Abstract
Background
People with Multiple Sclerosis (PwMS) suffer from an increased risk of unemployment during the course of the disease. In recent years progress has been made in increasing the time until patients have to leave the workforce permanently. Such a retirement is often associated with MS but the driving factors including disability progression, support measures at the workplace, and societal aspects are not yet fully understood.
Methods
We consolidated data from four European MS databases from Germany, Poland, Sweden, and the United Kingdom, which were able to provide data on working status, disability progression and quality of life in accordance with the data harmonization framework of the EUReMS (European Registry in Multiple Sclerosis) project.
Results
Factors strongly associated with unemployment are disability progression, low quality of life and being close to the statutory retirement age. Overall, highest employment rate (77%) and lowest effects of gender and disease duration were found in Sweden.
Conclusions
We found remarkable differences between the European registers and the countries studied, which may indicate inequalities at European level. Furthermore, our findings suggest that it is feasible and useful to combine data from different MS registers in Europe, albeit the data structures are heterogeneous.
Keywords: multiple sclerosis, harmonization, registries, employment
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the central nervous system that may have a huge impact on the life of persons affected by the disease. People with MS (PwMS) often suffer from motor disability and gait problems, visual disturbances, fatigue, depression, cognitive dysfunction, sensory loss and bladder problems that all may influence activities of daily living, work ability, social participation and ultimately health-related quality of life (HRQoL). Although the frequency of inability to work seems to decrease over time (from 70–80% within 5 years of diagnosis 1 to 40–50% within 10 years of onset in more recent studies2–4), loss of employment is still one of the most troubling consequences of MS and contributes to the economic burden of the disease on the societal and personal level. 5 Work ability is critical not only for generating income, but also for sense of identity, self-esteem and social contact 6 and certainly has a profound effect on HRQoL. In fact, early retirement has been associated with lower HRQoL levels in one analysis of the UK MS registry 7 as well as in one small study from Germany. 8 In Sweden, several risk factors for unemployment have been found,9–11 and also the association with the income level has been quantified. 12 Apart from demographic and disease-related issues, country-specific factors may also have an impact on employment status. The MS Barometer estimates the proportion of PwMS in active work ranges from 5% (Belarus, Hungary) to 50% (Italy, Luxembourg, and Spain), and 0% (Bosnia-Herzegovina) to 60% (Slovenia) for full-time and part-time jobs, respectively. 13 These large discrepancies in employment status within Europe may be accounted for by differences in social security systems, extent of public support programs for persons with disabilities, and legal aspects. Still, the role and interaction of demographics and disease characteristics have not been figured out. Therefore, a cross-national analysis of work eligibility taking individual factors, such as age, gender, disease course, disease duration and disability level into account, is needed to enable scientifically sound comparisons of employment status between European countries.
The EUReMS (European Registry in Multiple Sclerosis) project was an initiative of the European MS Platform (EMSP) which aimed at establishing the network and methodologies for sustainable collection, comparison and assessment of MS data across Europe. One of its missions is the assessment of quality of life, burden of symptoms and socio-economic aspects from the person's perspective across European countries. 14 In a European-wide survey, physician-based outcomes are used in all 13 MS registries that were analyzed, whereas patient-reported outcomes (PRO) were only collected in six registries, and only four of them used standardized HRQoL instruments at the time of assessment. 15 This underlines the need for a harmonized and standardized data collection system for measuring the impact of the disease from the person's perspective. The aim of this study was 1) to develop and establish a platform for integration and comprehensive analyses of (PRO) data from different MS data sources across Europe, 2) to investigate whether it is feasible to collect PRO data in a considerable number of people with MS on a European level, and 3) to compare HRQoL and employment in several European countries as part of a feasibility study.
Methods
A key factor of the study was dealing with heterogeneous data sources in a defined research context of: European MS registries; burden of MS; PRO data in relation to employment. Data acquisition ranges from the export framework of the EUReMS project from 2014 (Swedish MS Register) to export frameworks established later (German MS register and RejSM) to study specific data access via VPN (UK MS Register). A detailed overview is provided in Table 1.
Table 1.
Data providing MS registries.
| Data source (country) | Germany | Poland | Sweden | United Kingdom |
|---|---|---|---|---|
| Name of the register | German MS Register | RejSM | Svenska MS-registret (SMSreg) | UK MS Register |
| Institution | MS Forschungs- und Projekten twicklungs- gGmbH, Hanover, Germany | University of Science & Technology, Cracow, Poland | Karolinska Institutet, Stockholm, Sweden |
Swansea University, Swansea, UK |
| Cohort extracted for PRO analysis | PwMS eligible to PRO documentation (pilot phase) | Recruited MSIS29 cohort | Cohort from EUReMS framework | PwMS eligible to PRO documentation and international collaboration |
| Cohort size | 6105 | 246 | 13,102 | 12,971 |
Research questions
The following research hypotheses regarding the ability to work were addressed in the framework of this study:
The employment status of PwMS differs among European countries
The association of employment status and HRQoL differs among European countries
The influence of age / age at onset / education / fatigue / gender / disease duration / disease course / disability (Expanded Disability Status Scale [EDSS], MS Walking Scale [MSWS]) on employment status differs among European countries.
Inclusion and exclusion criteria
Persons with MS were excluded if they had missing or contradicting information in the baseline variables gender or year of birth or when both the date of onset and the date of diagnosis were missing. Patient data was assessed at the time whenever (longitudinal) information on working status was available or in a prespecified time interval before (see next subsection for details). If the working status was not assessed (with a specific date) or could not be linked to a definite MS disease course, the participant was excluded from the study. If multiple valid assessments existed per participant, the most current one was used.
Since the legal age for retirement is at age 65 in Germany, Poland, Sweden, and the UK, we excluded all PwMS who were above this age. Also, PwMS younger than 25 years were excluded, because a large number of people in this age group are still in education.
Variable coding and missing values
The working status was categorized as ‘working’, i.e. income generating, and ‘not working’ only. If PwMS had a working position without generation of income they were classified as not working. This definition was comparable in all four data-providing countries.
If date of onset was missing but date of diagnosis was available, age of onset values were imputed or vice versa. Imputation was done with boosted regression models 16 based on additional covariates year of birth, gender, disease course at onset, country and all two-fold interactions, allowing for country specific estimates. Predicted values were then used to replace missing values. The method is used to impute a low number of missing dates and restrictions to achieve an ascending order of birth, disease onset, diagnosis, and assessment of the working status were enforced in all patients.
Longitudinal measurements of the working status were recorded by visit date along with EDSS and the disease course in data from Germany, Poland, and Sweden. For the UK register the MSWS needed to be assessed at the time of working status measurement, or at a limited time of up to 45 days beforehand. The latest update for the disease course needed to be a maximum of five months beforehand. Health related quality of life (HRQoL) was assessed with the MS specific questionnaire MSIS-29 17 broken into psychological (psy) and physical (phy) subscales, and a general quality of life questionnaire, the EQ-5D (European SSI index, 18 Validation in Sweden 19 ). When no quality of life measurement within 30 days (±15 additional days for data matching) prior to the assessment of working status was available then the variable was considered as missing.
Statistical methods
The main outcome was the working status assessed by logistic regression models, with various types of adjustment for covariates. Univariate and multivariate models were used to investigate the influence or association of different variables towards ability to work. The baseline variables used were gender (female), disease type at onset, and diagnostic delay, which is the time between disease onset and date of confirmed diagnosis, which may serve as an indicator of disease activity in the early years of the disease. Furthermore, a variable on whether conversion to secondary progressive MS had taken place was added. At the date of the working status assessment, the time until statutory retirement age (65) was used as a proxy variable for age along with disease duration. In the German, Polish, and Swedish data, the EDSS was used as a measure of disability progression, while in the UK the MSWS was used. For the latter, the MSWS scores were scaled such that the range from 0 to 100 of the MSWS reflects the EDSS range from 0 to 6.5. The range was adapted from 20 (see also Figure 1).
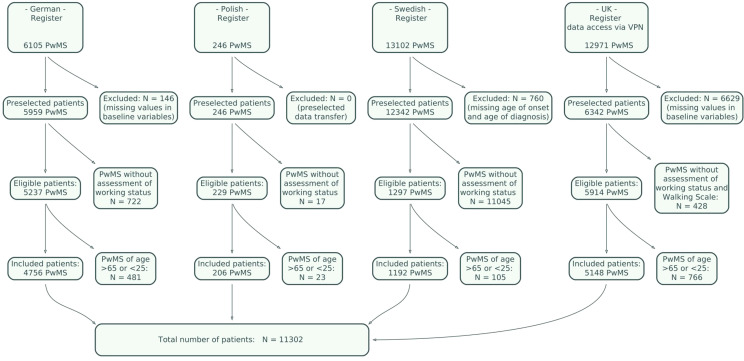
Figure 1.
Flowchart of inclusion criteria and number of PwMS that were included in the study.
All covariates were used in the multivariate model, so that we were able to adjust for baseline covariates and disease progression simultaneously. Additional HRQoL covariates like MSIS-29 (physical and psychological subscales) as well as the EQ-5D were specifically added to the model. The reference group was represented by a (hypothetical) male PwMS who is close to reaching the statutory retirement age (65 years). Furthermore, the reference is assumed to be newly diagnosed with a relapsing-remitting disease course and has not had disease progression so far (EDSS 0). Logistic regression models were carried out with a penalized likelihood method (Firth correction) to avoid bias in estimation. Estimation is done for each stratum by each country separately. Models on the pooled data were additionally fitted, i.e. boosted regression models to investigate interaction effects, functional forms of covariates, and missing values as sensitivity analyses.
Results
A total of 11,302 patients met the inclusion criteria of the study. Country-specific numbers of included and excluded patients are given in the flowchart in Figure 1. Imputation with boosted regression was carried out for missing values of date of onset in 313 cases in the German data, no cases in the Polish data, 27 cases in the Swedish data, and 42 cases in the UK data. Similarly, for the date of diagnosis the number of cases were 60, 0, 118, and 220.
Table 2 shows descriptive statistics for the four registries. PwMS were youngest in Sweden (44.3 years), had lowest disability (median EDSS of 2.5), and highest quality of life measures (median EQ-5D of 0.8).
Table 2.
Descriptive statistics by country reporting frequencies (%) for categorical data, means and standard deviation (sd) for metric variables, and median, the 25% and 75% quantiles (q25, q75) for ordinal data. Group comparisons were done in a random-effects meta-analytic approach reporting the pooled estimate and p-values of the Q-test for heterogeneity.
| Pooled estimate | Germany | Poland | Sweden | United Kingdom | p-value | |
|---|---|---|---|---|---|---|
| Sample size | n = 6154 | n = 4756 | n = 206 | n = 1192 | n = 5148 | |
| Age: mean (sd) | 46.4 | 45.6 (9.9) | 47.1 (10.4) | 44.3 (9.6) | 48.6 (9.6) | <0.01 |
| Females (%) | 71.7% | 71.8% | 66.0% | 71.1% | 74.3% | <0.01 |
| Disease Course: | (n = 4666) | (n = 206) | (n = 1192) | (n = 5148) | ||
| Relapsing Remitting (%) | 62.2% | 77.0% | 37.9% | 74.3% | 58.8% | <0.01 |
| Secondary Progress. (%) | 30.5% | 17.3% | 56.8% | 21.5% | 27.6% | <0.01 |
| Primary Progressive (%) | 7.3% | 5.7% | 5.3% | 4.2% | 13.6% | <0.01 |
| Year of birth: mean (sd) | 1970.4 (9.9) |
1966.9 (10.3) |
1968.9 (9.7) |
1965.6 (9.6) |
<0.01 | |
| Age of onset: mean (sd) | 32.7 | 32.2 (9.9) | 34.1 (8.3) | 31.1 (9.2) | 33.4 (9.7) | <0.01 |
| Diagnostic delay: median (q25,q75) |
1.0 (0.0,2.8) | 1.0 (1.0,2.0) | 2.0 (1.0,5.0) | 1.7 (0.4,5.0) | ||
| Disease duration: mean (sd) | 13.7 | 13.2 (8.8) | 12.9 (7.5) | 13.2 (8.2) | 15.2 (10.1) | <0.01 |
| EDSS / MSWS: mean (sd) | (excl. UK) 3.3 | (n = 4756) 3.1 (2.2) |
(n = 206) 3.9 (1.9) |
(n = 1192) 2.7 (2.2) |
(MSWS; n = 4136) 59.9 (34.4) |
<0.01 |
| median (q25,q75) | 2.5 (1.5,4.5) | 3.5 (2.5,5.0) | 2.5 (1.0,4.0) | 69.0 (28.6,93.0) | ||
| MSIS-29 phy: median (q25,q75) |
(n = 55) 45.0 (28.8,53.1) |
(n = 203) 31.2 (13.1,51.2) |
(n = 252) 12.5 (3.8,28.8) |
(n = 2056) 48.3 (26.7,68.0) |
||
| MSIS-29 psy: median (q25,q75) | 38.9 (22.2,52.8) |
36.1 (25.0,52.8) |
19.4 (8.3,38.9) |
44.4 (25.9,63.0) |
||
| EQ-5D SSI: mean (sd) | 0.641 | (n = 206) 0.6 (0.3) |
(n = 393) 0.8 (0.2) |
(n = 2083) 0.6 (0.2) |
<0.01 | |
| median (q25,q75) | 0.6 (0.5,0.8) | 0.8 (0.7,1.0) | 0.6 (0.4,1) | |||
| Proportion Working (%) | 52.1% | 55.5% | 29.6% | 77.0% | 45.8% | <0.01 |
HRQoL scales
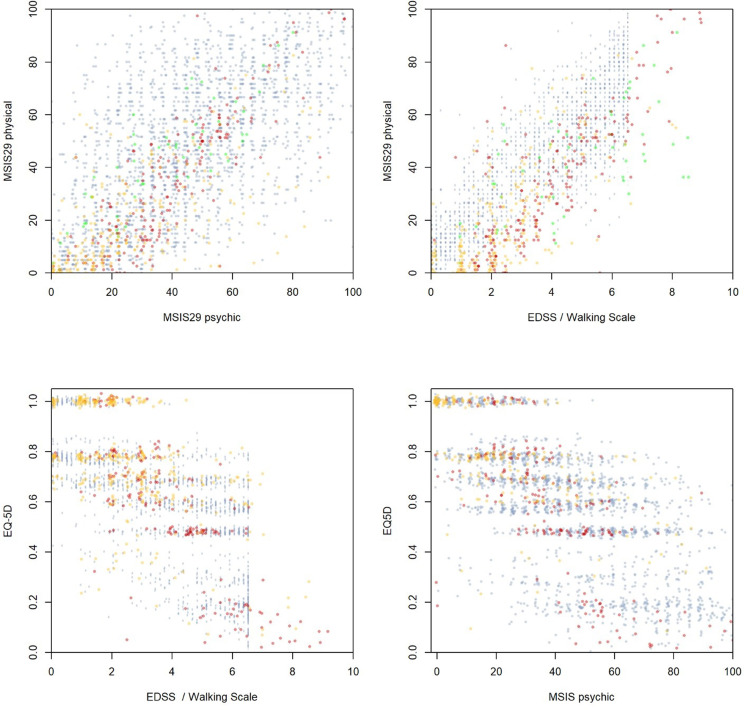
MSIS-29 with its physical and psychological subscales and EQ-5D (Europe SSI) were used as quality of life measurements. EQ-5D was not assessed in Germany. Figure 2 shows scatterplots displaying the range of the subscales, construct validity and correlation.
Figure 2.
Scatterplots of HRQoL outcomes for different countries (Germany in green; Poland in red; Sweden in yellow; United Kingdom in blue).
Table 3 shows the correlation between disability and quality of life measurements, stratified by data source, also allowing a direct comparison between EDSS and MS Walking Scale.
Table 3.
Correlations of HRQoL outcomes showing correlation coefficients by Pearson as well as Kendall's τ.
| Variable 1 | Variable 2 | Country | Pearson | Kendall's τ |
|---|---|---|---|---|
| EDSS | MSIS29 (phy) | Germany | 0.641 | 0.444 |
| EDSS | MSIS29 (phy) | Poland | 0.824 | 0.662 |
| EDSS | MSIS29 (phy) | Sweden | 0.719 | 0.547 |
| MSWS | MSIS29 (phy) | UK | 0.873 | 0.702 |
| EDSS | EQ-5D | Poland | -0.822 | -0.671 |
| EDSS | EQ-5D | Sweden | -0.559 | -0.413 |
| MSWS | EQ-5D | UK | -0.695 | -0.542 |
| MSIS29 (psy) | EQ-5D | Poland | -0.735 | -0.597 |
| MSIS29 (psy) | EQ-5D | Sweden | -0.651 | -0.536 |
| MSIS29 (psy) | EQ-5D | UK | -0.650 | -0.482 |
Multivariate analyses
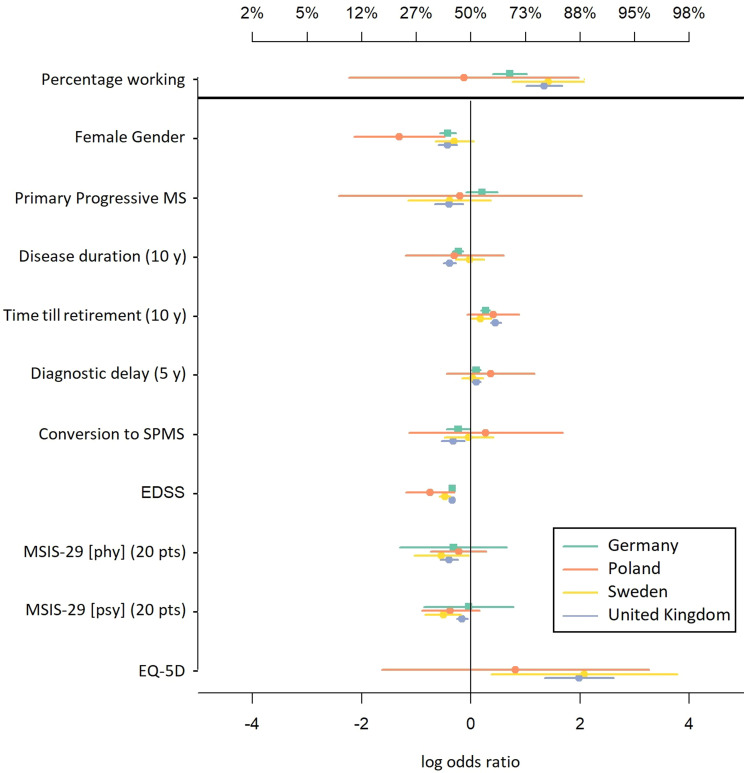
Multivariate logistic regressions showed that the overall frequency of PwMS to be working differs between the registers, with the highest rate of employment being found in the Swedish data having an adjusted employment rate for the reference group of 80.2%, followed by 79.3% in the UK, 67.1% in Germany and being with 46.8% lowest in Poland, as displayed in Figure 3. Female PwMS were more likely to be unemployed than males. The difference in employment between men and women was smallest in Sweden with a gender odds ratio of 0.74. In the UK and the German data the gender odds ratio was 0.65 and in Poland it was estimated lowest as of 0.27. While the chance of employment was generally lower for PwMS suffering from progressive disease forms, these effects were only partly present when adjustment for the actual disease progression and time was done. Effects of secondary progressive and primary progressive MS were only found to be statistically significant in the UK, with the proviso that the MSWS was used to measure disease progression.
Figure 3.
Summary of effect estimates of the multivariate logistic regression displayed as log odds ratios including 95% confidence intervals. At the top ‘percentage working’ shows the likelihood of employment for the reference group (65 years old, male PwMS with little disability). Values greater zero (x-axis) show positive associations of the covariates with employment (for the reverse-scaled EQ-5D higher values indicate higher QoL).
Disease duration and time until retirement showed small effects when adjustment for disease progression took place. A slight heterogeneity among the four countries in the estimates for disease duration was found (p = 0.03) with the effect being smallest in Sweden. The diagnostic delay did not have any significant effect on employment status in the model that adjusts for the disease progression, unlike unadjusted models, since diagnostic delay may serve as a proxy variable for disease activity. When using EDSS and MSWS as a direct measure of disease progression they showed highly significant effects (p < 0.01) on the employment status with an odds ratio of 0.67 per EDSS point and, additionally to the EDSS, quality of life outcomes did so as well. The odds ratio for the MSIS-29 was 0.67 per 20 points for the physical subscale and 0.78 for the psychological subscale, and for the (reverse-scaled) EQ-5D it was 1.92 per point.
Discussion
Research and clinical metrics mostly focus on physician-driven outcomes such as the EDSS or the Multiple Sclerosis Functional Composite (MSFC) which are heavily weighted towards physical dysfunction and are not useful to cover other aspects of the disease.21,22 Moreover, it is well known that the perspectives of physicians and patients differ: whereas physicians consider physical dysfunction as more important, patients are predominantly troubled by the psychological consequences of the disease. 23 Therefore, patient-reported outcomes (PRO), particularly disease-specific HRQoL scales, have increasingly been advocated to properly assess the patient's perspective.24,25 In the current study we found that patient-reported outcomes are strongly related to ability to work, in addition to the degree of disability recorded through the EDSS. Explicitly, the patient-reported physical subscale of the MSIS-29 (per 20 points) adds the same explanatory value (OR of 0.67) additional to the neurologically assessed EDSS as the EDSS provides as single measure of physical disability.
From a methodological view one important outcome of the PRO study is that data pooling among European registers is possible, comparable with efforts and results from similar projects.26,27 A crucial challenge in the project was dealing with the heterogeneity of data sources. For instance, the registries from Germany, Poland, and Sweden are clinician based compared to the UK MS Register for which at time of data access only patient reported data was available. Furthermore, there are obvious differences in the patient selection mechanisms of the participating data providers including cohort sizes for this study. The Swedish registry has been operative for the longest time and has the highest national coverage compared to other registries. Registries themselves undergo technical and dataset revisions resulting in temporal changes in the patient cohorts. These difficulties were not only of theoretical nature but perceptible through different patient characteristics. Thus, evaluating ‘real world’ data needs to adjust for relevant confounders and all different data sources should be pooled in a way that allows for heterogeneity. In this study, the meta-analytic approach with random effects for the multivariable regression estimates was able to adjust for these differences quite convincingly. Our model, which adjusted for baseline characteristics and disease progression simultaneously, yielded more homogeneous estimates which were in ranges that can be regarded as credible. In this study the meta-analytic approach also allowed to use different measures to adjust for physical deficits through disease progression. In this regard, the UK data showed that the MSWS can be an effective surrogate for EDSS, confirming previous findings. 20 In this context, there may be additional upcoming possibilities to complement classical EDSS documentation, since nowadays activity levels can be monitored by hand-held devices or activity trackers. 28 Long-term benefit, objectivity, and sustainability of such solutions has yet to be demonstrated. 29
The association to other patient-reported health measures seems similar as well as effect estimates in the logistic regression were found to result in similar overall models. However, different cohort sizes and thus different precision in the estimates are a limitation in making direct comparisons between data sources. As found in other studies, the association between the working status and physical impairment on the one side, 26 but also psychological measures as the respective subscale of MSIS-29 or EQ-5D on the other side 11 were found to be strong.
Some notable differences between the data sources were seen when we compared the multivariate effect estimates for covariates which may relate to country-specific effects. Our results regarding female-to-male employment ratio needs to be viewed in relation to the general population of these countries. The generally high female employment rate in Scandinavian countries 30 could also be found in the Swedish data. Still, employment is lower in females. The overall employment chances for PwMS seem to be highest in Sweden and the UK while they seem to be less in Germany and lowest in Poland. 26 The association of the disease course decreased substantially when adjustment for the disease progression is carried out. Similar results were obtained in the study by Kavaliunas et al. 31 in which the level of income was evaluated. Impact of disease duration and time to retirement was found to be least in Sweden, indicating that the ‘true’ disease progression is the relevant factor for unemployment. The use of the Walking Scale in the UK data may explain some differences in the effects seen, reflecting fewer functional systems as the EDSS does.
Overall, our multivariate model to assess the employment status showed plausible results incorporating country-specific differences where the fundamental relationships in the data appeared to be similar though. Univariate models showed less of such a stable behavior, which is not surprising since those are unable to adjust for various kinds of selection biases that arise through different type of data sources. Regarding the HRQoL measures, the country-specific differences in correlation coefficients between the psychological and physical subscales of the MSIS-29 and the EDSS respectively are of particular interest, being highest in Poland and much lower in UK and Germany. This means that in the latter countries other factors than physical disability may impact psychological well-being more than in Poland, for instance.
Limitations of the study were lack of information about education and individual's fatigue, which therefore could not be investigated. Also, the inference of the study was limited to the explanation of the ability of work in dependence of the patient's health status since quality of life comparisons between countries are expected to be very sensitive to patient selection. Furthermore, the comparability of data sources may be limited by some unmeasured selection biases that may be attributable to patient recruitment, cohort sizes or other factors. However, taken these shortcomings into account, the results of our study show that there is added value in combining data from different MS registries in Europe, despite the obstacle of dealing with heterogeneous data structures. Further efforts are needed to standardize and harmonize data collection on a European level in order to assess, compare and ultimately improve quality of life of PwMS across Europe.
Footnotes
Data availability: Anonymized data will be made available upon reasonable request from any qualified investigator subject to the terms and conditions of the registries’ use-and-access policies and the informed consents of the patients.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DE, TP, RM, WB have nothing to disclose. JH has received honoraria for serving on advisory boards for Biogen, Bristol Myers Squibb, Sanofi-Genzyme, Merck KGaA, Novartis and Sandoz and speaker’s fees from Biogen, Novartis, Merck KGaA, Teva and Sanofi-Genzyme. He has served as P.I. for projects, or received unrestricted research support from Biogen, Bristol Myers Squibb, Merck KGaA, Novartis, Roche, and Sanofi-Genzyme. His MS research was funded by the Swedish Research Council and the Swedish Brain foundation. None resulted in a conflict of interest. AS has no personal pecuniary interests to disclose, other than being the lead of the German MS Registry, which receives funding from a range of public and corporate sponsors, recently including The German Innovation Fund (G-BA), The German MS Trust, German MS Society, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, and Sanofi. None resulted in a conflict of interest. CT is member of advisory boards of Merck, Novartis and the multi-sponsored “Brain Health Initiative” of Gavin Giovannoni. PF has received speaker’s fees and honoraria for advisory boards from Almirall, Bayer, Biogen Idec, Bristol Myers Squibb, Genzyme, Novartis, Merck-Serono, Roche and Teva. None resulted in a conflict of interest.
Funding: This work on patient reported outcomes and employment was initiated as part of the EUReMS project which has received (1) co-funding from the European Union, in the framework of the Second Health Programme 2008-2013, Priority Area: 3.3.2 Promote health – Promote healthier ways of life and reduce major diseases and injuries – Action: 3.3.2.7 Prevention of major and chronic diseases and rare diseases and (2) from the following sponsors: Hoffmann La Roche, BayerSchering, Biogen Idec, Merck serono, Almirall, Sanofi-Aventis, TEVA, Genzyme, Medtronic Foundation, Novartis, ECRIMS. Co-funding by Biogen allowed continuation of the previously unfinished analysis of already existing and newly collected PRO data, being provided by additional registries joining the project.
ORCID iDs: David Ellenberger https://orcid.org/0000-0002-2274-5025
Waldemar Brola https://orcid.org/0000-0002-7955-3454
Rod Middleton https://orcid.org/0000-0002-2130-4420
Alexander Stahmann https://orcid.org/0000-0001-5308-105X
Publication history: Neither this manuscript nor its contents have been previously published.
Statistical analysis: Data was analyzed by David Ellenberger employed as a statistician at the Registry Custiodian, MS Forschungs- und Projektentwicklungs-gGmbH, Hanover, Germany
Contributor Information
Tina Parciak, University Medical Center, Georg-August-Universität Göttingen, Germany.
Waldemar Brola, Collegium Medicum, Jan Kochanowski University, Kielce, Poland Kielce, Poland.
Jan Hillert, Karolinska Institute, Stockholm, Sweden.
Rod Middleton, Swansea University Medical School, Swansea, UK.
Alexander Stahmann, German MS Register, MS Forschungs- und Projektentwicklungs-gGmbH, Hannover, Germany.
Christoph Thalheim, European Multiple Sclerosis Platform, Brussels, Belgium.
Peter Flachenecker, Neurological Rehabilitation Center Quellenhof, Bad Wildbad, Germany.
References
- 1.Kornblith AB, La Rocca NG, Baum HM. Employment in individuals with multiple sclerosis. Int J Rehabil Res Int Z Rehabil Rev Int Rech Readaptation 1986; 9: 155–165. [DOI] [PubMed] [Google Scholar]
- 2.Flachenecker P, Stuke K, Elias W, et al. Multiple Sclerosis Registry in Germany – Results of the Extension Phase 2005/2006. Dtsch Aerzteblatt Online [Internet]. 2008 Feb 15 [cited 2019 Dec 18]; Available from: https://www.aerzteblatt.de/10.3238/arztebl.2008.0113 [DOI] [PMC free article] [PubMed]
- 3.Julian LJ, Vella L, Vollmer Tet al. et al. Employment in multiple sclerosis: exiting and re-entering the work force. J Neurol 2008; 255: 1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baneke P. MSIF Survey on employment. 2010.
- 5.Kobelt G, Thompson A, Berg Jet al. et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler J 2017 Jul 1; 23: 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KL, Yorkston KM, Klasner ERet al. et al. The cost and benefits of employment: a qualitative study of experiences of persons with multiple sclerosis. Arch Phys Med Rehabil 2004; 85: 201–209. [DOI] [PubMed] [Google Scholar]
- 7.Jones KH, Ford DV, Jones PA, et al. How people with multiple sclerosis rate their quality of life: an EQ-5D survey via the UK MS register. PLoS One 2013; 8: e65640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause I, Kern S, Horntrich Aet al. et al. Employment status in multiple sclerosis: impact of disease-specific and non-disease-specific factors. Mult Scler J 2013; 19: 1792–1799. [DOI] [PubMed] [Google Scholar]
- 9.Gyllensten H, Wiberg M, Alexanderson Ket al. et al. Comparing costs of illness of multiple sclerosis in three different years: a population-based study. Mult Scler J 2018; 24: 520–528. [DOI] [PubMed] [Google Scholar]
- 10.Gyllensten H, Wiberg M, Alexanderson Ket al. et al. How does work disability of patients with MS develop before and after diagnosis? A nationwide cohort study with a reference group. BMJ Open 2016; 6: e012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavaliunas A, Danylaite Karrenbauer V, Gyllensten H, et al. Cognitive function is a major determinant of income among multiple sclerosis patients in Sweden acting independently from physical disability. Mult Scler J 2019; 25: 104–112. [DOI] [PubMed] [Google Scholar]
- 12.Wiberg M, Friberg E, Stenbeck M, et al. Sources and level of income among individuals with multiple sclerosis compared to the general population: a nationwide population-based study. Mult Scler J 2015; 21: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 13.Kasilingam E. MS Barometer 2011. 2011.
- 14.Pugliatti M, Eskic D, Mikolcić T, et al. Assess, compare and enhance the status of persons with M ultiple S clerosis (MS) in E urope: a E uropean R egister for MS. Acta Neurol Scand 2012; 126: 24–30. [DOI] [PubMed] [Google Scholar]
- 15.Flachenecker P, Buckow K, Pugliatti M, et al. Multiple sclerosis registries in Europe–results of a systematic survey. Mult Scler J 2014; 20: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 16.Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol 2008; 77: 802–813. [DOI] [PubMed] [Google Scholar]
- 17.Hobart J, Lamping D, Fitzpatrick Ret al. et al. The multiple sclerosis impact scale (MSIS-29) a new patient-based outcome measure. Brain 2001; 124: 962–973. [DOI] [PubMed] [Google Scholar]
- 18.Group TE. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 19.Ernstsson O, Tinghög P, Alexanderson Ket al. et al. The external validity of mapping MSIS-29 on EQ-5D among individuals with multiple sclerosis in Sweden. MDM Policy Pract 2017; 2: 2381468317692806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler J 2008; 14: 383–390. [DOI] [PubMed] [Google Scholar]
- 21.Fischer JS, Rudick RA, Cutter GRet al. et al. The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 22.Sharrack B, Hughes RA. Clinical scales for multiple sclerosis. J Neurol Sci 1996; 135: 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell PM, McDowell Z, Wong CKet al. et al. Doctors and patients don’t agree: cross sectional study of patients’ and doctors’ perceptions and assessments of disability in multiple sclerosis. Br Med J 1997; 314: 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayo NE, Hum S, Kuspinar A. Methods and measures: what’s new for MS? Mult Scler J 2013; 19: 709–713. [DOI] [PubMed] [Google Scholar]
- 25.Riazi A. Patient-reported outcome measures in multiple sclerosis. Int MS J 2006 Nov; 13: 92–99. [PubMed] [Google Scholar]
- 26.Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment. Mult Scler J 2021; 27(2): 281–289. [DOI] [PubMed] [Google Scholar]
- 27.Iaffaldano P, Lucisano G, Butzkueven H, et al. Early treatment delays long-term disability accrual in RRMS: Results from the BMSD network. Mult Scler J 2021; 27(10): 1543–1555. [DOI] [PubMed]
- 28.Maillart E, Labauge P, Cohen M, et al. MSCopilot, a new multiple sclerosis self-assessment digital solution: results of a comparative study versus standard tests. Eur J Neurol 2020; 27: 429–436. [DOI] [PubMed] [Google Scholar]
- 29.Siristatidis C, Pouliakis A, Karageorgiou Vet al. et al. Mobile apps for helping patient-users: is it still far-fetched? Sustainability 2020 Jan; 12: 106. [Google Scholar]
- 30.OECD - Closing the Gender Gap Sweden. 2011.
- 31.Kavaliunas A, Manouchehrinia A, Karrenbauer VD, et al. Income in multiple sclerosis patients with different disease phenotypes. PloS One 2017; 12: e0169460. [DOI] [PMC free article] [PubMed] [Google Scholar]