditions were employed as previously described (33). PCR products were sequenced using the dideoxy chain termination method (37).
In vitro pharmacodynamic models.
THB-Y was utilized for the in vitro pharmacodynamic models, and tryptic soy agar supplemented with 5% sheep blood (TSA-SB) was used to determine colony counts (20). The in vitro model consisted of a 250-ml one-compartment glass chamber with ports for the addition and/or removal of media, antibiotics, and samples (19, 20). Prior to each experiment, colonies from an overnight growth on TSA-SB were added to THB-Y as necessary to obtain a suspension of ∼1 × 108 CFU/ml. A 2.5-ml volume of this suspension was added to the chamber to produce a starting inoculum of ∼1 × 106 CFU/ml. Fresh stock solutions of fluoroquinolones were prepared on the first day of the experiment and were stored at 2 to 8°C between dosage administration times. Dosing regimens included ciprofloxacin at 400 mg every 12 h (peak concentration, 5 μg/ml), gatifloxacin at 400 mg every 24 h (peak concentration, 3.5 μg/ml), grepafloxacin at 600 mg every 24 h (peak concentration, 2.5 μg/ml), levofloxacin at 500 mg every 24 h (peak concentration, 6 μg/ml), moxifloxacin at 400 mg every 24 h (peak concentration, 5 μg/ml), and trovafloxacin at 200 mg every 24 h (peak concentration, 3 μg/ml) for 48 h. Antibiotics were administered as a bolus into the models over 30 s using a hypodermic syringe. A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) was used to displace antibiotic-containing media to simulate the half-lives of ciprofloxacin (3 h), gatifloxacin (8 h), grepafloxacin (15 h), levofloxacin (6 h), moxifloxacin (10 h), and trovafloxacin (12 h) (16, 35, 38). Each model apparatus was placed in a water bath and maintained at 37°C for the entire study period. Model experiments were performed in duplicate to ensure reproducibility.
Pharmacokinetic analysis.
Samples (0.5 ml) from each model experiment were obtained at 0, 0.5, 2, 4, 8, 24, 28, 32, and 48 h for the determination of antibiotic concentrations and were stored at −70°C until analysis. Concentrations of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin were determined by bioassay using Klebsiella pneumoniae ATCC 10031 as the indicator organism. Blank 1/4-in. paper disks were spotted with 20 μl of samples or standards, which then were placed in triplicate on Mueller-Hinton agar plates preswabbed with a 0.5-McFarland standard suspension of indicator organism. Concentrations of fluoroquinolones used as standards were 5, 1.25, and 0.3125 μg/ml. Plates were incubated for 18 to 24 h at 37°C. All plates achieved a correlation coefficient of ≥0.95. For all fluoroquinolones, the between-day coefficient of variation was 3.1 to 6.8%. Antibiotic half-lives were calculated from the slopes of the drug concentration versus time plots. The AUC obtained from the drug concentration versus time plot was calculated by the linear trapezoidal rule using the PK Analyst programs (Micromath, Salt Lake City, Utah).
Pharmacodynamic analysis.
Samples (0.5 ml) were removed from each model at the same time points used for the pharmacokinetic analysis, except that samples also were obtained at 1 and 6 h. Each sample was serially diluted in cold 0.9% sodium chloride, and bacterial counts were determined by placing 20-μl spots of dilutions onto TSA-SB. Colonies were counted following incubation for 24 h at 37°C. It has been determined previously that these methods have a limit of detection of 2 log10 CFU/ml (9). The total reduction in log10 CFU/ml over 48 h was determined by plotting time-kill curves, and the time to achieve a 99.9% reduction in the initial inoculum was determined by visual inspection of these curves. To avoid antibiotic carryover, all samples were diluted sufficiently prior to plating, such that antibiotic concentrations were reduced below the MICs of the drugs for each organism. The AUC0–24/MIC and Cmax/MIC ratios were calculated for each fluoroquinolone. The development of raised MICs of each antibiotic during experiments was investigated by plating 100 μl of the 24- and 48-h samples onto TSA-SB containing two and four times the MIC of the appropriate fluoroquinolone. These plates were examined for growth after incubation for 48 h at 37°C. Changes in MICs also were checked for each model at 48 h via Etest (AB Biodisk, Solna, Sweden).
Statistical analysis.
Changes in log10 CFU/ml at 48 h plus time to 99.9% killing were compared by analysis of variance with Tukey's post hoc test for multiple comparisons. Relationships between AUC0–24/MIC, Cmax/MIC, and log10 CFU/ml at 48 h were determined by Pearson's correlation. A P value of ≤0.05 was considered significant.
RESULTS
Susceptibility testing.
MIC data for study strains are summarized in Table 1. The ciprofloxacin MICs for strains R919 and R921 were unchanged following seven serial passages on antibiotic-free media. Overall, there were no differences in MICs determined in SMHB-LHB or THB-Y. Trovafloxacin was the most active fluoroquinolone against R919 and R921, followed by grepafloxacin, moxifloxacin, gatifloxacin, and finally levofloxacin. Ciprofloxacin and norfloxacin MICs were increased 8- to 16-fold and 4- to 8-fold for R919 and R921, respectively. The presence of albumin did not affect the MIC results observed for trovafloxacin.
TABLE 1.
Susceptibilities of study strains, determined in THB-Y
| Antimicrobial agenta | MIC (μg/ml) for:
|
||
|---|---|---|---|
| 79 | R919 | R921 | |
| Ciprofloxacin | 0.5 | 4.0 | 8.0 |
| Ciprofloxacin + R | 0.25 | 0.25 | 0.5 |
| Norfloxacin | 8.0 | 32.0 | 64.0 |
| Norfloxacin + R | 0.5 | 2.0 | 2.0 |
| Gatifloxacin | 0.25 | 0.5 | 0.5 |
| Levofloxacin | 1.0 | 2.0 | 4.0 |
| Grepafloxacin | 0.125 | 0.25 | 0.5 |
| Moxifloxacin | 0.25 | 0.25 | 0.5 |
| Trovafloxacin | 0.06 | 0.125 | 0.25 |
| Trovafloxacin + albumin | 0.06 | 0.25 | 0.25 |
| Reserpine | ≥200 | ≥200 | ≥200 |
| EtBr | 4.0 | 32.0 | 32.0 |
| EtBr + R | 0.25 | 0.25 | 0.25 |
| Acriflavine | 8.0 | 8.0 | 8.0 |
| BAC | 0.5 | 2.0 | 2.0 |
| Cetrimide | 0.5 | 1.0 | 1.0 |
| TPP | 64.0 | 64.0 | 64.0 |
R, reserpine (10 μg/ml); EtBr, ethidium bromide; BAC, benzalkonium chloride; TPP, tetraphenylphosphonium bromide.
The MICs of ethidium bromide and benzalkonium chloride were increased 16- and 4-fold, respectively, for both mutant strains, compared to strain 79. The addition of reserpine resulted in a reduction in the MICs of ciprofloxacin, norfloxacin, and ethidium bromide for all strains. These MICs were reduced 2-, 16-, and 16-fold, respectively, for strain 79. For R919 and R921, the MIC reductions were 16- to 32-fold for ciprofloxacin and norfloxacin and 128-fold for ethidium bromide.
QRDR sequencing.
Strain R919 was found to have a mutation resulting in an S114G substitution in GyrA. No mutations resulting in amino acid substitutions were found in the gyrB QRDR of R919 or the gyrA and gyrB QRDRs of R921. All strains were found to have mutations resulting in a K137N substitution in ParC and an I460V substitution in ParE.
Pharmacodynamic and pharmacokinetic studies.
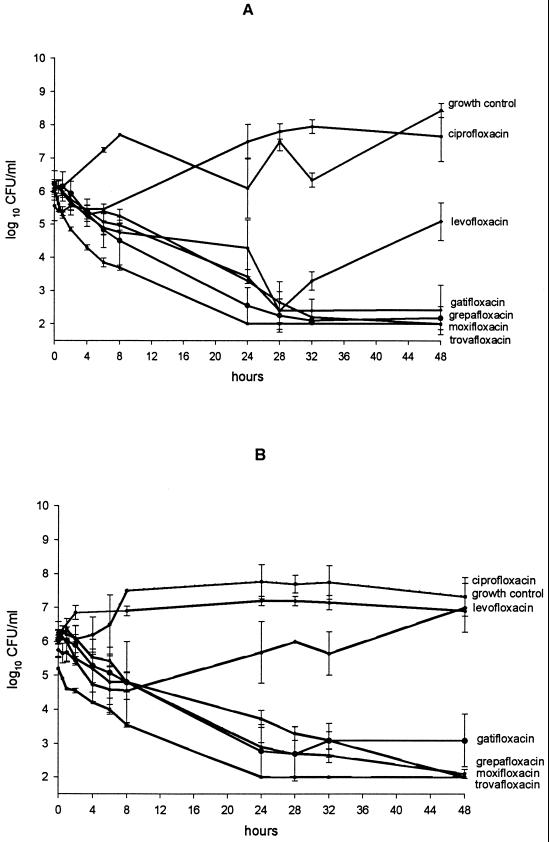
The results of model experiments are shown in Fig. 1. All drugs but ciprofloxacin achieved 99.9% killing in approximately 28 h against isolate R919. Regrowth at 48 h was minimal with gatifloxacin, grepafloxacin, moxifloxacin, and trovafloxacin; each maintained 99.9% killing at this time point. Against isolate R921, all but ciprofloxacin and levofloxacin achieved 99.9% killing by 28 h, with gatifloxacin, moxifloxacin, and trovafloxacin killing to the limit of detection (2 log10 CFU/ml) at 48 h. Modest, statistically insignificant regrowth was observed with gatifloxacin at 48 h. Although levofloxacin initially showed activity against both R919 and R921, by 48 h significant regrowth was seen (P < 0.05). Ciprofloxacin had no inhibitory or killing effect against either R919 or R921.
FIG. 1.
In vitro pharmacodynamic results. (A) R919; (B) R921. Data are means (± standard deviations) of multiple (four to six) determinations.
Pharmacodynamic parameters can be found in Table 2. AUC0–24/MIC ratios ranged from 5.8 to 442.4, with trovafloxacin having the highest ratio followed by moxifloxacin, grepafloxacin, gatifloxacin, levofloxacin, and then ciprofloxacin. Resistance (see below) and/or significant regrowth was observed with AUC0–24/MIC ratios of ≤31.7. Bacterial counts were reduced to the limits of detection without the presence of regrowth when AUC0–24/MIC ratios were ≥82. Cmax/MIC ratios ranged from 0.68 to 31.3, with trovafloxacin again showing the highest ratio followed by moxifloxacin, grepafloxacin, gatifloxacin, levofloxacin, and then ciprofloxacin. Resistance and/or significant regrowth was observed in cases where the Cmax/MIC ratio was ≤3.1. The mean peak, half-life, and AUC0–24 are shown in Table 3. A significant correlation (P < 0.05) was found for both Cmax/MIC and AUC0–24/MIC in relationship to decreases in colony counts at both 24 and 48 h when all drugs and both organisms were compared.
TABLE 2.
Pharmacodynamic parameters of evaluated fluoroquinolones
| Fluoroquinolone | AUC0–24/MIC
|
Cmax/MIC
|
||
|---|---|---|---|---|
| R919 | R921 | R919 | R921 | |
| Ciprofloxacin | 11.6 ± 1.9a | 5.8 ± 1.0a | 1.35 ± 0.01a | 0.68 ± 0.01a |
| Gatifloxacin | 75.9 ± 9.5 | 75.9 ± 9.5 | 7.4 ± 1.0 | 7.4 ± 1.0 |
| Grepafloxacin | 163.3 ± 21.6 | 81.7 ± 10.8 | 11.9 ± 1.6 | 5.9 ± 0.8 |
| Levofloxacin | 31.7 ± 3.6a | 15.9 ± 1.8a | 3.1 ± 0.2a | 1.6 ± 0.1a |
| Moxifloxacin | 213.5 ± 34.9 | 106.8 ± 17.4 | 18.9 ± 1.3 | 9.4 ± 0.6 |
| Trovafloxacin | 442.4 ± 79.5 | 221.2 ± 39.7 | 31.3 ± 3.0 | 15.7 ± 1.5 |
Associated with resistance and/or significant (P < 0.05) regrowth.
TABLE 3.
Pharmacokinetic parameters of evaluated fluoroquinolones
| Fluoroquinolone | AUC0–24 (μg · h/ml) | Peak concn (μg/ml) | Half-life (hour) | Trough concn (μg/ml) |
|---|---|---|---|---|
| Ciprofloxacin | 46.2 ± 7.7 | 5.4 ± 0.04 | 3.2 ± 0.7 | 0.81 ± 0.15 |
| Gatifloxacin | 37.9 ± 4.7 | 3.7 ± 0.5 | 8.5 ± 1.9 | 0.44 ± 0.25 |
| Grepafloxacin | 40.8 ± 5.4 | 2.9 ± 0.4 | 13.9 ± 1.5 | 0.79 ± 0.44 |
| Levofloxacin | 63.5 ± 7.1 | 6.3 ± 0.4 | 8.03 ± 0.9 | 0.57 ± 0.25 |
| Moxifloxacin | 53.4 ± 8.7 | 4.7 ± 0.3 | 9.9 ± 3.5 | 0.76 ± 0.47 |
| Trovafloxacin | 63.5 ± 8.9 | 5.2 ± 1.1 | 11.6 ± 3.6 | 1.0 ± 0.55 |
Resistance.
Samples from each model were evaluated for resistance at 24 and 48 h by plating aliquots onto solid media containing two and four times the MIC of the appropriate drug. Increases in MICs of ciprofloxacin (≥32 μg/ml) were observed at 24 and 48 h for both R919 and R921. Slight increases in MICs (one- to twofold) of levofloxacin were observed at 48 h for both mutants. No changes in MICs of any of the other fluoroquinolones were observed.
DISCUSSION
The incidence of penicillin resistance in S. pneumoniae has increased significantly in the United States over the last 2 decades (4, 12, 17, 40). Resistance to other antimicrobial agents, such as cephalosporins, macrolides, and trimethoprim-sulfamethoxazole, also is increasing (17, 40). The emergence of multidrug-resistant S. pneumoniae has prompted the need for the development of alternative treatments. The newer fluoroquinolones are viable options, as they have greater activity against gram-positive organisms. In fact, recent treatment guidelines for community-acquired pneumonia list newer fluoroquinolones such as levofloxacin, grepafloxacin, and trovafloxacin as alternatives for treatment of this condition (5).
There has been a rapid emergence of resistance to the fluoroquinolones in gram-positive bacteria, especially in S. aureus (11, 13, 24, 42). Reported resistance mechanisms in that organism include alterations in topoisomerase IV and DNA gyrase and/or active efflux of the drug (21–26, 33, 42). Similar resistance mechanisms can be found in S. pneumoniae. Furthermore, ciprofloxacin has not proven to be consistently reliable for treating infections caused by S. pneumoniae. Ciprofloxacin-intermediate (MIC, ≥1 to ≥2 μg/ml) and -resistant (MIC, >2 μg/ml) strains have already been reported (3, 10, 13, 14, 21, 33, 42).
To date, the incidence of fluoroquinolone-resistant S. pneumoniae has been relatively low (<2%). However, an increase in the usage of fluoroquinolones for therapy of community-acquired pneumonia and other infections may result in increased resistance. Two recent reports from Hong Kong and Canada found resistance to be present in 12.1 and 1.7% of strains, respectively. Interestingly, resistance was shown not only to the older fluoroquinolones, such as ciprofloxacin, but also to levofloxacin and trovafloxacin (10, 21).
Strain R919 was found to have a mutation resulting in an S114G substitution in GyrA. This substitution has been described previously in ciprofloxacin-resistant clinical isolates of S. pneumoniae, but its relation to the resistance phenotype is uncertain, as no genetic analysis of it has been done (15). All strains, including the ciprofloxacin-susceptible parent strain, had single amino acid substitutions in both ParC and ParE. The fact that these substitutions were found in the susceptible parent strain indicates that they are not responsible for the raised fluoroquinolone MICs observed in R919 and R921. MIC data revealed that both R919 and R921 have multidrug resistance phenotypes reversible by reserpine. This indicates the likely presence of a multidrug efflux pump which, based on the nearly complete reversal of resistance by reserpine, is the main resistance mechanism in R919 and R921. The multidrug resistance phenotypes of R919 and R921 may represent activity of PmrA, a recently described S. pneumoniae multidrug efflux pump (18). The fact that reserpine resulted in a decrease in MICs of various compounds in strain 79 as well indicates that this pump is active in wild-type susceptible strains.
Our experiments were designed to evaluate the efficacy of the newer fluoroquinolones against ciprofloxacin-resistant strains of S. pneumoniae. As predicted, ciprofloxacin was not effective against either R919 or R921. Although levofloxacin displayed activity against R919 for up to 28 h, significant regrowth was noted at 48 h and minimal short-term activity was seen against R921. Gatifloxacin, grepafloxacin, moxifloxacin, and trovafloxacin each had significant activity against both isolates, with greater than 99.9% killing being observed at 48 h. Both isolates displayed increases in the level of resistance to ciprofloxacin and levofloxacin upon exposure to either drug, with increases in MICs seen as early as 24 h for ciprofloxacin.
Most of the definitive studies with respect to the pharmacodynamic effects of the fluoroquinolones have concentrated on gram-negative infections, where an AUC0–24/MIC ratio of >100 or a Cmax/MIC ratio of >8 appears to be predictive of a good therapeutic response (16, 28, 36, 39). There are limited data, however, examining this relationship in gram-positive bacteria. Andes and Craig evaluated 19 publications examining the use of fluoroquinolones in various models of experimental endocarditis (1). The data suggested that a 24-h AUC/MIC ratio of ≥100 may be the best predictor of successful outcome. In contrast, a study by Wright et al. examining pharmacodynamic outcome parameters for levofloxacin and ciprofloxacin versus S. pneumoniae in an in vitro pharmacodynamic model found no significant difference in the relationship of response to AUC0–24/MIC or Cmax/MIC. Ciprofloxacin AUC0–24/MIC ratios of 17.4 and 8.7 were associated with regrowth at 24 h. AUC0–24/MIC ratios of >34.8 were not associated with regrowth (D. H. Wright, M. L. Peterson, L. B. Hovde, G. Brown, and J. C. Rotschafer, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-111a, 1998). Previously, an attempt by our laboratory to describe a relationship between the AUC0–24/MIC ratio for fluoroquinolones and S. pneumoniae was not successful (20). This likely was due to the use of fluoroquinolone-sensitive strains in our experiment, where the AUC0–24/MIC ratio was optimized even with some of the older fluoroquinolones. Evaluating strains with lower susceptibility to fluoroquinolones increases the ability to find a correlation. In the present study, we did observe a significant (P < 0.05) correlation with AUC0–24/MIC and Cmax/MIC ratios in relationship to killing and resistance; however, a specific value for these two parameters was not determined. It should be noted that our pharmacodynamic experiment did not model pneumococcal pneumonia and that the contribution of immune factors, such as neutrophils, was absent. Therefore, our results should be extrapolated with caution. Significant regrowth and resistance were observed with an AUC0–24/MIC ratio of ≤31.7 and Cmax/MIC ratio of ≤3.1, and bacterial counts remained at the limit of detection for AUC0–24/MIC ratios of ≥82. We did not have AUC0–24/MIC ratios that ranged between >31.7 and ≤75.9. Therefore, we cannot pinpoint the exact AUC0–24/MIC ratio needed to prevent regrowth and resistance. It is also important to note that the significance of regrowth in our model is unknown. Although there was a significant relationship between the AUC0–24/MIC and Cmax/MIC ratios and regrowth, it is not known whether the regrowth could be extrapolated to clinical situations, especially in light of the fact that for most of the time there were no changes in MICs from baseline for the organisms. In order to determine more definitively a specific AUC0–24/MIC and/or Cmax/MIC ratio predictive of outcome, studies examining numerous isolates for which MICs are close to the NCCLS breakpoints are needed.
ACKNOWLEDGMENTS
This project was supported by grants from Pfizer Inc. and Bayer Corporation.
REFERENCES
- 1.Andes D R, Craig W A. Pharmacodynamics of fluoroquinolones in experimental models of endocarditis. Clin Infect Dis. 1998;27:47–50. doi: 10.1086/514624. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumonia: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Barry A L, Fuchs P C, Brown S D. In vitro activities of five fluoroquinolone compounds against strains of Streptococcus pneumoniae with resistance to other antimicrobial agents. Antimicrob Agents Chemother. 1996;40:2431–2433. doi: 10.1128/aac.40.10.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry A L, Brown S D, Fuchs P C. Fluoroquinolone resistance among recent clinical isolates of Streptococcus pneumoniae. J Antimicrob Chemother. 1999;43:428–429. doi: 10.1093/jac/43.3.428. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett J G, Breiman R F, Mandell L A, File T M., Jr Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 6.Bédos J-P, Rieux V, Bauchet J, Muffat-Joly M, Carbon C, Azoulay-Dupuis E. Efficacy of trovafloxacin against penicillin-susceptible and multiresistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1998;42:862–867. doi: 10.1128/aac.42.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer R, Pestova E, Millichap J J, Stosor V, Noskin G A, Peterson L R. A convenient assay for estimating the possible involvement of efflux of fluoroquinolones by Streptococcus pneumoniae and Staphylococcus aureus: evidence for diminished moxifloxacin, sparfloxacin, and trovafloxacin efflux. Antimicrob Agents Chemother. 2000;44:798–801. doi: 10.1128/aac.44.3.798-801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brueggemann A B, Kugler K C, Doern G V. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappelletty D M, Rybak M J. Bactericidal activities of cefprozil, penicillin, cefaclor, cefixime, and loracarbef against penicillin-susceptible and -resistant Streptococcus pneumoniae in an in vitro pharmacodynamic infection model. Antimicrob Agents Chemother. 1996;40:1148–1152. doi: 10.1128/aac.40.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D K, McGeer A, De Azavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 11.Cormican M G, Jones R N. Cross-resistance analysis for clinafloxacin compared with ciprofloxacin, fleroxacin, ofloxacin, and sparfloxacin using a predictor panel of ciprofloxacin-resistant bacteria. J Antimicrob Chemother. 1995;36:431–434. doi: 10.1093/jac/36.2.431. [DOI] [PubMed] [Google Scholar]
- 12.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ednie L M, Jacobs M R, Appelbaum P C. Comparative activities of Clinafloxacin against gram-positive and -negative bacteria. Antimicrob Agents Chemother. 1998;42:1269–1273. doi: 10.1128/aac.42.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endtz H P, Mouton J W, den Hollander J G, van den Braak N, Verbrugh H A. Comparative in vitro activities of trovafloxacin (CP-99,219) against 445 gram-positive isolates from patients with endocarditis and those with other bloodstream infections. Antimicrob Agents Chemother. 1997;41:1146–1149. doi: 10.1128/aac.41.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrándiz M J, Fenoll A, Liñares J, de la Campa A G. Horizontal transfer of parC and gyrA in fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:840–847. doi: 10.1128/aac.44.4.840-847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedland I R, Med M, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Eng J Med. 1999;331(6):377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 18.Gill M J, Brenwald N P, Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:187–189. doi: 10.1128/aac.43.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gootz T D, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer R J. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. doi: 10.1128/aac.40.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershberger E, Rybak M J. Activities of trovafloxacin, gatifloxacin, clinafloxacin, sparfloxacin, levofloxacin, and ciprofloxacin against penicillin-resistant Streptococcus pneumoniae in an in vitro infection model. Antimicrob Agents Chemother. 2000;44:598–601. doi: 10.1128/aac.44.3.598-601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho P-L, Que T-L, Tsang D N-C, Ng T-K, Chow K-H, Seto W-H. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1999;43:1310–1313. doi: 10.1128/aac.43.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoellman D B, Lin G, Jacobs M R, Appelbaum P C. Anti-pneumococcal activity of gatifloxacin compared with other quinolone and non-quinolone agents. J Antimicrob Chemother. 1999;43:645–649. doi: 10.1093/jac/43.5.645. [DOI] [PubMed] [Google Scholar]
- 23.Hoogkamp-Korstanje J A A. In-vitro activities of ciprofloxacin, levofloxacin, lomefloxacin, ofloxacin, pefloxacin, sparfloxacin, and trovafloxacin against gram-positive and gram-negative pathogens from respiratory tract infections. J Antimicrob Chemother. 1997;40:427–431. doi: 10.1093/jac/40.3.427. [DOI] [PubMed] [Google Scholar]
- 24.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanematsu E, Deguchi T, Yasuda M, Kawamura T, Nishino Y, Kawada Y. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of DNA topoisomerase IV associated with quinolone resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1998;42:433–435. doi: 10.1128/aac.42.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafredo S C, Foleno B D, Fu K P. Induction of resistance of Streptococcus pneumoniae to quinolones in-vitro. Chemotherapy. 1993;39:36–39. doi: 10.1159/000238971. [DOI] [PubMed] [Google Scholar]
- 27.Legg J M, Bint A J. Will pneumococci put quinolones in their place? J Antimicrob Chemother. 1999;44:425–427. doi: 10.1093/jac/44.4.425. [DOI] [PubMed] [Google Scholar]
- 28.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–39. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 29.Lorian V. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams & Wilkins; 1996. pp. 482–483. [Google Scholar]
- 30.Markham P N. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob Agents Chemother. 1999;43:988–989. doi: 10.1128/aac.43.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd rd. Approved standard M7–A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 32.Norrby S R. Grepafloxacin in respiratory tract infections: are we ready to accept a quinolone for empirical treatment? J Antimicrob Chemother. 1997;40(Suppl. A):99–101. doi: 10.1093/jac/40.suppl_1.99. [DOI] [PubMed] [Google Scholar]
- 33.Pan X-S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pankuch G A, Jacobs M R, Appelbaum P C. Activity of CP99,219 compared with DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin, and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J Antimicrob Chemother. 1995;35:230–232. doi: 10.1093/jac/35.1.230. [DOI] [PubMed] [Google Scholar]
- 35.Perry C M, Barman Balfour J A, Lamb H M. Gatifloxacin. Drugs. 1999;58:683–696. doi: 10.2165/00003495-199958040-00010. [DOI] [PubMed] [Google Scholar]
- 36.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin, a new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 37.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan J T, Woodruff M, Lettieri J, Agarwal V, Krol G J, Leese P T, Watson S, Heller A H. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother. 1999;43:2793–2797. doi: 10.1128/aac.43.11.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas J K, Forrest A, Bhavnani S M, Hyatt J M, Cheng A, Ballow C H, Schentag J J. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42:521–527. doi: 10.1128/aac.42.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thornsberry C, Jones M E, Hickey M L, Mauriz Y, Kahn J, Sahm D F. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997–1998. J Antimicrob Chemother. 1999;44:749–759. doi: 10.1093/jac/44.6.749. [DOI] [PubMed] [Google Scholar]
- 41.Visalli M A, Jacobs M R, Appelbaum P C. MIC and time-kill study of activities of DU-6859a, ciprofloxacin, levofloxacin, sparfloxacin, cefotaxime, imipenam, and vancomycin against nine penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1996;40:362–366. doi: 10.1128/aac.40.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeller V, Janoir C, Kitzis M D, Gutmann L, Moreau N J. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1973–1978. doi: 10.1128/aac.41.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]