Abstract
Hepatitis C virus (HCV) infection is the most common blood-borne chronic infection in the United States. Chronic lymphocytic sialadenitis and sicca syndrome have been reported in chronic HCV infection. Up to 55% of these patients may have xerostomia; the mechanisms of the xerostomia and salivary gland (SG) hypofunction remain controversial. The objectives of this project are to establish if xerostomia associates with SG and HCV infection and to characterize the structural changes in SG and saliva composition. Eighteen HCV-infected patients with xerostomia were evaluated for SG dysfunction; 6 of these patients (patients 1–6) were further evaluated for SG histopathological changes and changes in saliva composition. The techniques used include clinical and laboratory assessment, SG ultrasonography, histological evaluation, sialochemical and proteomics analysis, and RNA in situ hybridization. All the HCV patients had low saliva flow, chronic sialadenitis, and SG fibrosis and lacked Sjögren syndrome (SS) characteristic autoantibodies. Further evaluation of a subgroup of 6 HCV patients (patients 1–6) demonstrated diffuse lymphocytic infiltrates that are predominantly CD8+ T cells with a significant increase in the number of inflammatory cells. Alcian Blue/periodic acid–Schiff staining showed significant changes in the ratio and intensity of the acinar secretory units of the HCV patients’ minor SG. The submandibular glands showed significant ultrasonographic abnormalities in the parenchyma relative to the parotid glands. Significant changes were also observed in the concentration of sodium and mucin 5b. Although no significant correlation was observed between the lymphocytic infiltrates and the years of HCV chronic infection, a positive correlation was observed between HCV RNA–positive epithelial cells and the years of HCV infection. Consistent with the low saliva flow and xerostomia, patients showed changes in several markers of SG acinar and ductal function. Changes in the composition of the saliva suggest that HCV infection can cause xerostomia by mechanisms distinct from SS.
Keywords: Sjögren’s syndrome, xerostomia, sialadenitis, salivary hypofunction, chronic infection, sicca syndrome
Introduction
Hepatitis C virus (HCV) infection is the most common blood-borne chronic infection in the United States, with an estimated prevalence of 2.4 million people in the United States and up to 170 million globally (Hofmeister et al. 2019). Approximately 15% to 25% of HCV-infected people clear the virus on their own, but most HCV infections are chronic with detectable viral load unless specifically treated (Thomas and Seeff 2005).
Multiple HCV extrahepatic manifestations (EHMs) have been reported in patients with chronic HCV infection, including chronic lymphocytic sialadenitis and sicca syndrome indistinguishable from primary Sjögren syndrome (SS) (Aceti et al. 1992; Zignego and Craxi 2008). Xerostomia, the subjective impression of oral dryness, has been reported in 5% to 55% of HCV-infected individuals (Castro Ferreiro et al. 2002; Ramos-Casals et al. 2003; Giordano et al. 2005; Grossmann Sde et al. 2010; Nawito et al. 2011). Early studies posited an association between HCV infection and SS development (Haddad et al. 1992) and showed that expression of the E1 and E2 HCV envelope proteins elicits an exocrinopathy resembling SS in mice (Koike et al. 1997). In addition, SS-like focal lymphocytic infiltrates in the salivary glands (SGs) were observed in approximately 50% of HCV-infected patients but with less glandular tissue damage as compared to SS (Loustaud-Ratti et al. 2001; Carrozzo 2008). The SG lymphocytic infiltrate in HCV-infected patients was generally diffuse and predominantly composed of CD8+ T cells, but studies have also reported a predominance of CD4+ T cells but to a lesser degree than in SS (Freni et al. 1995). HCV-infected individuals present a higher prevalence of liver involvement and cryoglobulinemia than SS patients, in addition to usually lacking characteristic primary SS antibodies (i.e., anti-SSA and anti-SSB) (Ramos-Casals et al. 2005; Brito-Zerón et al. 2015), as well as high levels of autoantibodies, including antinuclear antigen (ANA), anticardiolipin antibodies (ACA-IgG/IgM), double-stranded DNA (dsDNA), and rheumatoid factor (RF) (Navarta et al. 2018). The 2016 American–European Consensus Criteria for the classification of primary SS determined that evidence of HCV infection is an exclusion criterion for the classification of a patient as having SS (Shiboski et al. 2017). Mechanistically, it is suggested that HCV is directly involved in the pathogenesis of SG dysfunction, but the exact pathogenic mechanism driving the xerostomia has not been elucidated. In clinical practice, these similarities may generate confusion in the diagnosis and proper treatment of the patients. Therefore, investigation of this patient population may be informative at both a clinical and a mechanistic level on the development, diagnosis, and treatment of SS.
Changes in the perception of oral dryness may be caused by viral infections of the SG that are associated with reduced saliva production, changes in the composition of saliva, glandular swelling, and/or diffuse infiltrative lymphocytic syndrome (Jeffers and Webster-Cyriaque 2011). However, the molecular mechanisms of the associated xerostomia are not well understood and likely context dependent. In addition to HCV, other viruses are associated with SG pathology, including hepatitis D virus, human immunodeficiency virus type 1, human T-cell leukemia virus type 1 (reviewed in Rahayuningtyas and Setiadhi 2019), and severe acute respiratory syndrome coronavirus 2 (Huang et al. 2021). HCV can directly infect SG epithelial cells (Arrieta et al. 2001; Grossmann Sde et al. 2010), and the xerostomia experienced by HCV patients may be the result of glandular tissue inflammation, destruction of the SG epithelium, and/or infection of the SG epithelium and resultant dysfunction of the secretory and water permeability pathways.
In this study, 18 HCV-infected patients were evaluated for xerostomia, and the clinical parameters and sialometry were assessed (Fig. 1A); further analyses were performed on 6 of the HCV patients and age-matched healthy volunteers (HVs). Consistent with the low saliva flow and xerostomia, patients showed changes in several markers of SG acinar and ductal function, as well as changes in the structure of the submandibular salivary gland (SMG). These findings suggest a unique mechanism associated with the change in gland activity and will aid in the management of HCV-associated oral disease.
Figure 1.
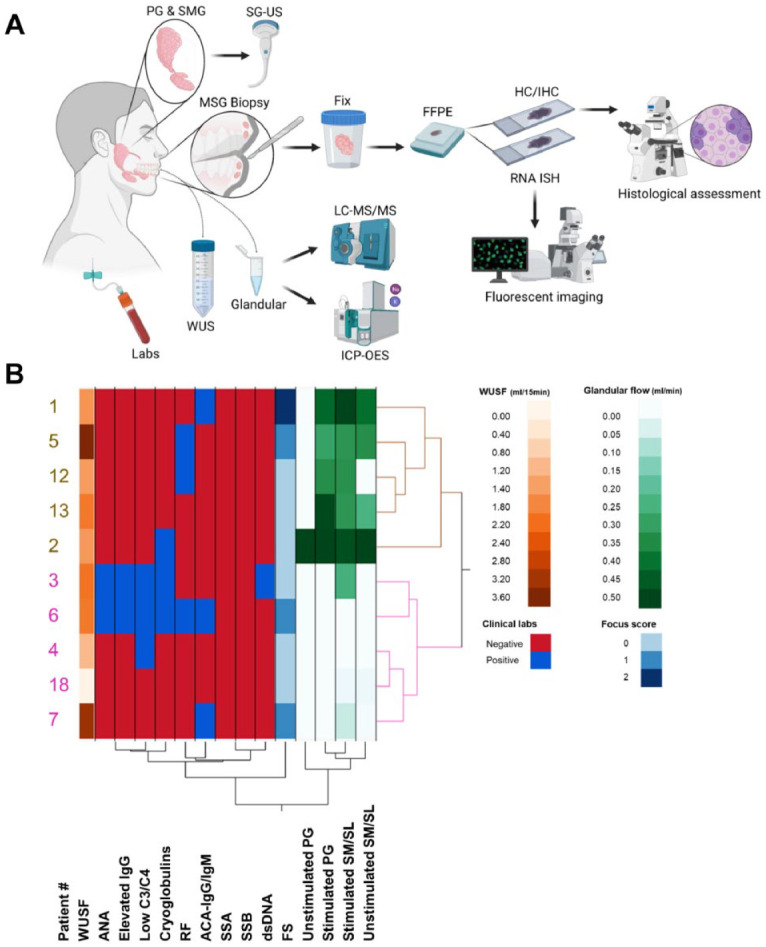
Study design, clinical parameters, and sialometry. This study evaluated and enrolled 18 HCV–positive patients with complaints of xerostomia to understand the role that viruses play in the development of inflammation and dysfunction of the SG. Diagram depicts collection of blood, saliva fluids, and SG specimens from HVs and HCV patients for evaluation. Submandibular/sublingual saliva was used for sialometry and sialochemistry (i.e., sodium, potassium, and protein composition). Salivary gland ultrasonography was performed in the PG and SMG of study participants. Lower lip MSG biopsies were collected and fixed in formalin, and tissue sections were prepared from FFPE blocks for staining (i.e., H&E, TRI, PAB, IHC, RNA ISH) and histologically assessed (A). Hierarchical clustering analysis was used to identify groupings of patients who shared clinical features and chose representative patients of each group (patients with limited clinical data may not be shown in the diagram) (B). Two distinct clusters of patients were found among the HCV patients evaluated in this study (depicted in brown and pink), those with and without measurable glandular saliva flow. Patients 1, 2, and 5 represent patients from the group with measurable glandular saliva flow; patients 3, 4, and 6 represent patients from the group without measurable glandular saliva flow. Diagram made with Biorender. ACA, anticardiolipin antibodies; ANA, antinuclear antigen; FFPE, formalin fixed, paraffin embedded; FS, focus score; HCV, hepatitis C virus; HV, healthy volunteer; H&E, hematoxylin and eosin; IHC, immunohistochemistry; ISH, in situ hybridization; MSG, minor salivary gland; PAB, Alcian Blue/periodic acid–Schiff; PG, parotid gland; RF, rheumatoid factor; SG, salivary gland; SGUS, salivary gland ultrasonography; SLG, sublingual gland; SM, submandibular; SMG, submandibular gland; SS-A, Sjögren antibody A; SS-B, Sjögren antibody B; TRI, Masson’s trichrome; WUSF, whole unstimulated saliva flow.
Materials and Methods
Materials and methods are reported in the supplemental section.
Results
Patient Characteristics and Clinical Parameters
The patient cohort consisted of 14 women and 4 men, with a median age of 59 ± 12 y (range, 43–81). All patients had active HCV infection and presented with xerostomia.
A summary of the patients (n = 18) is found in Appendix Table 1. Of the patients, 5 had RF, 4 had ANA, 4 had ACA-IgG/IgM, 2 had dsDNA antibodies, 5 had cryoglobulins, 1 had SSA antibodies, and 2 had SSB antibodies. Six patients had low complement proteins C3 and C4, and 3 had elevated serum IgG levels (>1,445). On minor salivary gland biopsy, one-third of the patients (n = 6) had focal lymphocytic infiltrates with focus scores (FSs) ranging between 1 (n = 3) and 2 (n = 3). Hierarchical clustering analysis (HCA) (Fig. 1B), used to organize the patients in nested clusters based on similarities between clinical features, showed that most of the HCV patients evaluated lacked markers of autoimmune or systemic diseases, including those associated with SS (Fig. 1B; Appendix Table 1).
Hierarchical analysis showed 2 distinct clusters of patients demarcated by the glandular saliva (i.e., parotid [PG], submandibular [SM], and sublingual [SL]) flow rates (Fig. 1B). Two of the HCV patients had severe salivary hypofunction, defined as whole unstimulated saliva flow (WUSF) less than 1.5 mL/15 min (Navazesh et al. 1992; Vitali et al. 1994). The median WUSF was 1.95 mL/15 min (range, 1.05–3.40). Most of the patients had low unstimulated PG (median flow of 0.09 mL/min) and SM/SL (median flow of 0.15 mL/min) saliva flow. Stimulation with citric acid increased the median flow from 0.17 to 0.31 mL/min. The lack of severe hypofunction in general and responsiveness to citric acid stimulation suggest that saliva flow rate alone may not be sufficient to explain the HCV-associated xerostomia.
A representative subgroup of 6 patients (patients 1–6) based on the measured glandular saliva flow rate (normal vs. low) and autoantibody status (positive or negative) was chosen for in-depth analysis (Appendix Table 1). These patients had a time interval between HCV infection and minor salivary gland biopsy (MSGB) of 10 to 50 y (Appendix Table 1). They also represented a wide range of HCV RNA serum concentration (i.e., 5.5 × 105 to 3.1 × 107 genome copies/mL) and HCV genotypes (Appendix Table 1), and few had comorbidities (i.e., osteoarthritis and diabetes type 2). In addition, 4 patients had aqueous tear deficiency, as determined by a Schirmer’s test result of ≤5 mm wetting/5 min in at least 1 eye (Appendix Table 1). Neither the HCV genotype nor serum viral load correlated with the SG function.
HCV RNA Detection in SG by In Situ Hybridization
RNA in situ hybridization (ISH) was performed on MSGB tissue sections (patients 1–6) to assess the presence of HCV genomes using RNAscope probe sets (Materials and Methods; Fig. 2A–C). HCV RNA signal was observed in the epithelial cells of the acini (Fig. 2′A) and ducts (Fig. 2′B), as well as in the interlobular infiltrative cells (Fig. 2′C). HCV was detected in all the HCV MSGB but not in those of HV MSGB. The mean percentage of HCV-infected cells was 59% ± 6.8% (range, 53%–71%) for acinar epithelial cells, 31% ± 15.3% (range, 12%–54%) for ductal epithelial cells, and 51% ± 10.7% (range, 35%–65%) for infiltrating inflammatory cells.
Figure 2.
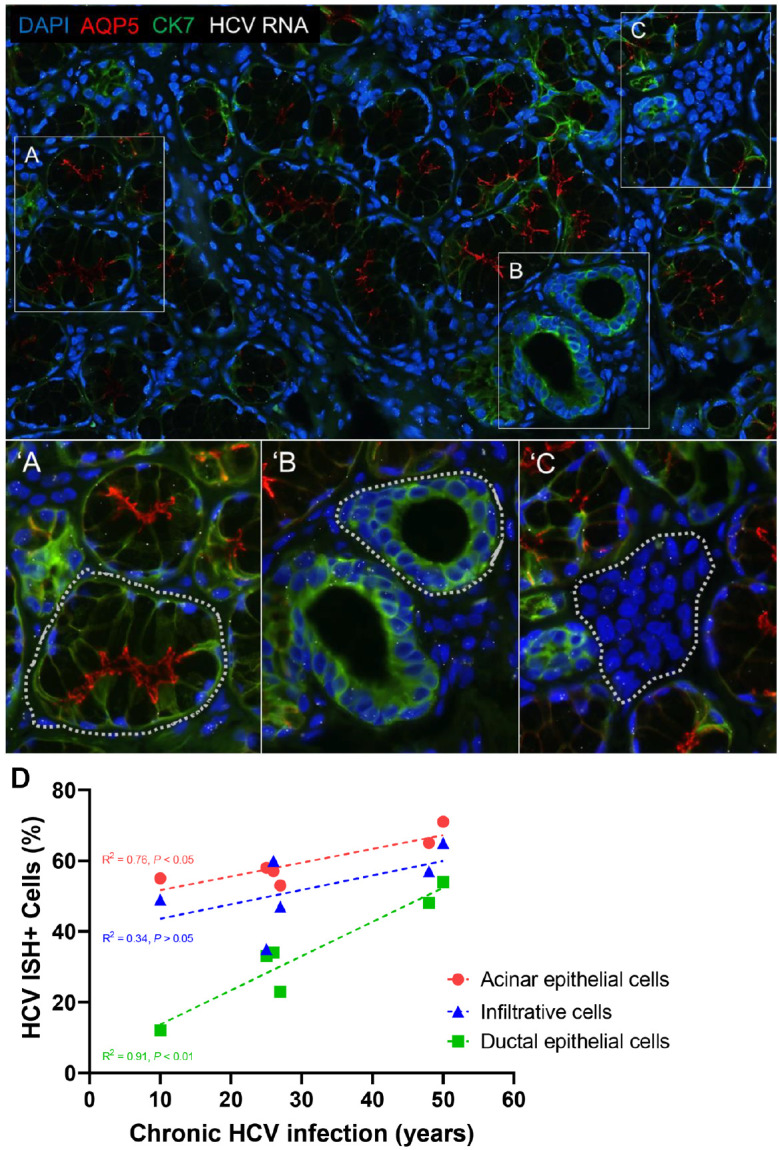
Lip salivary gland biopsy HCV–RNA in situ hybridization. RNA ISH was used to investigate the presence and tropism of HCV in the salivary glands. Representative images of HCV RNA detection by RNAscope assay in acinar epithelial cells (A), ductal epithelial cells (B), and interlobular inflammatory cells (C). The percentage of acinar (′A), ductal (′B), and inflammatory (′C) cells expressing 1 or more HCV RNA ISH dots was calculated from the regions demarcated with a dotted line. Correlation of HCV RNA ISH signal and years of chronic HCV infection (D) with a XY graph illustrating the association between percentage of salivary gland (SG) acinar epithelial cells (red; P < 0.05; r = 0.8706), ductal epithelial cells (green; P < 0.01; r = 0.9537), and infiltrative cells (blue; P > 0.05; r = 0.5791) staining positive for HCV genomic RNA and the years of HCV infection. Each dot represents an individual case, and the colored dotted line represents a linear regression of the data. This observation suggests that during the course of the chronic infection, the virus is not static and continues to spread, a correlation that, to our knowledge, has not been reported for chronic liver infection and the percentage of HCV RNA–positive hepatocytes. Our study did not find a direct correlation between HCV RNA–positive SG epithelial cells and SG hypofunction or sialadenitis, as well as no correlation between the serum levels of HCV RNA and the percentage of SG HCV RNA ISH–positive cells. The brightness and contrast were adjusted equally among all the images for clarity. Blue, DAPI stain; green, cytokeratin 7 stain; red, AQP5 stain; white, HCV RNA; HCV, hepatitis C virus; ISH, in situ hybridization. Significance was set at P ≤ 0.05.
To understand the relationship between the length of infection and the number of positive cells in SG, viral loads were compared across the cell types infected with HCV. In HCV patients with sicca, there was a statistically significant positive correlation between the years of HCV infection and the percentage of acinar and ductal epithelial cells positive for HCV RNA ISH signals (R2 = 0.76; P = 0.02; R2 = 0.91; P = 0.003, respectively), but there was no significant correlation between infiltrating inflammatory cells and years of HCV infection (R2 = 0.34; P = 0.23) (Fig. 2D). No correlation was also found between the percentage of HCV-positive acinar cells, ductal cells, or infiltrating inflammatory cells and the serum levels of HCV RNA (R2 = 0.00; P = 0.96) or WUSF (R2 = 0.00; P = 0.96). Furthermore, no statistically significant correlation was found between the serum levels of HCV RNA and the WUSF (R2 = 0.46; P = 0.14) or the years of HCV infection (R2 = −0.04;P = 0.69). These results confirmed the presence of HCV genome in both acinar and ductal epithelial cells and that the level of HCV detected in the SG correlates with years of infection and suggests evolving disease.
Glandular Histopathology
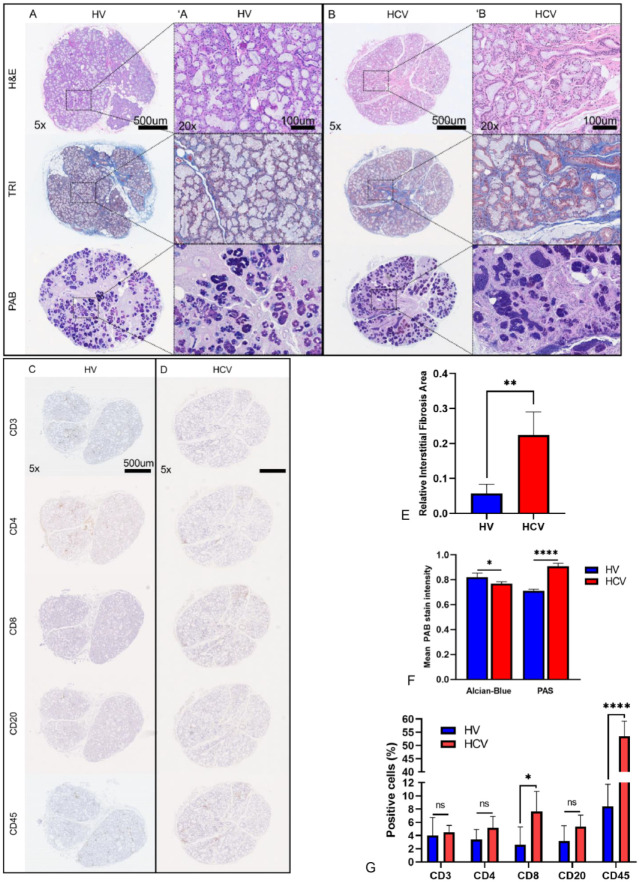
The glandular histopathology of HCV patients was compared relative to HVs (Fig. 3A, ′A). Two general histopathologic patterns were present: nonspecific chronic sialadenitis (FS = 0; n = 3) (Fig. 3B, ′B) and mild chronic sialadenitis with focal lymphocytic sialadenitis (FLS) (FS = 1, n = 2; FS = 2, n = 1) (Appendix Table 1) (Daniels et al. 2011). The lymphocytic infiltrate was further evaluated with a basic immune panel stain (i.e., CD3, CD4, CD8, CD20, and CD45 cell markers) (Fig. 3C, D). The Masson’s trichrome (TRI)–stained HCV MSGB had significantly higher levels of fibrosis (relative interstitial fibrosis area [RIFA] = 0.22; P = 0.0048) when compared with HV (RIFA = 0.06) (Fig. 3E); histologic analysis of the MSGB by Alcian Blue/periodic acid–Schiff (PAB) staining suggested a shift in the cellular composition of the MSG following HCV infection. Alcian Blue stain intensity, which stains for acidic mucins, was significantly reduced (P = 0.0110), and periodic acid–Schiff (PAS) stain intensity, which stains neutral mucins and glycogen, was significantly increased relative to HVs (P = 0.001) (Fig. 3F). This shift in staining indicates a greater abundance of serous- versus mucin-secreting cells, suggesting dedifferentiation or remodeling of glandular tissue following HCV infection.
Figure 3.
Glandular histopathology. Histological comparison of representative MSGB for each of the staining (A, B) and a basic immune panel (C, D) from HV and patients with HCV-induced xerostomia with mild chronic sialadenitis are shown, respectively. A 20× magnified region of the H&E-, TRI-, and PAB-stained tissue sections is shown for the HV (′A) and HCV (′B) patient, respectively. Increased interlobular fibrosis as highlighted by TRI staining (blue) relative to the HV is evident. All HCV patients presented a significant increase in RIFA compared to HV (E). Acinar atrophy and disorganization are evident in the HCV group, as highlighted by H&E and PAB staining (B, ′B). Increased glycoprotein expression as highlighted by PAS staining (dark purple) relative to the HV is evident. The HCV-induced xerostomia patients’ biopsies have a significant decrease in mucinous acini and a significant increase in serous acini as highlighted by PAB staining (F) and demonstrate a diffuse lymphocytic infiltration composed mainly of CD8+ T cells and a large population of histiocytes (D, G). H&E, hematoxylin and eosin; HCV, hepatitis C virus; HV, healthy volunteer; MSGB, minor salivary gland biopsy; PAB, Alcian Blue/PAS; PAS, periodic acid-Schiff; RIFA, relative interstitial fibrosis area; TRI, Masson’s trichrome. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001.
Consistent with histopathological assessment, immunohistochemistry demonstrated a diffuse infiltrate in HCV patients’ MSGB with a significant increase in the total number of infiltrating inflammatory cells (CD45+; P < 0.0001) (Fig. 3G). Although there were no significant differences in the number of CD3+ T cells, CD4+ T cells, and CD20+ B cells (Fig. 3G), a significant increase in CD8+ T cells was observed (P = 0.0297). The increase in CD8+ T cells is consistent with previous studies that showed large, sustained populations of persistent dysfunctional CD8+ T cells in the context of HCV infection (Gruener et al. 2001).
Glandular Imaging
To noninvasively assess the PG and SMG, Salivary gland ultrasonography (SGUS) was performed and scored using previously reported parameters (Theander and Mandl 2014; Jousse-Joulin et al. 2017). Salivary gland ultrasonography revealed PG and SMG with normal homogeneity and echogenicity without hypoechoic lesions for 2 patients (patients 3 and 4). The other patients (patients 1, 2, 5, and 6) presented consistent, generally mild changes in SMG, including hyperechoic bands, and abnormal echogenicity and homogeneity but with normal PG imaging. Representative SGUS images for the PG (Fig. 4A, B) and SMG (Fig. s4C, D) are shown for HV and HCV patients, respectively. A simplified SGUS scoring system (Theander and Mandl 2014) was used to assess parenchyma dyshomogeneity: normal (score 0) or unspecific (score 1) and abnormal (scores 2 and 3). The sum SGUS score of the HCV PG and SMG was not significantly different compared with HVs, but the sum SGUS score of the SMG of the HCV patients was significantly higher than that of the HCV PG (Fig. 4E). Scoring using the comprehensive scaled scoring system showed a similar trend (Jousse-Joulin et al. 2017) (Appendix Fig. 2). These results suggest that HCV infection may preferentially affect the SMG.
Figure 4.
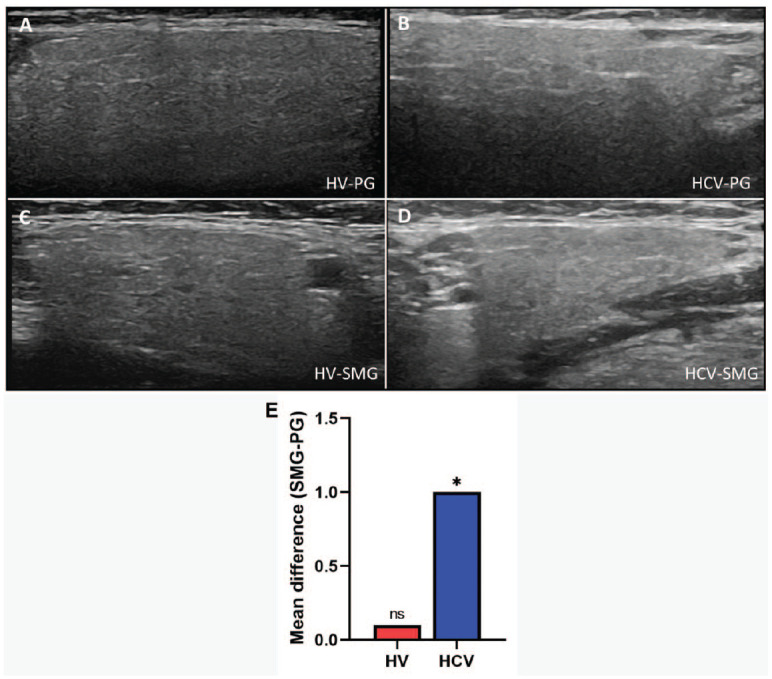
Salivary gland ultrasonography. Ultrasonography images of a PG of HV (A) and HCV (B) and of a SMG of HV (C) and HCV (D) are shown. Normal or abnormal parenchyma of the SG was evaluated from the entire image, including visibility of the PG posterior border. A simplified SGUS scoring system was used to assess parenchyma dyshomogeneity (Theander and Mandl 2014). The mean difference of the sum SMG and PG US score is shown (E). The HCV group SMG had a significantly higher SGUS score as compared to the HCV PG; the HV’s SMG showed a slightly higher SGUS score relative to the HV PG. Significance was set at P ≤ 0.05. HCV, hepatitis C virus; HV, healthy volunteer; SG, salivary gland; SGUS, salivary gland ultrasonography; SMG, submandibular gland; PG, parotid gland; US, ultrasonography. *P ≤ 0.05.
Sialometry
The above data support reorganization and change in cellular composition of the SG of HCV patients. To assess if these changes resulted in a change in saliva flow, WUSF and glandular saliva flow were compared in the subset of 6 patients. Three patients (patients 2, 3, and 5) reported onset of dry mouth during the 5 y prior to the oral evaluation by our team, while the other 3 patients (patients 1, 4, and 6) reported chronic symptoms of dry mouth. One patient (patient 4) had salivary hypofunction with WUSF of 1.05 mL/15 min; 2 patients (patients 1 and 2) had borderline salivary hypofunction with WUSF of 1.56 and 1.52 mL/15 min, respectively; and the other 3 patients had no salivary hypofunction (patients 3, 5, and 6) with a median WUSF of 2.68 mL/15 min (range, 1.95–3.40) (Appendix Table 1). Only 3 patients (patients 1, 2, and 5) had any measurable citric acid–stimulated saliva flow in either PG with a median flow of 0.45 mL/min/gland (range, 0.09–0.21). Unstimulated SM/SL saliva flow was measurable in 3 patients (patients 1, 2, and 5) with a median flow of 0.16 mL/min (range, 0.11–0.21) and stimulated flow with citric acid in 4 patients (patients 1, 2, 3, and 5) with a median flow of 0.40 (range, 0.30–0.49) (Appendix Table 1). Most of the patients had increased SM/SL saliva flow after stimulation; thus, we probed if the composition of saliva was altered.
Sialochemistry
The above histologic and echoic changes in the structure of the gland and lack of defined salivary hypofunction suggest that a change in salivary composition may be associated with the xerostomia. Sialochemical analysis was performed on unstimulated and stimulated SM/SL saliva of HVs and HCV patients (patients 1–6). Although potassium (K+) was slightly decreased in the SM/SL saliva in the HCV patients, it was not statistically significant (Fig. 5A). In contrast, a significant increase in Na+ concentration was observed in the stimulated SM/SL saliva of the HCV group compared with the HV group (P = 0.0096) (Fig. 5B). These results suggest altered ductal function in the reabsorption of Na+.
Figure 5.
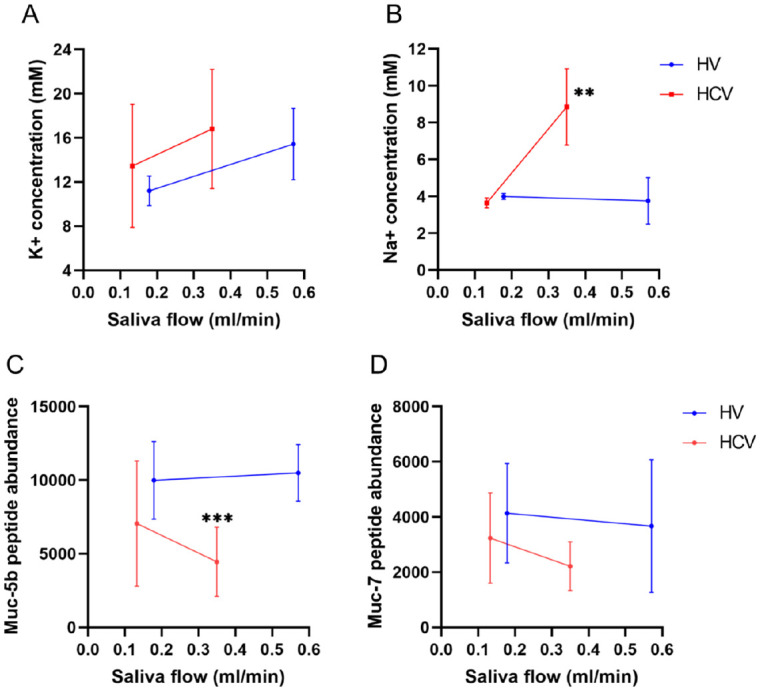
Sialochemistry. Limited sialochemical assessment was completed via ICP-OES analysis. An average of 2 values is reported (mM). The concentration of potassium (A) and sodium (B) in unstimulated saliva and stimulated saliva from HVs and HCV patients is shown in relation to the mean saliva flow (mL/min). The concentration of potassium was slightly increased during stimulation of saliva, while sodium significantly increased in the stimulated saliva of HCV patients relative to HVs, indicative of ductal epithelial dysregulation. Mass spectroscopy analysis was performed to assess changes in mucins concentration, which are thought to play an important role in the subjective sensation of moisture and lubricity, mainly mucin 5b and mucin 7. In this study, mucin 5b (C) and mucin 7 (D) were decreased in the saliva of the HCV patients. Mucin 5b was significantly decreased in the stimulated saliva of the HCV patients. Mucin 5b is a sticky, gel-forming high molecular weight mucin that forms multimers and a viscous coating that traps water molecules, which provides lubrication and moistening of the oral cavity. Mucin 7, a watery, monomeric low molecular weight mucin, absorbs much less water than mucin 5b. Thus, changes in the quantity, quality, or hydration of mucins may play a role in the perception of oral dryness and desiccation. Significance was set at P ≤ 0.05. HCV, hepatitis C virus; HV, healthy volunteer; ICP-OES, inductively coupled plasma optical emission spectroscopy; K+, potassium; mM, millimolar; MS, mass spectroscopy; muc-5b, mucin 5b; muc-7, mucin 7; Na+, sodium. **P ≤ 0.01; ***P ≤ 0.001.
The protein and mucin content of the unstimulated and stimulated saliva of HVs and HCV patients was assessed using proteomics analysis (Appendix Fig. 3A, B). Mucin 5b and mucin 7 were reduced in all saliva samples in the HCV group (Fig. 5C, D). Significant reductions in the concentration of mucin 5b was observed in the stimulated SM/SL saliva of the HCV patients (P = 0.0005) (Fig. 5C). These results support our observations of glandular acini dedifferentiation or remodeling to less mucinous saliva and possible loss of mucous cells.
Discussion
Hepatitis C virus chronic infection is frequently associated with several EHMs that include rheumatologic autoimmune and inflammatory manifestations, as well as complex oral complications (Aceti et al. 1992; Zignego and Craxi 2008). Chronic HCV infection may be treatable, but the long-term effects on EHM are not clear; to better assess if HCV was causal of the oral complications or the result of secondary changes in the immune system, we studied the oral complications in a group of patients with chronic HCV infection.
A portion of the HCV-positive patients evaluated in this study had clinical and histopathological features similar to SS and lacked markers of autoimmune disease unrelated to chronic HCV infection. While we did have some patients with ANA (22%), the prevalence of ANA in HCV patients is 20% to 35% and is 6% to 14% in the general population (Khairy et al. 2013; Li et al. 2019). Patients with HCV chronic infection have high levels of circulating autoantibodies and can be found in 53% of the HCV-positive patients. These autoantibodies may also develop as a consequence of aging and extensive liver fibrosis. About one-third of the 18 HCV patients evaluated in this study, including the subgroup of 6 HCV patients, were positive for 1 or more of these autoantibodies and RF. However, none of our subjects had autoantibodies characteristic of SS (Fig. 1B).
The normal flow of unstimulated whole saliva is 0.3 to 0.5 mL/min; thus, a flow above 0.3 mL/min is considered normal and a flow <0.1 mL/min is considered clinical SG hypofunction and may result in xerostomia (Sreebny and Valdini 1987; Speight et al. 1992). All 18 patients evaluated in this study presented with subjective complaint of dry mouth, but most of the patients had WUSF above 0.1 mL/min and over half had an increase in PG and SM/SL saliva flow after citric acid stimulation (Fig. 1B; Appendix Table 1). Thus, over half of the measurable stimulated glandular saliva was within the normal range, suggesting that other parameters than saliva flow, such as lubricity or buffering capacity of the saliva, may be important in the sensation of oral wetness. In addition to SG involvement, 4 of the 6 patients in the subgroup had lacrimal secretion deficiencies as shown by the Schirmer’s test (Appendix Table 1), an observation similar to SS-associated sicca. The mechanism of lacrimal gland dysfunction and HCV tropism to these glands remains unknown.
Histopathological evaluation of MSGB of patients 1 to 6 showed mild SG atrophy (Fig. 3B, ′B), increased levels of fibrosis (Fig. 3E), and diffuse lymphocytic infiltrate mainly composed of CD8+ T cells (Fig. 3G) as compared to HVs with the occasional foci of inflammation. The composition of the lymphocytic infiltrate is highly suggestive of a chronic antiviral immune response. It was noted that the atrophy, fibrosis, and pattern of inflammation were predominantly diffuse throughout the glandular tissues and did not correlate with the patient’s age or years of chronic HCV infection. Histochemical evaluation showed changes in the expression and distribution of acinar secretory units with decreased levels of mucinous acini (Fig. 3F; Appendix Fig. 4). These observations associate with decreased levels of mucin 5b (Fig. 5C). Loss of the mucous-producing cells in the MSG, if applied to other glands with similar types of cells (e.g., the SMG), might suggest a change in the types and amount of mucins being secreted. These changes may explain the discordant yet consistent finding of reports of dry mouth but without objective salivary gland dysfunction by WUSF.
Salivary gland ductal epithelia play an important role in the composition of salivary secretions by secreting and reabsorbing ions as the saliva is transported into the oral cavity (Pedersen et al. 2018). Sialochemical analysis showed that the concentration of Na+, which is extensively reabsorbed by the ductal cells, was significantly increased in the stimulated saliva of HCV patients compared to HVs (Fig. 5B). Given the lower saliva flow rate in the HCV group and the higher concentration of Na+, it suggests the ductal epithelial cells have reduced capacity to reabsorb Na+ compared to HVs. Increased concentration of Na+, unaltered levels of K+, and lower flow rates of saliva have also been reported in SS patients (Kalk et al. 2001) and are indicative of ductal epithelial dysregulation. The mechanism of K+ transport differs from the active ductal transport of Na+. Although the periductal lymphocytic infiltrate may affect the electrolyte transport function of the ductal epithelia, the level of infiltrate did not correlate with the change in saliva composition.
Saliva is rich in proteins, including mucins, statherins, histatins, proline-rich proteins, and amylases (Appendix Fig. 3). Mass spectroscopy analysis showed that the concentration of mucins was decreased in the stimulated saliva of the HCV patients relative to the HVs. A link between low levels of mucin 5b in the SMG and xerostomia has been reported in radiation-induced xerostomia patients (Dijkema et al. 2012), as well as altered sulfation of mucin 5b in SS patients rather than low levels of the mucin 5b (Alliende et al. 2008). Our findings support a possible mechanism for the xerostomia in HCV patients as a result of decreased mucins produced by the SMG.
The changes in the saliva composition described in this study suggest a novel mechanism distinct from SS that may explain the xerostomia in the HCV patients; furthermore, it supports a role for virus infection in salivary gland dysfunction. Our results show that while HCV sicca closely phenotypes SS, not all HCV-infected patients have autoimmune epithelitis. It is more likely that the inflammation is driven by the presence of HCV RNA in the glands, as evidenced by increased numbers of CD8+ T cells, a hypothesis we are currently testing. Further large-scale studies will be needed to support the findings of this study and better understand the molecular mechanism associated with HCV infection and xerostomia. Newer generations of direct-acting antiviral (DAA) therapy for chronic HCV infection are effective in eliminating the virus in most people treated with over 95% of virological response (Baumert et al. 2019). Thus, evaluating HCV-infected patients after HCV DAA treatment would be informative to determine if the HCV-associated SG disturbances resolve and the perception of oral dryness improves.
Author Contributions
J.O. Maldonado, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M.E. Beach, Y. Wang, P. Perez, H. Yin, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; E. Pelayo, S. Fowler, M. Grisius, A.N. Baer, V. De Giorgi, contributed to data acquisition, critically revised the manuscript; I. Alevizos, contributed to conception, design, and data acquisition, critically revised the manuscript; B. Walitt, contributed to conception and data interpretation, critically revised the manuscript; H.J. Alter, contributed to conception and data acquisition, critically revised the manuscript; B.M. Warner, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; J.A. Chiorini, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211049395 for HCV Infection Alters Salivary Gland Histology and Saliva Composition by J.O. Maldonado, M.E. Beach, Y. Wang, P. Perez, H. Yin, E. Pelayo, S. Fowler, I. Alevizos, M. Grisius, A.N. Baer, B. Walitt, V. De Giorgi, H.J. Alter, B.M. Warner and J.A. Chiorini in Journal of Dental Research
Acknowledgments
We thank the patients for their participation in our clinical studies. We acknowledge the National Institutes of Health (NIH) Clinic staff for their support in caring for the participating patients. The sialochemical analysis was performed by Dr. Heather Kalish from the Trans-NIH Shared Resource on Biomedical Engineering and Physical Science, National Institute of Biomedical Imaging and Bioengineering. The MSGB specimens were interpreted by David E. Kleiner, a board-certified surgical pathologist from the Anatomic Pathology Laboratory of the National Cancer Institute, NIH, Bethesda, MD.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Dental and Craniofacial Research Grants to J.A. Chiorini (1ZIADE000695) and B.M. Warner (1ZIADE000704), and, in part, by the NIDCR Mass Spectrometry Facility Core, Imaging Core (ZIC DE000750-01), and Combined Technical Research Core (ZIC DE000729-09).
ORCID iDs: J.O. Maldonado  https://orcid.org/0000-0003-1226-7119
https://orcid.org/0000-0003-1226-7119
References
- Aceti A, Taliani G, Sorice M, Amendolea MA. 1992. HCV and Sjögren’s syndrome. Lancet. 339(8806):1425–1426. [DOI] [PubMed] [Google Scholar]
- Alliende C, Kwon YJ, Brito M, Molina C, Aguilera S, Perez P, Leyton L, Quest AF, Mandel U, Veerman E, et al. 2008. Reduced sulfation of MUC5B is linked to xerostomia in patients with Sjögren syndrome. Ann Rheum Dis. 67(10):1480–1487. [DOI] [PubMed] [Google Scholar]
- Arrieta JJ, Rodriguez-Inigo E, Ortiz-Movilla N, Bartolome J, Pardo M, Manzarbeitia F, Oliva H, Macias DM, Carreno V. 2001. In situ detection of hepatitis C virus RNA in salivary glands. Am J Pathol. 158(1):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumert TF, Berg T, Lim JK, Nelson DR. 2019. Status of direct-acting antiviral therapy for hepatitis C virus infection and remaining challenges. Gastroenterology. 156(2):431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito-Zerón P, Gheitasi H, Retamozo S, Bové A, Londoño M, Sánchez-Tapias J-M, Caballero M, Kostov B, Forns X, Kaveri SV. 2015. How hepatitis C virus modifies the immunological profile of Sjögren syndrome: analysis of 783 patients. Arthritis Res Ther. 17(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozzo M. 2008. Oral diseases associated with hepatitis C virus infection. Part 1. Sialadenitis and salivary glands lymphoma. Oral Dis. 14(2):123–130. [DOI] [PubMed] [Google Scholar]
- Castro Ferreiro M, Hermida Prieto M, Barral Rodriguez S, Laredo Vazquez R, Castro Iglesias A, Diz Dios P. 2002. Whole stimulated salivary flow in patients with chronic hepatitis C virus infection. J Oral Pathol Med. 31(2):117–120. [DOI] [PubMed] [Google Scholar]
- Daniels TE, Cox D, Shiboski CH, Schiødt M, Wu A, Lanfranchi H, Umehara H, Zhao Y, Challacombe S, Lam MY. 2011. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 63(7):2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema T, Terhaard CH, Roesink JM, Raaijmakers CP, van den Keijbus PA, Brand HS, Veerman EC. 2012. MUC5B levels in submandibular gland saliva of patients treated with radiotherapy for head-and-neck cancer: a pilot study. Radiat Oncol. 7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freni MA, Artuso D, Gerken G, Spanti C, Marafioti T, Alessi N, Spadaro A, Ajello A, Ferrau O. 1995. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology. 22(2):389–394. [PubMed] [Google Scholar]
- Giordano N, Amendola A, Papakostas P, Cipolli F, Agate VM, Battisti E, Marchi B, Nuti R. 2005. Immune and autoimmune disorders in HCV chronic liver disease: personal experience and commentary on literature. New Microbiol. 28(4):311–317. [PubMed] [Google Scholar]
- Grossmann Sde M, Teixeira R, Oliveira GC, Gleber-Netto FO, Araujo FM, Araujo FM, Carmo MA. 2010. Xerostomia, hyposalivation and sialadenitis in patients with chronic hepatitis C are not associated with the detection of HCV RNA in saliva or salivary glands. J Clin Pathol. 63(11):1002–1007. [DOI] [PubMed] [Google Scholar]
- Gruener NH, Lechner F, Jung M-C, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 75(12):5550–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J, Deny P, Munz-Gotheil C, Ambrosini JC, Trinchet JC, Pateron D, Mal F, Callard P, Beaugrand M. 1992. Lymphocytic sialadenitis of Sjögren’s syndrome associated with chronic hepatitis C virus liver disease. Lancet. 339(8789):321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, Edlin BR, Mermin J, Ward JW, Ryerson AB. 2019. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology. 69(3):1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al. 2021. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 27(5):892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers L, Webster-Cyriaque J. 2011. Viruses and salivary gland disease (SGD) lessons from HIV SGD. Adv Dent Res. 23(1):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse-Joulin S, Nowak E, Cornec D, Brown J, Carr A, Carotti M, Fisher B, Fradin J, Hocevar A, Jonsson MV, et al. 2017. Salivary gland ultrasound abnormalities in primary Sjögren’s syndrome: consensual US-SG core items definition and reliability. RMD Open. 3(1):e000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, Nieuw Amerongen AV. 2001. Sialometry and sialochemistry: diagnostic tools for Sjögren’s syndrome. Ann Rheum Dis. 60(12):1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairy M, El-Raziky M, El-Akel W, Abdelbary MS, Khatab H, El-Kholy B, Esmat G, Mabrouk M. 2013. Serum autoantibodies positivity prevalence in patients with chronic HCV and impact on pegylated interferon and ribavirin treatment response. Liver Int. 33(10):1504–1509. [DOI] [PubMed] [Google Scholar]
- Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, Kurokawa K, Matsuura Y, Miyamura T. 1997. Sialadenitis histologically resembling Sjögren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci U S A. 94(1):233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu X, Cui J, Song W, Liang Y, Hu Y, Guo Y. 2019. Epidemiological survey of antinuclear antibodies in healthy population and analysis of clinical characteristics of positive population. J Clin Lab Anal. 33(8):e22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loustaud-Ratti V, Riche A, Liozon E, Labrousse F, Soria P, Rogez S, Babany G, Delaire L, Denis F, Vidal E. 2001. Prevalence and characteristics of Sjögren’s syndrome or Sicca syndrome in chronic hepatitis C virus infection: a prospective study. J Rheumatol. 28(10):2245–2251. [PubMed] [Google Scholar]
- Navarta LM, Espul CA, Acosta-Rivero N. 2018. High prevalence of a variety of autoantibodies in a population of hepatitis C virus-infected individuals. APMIS. 126(6):515–522. [DOI] [PubMed] [Google Scholar]
- Navazesh M, Christensen C, Brightman V. 1992. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 71(7):1363–1369. [DOI] [PubMed] [Google Scholar]
- Nawito Z, Amin A, El-Fadl SA, Abu El, Einen K. 2011. Sicca complex among Egyptian patients with chronic hepatitis C virus infection. Clin Rheumatol. 30(10):1299–1304. [DOI] [PubMed] [Google Scholar]
- Pedersen AML, Sorensen CE, Proctor GB, Carpenter GH, Ekstrom J. 2018. Salivary secretion in health and disease. J Oral Rehabil. 45(9):730–746. [DOI] [PubMed] [Google Scholar]
- Rahayuningtyas ED, Setiadhi R. 2019. Virus as a cause of salivary gland diseases. ODONTO Dent J. 6(1):37–42. [Google Scholar]
- Ramos-Casals M, Loustaud-Ratti V, De Vita S, Zeher M, Bosch J-A, Toussirot E, Medina F, Rosas J, Anaya J-M, Font J. 2005. Sjögren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine. 84(2):81–89. [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M, Trejo O, Garcia-Carrasco M, Font J. 2003. Therapeutic management of extrahepatic manifestations in patients with chronic hepatitis C virus infection. Rheumatology (Oxford). 42(7):818–828. [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ. 2017. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 69(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight PM, Kaul A, Melsom RD. 1992. Measurement of whole unstimulated salivary flow in the diagnosis of Sjögren’s syndrome. Ann Rheum Dis. 51(4):499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreebny LM, Valdini A. 1987. Xerostomia. A neglected symptom. Arch Intern Med. 147(7):1333–1337. [DOI] [PubMed] [Google Scholar]
- Theander E, Mandl T. 2014. Primary Sjögren’s syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res (Hoboken). 66(7):1102–1107. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Seeff LB. 2005. Natural history of hepatitis C. Clin Liver Dis. 9(3):383–398, vi. [DOI] [PubMed] [Google Scholar]
- Vitali C, Moutsopoulos HM, Bombardieri S. 1994. The European community study group on diagnostic criteria for Sjögren’s syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren’s syndrome. Ann Rheum Dis. 53(10):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zignego AL, Craxi A. 2008. Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis. 12(3):611–636, ix. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211049395 for HCV Infection Alters Salivary Gland Histology and Saliva Composition by J.O. Maldonado, M.E. Beach, Y. Wang, P. Perez, H. Yin, E. Pelayo, S. Fowler, I. Alevizos, M. Grisius, A.N. Baer, B. Walitt, V. De Giorgi, H.J. Alter, B.M. Warner and J.A. Chiorini in Journal of Dental Research