Abstract
The extracellular matrix (ECM) is a highly dynamic amalgamation of structural and signaling molecules whose quantitative and qualitative modifications drive the distinct programmed morphologic changes required for tissues to mature into their functional forms. The craniofacial complex houses a diverse array of tissues, including sensory organs, glands, and components of the musculoskeletal, neural, and vascular systems, alongside several other highly specialized tissues to form the most complex part of the vertebrate body. Through cell-ECM interactions, the ECM coordinates the cell movements, shape changes, differentiation, gene expression changes, and other behaviors that sculpt developing organs. In this review, we focus on several common key roles of the ECM to shape developing craniofacial organs and tissues. We summarize recent advances in our understanding of the ability of the ECM to biochemically and biomechanically orchestrate major events of craniofacial development, and we discuss how dysregulated ECM dynamics contributes to disease and disorders. As we expand our understanding of organ-specific matrix functionality and composition, we will improve our ability to rationally modify matrices to promote regeneration and/or prevent degenerative outcomes in vitro and in vivo.
Keywords: craniofacial biology, craniofacial genetics, embryology, developmental biology, cell-matrix interactions, matrix biology
Introduction
The extracellular matrix (ECM) is a composite of proteins, polysaccharides, and water that provides critical biochemical and structural support to cells and tissues. The specific balance of these 3 major components is unique to, and reflects the functional requirements of, each tissue type. Craniofacial organs and tissues are intricate and complex structures derived from a variety of embryonic sources, including the cranial neural crest, embryonic ectoderm, and prechordal mesoderm. Throughout craniofacial development, the ECM undergoes a series of quantitative and qualitative changes to cater to the unique requirements of each developing tissue. Integrin- and other transmembrane protein–mediated cell-ECM interactions allow cells to sense and respond to these changes in matrix dynamics by migrating, proliferating, and/or differentiating. These interactions allow embryonic tissues to develop into craniofacial structures such as the teeth, palate, cranium, sensory organs, glands, muscles, and temporomandibular joints.
Craniofacial structures consist of a large variety of tissue types, each with a unique ECM composition reflecting the functional roles of each tissue—ranging from the fibrous interstitial connective tissue matrices of tooth enamel and dentin to the sheet-like specialized basement membranes of salivary glands (Young and Krebsbach 2006; Zhang and Yelick 2018; Moradian-Oldak and George 2021; Fig. 1). Although the matrix of each tissue is unique, common roles of the ECM in organ development and homeostasis have emerged. As depicted in Figure 2, the ECM can serve as a pathway or road for cell migration, a template for mineralization, a microenvironmental niche for differentiation, or a guide for tissue growth, among others, as we highlight in this review. When the ECM fails to serve these roles, the result is often a disease or disorder, as exemplified by the abundance of tooth ECM protein mutations associated with amelogenesis and dentinogenesis imperfecta (Table 1; Yoshizaki and Yamada 2013; Smith et al. 2017).
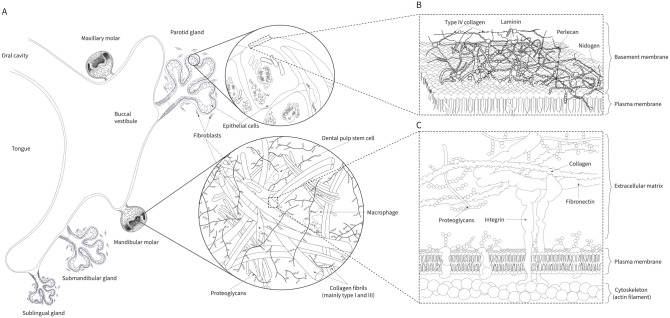
Figure 1.
Extracellular matrices of craniofacial tissues. The examples shown indicate the molecular composition of the loose fibrillar interstitial connective tissue of the dental pulp and the sheet-like basement membrane of the salivary gland. (A) A coronal slice of the developing oral cavity shows the tongue, palate, and developing molars with the parotid, submandibular, and sublingual salivary glands. (B) In contrast to the loose interstitial matrix of the dental pulp, the basement membrane is a dense ultrathin sheet structurally defined by laminin and type IV collagen. These 2 polymeric structural components are connected by proteins such as perlecan, nidogen/entactin, and others. The basement membrane defines developing tissue boundaries and provides plasticity and rigidity to guide growth while protecting tissues from mechanical damage. (C) The pulp connective tissue is composed of collagens, fibronectin, proteoglycans, glycosaminoglycans, and several other noncollagenous proteins. Although type I collagen predominates, type III collagen represents a relatively large proportion of the pulp fibrous matrix. This loose connective tissue houses numerous cell types, including fibroblasts, odontoblasts, various immune defense cells, and stem cells. Integrins and other transmembrane proteins link extracellular matrix proteins to the cellular cytoskeleton and allow cells to respond to extracellular cues.
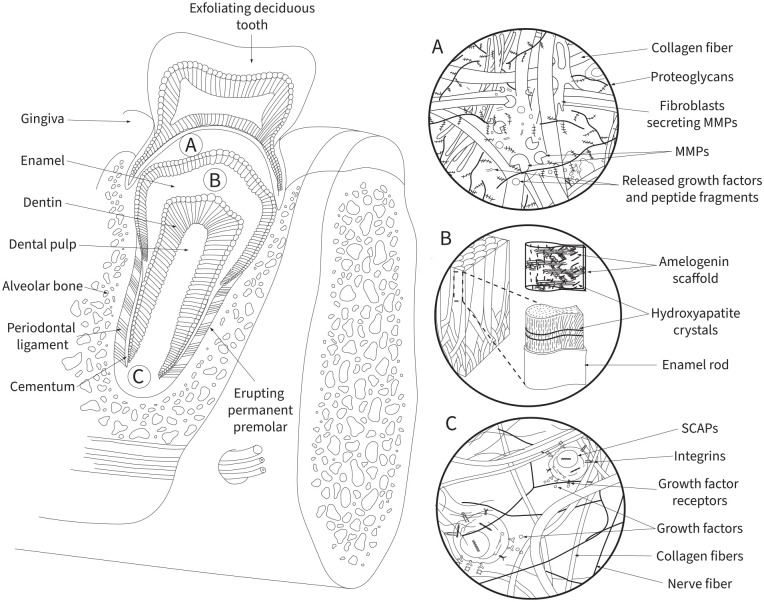
Figure 2.
The extracellular matrix (ECM) in craniofacial development. With the erupting tooth as an example, illustrated here are major roles of the ECM: serving as a mechanical guide or director, controlling cell differentiation/fate specification, and providing a scaffold for developing craniofacial tissues. (A) ECM remodeling is a finely tuned and carefully regulated process crucial for multiple steps of craniofacial development. A balance between matrix proteins such as matrix metalloproteases, the ADAM (a disintegrin and metalloprotease) family, lysyl oxidases, and their inhibitors control the extent of matrix degradation for tissue health. In addition to providing physical space for cell/tissue growth and movement, matrix degradation releases various growth factors and other key molecules for tissue development and homeostasis. (B) Unlike most mineralized tissues in which collagens serve as the main scaffolding proteins, amelogenin guides the hierarchical deposition of apatite crystals during enamel maturation. Self-assembled amyloid-like beta sheet deposits of amelogenin are transiently assembled to provide structural support for enamel mineralization. (C) The human tooth houses various types of stem cells, including those of the apical papilla. The ECM provides mechanical and chemical cues to drive stem cell self-renewal, maintenance, proliferation, and differentiation. Stem cells sense physical cues (e.g., stiffness, elasticity, topography) predominantly via integrins, as well as chemical cues via cell surface receptors and coreceptors (e.g., numerous types of growth factor receptors, frizzled 7/syndecan 4 coreceptor complexes, syndecans). These ECM-driven cues mediate cell anchorage and various intracellular signaling cascades to regulate stem cell dynamics. MMPs, matrix metalloproteases; SCAPs, stem cells of the apical papilla.
Table 1.
Extracellular Matrix Proteins Mutated in Human Craniofacial Diseases.
| Gene | Protein | Phenotype | OMIM |
|---|---|---|---|
| HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein (perlecan) | Schwartz-Jampel syndrome, type I (various myopathies and osteopathies, microcephaly, facial abnormalities) | 255800 |
| Dyssegmental dysplasia, Silverman-Handmaker type (lethal, flat face, micrognathia, cleft palate, encephalocele) | 224410 | ||
| ACAN | Aggrecan core protein | Short stature and advanced bone age, with or without early-onset osteoarthritis and/or osteochondritis dissecans, midface hypoplasia | 165800 |
| Spondyloepimetaphyseal dysplasia, aggrecan type (craniofacial abnormalities included relative macrocephaly, severe midface hypoplasia with almost absent nasal cartilage, relative prognathism, and slightly low-set posteriorly rotated ears) | 612813 | ||
| LAMA3 | Laminin subunit alpha 3 | Laryngoonychocutaneous syndrome (hoarseness, tooth dysmorphia, crusted lesions of face, chronic bleeding; often present with hypoplastic pitted enamel) | 245660 |
| Epidermolysis bullosa (often present with hypoplastic pitted enamel) | 226650 | ||
| LAMB1 | Laminin subunit beta 1 | Lissencephaly 5 (hydrocephalus and other cranial malformations) | 615191 |
| LAMB3 | Laminin subunit beta 3 | Amelogenesis imperfecta, type IA | 104530 |
| Epidermolysis bullosa, junctional, Herlitz type | 226700 | ||
| Epidermolysis bullosa, junctional, non-Herlitz type | 226650 | ||
| COL17A1 | Collagen type XVII, alpha 1 chain | Epidermolysis bullosa, junctional, non-Herlitz type (often present with hypoplastic pitted enamel) | 226650 |
| FBN1 | Fibrillin 1 | Acromicric dysplasia (growth delay and distinct facial dysmorphism) | 102370 |
| FRAS1 | Extracellular matrix organizing protein FRAS1 | Fraser syndrome 1 (missing lacrimal ducts, middle and outer ear malformations, high palate, cleaved nares and tongue, laryngeal stenosis, hypertelorism) | 219000 |
| MGP | Matrix Gla protein | Keutel syndrome (midface hypoplasia and ectopic abnormal calcifications including laryngotracheobronchial calcifications, cerebral calcifications, and others) | 245150 |
| COL7A1 | Collagen type VII, alpha 1 chain | Epidermolysis bullosa, several subtypes (blistering and scarring of skin and mucous membranes) | 120120 |
| COL18A1 | Collagen type XVIII, alpha 1 chain | Knobloch syndrome, type 1 (occipital bone and tissue defects, eye abnormalities) | 267750 |
| LTBP3 | Latent-transforming growth factor beta binding protein 3 | Dental anomalies and short stature (hypoplastic amelogenesis imperfecta, short stature, skeletal dysplasia) | 601216 |
| Geleophysic dysplasia 3 (distinct dysmorphic facial features, short stature, skeletal dysplasia, restricted movements of joints) | 617809 | ||
| LTBP4 | Latent-transforming growth factor beta binding protein 4 | Cutis laxa, autosomal recessive (Urban-Rifkin-Davis syndrome, microretrognathia, midface deficiency, wide fontanelles; disrupted development of craniofacial, gastrointestinal, and several other tissues) | 613177 |
| AMBN | Ameloblastin | Amelogenesis imperfecta, type IF | 616270 |
| AMELX | Amelogenin, X isoform | Amelogenesis imperfecta, type IE | 301200 |
| AMTN | Amelotin | Amelogenesis imperfecta, type IIIB | 617607 |
| ENAM | Enamelin | Amelogenesis imperfecta, type IB | 104500 |
| Amelogenesis imperfecta, type IC | 204650 | ||
| COL1A1 | Collagen type I, alpha 1 chain | Osteogenesis imperfecta, type I (often with dentinogenesis imperfecta) | 166200 |
| Osteogenesis imperfecta, type II (often with dentinogenesis imperfecta) | 166210 | ||
| Osteogenesis imperfecta, type III (often with dentinogenesis imperfecta) | 259420 | ||
| Osteogenesis imperfecta, type IV (often with dentinogenesis imperfecta) | 166220 | ||
| COL1A2 | Collagen type I, alpha 2 chain | Osteogenesis imperfecta, type II (often with dentinogenesis imperfecta) | 166210 |
| Osteogenesis imperfecta, type III (often with dentinogenesis imperfecta) | 259420 | ||
| Osteogenesis imperfecta, type IV (often with dentinogenesis imperfecta) | 166220 | ||
| DMP1 | Dentin matrix acidic phosphoprotein 1 | Hypophosphatemic rickets (presents with dentin defects and craniofacial developmental abnormalities) | 241520 |
| DSPP | Dentin sialophosphoprotein | Dentin dysplasia, type II | 125420 |
| Dentinogenesis imperfecta, type II | 125490 | ||
| Dentinogenesis imperfecta, type III | 125500 | ||
| Deafness, autosomal dominant 39, with dentinogenesis imperfecta type I | 605594 | ||
| BGN | Biglycan | Meester-Loeys syndrome (dysmorphic facial features including frontal bossing, malar hypoplasia, hypertelorism, and proptosis; aneurysms and nonspecific connective tissue abnormalities) | 300989 |
A noncomprehensive list of extracellular matrix proteins mutated in various human craniofacial diseases and disorders.OMIM, Online Mendelian Inheritance in Man.
The advent of novel biochemical, biomechanical, imaging, and other experimental techniques has deepened our understanding of how cells sense and respond to environmental cues during craniofacial morphogenesis. Beyond unveiling the interplay between cells and their extracellular surroundings, these studies reveal potentially targetable molecular pathways and guiding principles for novel therapeutic intervention and regeneration strategies. Structured around the common roles of the ECM serving as a scaffold, an orchestrator of stem cell decision making, and a regulator of mechanics in tissue morphogenesis, this review focuses on very recent advances in our understanding of cell-ECM dynamics in craniofacial development, discusses how dysregulation of ECM composition and structure can lead to disease, and highlights ECM-related targets and biomimetics with therapeutic potential.
ECM Scaffold: Migration and Mineralization
Two fundamental roles of the ECM are to serve as a physical scaffold and to provide biochemical signals to cells. Cells interact with multiple ECM molecules (e.g., collagens, fibronectin, laminins, proteoglycans) to migrate and organize into functional tissues (Hynes and Yamada 2012). Organic ECM fibers serve as scaffolds for the deposition of inorganic compounds during tissue mineralization. As reviewed in the following section, when these elemental roles of the ECM go awry, the outcome is often a human developmental abnormality.
Roads and Traffic Lights for Migration and Tissue Morphogenesis
From the early stages of neural crest formation to the budding of salivary glands and the final positioning of mandibular molar progenitor populations, coordinated cell migration and tissue remodeling are critical for the proper positioning and development of craniofacial structures (Abramyan and Richman 2018). Although extensive research has characterized cell migration and tissue movements during craniofacial development, as in organ formation and palatogenesis (see Appendix for discussion), our understanding of these complex processes continues to be challenged and refined each year. For example, bending of epithelia during salivary gland and tooth development for epithelial invagination is not only associated with the previously described columnar-to-wedge shape changes of cells but is driven by newly identified critical roles acting in combination: 1) the strong adhesion of peripheral cells to the basement membrane ECM through integrins, 2) the low adhesion of these cells to each other via E-cadherin, and 3) the low cell-matrix adhesion with high cell-cell adhesion in the centrally located cells (Li et al. 2020; Wang et al. 2021). Spatiotemporally regulated abundance and distribution of cell-ECM and cell-cell anchoring proteins (e.g., tooth-specific glycoproteins, collagens, laminins, fibronectin, cadherins) are similarly critical traffic lights in subsequent tooth development (e.g., Heymann et al. 2002; Klein et al. 2013; Yoshizaki and Yamada 2013). In a recent example, inner enamel epithelial cells overexpressing E-cadherin and failing to migrate are associated with an enamel hypoplasia phenotype (Chiba et al. 2019; He et al. 2019).
Although our understanding of the biological functions of ECM-cell interaction proteins continues to expand, we are still far from a comprehensive understanding of the intricate ECM-cell interplay underlying proper cranial neural crest migration. For example, even though MAPRE2 is a member of the microtubule plus-end tracking protein family, whose members are well known for regulating microtubule growth and stabilization, it regulates the adhesion of migrating cranial neural crest cells to the roads provided by ECM fibers (Thues et al. 2021). This recently identified role of MAPRE2 may help to explain the craniofacial dysmorphism, cleft palate, and other facial anomalies found in the polymalformative syndromes caused by MAPRE2 mutations (Thues et al. 2021). Novel experimental approaches (e.g., reconstructing the genome-wide human regulatory network of cranial neural crest cells through combining paired gene expression and chromatin accessibility data; Feng et al. 2021) further our knowledge about the genetic landscape of craniofacial diseases and may provide a more comprehensive understanding of the ECM’s roles in regulating this developmentally crucial cell population.
Templates for Mineralization
A complex process of spatiotemporally regulated bone resorption and mineralization is required for craniofacial development. Numerous signaling cascades and feedback loops coordinate the processes of ECM deposition, organization, and resorption required for the development and homeostasis of bone, enamel, and other mineralized tissues of the head/neck. When this balance is lost (e.g., through mutations in the mineralization inhibitor matrix Gla protein or the various enamel and dentin matrix proteins), the results are craniofacial developmental defects (e.g., Keutel syndrome associated with matrix Gla protein mutations [Marulanda et al. 2017; OMIM 245150] and the extensively characterized mutations associated with amelogenesis imperfecta and dentinogenesis imperfecta; Table 1).
Collagen Organization and Cross-linking
As emphasized throughout this review, the fibrous architecture of the ECM serves a variety of roles during development and homeostasis, ranging from offering pathways for cell migration to providing scaffolding for mineralization. The ability to alter matrix protein cross-linking and organization is fundamental to several current dental/medical therapies. Indeed, conventional dental restorative procedures exploit therapeutic tooth ECM cross-linking to bond dental materials to sound tooth structure (Maravic et al. 2021). Accordingly, when the processes involved in producing an ordered cross-linked matrix go awry, development and homeostasis are impaired. For example, conditional knockout of Yap/Taz (transcriptional regulators whose downstream targets include various collagen cross-linking proteins) in the palatal shelf mesenchyme of mice results in failed collagen cross-linking, impaired palatal shelf elevation, and cleft palate (Goodwin et al. 2020), though loss of Yap/Taz could have affected more than collagen cross-linking. Several ECM structural proteins must work in harmony to form an ordered matrix. The importance of this harmonious function in craniofacial development is highlighted by the irregular collagen fiber deposition and aggregation observed after depleting chondroitin sulfate proteoglycans in the craniofacial complex—resulting in impaired connective tissue organization, failed intramembranous ossification of the skull, cleft palate, and severe facial dysmorphism (Ida-Yonemochi et al. 2018).
Enamel Mineralization
As the hardest and most mineralized vertebrate tissue, mature enamel is composed of highly organized crystalline apatite fibers that are several microns long (Fig. 2B). Although it is generally accepted that amelogenin and other ECM proteins are critical for developing enamel’s complex uniaxial organization of fibrous apatite crystals (Lacruz et al. 2017), the normal rapid degradation of organic enamel content to produce the final mineralized tissue has made characterizing this transient developmental process difficult (Bai et al. 2020). The intricate interplay between matrix degradation and development required for dental hard tissue homeostasis has been reviewed elsewhere (e.g., see Margolis et al. 2014). Consequently, we have limited this section to very new concepts and refer readers to the reviews cited earlier. Using mouse models lacking degraders of enamel matrix proteins (e.g., KLK4), state-of-the-art microscopy techniques, in vitro mineralization assays, proteomic assays, and structural modeling, recent reports have characterized amelogenin-ameloblastin interactions in detail to show that full-length amelogenin first self-assembles into amyloid-like structures before forming beta-sheet nanoribbon templates to guide oriented growth of fibrous apatite nanocrystals (Bai et al. 2020; Bapat et al. 2020). Human mutations resulting in either failed production of amelogenin or extracellular secretion of amelogenin disrupt this assembly process and result in the enamel hypoplasia associated with hypoplastic/hypomaturation amelogenesis imperfecta (OMIM 301200; Table 1). Our ever-improving understanding of the roles of ECM in tooth mineralization has led to a surge in successful research developing biomimetic materials capable of mineralizing damaged enamel (Wang et al. 2020).
ECM Niche: Cell Differentiation and Fate Specification
The ECM guides the differentiation and fate specification of numerous cell types throughout craniofacial development and homeostasis. From neural crest cells to oral/maxillofacial stem cells, the ECM serves as a cellular niche providing structural, physical, and biochemical cues to guide cell fate. Significant craniofacial defects result when neural crest cells fail to receive ECM cues mediated by integrins and other cell surface proteins (Leonard and Taneyhill 2020). Focusing on these cell-ECM interactions, investigators are exploiting critical ECM cues to control stem cell dynamics for treatment of oral and maxilla facial disease (e.g., Yang et al. 2021). There are many excellent reviews on the potential for stem cell tissue engineering by controlling stem cell fate (e.g., Tewary et al. 2018), so we will focus on craniofacial stem cells.
Oral and Maxillofacial Stem Cells
The past decade has seen a dramatic expansion of research seeking to exploit the self-renewal and multiple differentiation potential of mesenchymal stem cells (MSCs) for treating oral diseases. The oral and maxillofacial region is replete with sources of MSCs, including MSCs derived from teeth (e.g., dental pulp stem cells, stem cells from the apical papilla, stem cells from human exfoliated deciduous teeth) and MSCs derived from salivary glands and periodontal tissues (e.g., periodontal ligament stem cells, gingiva-derived stem cells, salivary gland–derived stem cells).
Dental Stem Cells
Stem cell maintenance, proliferation, and differentiation are driven by the structural and chemical characteristics of the ECM niche (Fig. 2C). As highlighted in Table 2, the ECM composition of each dental tissue provides unique microenvironmental stimuli to precursor cells. Dental pulp stem cells are becoming promising regenerative tools because—depending on their microenvironmental niche—they not only can contribute to pulp homeostasis and replenishing damaged odontoblasts but can also differentiate into functionally active glial and neural cells (Laudani et al. 2020). This ability to differentiate into multiple tissue types is shared by their counterpart population of stem cells derived from the pulp tissue of exfoliated deciduous teeth (SHED; Xuan et al. 2018). The fate of dental pulp stem cells and SHED can be expanded to include osteogenic potential when the cells are surrounded by an extracellular microenvironment rich in granular hydroxyapatite deposits (Hagar et al. 2021). Most remarkably, a recent randomized controlled clinical trial highlighted the tremendous therapeutic potential of SHED when surrounded by the remnant matrix of trauma-induced necrotic dental pulp. In animal models and recruited humans, the implanted SHED regenerated the dental pulp, reforming vascular and nervous tissues and allowing continued root growth and apex closure (Xuan et al. 2018; see Appendix for discussion). These and similar reports suggest that even though each type of dental-derived stem cell has significant therapeutic potential, each population may be better suited for specific applications in tooth regeneration and beyond.
Table 2.
Extracellular Matrix Proteins of the Human Tooth.
| Tissue: Gene | Protein Name |
|---|---|
| Enamel | |
| AMBN | Ameloblastin |
| AMELX | Amelogenin, X isoform |
| AMELY | Amelogenin, Y isoform |
| AMTN | Amelotin |
| ENAM | Enamelin |
| ODAM (APIN) | Odontogenic ameloblast-associated protein (apin) |
| COL1 | Collagen type 1 |
| COL3A1 | Collagen type III, alpha 1 chain |
| Dentin | |
| COL1 | Collagen type 1 |
| COL3A1 | Collagen type III, alpha 1 chain |
| COL5A1 | Collagen type V, alpha 1 chain |
| BSP (IBSP) | Bone sialoprotein (integrin-binding sialoprotein) |
| DMP1 | Dentin matrix acidic phosphoprotein 1 |
| DSPP | Dentin sialophosphoprotein |
| FBLN7 | Fibulin 7 |
| MEPE | Matrix extracellular phosphoglycoprotein |
| OPN (SPP1) | Osteopontin (secreted phosphoprotein 1) |
| MGP | Matrix Gla protein |
| SPARC | SPARC (osteonectin) |
| BGLAP | Bone Gla protein (osteocalcin) |
| BGN | Biglycan |
| DCN | Decorin |
| FMOD | Fibromodulin |
| LUM | Lumican |
| OMD | Osteomodulin |
| AMELX | Amelogenin, X isoform |
| AMBN | Ameloblastin |
| EMILIN1, 2, and 3 | Emilin 1, 2, and 3 |
| Cementum | |
| COL1 | Collagen type 1 |
| BSP (IBSP) | Bone sialoprotein (integrin binding sialoprotein) |
| OPN (SPP1) | Osteopontin (secreted phosphoprotein 1) |
| BGLAP | Bone Gla protein (osteocalcin) |
| AHSG | Alpha-2-HS-glycoprotein |
| FMOD | Fibromodulin |
| LUM | Lumican |
| BGN | Biglycan |
| DCN | Decorin |
| VCAN | Versican |
| OMD | Osteomodulin |
| MGP | Matrix Gla protein |
| PTPLA/CAP | Protein-tyrosine phosphatase-like member A / cementum-attachment protein |
| Pulp | |
| COL1 | Collagen type 1 |
| COL3A1 | Collagen type III, alpha 1 chain |
| COL5A1 | Collagen type V, alpha 1 chain |
| COL6A1 | Collagen type VI, alpha 1 chain |
| FN1 | Fibronectin |
| CSPG4 | Chondroitin sulfate proteoglycan 4 |
| CSPG6 (SMC-3) | Chondroitin sulfate proteoglycan 6 (structural maintenance of chromosomes protein 3) |
| BGN | Biglycan |
| DCN | Decorin |
| VCAN | Versican |
| OPN (SPP1) | Osteopontin (secreted phosphoprotein 1) |
| BSP (IBSP) | Bone sialoprotein (integrin-binding sialoprotein) |
| EMILIN1, 2, and 3 | Emilin 1, 2, and 3 |
A noncomprehensive list of extracellular matrix proteins found in human teeth.
Stem Cells from Salivary Glands and Periodontal Tissues
As for dental stem cells, the ECM provides structural and biochemical inputs to guide the behavior of salivary gland– and periodontal tissue–derived stem cells. Stem cells derived from the periodontal ligament have gained interest due to their potential to regenerate mineralized and nonmineralized tissues when the ECM microenvironment provides calcium phosphate and growth factors to regenerate dental cementum—a promising finding for potentially developing more comprehensive therapy for the tooth-periodontal complex (Park et al. 2020). Remarkably, the cultured gingival fibroblasts derived from gingival tissues harbor a unique population of gingival MSCs that have an even more diverse potential for differentiation and therapeutic application that can be guided by providing appropriate extracellular scaffolds and signals. Indeed, gingival fibroblasts derived from gingival tissues with their associated gingival MSCs can restore 1) periodontal defects when provided with a collagen scaffold (Abdal-Wahab et al. 2020), 2) calvarial bony defects when provided with degradable polylactide scaffolds mimicking the mechanical properties of the bony matrix (Diomede et al. 2018), and 3) tongue defects when provided with a decellularized xenograft material (Zhang et al. 2019).
Beyond the tooth complex, salivary gland–derived stem cells may become an attractive therapeutic approach to replace functional salivary gland tissue. As ongoing research defines the physical and biochemical extracellular cues best suited to guide salivary gland stem cells to develop into functional gland tissue, we become closer than ever before to potentially adding stem cell transplantation to the list of xerostomia treatments alongside saliva substitutes and stimulants. By creating extracellular structures mimicking the properties of salivary glands—physical (e.g., fiber stiffness, density, topography) and biochemical (e.g., availability of FGF10)—several groups have reported successful production of functional salivary gland tissue and are even exploring the feasibility of human transplantation (Sui et al. 2020).
The ability of ECMs to control cell fate extends beyond multipotent stem cell populations. ECM mechanical cues, collagen compositions, and signaling gradients play critical roles in palate formation. For example, matrix collagen VI content and stiffness maintain mesenchymal cell differentiation in tooth bud formation (Mammoto et al. 2015), and ECM mechanical stretch and composition can promote calvarial osteoblast viability and differentiation in craniofacial suture remodeling (Liang et al. 2021).
Mechanical Regulation of Craniofacial Development
Our understanding about how the ECM mechanically orchestrates craniofacial development has been deepened in recent years by methodologies for delivering stem cells to damaged orofacial tissues: electrostatic microdroplet-generated hydrogel microspheres (Yang et al. 2021), finite element modeling of oral cavity forces (Brunt et al. 2015), measuring the 3-dimensional space distribution of forces in living tissues with magnetic fields (Zhu, Tao, et al. 2020), and other novel biomechanical tissue engineering and analysis techniques. The diverse biophysical and mechanical roles of the ECM in craniofacial development are reviewed in turn—from generating forces to forming impenetrable barriers.
Generating Forces
Changes in ECM composition and distribution can produce forces that drive tissue movements during development. For example, a major component of the palatal shelf ECM is the glycosaminoglycan hyaluronic acid (HA; Lan et al. 2019). During palatal shelf elevation, HA hydration-driven ECM expansion creates a pressure gradient to drive elevation of the palatal shelves. Deletion of the gene encoding HA synthase 2 in the palatal mesenchyme results in failed palatal shelf elevation and fusion (Lan et al. 2019). HA hydration and the associated increase in ECM-generated pressure gradients are similarly required for proper tongue and mandible morphogenesis (Lan et al. 2019; Yonemitsu et al. 2020). These spatiotemporal changes in ECM-generated pressure gradients orchestrate tissue movements for posterior displacement of the tongue, elongation of the mandibular arch, and elevation of the palatal shelves.
Turning to the cranium, pressure gradients caused by brain enlargement deform the ECM of the dura mater, creating tensile forces that are then transmitted to the bones and sutures of the cranium (Moazen et al. 2016; Marghoub et al. 2018). This increase in suture tension stimulates several biochemical signaling processes (e.g., growth factor and BMP signaling; Ikegame et al. 2016) in the suture mesenchyme that lead to cell proliferation, differentiation, and suture fusion. Indeed, abnormally reduced intracranial pressure and the associated reduction in cranial-ECM tension can result in premature suture fusion (craniosynostosis) and resulting microcephaly (Park and Yoon 2017).
Removing to Improve
Throughout development and homeostasis, the ECM is dynamically remodeled to permit tissue growth and oriented movement. This crucial process requires the balanced spatiotemporal expression of enzymes capable of degrading matrix proteins (e.g., MMPs [matrix metalloprotease], ADAMs [a disintegrin and metalloprotease], and ADAMTS [a disintegrin and metalloprotease with thrombospondin motifs]) as well as TIMPs (tissue inhibitor of metalloprotease; Hynes and Yamada 2012; Bonnans et al. 2014). Failure of this coordinated matrix degradation can result in severe developmental defects. For example, a combination of aberrant dentin formation and mineralization, alveolar bone remodeling, and periodontal ligament formation caused by loss of membrane-type matrix metalloproteinase 1 activity in the dental mesenchyme of developing teeth results in failed root formation and tooth eruption (Xu et al. 2016).
Recent reports have extended beyond characterizing the physical results of ECM remodeling during odontogenesis to scrutinize the roles of the various bioactive molecules that are released upon matrix degradation. For example, digested dentin matrix components released during dentin degradation stimulate tertiary (reparative) dentin formation to facilitate wound healing of the dentin-pulp complex (Okamoto et al. 2018). Furthermore, dentin sialoprotein (DSP) in the dentin matrix was recently reported to be a substrate of MMP9, and such DSP processing is critical for dentinogenesis—MMP9 knockout results in a dentinogenesis imperfecta–like phenotype (Yuan et al. 2017; Gou et al. 2020). Returning to palate formation, not only are HA-driven increases in ECM volume required but so is coordinated ECM remodeling to produce the forces needed to elevate the palatal shelves. Reduced collagen protein expression and cross-linking due to altered lysyl-oxidase-like 3 and 4 (LOXL3, 4) activity are associated with delayed or failed palatal shelf elevation (Zhang et al. 2015; Goodwin et al. 2020).
Directing Growth
As highlighted here, the ECM can serve as a physical barrier to restrict and guide cell/tissue movements. Not only temporal variation (as discussed in Removing to Improve) but also spatial differences in ECM stiffness and composition guide tissue growth. For example, comparatively higher ECM stiffness and fibronectin content in the distal versus medial portions of the developing first pharyngeal arch limit cell rearrangement, provide directionality to cell migration, and drive arch elongation (Zhu, Zhang, et al. 2020).
The ECM continues to direct growth as the derivatives of the first pharyngeal arch develop. For example, the bony matrix that envelopes developing multicuspid teeth determines the amount of intercuspal spacing and cusp alignment through physically restricting cusp growth (Renvoise et al. 2017). Moreover, a tenascin meshwork surrounds and aligns with actin bundles of the cells in the middle and posterior palatal shelf mesenchyme during palatal shelf elevation. This combination of oriented ECM and cellular cytoskeleton drives directional actin-dependent cell contractility and tissue movements during palate elevation (Chiquet et al. 2016). Proliferating mesenchymal cells of the fusing calvarial suture similarly respond to oriented extracellular tensile forces to direct growth of the expanding skull (Ikegame et al. 2019).
The studies reviewed here demonstrate how the ECM provides spatiotemporally regulated forces of varying types and magnitudes to drive growth and development of the craniofacial complex. Future research merging genetic, biochemical, and biomechanical approaches to understand the mechanical roles of ECM in controlling head and neck development promise to allow us to apply increasingly sophisticated mechanical manipulations and novel bioengineering platforms to therapeutically regenerate craniofacial tissues.
Conclusions and Perspectives
The craniofacial complex is composed of a variety of distinct tissue types, and appropriate development of each unique structure requires tight control of ECM degradation, assembly, modification, and signaling. In this review, we explore how the physical and biochemical properties of the ECM guide cell fate/differentiation, mineralization, cell/tissue migration, and tissue growth. ECMs can serve conceptually similar roles controlling tissue development and homeostasis, as well as organ-specific mechanisms. Many questions remain unanswered, providing rich opportunities for future research and therapy (e.g., see Oliver et al. 2021).
How the ECM regulates the dental stem cell niche needs extensive characterization. We have seen that dental pulp stem cells can regenerate odontoblasts, vascular tissue, and nervous tissue when implanted into trauma-induced necrotic human pulp tissue (Xuan et al. 2018). Although these studies successfully regenerated nervous tissue and the teeth responded to electric pulp testing, are these regenerated pulpal tissues capable of responding to thermal stimuli in a manner similar to virgin pulp? If not, why not? At present, regenerative studies involve traumatized single-rooted incisors. Can these studies be replicated in multirooted molars? Which components of the pulp matrix must remain for such implantation to regenerate necrotic pulp successfully? Answers to questions such as these may allow us to eventually regenerate healthy functional pulp from caries-induced necrotic pulp tissue.
Exactly how do amelogenin and ameloblastin interact structurally in vivo to template complex patterns of enamel mineralization? How do crystallization mechanisms differ at each stage of enamel mineralization? Answers to these and similar questions are crucial as we continue to develop biomaterials to remineralize damaged tooth structures. Furthermore, how do products of dentin matrix degradation stimulate new matrix deposition, and can these molecules be produced synthetically for potential therapeutic application?
More generally, what feedback mechanisms modulate the extent of matrix degradation as well as the direction and magnitude of ECM-provided forces to achieve appropriate cellular and tissue-level changes during craniofacial development? Though well appreciated in certain developmental events (e.g., palate elevation, as discussed earlier), how mechanical forces help guide normal craniofacial development requires deeper investigation. Organotypic 3-dimensional tissue culture models incorporating native ECM and ongoing genetic studies are improving our understanding of ECM dynamics in development. Nevertheless, we must continue to develop novel methods to investigate ECM dynamics in vivo to make strides in understanding diseased ECM states and to uncover new targets and methods for future therapy.
Author Contributions
D.A. Cruz Walma, K.M. Yamada, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211052982 for Extracellular Matrix in Human Craniofacial Development by D.A. Cruz Walma and K.M. Yamada in Journal of Dental Research
Acknowledgments
We apologize for not citing numerous publications to remain within reference limits and to focus on very recent publications.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research in the authors’ laboratory is supported by the Intramural Program of the National Institutes of Health, National Institute of Dental and Craniofacial Research, ZIA DE000524 and ZIA DE000525.
ORCID iDs: D.A. Cruz Walma  https://orcid.org/0000-0002-7781-7620
https://orcid.org/0000-0002-7781-7620
K.M. Yamada  https://orcid.org/0000-0003-1512-6805
https://orcid.org/0000-0003-1512-6805
References
- Abdal-Wahab M, Abdel Ghaffar KA, Ezzatt OM, Hassan AAA, El Ansary MMS, Gamal AY. 2020. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial). J Periodontal Res. 55(3):441–452. [DOI] [PubMed] [Google Scholar]
- Abramyan J, Richman JM. 2018. Craniofacial development: discoveries made in the chicken embryo. Int J Dev Biol. 62(1–3):97–107. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yu Z, Ackerman L, Zhang Y, Bonde J, Li W, Cheng Y, Habelitz S. 2020. Protein nanoribbons template enamel mineralization. Proc Natl Acad Sci U S A. 117(32):19201–19208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat RA, Su J, Moradian-Oldak J. 2020. Co-immunoprecipitation reveals interactions between amelogenin and ameloblastin via their self-assembly domains. Front Physiol. 11:622086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z. 2014. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 15(12):786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt LH, Norton JL, Bright JA, Rayfield EJ, Hammond CL. 2015. Finite element modelling predicts changes in joint shape and cell behaviour due to loss of muscle strain in jaw development. J Biomech. 48(12):3112–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, He B, Yoshizaki K, Rhodes C, Ishijima M, Bleck CKE, Stempinski E, Chu EY, Nakamura T, Iwamoto T, et al. 2019. The transcription factor amelod stimulates epithelial cell motility essential for tooth morphology.J Biol Chem. 294(10):3406–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Blumer S, Angelini M, Mitsiadis TA, Katsaros C. 2016. Mesenchymal remodeling during palatal shelf elevation revealed by extracellular matrix and F-actin expression patterns. Front Physiol. 7:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A, Nanci A, et al. 2018. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther. 9(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Duren Z, Xiong Z, Wang S, Liu F, Wong WH, Wang Y. 2021. hReg-CNCC reconstructs a regulatory network in human cranial neural crest cells and annotates variants in a developmental context. Commun Biol. 4(1):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AF, Chen CP, Vo NT, Bush JO, Klein OD. 2020. YAP/TAZ regulate elevation and bone formation of the mouse secondary palate. J Dent Res. 99(12):1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X, Xue Y, Zheng H, Yang G, Chen S, Chen Z, Yuan G. 2020. Gelatinases cleave dentin sialoprotein intracellularly. Front Physiol. 11:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar MN, Yazid F, Luchman NA, Ariffin SHZ, Wahab RMA. 2021. Comparative evaluation of osteogenic differentiation potential of stem cells derived from dental pulp and exfoliated deciduous teeth cultured over granular hydroxyapatite based scaffold. BMC Oral Health. 21(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Chiba Y, Li H, de Vega S, Tanaka K, Yoshizaki K, Ishijima M, Yuasa K, Ishikawa M, Rhodes C, et al. 2019. Identification of the novel tooth-specific transcription factor amelod. J Dent Res. 98(2):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann R, About I, Lendahl U, Franquin JC, Obrink B, Mitsiadis TA. 2002. E- and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am J Pathol. 160(6):2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Yamada KM. 2012. Extracellular matrix biology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [Google Scholar]
- Ida-Yonemochi H, Morita W, Sugiura N, Kawakami R, Morioka Y, Takeuchi Y, Sato T, Shibata S, Watanabe H, Imamura T, et al. 2018. Craniofacial abnormality with skeletal dysplasia in mice lacking chondroitin sulfate N-acetylgalactosaminyltransferase-1. Sci Rep. 8(1):17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame M, Ejiri S, Okamura H. 2019. Expression of non-collagenous bone matrix proteins in osteoblasts stimulated by mechanical stretching in the cranial suture of neonatal mice. J Histochem Cytochem. 67(2):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame M, Tabuchi Y, Furusawa Y, Kawai M, Hattori A, Kondo T, Yamamoto T. 2016. Tensile stress stimulates the expression of osteogenic cytokines/growth factors and matricellular proteins in the mouse cranial suture at the site of osteoblast differentiation. Biomed Res. 37(2):117–126. [DOI] [PubMed] [Google Scholar]
- Klein OD, Oberoi S, Huysseune A, Hovorakova M, Peterka M, Peterkova R. 2013. Developmental disorders of the dentition: an update. Am J Med Genet C Semin Med Genet. 163C(4):318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Habelitz S, Wright JT, Paine ML. 2017. Dental enamel formation and implications for oral health and disease. Physiol Rev. 97(3):939–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Qin C, Jiang R. 2019. Requirement of hyaluronan synthase-2 in craniofacial and palate development. J Dent Res. 98(12):1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudani S, La Cognata V, Iemmolo R, Bonaventura G, Villaggio G, Saccone S, Barcellona ML, Cavallaro S, Sinatra F. 2020. Effect of a bone marrow–derived extracellular matrix on cell adhesion and neural induction of dental pulp stem cells. Front Cell Dev Biol. 8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CE, Taneyhill LA. 2020. The road best traveled: neural crest migration upon the extracellular matrix. Semin Cell Dev Biol. 100:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Economou AD, Vacca B, Green JBA. 2020. Epithelial invagination by a vertical telescoping cell movement in mammalian salivary glands and teeth. Nat Commun. 11(1):2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Ding P, Li G, Lu E, Zhao Z. 2021. Hydroxyapatite nanoparticles facilitate osteoblast differentiation and bone formation within sagittal suture during expansion in rats. Drug Des Devel Ther. 15:905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, Mammoto A, Jiang A, Jiang E, Hashmi B, Ingber DE. 2015. Mesenchymal condensation-dependent accumulation of collagen vi stabilizes organ-specific cell fates during embryonic tooth formation. Dev Dyn. 244(6):713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravic T, Mancuso E, Comba A, Checchi V, Generali L, Mazzitelli C, Josic U, Hass V, Reis A, Loguercio AD, et al. 2021. Dentin cross-linking effect of carbodiimide after 5 years. J Dent Res. 100(10):1090–1098. [DOI] [PubMed] [Google Scholar]
- Marghoub A, Libby J, Babbs C, Pauws E, Fagan MJ, Moazen M. 2018. Predicting calvarial growth in normal and craniosynostotic mice using a computational approach. J Anat. 232(3):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HC, Kwak SY, Yamazaki H. 2014. Role of mineralization inhibitors in the regulation of hard tissue biomineralization: relevance to initial enamel formation and maturation. Front Physiol. 5:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marulanda J, Eimar H, McKee MD, Berkvens M, Nelea V, Roman H, Borras T, Tamimi F, Ferron M, Murshed M. 2017. Matrix Gla protein deficiency impairs nasal septum growth, causing midface hypoplasia. J Biol Chem. 292(27):11400–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazen M, Alazmani A, Rafferty K, Liu ZJ, Gustafson J, Cunningham ML, Fagan MJ, Herring SW. 2016. Intracranial pressure changes during mouse development. J Biomech. 49(1):123–126. [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak J, George A. 2021. Biomineralization of enamel and dentin mediated by matrix proteins. J Dent Res. 100(10):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Takahashi Y, Komichi S, Cooper PR, Hayashi M. 2018. Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Sci Rep. 8(1):10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, Jia S, Halpern LR, Graham EM, Turner EC, Colombo JS, Grainger DW, D’Souza RN. 2021. Innovative molecular and cellular therapeutics in cleft palate tissue engineering. Tissue Eng Part B Rev. 27(3):215–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DH, Yoon SH. 2017. The total calvarial transsutural distraction osteogenesis for 26 children with slit ventricle, craniosynostosis, or microcephaly after shunt operation. World Neurosurg. 97:701–709.e1. [DOI] [PubMed] [Google Scholar]
- Park JY, Park CH, Yi T, Kim SN, Iwata T, Yun JH. 2020. rhBMP-2 pre-treated human periodontal ligament stem cell sheets regenerate a mineralized layer mimicking dental cementum. Int J Mol Sci. 21(11):3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvoise E, Kavanagh KD, Lazzari V, Hakkinen TJ, Rice R, Pantalacci S, Salazar-Ciudad I, Jernvall J. 2017. Mechanical constraint from growing jaw facilitates mammalian dental diversity. Proc Natl Acad Sci U S A. 114(35):9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CEL, Poulter JA, Antanaviciute A, Kirkham J, Brookes SJ, Inglehearn CF, Mighell AJ. 2017. Amelogenesis imperfecta; genes, proteins, and pathways. Front Physiol. 8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Zhang S, Li Y, Zhang X, Hu W, Feng Y, Xiong J, Zhang Y, Wei S. 2020. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res Ther. 11(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewary M, Shakiba N, Zandstra PW. 2018. Stem cell bioengineering: building from stem cell biology. Nat Rev Genet. 19(10):595–614. [DOI] [PubMed] [Google Scholar]
- Thues C, Valadas JS, Deaulmerie L, Geens A, Chouhan AK, Duran-Romana R, Schymkowitz J, Rousseau F, Bartusel M, Rehimi R, et al. 2021. MAPRE2 mutations result in altered human cranial neural crest migration, underlying craniofacial malformations in CSC-KT syndrome. Sci Rep. 11(1):4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Matsumoto K, Lish SR, Cartagena-Rivera AX, Yamada KM. 2021. Budding epithelial morphogenesis driven by cell-matrix versus cell-cell adhesion. Cell. 184(14):3702–3716.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hu D, Cui J, Zeng Y, Gan X, Chen Z, Ren Q, Zhang L. 2020. Unraveling the mechanism for an amelogenin-derived peptide regulated hydroxyapatite mineralization via specific functional domain identification. J Mater Chem B. 8(45):10373–10383. [DOI] [PubMed] [Google Scholar]
- Xu H, Snider TN, Wimer HF, Yamada SS, Yang T, Holmbeck K, Foster BL. 2016. Multiple essential MT1-MMP functions in tooth root formation, dentinogenesis, and tooth eruption. Matrix Biol. 52–54:266–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, et al. 2018. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 10(455): eaaf3227. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang Q, Xie L, Zhang R, Qian R, Tian Y, Chen G, Tian W. 2021. hDPSC-laden GelMA microspheres fabricated using electrostatic microdroplet method for endodontic regeneration. Mater Sci Eng C Mater Biol Appl. 121:111850. [DOI] [PubMed] [Google Scholar]
- Yonemitsu MA, Lin TY, Yu K. 2020. Hyaluronic acid is required for palatal shelf movement and its interaction with the tongue during palatal shelf elevation. Dev Biol. 457(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Yamada Y. 2013. Gene evolution and functions of extracellular matrix proteins in teeth. Orthod Waves. 72(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF, Krebsbach PH. 2006. Extra-cellular matrix in the craniofacial complex. Basel (Switzerland): S. Karger. [Google Scholar]
- Yuan G, Chen L, Feng J, Yang G, Ni Q, Xu X, Wan C, Lindsey M, Donly KJ, MacDougall M, et al. 2017. Dentin sialoprotein is a novel substrate of matrix metalloproteinase 9 in vitro and in vivo. Sci Rep. 7:42449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang R, Liu Z, Hou C, Zong W, Zhang A, Sun X, Gao J. 2015. Loss of lysyl oxidase-like 3 causes cleft palate and spinal deformity in mice. Hum Mol Genet. 24(21):6174–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yelick PC. 2018. Craniofacial tissue engineering. Cold Spring Harb Perspect Med. 8(1):a025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shi S, Xu Q, Zhang Q, Shanti RM, Le AD. 2019. SIS-ECM laden with GMSC-derived exosomes promote taste bud regeneration. J Dent Res. 98(2):225–233. [DOI] [PubMed] [Google Scholar]
- Zhu M, Tao H, Samani M, Luo M, Wang X, Hopyan S, Sun Y. 2020. Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. Proc Natl Acad Sci U S A. 117(9):4781–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Zhang K, Tao H, Hopyan S, Sun Y. 2020. Magnetic micromanipulation for in vivo measurement of stiffness heterogeneity and anisotropy in the mouse mandibular arch. Research. 2020:7914074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211052982 for Extracellular Matrix in Human Craniofacial Development by D.A. Cruz Walma and K.M. Yamada in Journal of Dental Research