Abstract
Neuroendocrine prostate cancer (NEPC) is a rare entity. De novo NEPC is extremely rare; other cases are usually adenocarcinoma previously treated with hormonal therapies transforming to NEPC. Most of the cases are metastatic at diagnosis and regardless of the histology types, the prognosis is poor. In this report, we reviewed the checkpoint inhibitor (CPI) immunotherapies used for neuroendocrine tumors of the prostate. Very limited data with only a few cases were published which showed a limited activity by immunotherapy; therefore, we present our experience of 2 cases: (1) adenocarcinoma with foci of NEPC and (2) adenocarcinoma transforming to NEPC after treatment with androgen deprivation therapy (ADT); both of which were initially managed with ADT, chemotherapy followed by immunotherapy with durvalumab, a programmed death ligand 1 inhibitor. In these 2 cases, CPI therapy showed limited efficacy, suggesting that neuroendocrine histology is not very responsive to CPI treatment, regardless if onset is early or late. Other therapies need to be explored for the treatment of NEPC.
Keywords: neuroendocrine tumor of the prostate, castrate-resistant prostate tumor, immunotherapy
Introduction
Among men, prostate cancer comes as the second most common cancer.¹ Mostly, prostate cancers are adenocarcinomas with tumor cells showing luminal differentiation including expression of prostate-specific antigen (PSA) and androgen receptor (AR).
An aggressive rare variant of prostate cancer, neuroendocrine prostate cancer (NEPC), can be divided into 2 types: (1) de novo NEPC (primary), derived from neural crest cells and (2) therapy-related NEPC, developed from patients with adenocarcinoma, previously treated with hormone therapies. In lethal metastatic castrate-resistant prostate cancers (mCRPCs), the incidence of neuroendocrine phenotypes is 25% to 30%. 1 In 1 study, the median age at the time of diagnosis of NEPC was 6 months to 1 year. 2 Prostatic small-cell carcinoma (PSCC) is a rare variant of NEPC that accounts for 0.5% to 2% of all cases of prostate cancer. 3
Neuroendocrine differentiation of primary prostatic adenocarcinoma commonly develops after months or years of hormone manipulations as a part of treatment for prostatic adenocarcinoma. In 1 study, the median time from adenocarcinoma to treatment-related NEPC diagnosis was 39.7 months. 4 The clinical course for neuroendocrine prostatic tumor is very aggressive and has a poor prognosis. It does not express the AR and it is considered clinically hormone refractory. With the introduction of new highly potent AR-targeted agents such as abiraterone acetate and enzalutamide, treatment-related NEPC is becoming an even more important disease to recognize. 5 Most of the time, patients are presented with locally advanced or metastatic disease. Common sites of metastasis included bone, lymph node, and viscera.
At present, the standard therapy is similar to the treatment of small cell lung carcinoma, that is first-line carboplatin and docetaxel or cabazitaxel, or etoposide and cisplatin or carboplatin. 6 National Comprehensive Cancer Network (NCCN) guidelines suggest a combination of etoposide with either cisplatin or carboplatin in patients with pure small cell carcinoma of the prostate. Now, immune checkpoint inhibitors (CPIs) are used as a new treatment modality in patients with small cell lung cancer, and it has shown some promising results in patients with extrapulmonary neuroendocrine cancers as well. 2 Checkpoint inhibitor immunotherapy such as programmed death ligand 1 (PDL-1) inhibitors like durvalumab,7,8 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors like tremelimumab 7 has been tried in metastatic prostate tumors and neuroendocrine tumors of the lung and gastrointestinal origin, but not in NEPC. Only 2 CPI immunotherapy studies were published in the literature (Table 1). We like to share our experience in using durvalumab in combination with chemotherapy in treating patients with NET of the prostate and review the literature on immunotherapies tried in neuroendocrine tumors (NET) in prostate carcinoma.
Table 1.
| Therapeutic agent | Patient population | Number of patients | Chemotherapy tried | Characteristics | Objective responses |
|---|---|---|---|---|---|
| Atezolizumab 9 | Small cell or neuroendocrine tumor of the prostate | 7 | • In 6 out of 7, carboplatin and etoposide were also used. | • 2 out of 7 patients: de novo small cell/neuroendocrine
pathology. • Other 5: transformation from a preexisting adenocarcinoma. |
Median follow-up of 6.5 months: Median PFS: 3.4 months Median OS:8.4 months. |
| Pembrolizumab 10 (2 mg/kg every 3 weeks) | Metastatic platinum-refractory small cell carcinoma of the prostate | 1 | • Carboplatin and etoposide | • Transformation from a preexisting adenocarcinoma after hormonal therapy. | • Restaging after 4 cycles revealed substantial improvement in
tumor burden. • Stable disease with 21 cycles of therapy. |
Abbreviations: PFS, progression-free survival; OS, overall survival.
Case Description
Case 1
A 65-year-old male patient with a history of benign prostatic hyperplasia presented initially with acute urinary retention and acute kidney injury. He was further evaluated by a non-contrast computed tomography (CT) scan of the abdomen and pelvis which revealed an enlarged prostate with bilateral hydronephrosis of each moiety of horseshoe kidney and stranding around the right kidney with a large amount of retroperitoneal fluid suggesting fornical rupture. His bladder wall was also thickened.
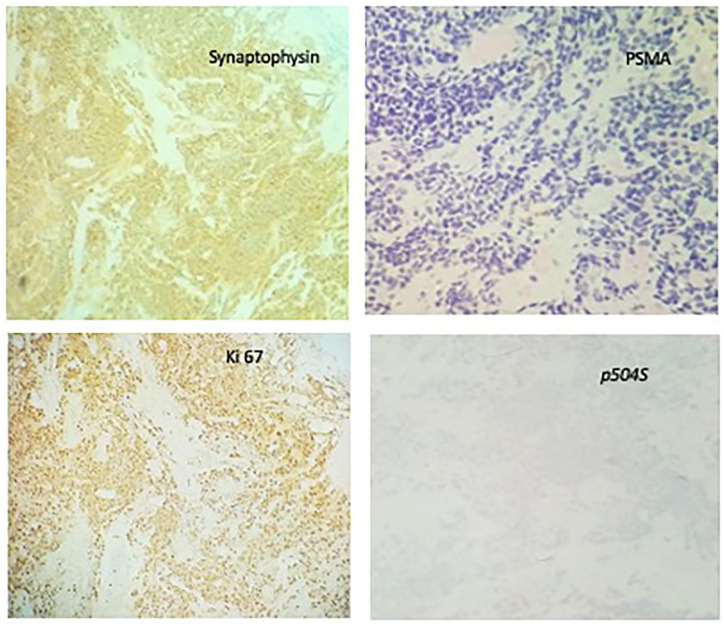
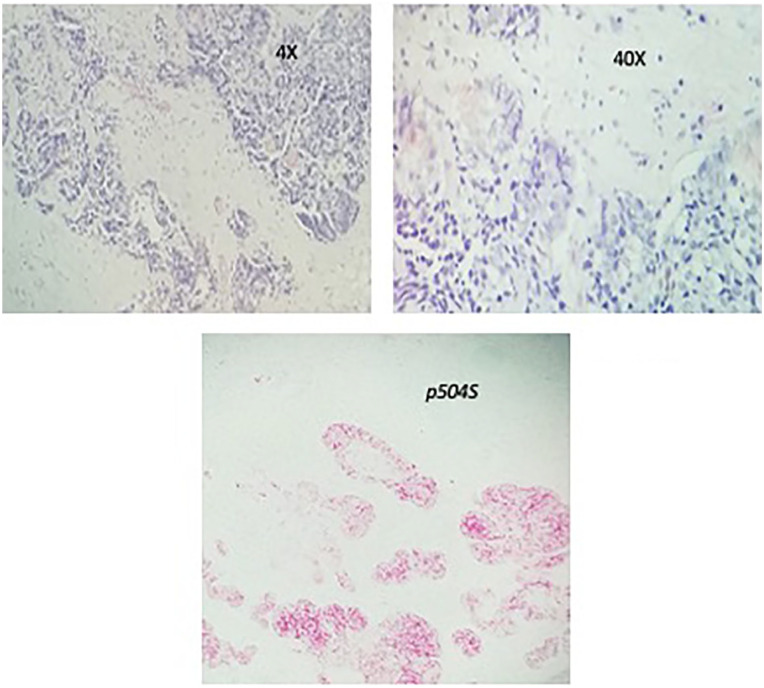
Subsequently, he had a cystoscopy which showed enlarged prostate with a mass infiltrating the trigone with ureteral obstruction. He underwent transurethral resection of the prostate and pathology revealed 2 pathologic types: one with high-grade prostatic adenocarcinoma and another component with a small cell of neuroendocrine differentiation (Figures 1 and 2). Immunohistochemistry showed of the small cell components were positive staining with CD 56, synaptophysin, and chromogranin A. Ki-67 was 40% to 50% (Figure 3). The adenomatous components were positive for prostate-specific membrane antigen (PSMA) and P504S (Figure 4). Initial PSA was 9.81 ng/mL and chromogranin was 208 ng/mL.
Figure 1.
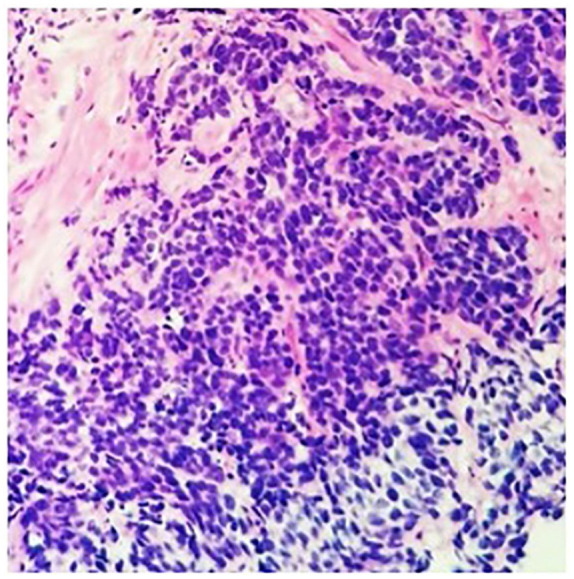
Prostate biopsy on case no. 1 showed an invasive high-grade carcinoma of 2 histomorphologic types: small cell neuroendocrine carcinoma (hematoxylin-eosin, original magnifications 4×).
Figure 2.
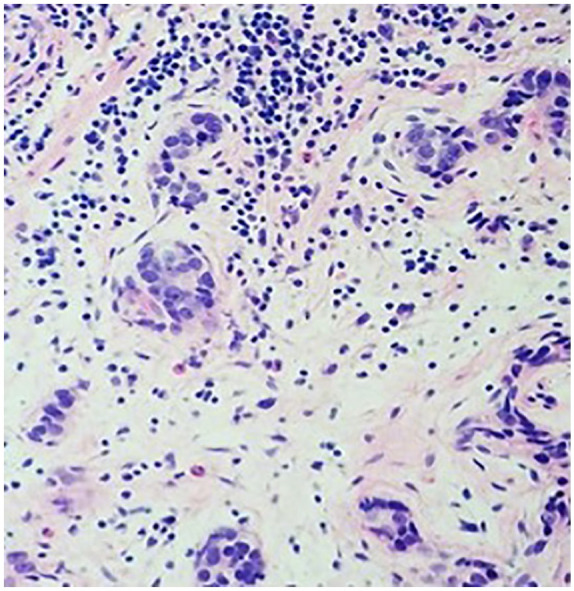
Prostate biopsy on case no. 1 showed an invasive high-grade carcinoma of 2 histomorphologic types: prostate adenocarcinoma above (hematoxylin-eosin, original magnifications 4×).
Figure 3.
Case no. 1, IHC studies reveal small cell component is positive for synaptophsyin and negative for PSMA; Ki67 index shows 30% to 40% positivity and negative for p504S.
Abbreviations: IHC, immunohistochemistry; PSMA, prostate-specific membrane antigen.
Figure 4.
Case no. 1, adenocarcinoma component showed original magnifications 4× [A]; magnifications 40×, and positive for p504S.
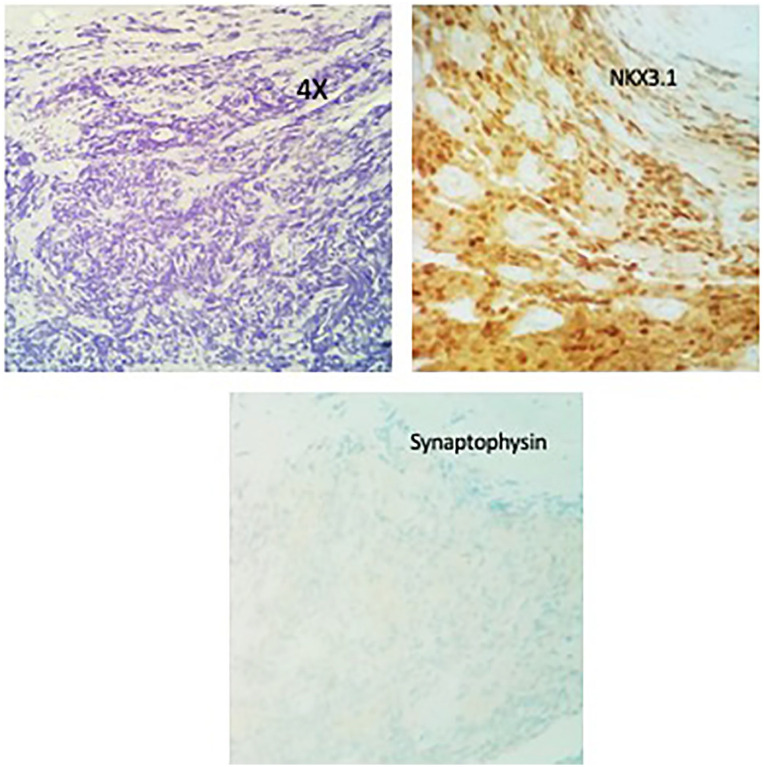
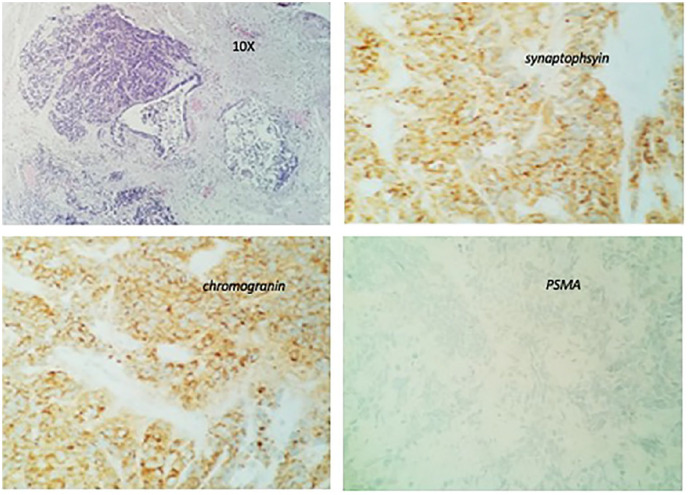
Further metastatic workup revealed him to have extensive bony metastasis. He was started on enzalutamide, leuprolide along irinotecan and carboplatin. His PSA and chromogranin A levels dropped to 0.1 and 109 ng/mL, respectively. After 6 cycles of treatment, the patient presented with a thoracic compression fracture with cord compression at T4 and T5. He underwent laminectomy which showed metastatic disease similar to his primary tumor; (Figures 5 and 6) a neuroendocrine component and an adenocarcinoma component. He received palliative radiation to the spine and was started on immunotherapy with durvalumab and cabazitaxel (20 mg/m2 every 3weeks). His CT of the chest, abdomen, and pelvis at 3 months revealed extensive bony sclerosis unchanged from the previous study . Later, his PSA and chromogranin levels started to go up and his general condition worsened. At this point, the family decided not to continue with aggressive treatment and he died a few days later.
Figure 5.
Case no. 1. Thoracic spine biopsy. H&E examination reveals metastatic thoracic tumor with 2 histogenesis: metastatic prostatic carcinoma in thoracic spine (10×), the adenocarcinoma was negative for synaptophysin and positive for NKX3.1.
Abbreviation: H&E, hematoxylin-eosin.
Figure 6.
Case no. 1. Thoracic spine biopsy. Thoracic metastastic tumor with the small cell component original magnifications (10×), IHC stain reveals strong positivity for synaptophsyin; strong and diffuse positivity for chromogranin, and negative for PSMA.
Abbreviations: IHC, immunohistochemistry; PSMA, prostate-specific membrane antigen.
Case 2
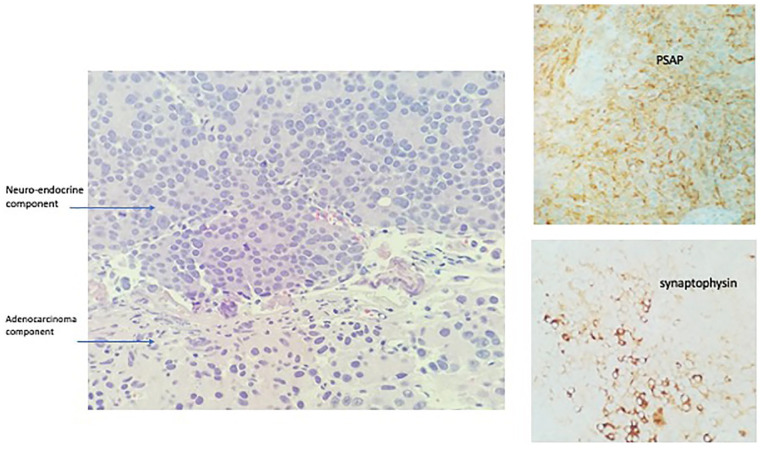
A 78-year-old male patient with a history of prostate cancer 19 years back (status post prostatectomy and leuprolide for 4 months after surgery) presented with hematuria. CT of the abdomen and pelvis revealed a lobulated mass in the urinary bladder and right-sided hydronephrosis. His PSA was elevated to 22.3 ng/mL. He had a biopsy of the prostate fossa and also had cystoscopy which revealed a large tumor extending from the bladder neck to the trigone which was resected. Pathology of the prostate bed and bladder tumor revealed invasive adenocarcinoma of the prostate with a Gleason score of 4 + 5 = 9 (Figures 7 and 8). Focal surface high-grade carcinoma was also seen in the bladder tumor. Immunohistochemistry revealed positive staining for adenocarcinomas PSMA and PSA and negative for urothelial markers GATA-3, Uroplakin, and CK903. Neuroendocrine marker synaptophysin showed weak positive staining, considered nonspecific and may be seen in adenocarcinomas. He was started on androgen deprivation therapy (ADT). A year later, the patient underwent another CT of the abdomen and pelvis which showed a lobulated mass in the urinary bladder. He underwent another transurethral resection of bladder tumor (TURBT) which revealed poorly differentiated prostatic carcinoma. Subsequently, a bone scan was done which showed metastatic disease to the L1 vertebra and right ischial ramus. He was started on enzalutamide for mCRPC. Two years later, he had a repeat bone scan which revealed that several skeletal metastases on the CT of the chest, abdomen, and pelvis with recurrence of mass in the bladder. He underwent another TURBT which revealed high-grade malignant neoplasm with neuroendocrine differentiation (Figure 9), prostate-specific acid phosphatase positive for adenocarcinoma component and synaptophysin positive for neuroendocrine component. He was started on abiraterone and prednisone. Positron emission tomography (PET)/CT done a year later showed an increase in the size of the endoluminal soft tissue mass in the bladder. Repeat TURBT was done, and pathology showed features consistent with prostatic adenocarcinoma of high-grade involving muscularis propria with focal neuroendocrine differentiation positive for synaptophysin as well as chromogranin.
Figure 7.
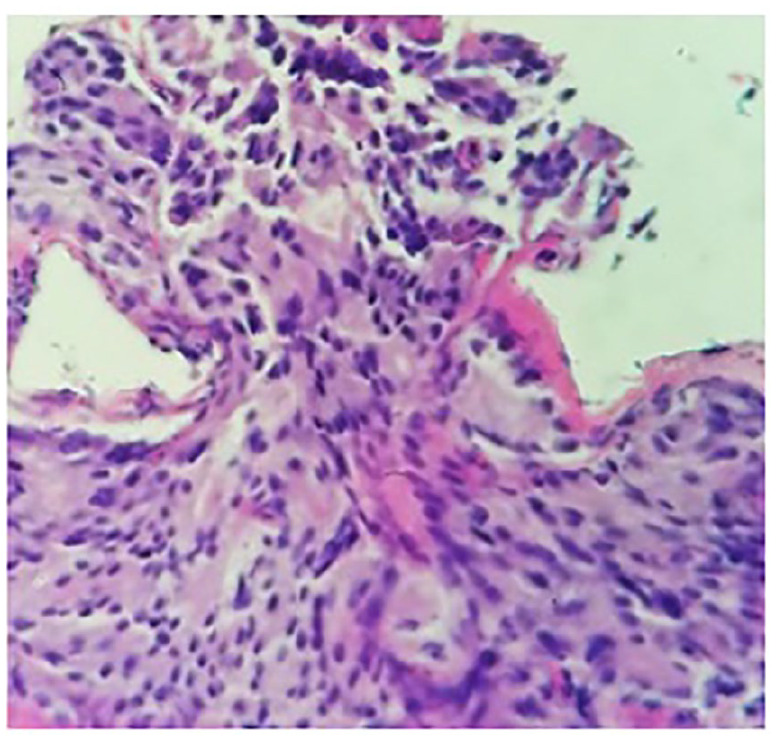
Case no. 2. (2014): Prostatic core biopsy, invasive adenocarcinoma 4 + 5 = 9/10; original magnifications 40×.
Figure 8.
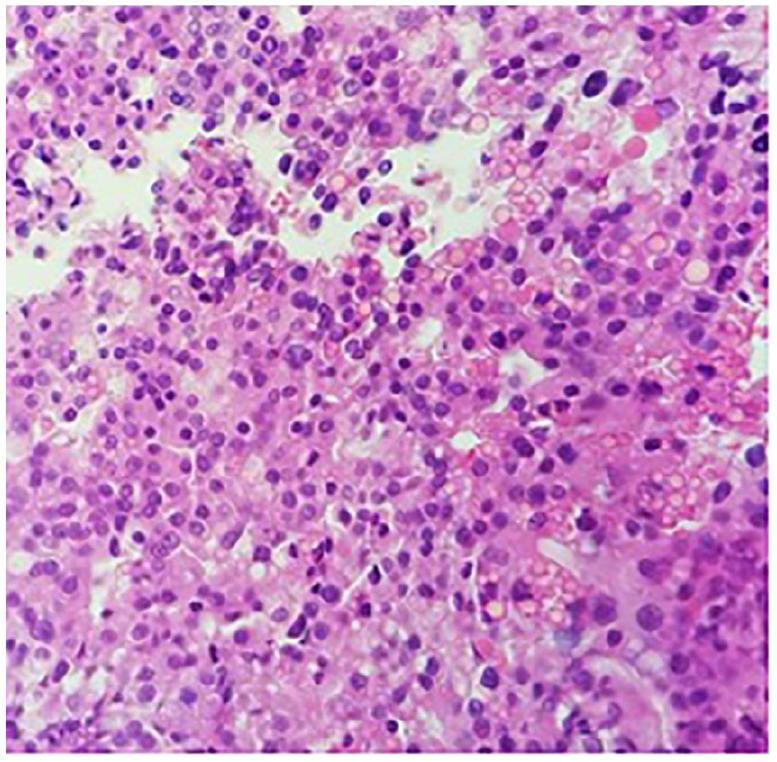
Case no. 2: Transurethral resection of bladder tumor showing invasive adenocarcinoma of prostate 4 + 5 = 9/10, original magnifications 40×. IHC stain showed tumor positive for prostate markers PSA and PSMA, and negative for urothelial markers GATA-3, Uroplakin, and CK903. Neuroendocrine marker synaptophysin showed weak positive staining, considered as nonspecific and may be seen in adenocarcinomas.
Abbreviations: IHC, immunohistochemistry; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen.
Figure 9.
Case no. 2. (2019): Microscopic examination of bladder tumor with patterns (hematoxylin-eosin, original magnifications 40× [A]) showed 2 components of pathology; one was high-grade adenocarcinoma and the other one was neuroendocrine component of urinary bladder tumor, high grade, involving the muscular propria. Neuroendocrine component was positive for synaptophysin and another was adenocarcinoma, positive for PSAP indicating prostate origin.
Abbreviation: PSAP, prostate-specific acid phosphatase.
Subsequently, the patient was started on cabazitaxel and durvalumab along with continuing ADT and bisphosphonates. But after 4 months of this treatment, a PET/CT scan showed progression of bladder mass. He then went to another doctor for receiving treatments.
Discussion
Prostate cancer is usually treated with surgery and radiotherapy. Initially, cancer survives by signaling through the ARs of tumor cells, which respond to testosterone. For this reason, therapies blocking testosterone and related hormones make a potent weapon against cancer that is not treated by surgery and/or chemotherapy. 11 Eventually, prostate tumors develop resistance to antiandrogen and neuroendocrine differentiation.
The NEPC has an aggressive clinical course. In patients with castration-resistant prostate cancer, treatment-related NEPC should be suspected in those who experience rapid progression with a low-serum PSA and metastases, especially in the setting of potent androgen deprivation treatment. 5 As the current knowledge about the optimal treatment of aggressive variant prostate carcinoma (AVPC) including prostate adenocarcinoma with neuroendocrine differentiation is incomplete, the guidelines of most medical societies make no specific treatment recommendations for this subset. In castrate-resistant prostate cancer with small-cell histology, cytotoxic chemotherapy has been associated with improved outcomes and is generally considered the preferred treatment option as mentioned above. Similar to small cell lung cancer platinum-based chemotherapy regimens: cisplatin/etoposide, carboplatin/etoposide, and docetaxel/carboplatin are recommended by NCCN. There is no clear consensus on the optimal first-line therapy in patients with clinical AVPC (putting aside pure small-cell histology), with 58% of the Advanced Prostate Cancer Consensus Conference 2017 voting in favor of standard mCRPC (24 months of suppression of testosterone) treatment and 42% of chemotherapy based on platinum drugs. 12
Reviewing CPI immunotherapy in the treatment of neuroendocrine tumors of the prostate, only very few studies have been published as outlined in Table 1. The first study was a single institute experience of using atezolizumab, a PDL-1 inhibitor for small cell or neuroendocrine component of prostate cancer in 7 patients. The combination of carboplatin, etoposide, and atezolizumab was the first-line treatment in 6 of the 7 patients. There seemed to be no additional benefit of adding immunotherapy; the conclusion deduced from the patients who received chemotherapy plus immunotherapy in the first-line setting as at a median follow-up of 6.5 months (range: 1.5-15.1 months), with median progression-free survival (PFS) of 3.4 months and median overall survival of 8.4 months. 9 In another case report, pembrolizumab was used in metastatic platinum-refractory small cell carcinoma of the prostate, in which restaging after 4 cycles revealed substantial improvement in tumor burden and had stable disease with 21 cycles of treatment. 10 In another study published as an abstract form of using avelumab, 13 19 patients were studied, but only 5 patients (27%) had neuroendocrine histology, with an overall response rate of 6.7% with 1 complete response (patient with high micro satellite instability), 0 partial response, 3 (20%) with stable disease, and 11 (73%) with progressive disease. So CPI immunotherapy so far published has been shown to have limited activity in treating neuroendocrine tumors of the prostate. In our 2 cases, we tried with cabazitaxel and durvalumab, another PDL-1 inhibitor, which has been tried previously in neuroendocrine tumors of gastrointestinal and lung origin but had not been used specifically for neuroendocrine prostate tumors before as far as we know. In both of the cases, the outcomes were not promising. Our initial idea was that chemotherapy in combination with CPI therapy had proven survival benefits over chemotherapy alone in extensive small cell carcinoma of the lung 14 ; therefore, CPI therapy was tried in neuroendocrine carcinoma of the prostate. The poor response of CPI therapy in NEPC as seen in our two cases and previous studies it seems like NEPC probably are different in underlying pathophysiology compared to other NET. Also, small cell lung carcinoma was associated with smoking, high tumor mutation burden (TMB), and formation of neo-antigens. 15 However, NEPCs were mainly driven by Aurora kinase A and N-My 16 which may not have high neo-antigens or TMB.
Aurora kinase A catalytic inhibitor, alisertib, has been used in phase II trials in patients with castration-resistant and NEPC. Out of 60 treated patients, 54% of evaluable patients (30/56) were classified as NEPC based on morphologic criteria. 17 The 6-month radiographic PFS was 13.4% and the median overall survival was 9.5 months (7.3-13 months). Exceptional responders have been identified; 2 cases of liver metastases completely resolved with treatment and 2 cases of stable disease (14 months and 3.8 years). 18
Other possible treatments for neuroendocrine tumor of the prostate include MEK inhibitor (encorafenib) 19 and histone deacetylase inhibitor (suberoylanilide hydroxamic acid). 20 Also, CD44, a glycoprotein that mediates cell-cell and cell-matrix adhesion, is expressed in 100% of the prostatic small cell cancer (PSCC) cases, and CD56 expression is noted in 83% to 92% of the patients with PSCC. Both of these may act as therapeutic targets and can be tried for the treatment of PSCC in the future. 21 More exploration has to be done with clinical trials in the future for these possible treatments.
Conclusion
Checkpoint inhibitors immunotherapy is a potentially promising method in cancer treatments. Neuroendocrine tumor of the prostate is a very aggressive tumor with a poor prognosis, the review of the literature and our experience of these 2 cases showed neuroendocrine histology in prostate carcinoma is not very responsive to CPI treatment, regardless if onset is early or late. Exploring other modalities of treatment such as Aurora kinase A catalytic inhibitor or others may be necessary.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethics approval to report this case was obtained from Brookdale Hospital IRB Review Board. Our institution does not require ethical approval for reporting individual case reports.
Informed Consent: Informed consent for patient information to be published in this article was obtained.
ORCID iD: Jen C. Wang  https://orcid.org/0000-0002-9623-6645
https://orcid.org/0000-0002-9623-6645
References
- 1. Gupta K, Gupta S. Neuroendocrine differentiation in prostate cancer: key epigenetic players. Transl Cancer Res. 2017;6(suppl 1):S104-S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stelwagen J, de Vries EG, Walenkamp AME. Current treatment strategies and future directions for extrapulmonary neuroendocrine carcinomas: a review. JAMA Oncol. 2021;7:759-770. [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conteduca V, Oromendia C, Eng KW, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlson RH. The treatment challenges of neuroendocrine prostate cancer. Oncology Times. 2015;37(4):118-119. [Google Scholar]
- 6. UroTodaycom. APCCC 2019: Neuroendocrine Prostate Cancer Spectrum—Diagnosis and Treatment. https://www.urotoday.com/conference-highlights/apccc-2019/114635-apccc-2019-neuroendocrine-prostate-cancer-spectrum-diagnosis-and-treatment.html. Accessed September, 2021.
- 7. Oncology T. Featured Clinical Focus. https://www.targetedonc.com/view/sunitinib-in-malignant-pheochromocytoma-paraganglioma-show-efficacy-may-change-future-practice. Accessed September, 2021.
- 8. Institute NC. Clinical Trial Information. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2016-01618. Accessed September, 2021.
- 9. Wee CE, Costello BA, Orme JJ, Quevedo JF, Pagliaro LC. Chemotherapy with atezolizumab for small cell or neuroendocrine carcinoma of the prostate: a single-institution experience. The Prostate. 2021;81(13):938-943. [DOI] [PubMed] [Google Scholar]
- 10. Salhab M, Donahue M, Walsh W. Pembrolizumab for platinum-refractory small cell carcinoma of the prostate, a case report. Hematology and Medical Oncol. 2018;3(4). doi: 10.15761/hmo.1000169. [DOI] [Google Scholar]
- 11. Richards S. Targeted immunotherapy for deadly prostate cancer shows promise in the preclinical test. https://www.fredhutch.org/en/news/center-news/2020/11/Lee-antibody-conjugate-neuroendocrine-prostate-cancer.html. Accessed April 5, 2022.
- 12. Moore SR, Reinberg Y, Zhang G. Small cell carcinoma of the prostate: effectiveness of hormonal versus chemotherapy. Urology. 1992;39:411-416. [DOI] [PubMed] [Google Scholar]
- 13. Brown LC, Halabi S, Humeniuk MS, et al. Efficacy of the PD-L1 inhibitor avelumab in neuroendocrine or aggressive variant prostate cancer: results from a phase II, single-arm study. J Clin Oncol. 2021;39(6 suppl):89-89. [Google Scholar]
- 14. Horn L, Mansfield AS, Szcz!sna A, et al. First!line atezolizumab plus chemotherapy in extensive!stage small!cell lung cancer. N Engl J Med. 2018;379(23):2220-2229. doi: 10.1056/nejmoa1809064. [DOI] [PubMed] [Google Scholar]
- 15. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1(6):487-495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38(6):756-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beltran H, Oromendia C, Danila DC, et al. A Phase II trial of the aurora kinase a inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin Cancer Res. 2019;25(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dicken H, Hensley PJ, Kyprianou N. Prostate tumor neuroendocrine differentiation via EMT: the road less traveled. Asian J Urol. 2019;6(1):82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu X, Huang YH, Li YJ, et al. Potential therapeutic effect of epigenetic therapy on treatment-induced neuroendocrine prostate cancer. Asian J Androl. 2017;19(6):686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar K, Ahmed R, Chukwunonso C, et al. Poorly differentiated small-cell-type neuroendocrine carcinoma of the prostate: a case report and literature review. Case Rep Oncol. 2018;11(3):676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]