Abstract
Objective
Bronchiectasis is a heterogeneous disease with distinct phenotypes. The post-tuberculosis (post-TB) bronchiectasis phenotype is prevalent in many countries but is under-studied. Our aim was to identify distinct phenotypic characteristics of post-TB bronchiectasis.
Methods
We recruited adults admitted between Jan 2010-Oct 2017 at Changi General Hospital, Singapore for bronchiectasis exacerbation. We collected demographics, symptoms, lung function, microbiology and FACED scores. Participants were followed-up until the next hospitalized exacerbation or end of study, whichever was sooner. Participants diagnosed by their attending respiratory specialist to have post-TB bronchiectasis were compared to those with bronchiectasis from other aetiologies.
Results
148 participants were included with mean±standard deviation age 63 ± 9 years; 46 (31.1%) had post-TB bronchiectasis and 102 (68.9%) other aetiologies. Compared to other aetiologies, participants with post-TB bronchiectasis had significantly lower body mass index (BMI), more frequent presentation with haemoptysis, lower forced expiratory volume in one second (FEV1), more frequent isolation of nontuberculous mycobacteria (NTM), and higher FACED scores indicating greater disease severity. Over a median follow-up of 21 months, post-TB bronchiectasis was associated with shorter time to next hospitalized exacerbation (49 vs 76 months, Log-Rank p = .01).
Conclusion
Post-TB bronchiectasis is a distinct entity with higher rates of haemoptysis and NTM isolation, more frequent exacerbations, and greater disease severity.
Keywords: Bronchiectasis, Tuberculosis, post-tuberculous, severity, exacerbation
Introduction
Bronchiectasis is a chronic respiratory disease characterised by abnormal dilatation of the airways and persistent respiratory symptoms such as cough, sputum production, haemoptysis and dyspnea. 1 The prevalence of bronchiectasis varies in different countries, ranging from about 50-70 per 100,000 population in Europe and North America to as high as 1200 per 100,000 in China among the population aged 40 years and above. 2 Prevalence increases with age and this disease poses a significant economic burden on healthcare services worldwide. 2
Bronchiectasis is not a uniform disease; rather, it is heterogenous with distinct phenotypes.3,4 Different phenotypes exist due to variability in aetiology, clinical characteristics, and treatment response.5-7 In countries where the burden of tuberculosis is significant, post-tuberculosis is thought to be one of the principal causes of bronchiectasis. 8 For example, in the Indian EMBARC cohort, the most frequent aetiology of bronchiectasis was post-TB at 35.5%. 9 Other studies conducted within Asia have also detected high rates of post-TB bronchiectasis such as in China (16%), Taiwan (12%), Thailand (32%), and Singapore (37%).10-13 Despite the significant burden of post-TB bronchiectasis, especially in low- and middle-income countries, the clinical characteristics and outcomes of post-TB bronchiectasis have been sparsely described. 14 Studies investigating real-world clinical characteristics and outcomes are needed to improve our understanding of patients’ needs in the treatment of post-TB bronchiectasis and provide the basis for formulating evidence-based recommendations for this condition. 15
The aim of the present study was to identify distinct phenotypic characteristics of post-TB bronchiectasis. Using a cohort of participants recruited from a tertiary hospital in Singapore, we compared clinical features and outcomes between post-TB bronchiectasis versus bronchiectasis due to other aetiologies.
Methods
Design
This was a single-centre, longitudinal, real-world observational study.
Participants and setting
The study enrolled adults who were admitted at Changi General Hospital, Singapore, for bronchiectasis exacerbation between 1 January 2010 and 31 October 2017. Participants were aged 21 years or older, with a diagnosis of bronchiectasis made by a specialist respiratory physician and supported by radiologic evidence of bronchiectasis (inner or outer airway-artery diameter ratio of 1.0 or more, lack of tapering of the airways, visibility of airways in the periphery) on computed tomography (CT) of the thorax. 16 Prisoners were excluded from the study because use of prisoners (who are vulnerable persons) as research subjects is highly controversial and it was possible to conduct the study among non-prisoners.
We recorded the aetiological diagnosis made by the attending respiratory specialist, following best practice recommendations from the 2010 British Thoracic Society guidelines applicable at the time. 17 This included clinical assessments for infective/post-infective causes, connective tissue diseases, immunodeficiencies, allergic bronchopulmonary aspergillosis, and reflux or aspiration. History of pulmonary tuberculosis was validated according to World Health Organization case definition for tuberculosis, 18 that is, either bacteriologically-confirmed (i.e., culture-positive) or clinically-diagnosed (in which a clinician or other medical practitioner had made a decision to give a full course of TB treatment, in the absence of bacteriologic confirmation).
Baseline data was extracted from the first admission within the study period. Participants were followed up until the next hospitalized exacerbation or end of the study period, whichever was sooner.
Variables
Data was extracted from medical records and consisted of participant demographics, smoking history, the Modified Medical Research Council (mMRC) dyspnoea scale, 19 respiratory rate and oxygen saturation at initial presentation, intubation rates, lung function, radiology (number of lung lobes affected by bronchiectasis on CT-thorax), and microbiology (bacterial cultures, mycobacterial cultures, and nucleic acid tests). The list of comorbidities (pulmonary and extrapulmonary) to extract were based on known or potential relevance to bronchiectasis.
Spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines 20 with predicted values obtained from spirometric standards for healthy non-smoking adults 21 and adjusted by a factor of 0.94 as recommended for Asians. 22
Statistical analysis
Data were expressed as mean ± standard deviation, median with 25–75% interquartile range, and n (%) for parametric data, nonparametric data and categorical data respectively.
Comparisons between post-TB versus other aetiologies were performed using Student’s t-test, Mann-Whitney U test, or Chi-squared test for parametric, nonparametric and categorical data respectively.
We analysed time to next hospitalized exacerbation using the Kaplan-Meier survival curve. Differences in exacerbation-free survival between post-TB versus other aetiologies were compared using the Log-Rank test.
To quantify disease severity, we used the FACED score, a validated disease severity index incorporating multidimensional (clinical, radiologic, physiologic, microbiologic) parameters. 23 The components of the FACED score are forced expiratory volume in one second (FEV1), age, Pseudomonas colonisation, number of lobes, and mMRC dyspnoea scale. For this analysis, we excluded participants with unknown values on any of the variables used to calculate the score.
All analyses were performed using SPSS version 25 for Windows.
Ethics
The study was granted waiver of informed consent by the SingHealth Centralised Institutional Review Board on the following grounds: the research was purely observational and did not interfere with participants' usual care, and use of data involved no more than minimal risk to the research subject. In addition, data was deidentified by an institution-appointed trusted third party according to institutional data protection requirements.
Results
The overall study population comprised of 148 participants (Table 1). The mean ± standard deviation age at first admission was 63 ± 9 years, 56.8% were male and 62.8% were never smokers. The most common aetiology of bronchiectasis was idiopathic (64.2%), followed by post-TB (31.1%), post-infective excluding post-TB bronchiectasis (4%) and rheumatoid arthritis (0.7%). Thus, aetiologies other than post-TB comprised of 102 (68.9%) participants.
Table 1.
Patient characteristics. Data are presented as N (%), mean ± standard deviation or median (interquartile range).
| Variable | All | Post-TB | Other aetiologies | p-value |
|---|---|---|---|---|
| N | 148 | 46 (31.1%) | 102 (68.9%) | |
| Demographics | ||||
| Age, years | 63 ± 9 | 63 ± 10 | 63 ± 8 | 0.93 |
| Male | 84 (56.8%) | 30 (65.2%) | 54 (52.9%) | 0.16 |
| Ethnicity | 0.34 | |||
| Chinese | 95 (64.2%) | 29 (63.0%) | 66 (64.7%) | |
| Malay | 35 (23.6%) | 9 (19.6%) | 26 (25.5%) | |
| Indian | 7 (4.7%) | 2 (4.3%) | 5 (4.9%) | |
| Others | 11 (7.4%) | 6 (13.0%) | 5 (4.9%) | |
| Body mass index (BMI), kg/m2 | 22.9 ± 6.0 | 21.2 ± 6.4 | 23.6 ± 5.7 | 0.04 |
| Unknown BMI | 21 (14.2%) | 9 (19.6%) | 12 (11.8%) | |
| Current or ex-smoker | 55 (37.2%) | 20 (43.5%) | 35 (34.3%) | 0.29 |
| Modified MRC dyspnoea scale | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.69 |
| Haemoptysis | 26 (17.6%) | 14 (30.4%) | 12 (11.8%) | 0.006 |
| Oxygen saturation, % | 98 ± 2 | 98 ± 2 | 98 ± 2 | 0.93 |
| Respiratory rate, breaths per minute | 19 ± 2 | 19 ± 3 | 19 ± 2 | 0.81 |
| Number of lobes affected | 2 (1–2) | 2 (1–3) | 1 (1–2) | 0.26 |
| Unknown number of lobes affected | 1 (0.7%) | 0 (0.0%) | 1 (0.9%) | |
| Required mechanical ventilation | 4 (2.7%) | 3 (6.5%) | 1 (1.0%) | 0.09 |
| Spirometry | ||||
| FEV1/FVC | 0.72 (0.70–0.74) | 0.72 (0.69–0.74) | 0.72 (0.70–0.74) | 0.42 |
| FEV1, % predicted | 75 (57–90) | 63 (40–82) | 78 (60–95) | 0.005 |
| FVC, % predicted | 76 (63–92) | 68 (61–81) | 80 (66–94) | 0.05 |
| Unknown lung function | 46 (31.1%) | 20 (43.4%) | 26 (25.5%) | |
| Comorbidities | ||||
| Pulmonary | ||||
| Chronic obstructive pulmonary disease | 33 (22.3%) | 12 (26.1%) | 21 (20.6%) | 0.46 |
| Asthma | 25 (16.9%) | 5 (10.9%) | 20 (19.6%) | 0.19 |
| Lung cancer | 1 (0.7%) | 0 (0%) | 1 (1.0%) | 1.00 |
| Extrapulmonary | ||||
| Ischemic heart disease | 16 (10.8%) | 5 (10.9%) | 11 (10.8%) | 0.99 |
| Diabetes | 19 (12.8%) | 4 (8.7%) | 15 (14.7%) | 0.53 |
| Liver disease | 1 (0.7%) | 1 (2.2%) | 0 (0%) | 0.31 |
| Chronic renal failure (moderate to severe CKD) | 3 (2.0%) | 1 (2.2%) | 2 (2.0%) | 1.00 |
| Connective tissue disease | 11 (7.4%) | 0 (0%) | 11 (10.8%) | 0.02 |
| Neurological diseases (stroke, dementia, hemiplegia) | 10 (6.8%) | 1 (2.2%) | 9 (8.8%) | 0.44 |
| Gastroesophageal reflux disease | 4 (2.7%) | 1 (2.2%) | 3 (2.9%) | 0.55 |
| Peptic ulcer disease | 3 (2.0%) | 0 (0%) | 3 (2.9%) | 0.55 |
| Sinonasal disease | 5 (3.4%) | 2 (4.3%) | 3 (2.9%) | 0.65 |
| Microbiology | ||||
| Organism identified | 37 (25.0%) | 22 (47.8%) | 15 (14.7%) | <0.001 |
| Non-tuberculous mycobacteria | 17 (11.5%) | 12 (26.1%) | 5 (4.9%) | <0.001 |
| Pseudomonas aeruginosa | 4 (2.7%) | 2 (4.3%) | 2 (2.0%) | 0.59 |
| Klebsiella pneumoniae | 4 (2.7%) | 2 (4.3%) | 2 (2.0%) | 0.59 |
| Haemophilus influenzae | 3 (2.0%) | 1 (2.2%) | 2 (2.0%) | 1.00 |
| Viruses | 7 (4.7%) | 4 (8.7%) | 3 (2.9%) | 0.20 |
| Aspergillus | 1 (0.7%) | 0 (0.0%) | 1 (1.0%) | 1.00 |
FEV1 = forced expiratory volume in one second; FVC = Forced Vital Capacity; MRC = Modified Medical Research Council dyspnoea scale.
Participants with post-TB bronchiectasis had significantly lower BMI (21.2 ± 6.4 vs 23.6 ± 5.7kg/m2, p = .04), more frequent presentations with haemoptysis (30.4% vs. 11.8%, p = .006), and lower FEV1 %predicted (median 63% vs. 78%, p = .005), compared to those with bronchiectasis due to other aetiologies. There were no significant differences detected for mMRC dyspnoea scores, respiratory rate, oxygen saturation, intubation rates, and number of lung lobes affected.
There was a higher rate of microbiological pathogens identified from respiratory specimens in participants with post-TB bronchiectasis versus other aetiologies (47.8% vs 14.7%, p < .001). This was mainly driven by a higher rate of NTM isolation (26.1% vs. 4.9%, p < .001). Among the 12 of 46 post-TB bronchiectasis participants in whom NTM was isolated, the exact species was not identified in 8, and the remaining 4 were Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium kansasii, and Mycobacterium avium complex respectively. There was also a nonsignificant trend toward higher rates of Pseudomonas aeruginosa, Klebsiella pneumoniae, and viruses isolated among post-TB bronchiectasis participants than in other aetiologies. Viruses isolated in post-TB bronchiectasis were Influenza virus (n = 4) and Rhinovirus (n = 1), whereas in other aetiologies, the viruses implicated were Parainfluenza virus (n = 1), Respiratory Syncytial Virus (n = 1) and Rhinovirus (=1).
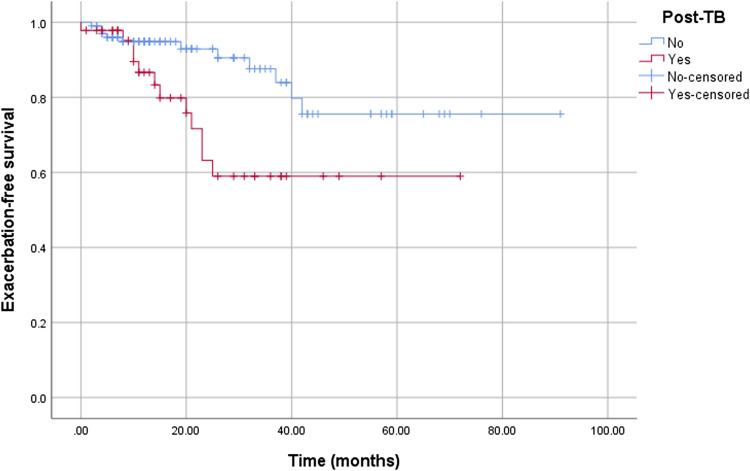
Exacerbation-free survival is depicted in Figure 1. Over a median follow-up of 21 months (interquartile range 11–40 months), post-TB bronchiectasis was associated with a shorter time to next exacerbation compared with other aetiologies (49 [95% confidence interval, CI: 39–59] vs 76 [95% CI: 67–84] months, Log-Rank p=0.01). This association was independent of NTM isolation (adjusted pooled Log-Rank p = .025).
Figure 1.
Kaplan Meier curve for exacerbation-free survival between post-TB bronchiectasis and other aetiologies of bronchiectasis.
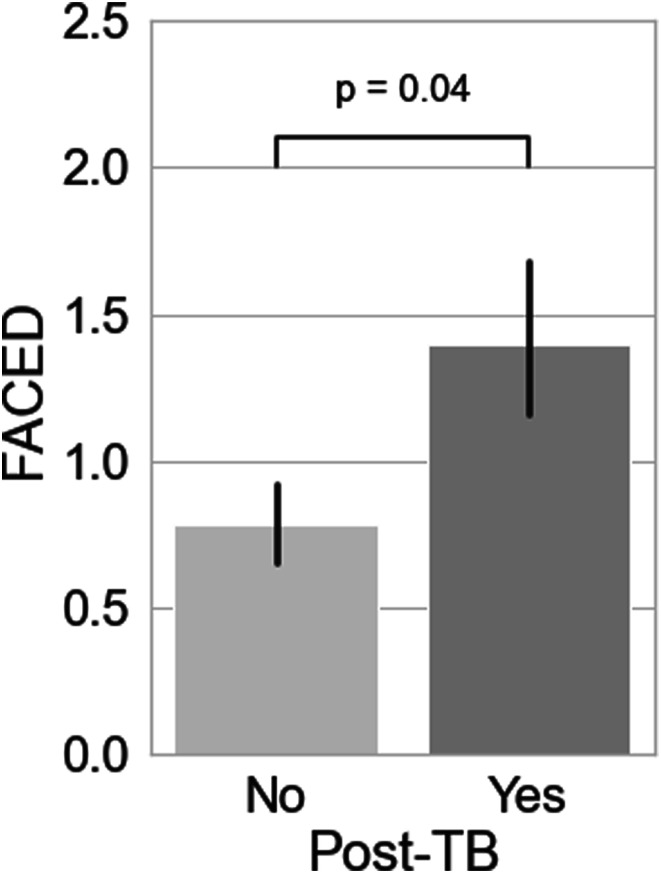
Out of the overall cohort, n = 100 had complete data for computing FACED scores. In this subset of participants, post-TB bronchiectasis aetiology was associated with significantly higher FACED scores compared to other aetiologies (Mann-Whitney U test, p = 0.04) (Figure 2).
Figure 2.
Comparison of mean FACED scores between post-TB bronchiectasis and other aetiologies of bronchiectasis.
Discussion
The objective of this real-world study was to elucidate the phenotypic characteristics of post-TB bronchiectasis, and to evaluate whether this phenotype is distinct from other aetiologies of bronchiectasis. Compared to other aetiologies, post-TB bronchiectasis was associated with lower BMI, more frequent presentation with haemoptysis, higher rates of NTM isolation from respiratory specimens, and lower FEV1. We also found that post-TB bronchiectasis was associated with shorter time to next hospitalized exacerbation and more severe disease (based on higher FACED scores). Our findings indicate that post-TB bronchiectasis is a more debilitating disease with increased frequency of exacerbations and potential hospital admission, and may pose a high burden to the healthcare system compared with other aetiologies of bronchiectasis, revealing an area of potential unmet need among people with this condition.
Post-TB was the identified aetiology in about one third of our cohort, in agreement with the prevalence of 37.5% found in another study which analysed a nationwide administrative database. 13 Our study has additionally elucidated detailed clinical characteristics and outcomes of the post-TB bronchiectasis phenotype, showing that this group experiences greater disease severity and higher risk of exacerbation. A study conducted in Saudi Arabia found that post-TB bronchiectasis had higher severity scores than other aetiologies of bronchiectasis. 24 The India-EMBARC also showed similar characteristics of post-TB bronchiectasis in terms of severity, demographics, and microbiology compared to idiopathic and non-TB post-infective causes. 9 The variability of the observed findings from these studies may represent the complex interplay of genetic and environmental factors that influence the disease expression of post-TB bronchiectasis.
The present study found an increased rate of NTM isolation of 26.1% in post-TB bronchiectasis, which was more than five-fold greater than other aetiologies of bronchiectasis. A study from Taiwan also found a significantly higher rate of NTM isolation in post-TB bronchiectasis than in other aetiologies, 11 although the rate of isolation (7%) was lower than in our study. Previous studies found that NTM co-isolation occurs in 7–11% of active TB patients and about 6% of bacteriologically-proven TB cases subsequently develop NTM pulmonary disease.25-27 Additionally, NTM cohorts reported in the literature have demonstrated a prevalence of 5–30% having had a history of pulmonary TB. 28 Taken together, all these data indicate that an elevated risk of NTM colonisation/infection and subsequent development of NTM lung disease may be a distinctive characteristic of the post-TB bronchiectasis phenotype. Although structural lung disease is a known predisposing factor for NTM, it is unknown why bronchiectasis due to previous TB portends a much greater risk of NTM compared to other aetiologies. It may be that anatomic and immunologic factors which enhance the risk to both TB and NTM form the causal link. Poorer outcomes seen with post-TB bronchiectasis may be in part mediated by NTM, but our analysis showed that, independent of NTM, post-TB bronchiectasis was associated with shorter time to next exacerbation. Future studies are needed to elucidate the mechanisms and clinical significance of NTM in post-TB bronchiectasis.
Post-TB bronchiectasis poses greater morbidity and health care utilization for afflicted individuals. 15 The underlying pathogenic mechanisms leading to more frequent haemoptysis and exacerbations as well as greater disease severity in post-TB bronchiectasis is not known. In our study, the number of lobes affected was not significantly different between post-TB and other aetiologies, making it unlikely the worse outcomes were due to differences in the radiologic extent of bronchiectasis. An intriguing possibility is that post-TB bronchiectasis may have more severe ongoing airway inflammation. Further epidemiologic and mechanistic studies are needed to confirm that post-TB bronchiectasis has worse outcomes and to elucidate the mechanisms mediating these worse outcomes. In bronchiectasis, current treatments for haemoptysis and reducing risk of exacerbations are not specific to post-TB bronchiectasis. It is hoped that understanding the mechanisms for worse outcomes in post-TB bronchiectasis may lead to specific interventions to reduce morbidity in this disease.
Our study has several limitations. First, there were unknown values for some participants on variables such as mMRC dyspnoea score, spirometry, and body mass index. This reflects the real-world nature of the data, where patients may not be evaluated in a uniform manner. For example, spirometry may not be performed for various reasons, such as haemoptysis, ongoing infection, or inability to cooperate with the Forced Vital Capacity (FVC) manoeuvre. Nevertheless, data on important outcomes such as time to next exacerbation were complete. Second, this study was single-centre, and hence the findings may not be generalizable. The study cohort also appears to have mild bronchiectasis. Therefore, future studies with larger cohorts and involving individuals with more severe disease are needed. Third, individuals with NTM isolation could have had NTM pulmonary disease instead of post-TB bronchiectasis. Nevertheless, the association of post-TB bronchiectasis with shorter time to next exacerbation remained significant even after adjusting for NTM. Fourth, participants recruited in the later part of the study had a shorter duration of follow up and may not have had sufficient time to experience the event of interest (i.e., exacerbation). This may have influenced the findings for time to next exacerbation or exacerbation-free survival, and is a potential source of bias known as censoring. To mitigate this source of bias, we used Kaplan Meier survival analysis and Cox regression, which analyse both censored and uncensored observations to compute parameter estimates. Fifth, the association between bronchial inflammation and exacerbation rates could not be determined as biomarkers of bronchial and systemic inflammation, as well as sputum characteristics, were not collected as part of the study.
Conclusion
Post-TB bronchiectasis was found to be a distinct phenotype with more severe features, such as lower FEV1, lower BMI, more frequent haemoptysis, higher FACED score, and shorter time to next admission for exacerbation compared to other aetiologies of bronchiectasis. There is a need for more inclusive, international studies to better understand this disease which affects large swathes of vulnerable populations worldwide
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Isaac Fong https://orcid.org/0000-0003-3257-2137
References
- 1.Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers 2018; 4(1): 45. [DOI] [PubMed] [Google Scholar]
- 2.Guan W, Han X, Rosa-Carrillo D, et al. The significant global economic burden of bronchiectasis: a pending matter. Eur Respir J 2019; 53(2): 1802392. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “Frequent Exacerbator Phenotype” in Bronchiectasis. Am J Respir Crit Care Med 2018; 197(11): 1410–1420. [DOI] [PubMed] [Google Scholar]
- 4.Aliberti S., Lonni S., Dore S., et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016; 47: 1113–1122. [DOI] [PubMed] [Google Scholar]
- 5.Chotirmall SH, Chalmers JD. RESPIRE: breathing new life into bronchiectasis. Eur Respir J 2018; 51(1): 1702444. [DOI] [PubMed] [Google Scholar]
- 6.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392(10150): 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018; 6: 715–726. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekaran R, Aogain MM, Chalmers JD, et al. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med 2018; 18: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhar, Singh S., Talwar D., et al. Bronchiectasis in India: results from the European multicentre bronchiectasis audit and research collaboration (EMBARC) and respiratory research network of India registry. Lancet Glob Heal 2019; 7: e1269–e1279. [DOI] [PubMed] [Google Scholar]
- 10.Qi Q, Wang W, Li T, et al. Aetiology and clinical characteristics of patients with bronchiectasis in a Chinese Han population: a prospective study. Respirology (Carlton, Vic) 2015; 20(6): 917–924. [DOI] [PubMed] [Google Scholar]
- 11.Huang HY, Chung FT, Lo C, et al. Etiology and characteristics of patients with bronchiectasis in Taiwan: a cohort study from 2002 to 2016. BMC Pulm Med 2020; 20(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palwatwichai A, Chaoprasong C, Vattanathum A, et al. Clinical, laboratory findings and microbiologic characterization of bronchiectasis in Thai patients. Respirology (Carlton, Vic) 2002; 7(1): 63–66. [DOI] [PubMed] [Google Scholar]
- 13.Phua H. P., Lim W.-Y., Ganesan G., et al. Epidemiology and economic burden of bronchiectasis requiring hospitalisation in Singapore. ERJ Open Res 2021; 7(4): 00334–02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Kampen SC, Wanner A, Edwards M, et al. International research and guidelines on post-tuberculosis chronic lung disorders: a systemic scoping review. BMJ Glob Health 2018; 3: e000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allwood B. W., Byrne A., Meghji J., et al. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021; 100(8): 751–763. [DOI] [PubMed] [Google Scholar]
- 16.Hill AT, Sullivan AL, Chalmers JD, et al. British thoracic society guideline for bronchiectasis in adults. Thorax 2019; 74: 1–69. [DOI] [PubMed] [Google Scholar]
- 17.Pasteur MC, Bilton D, Hill AT. British thoracic society (non-CF) guideline group. british thoracic society guideline for non-CF bronchiectasis. Thorax 2010; 65: i1–i58. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 19.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93(3): 580–586. [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26(2): 319–338. [DOI] [PubMed] [Google Scholar]
- 21.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 1971; 103(1): 57–67. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26(5): 948–968. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-García MÁ, De Gracia J, Relat MV, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43(5): 1357–1367. [DOI] [PubMed] [Google Scholar]
- 24.AL-Harbi A, Al-Ghamdi M, Khan M, et al. Performance of multidimensional severity scoring systems in patients with post‐tuberculosis bronchiectasis. Int J Chron Obstruct Pulmon Dis 2020; 15: 2157–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsing SC, Weng SF, Cheng KC, et al. Increased risk of pulmonary tuberculosis in patients with previous non-tuberculous mycobacterial disease. Int J Tuberc Lung Dis 2013; 17(7): 928–933. [DOI] [PubMed] [Google Scholar]
- 26.Jun HJ, Jeon K, Um SW, et al. Nontuberculous mycobacteria isolated during the treatment of pulmonary tuberculosis. Respir Med 2009; 103(12): 1936–1940. [DOI] [PubMed] [Google Scholar]
- 27.Lim AYH, Chotirmall SH, Fok ETK, et al. Profiling non-tuberculous mycobacteria in an Asian setting: characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm Med 2018; 18: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler SJ, French J, Screaton NJ, et al. Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J 2006; 28(6): 1204–1210. [DOI] [PubMed] [Google Scholar]