Among infants with acute lymphoblastic leukemia (ALL), approximately 70-75% have KMT2A rearrangement (KMT2A-r) and 25-30% have KMT2A-germline (KMT2A-g) leukemia. Event-free (EFS) and overall survival (OS) for KMT2A-g infant ALL are significantly better than those of KMT2A-r infant ALL, but inferior to outcomes in older children with ALL. Aside from the absence of KMT2A-r, the well-defined prognostic factors in older children with B-ALL (age, initial white blood cell [WBC] count, cytogenetics) are not clearly established, as KMT2A-g infant ALL accounts for only ~1% of childhood ALL. Pediatric cooperative group trials conducted between 1996 and 2016 shown that the 4-6-year EFS/OS for infants with KMT2A-g ALL have ranged from 60-74% and 75-87%, respectively.1-4 Although the Japanese Infant Leukemia Study Group and the Japanese Pediatric Leukemia/Lymphoma Study Group reported remarkable 5-year EFS of 96% and 93% in KMT2A-g infant ALL in MLL96/98 and MLL-10, respectively, the cohort sizes were small, the sex ratios were skewed and the results have not been replicated in other cooperative groups.5-7 Children’s Oncology Group (COG) AALL0631 (clinicaltrials gov. Identifier: NCT00557193) was a phase III clinical trial for infants with newly diagnosed ALL with or without KMT2A-r.8 The trial was approved by Institutional Review Boards at participating COG member institutions and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the parents or guardians according to federal and local regulations. The primary aim of AALL0631 was to test the safety and efficacy of the addition of the FLT3 inhibitor, lestaurtinib, to chemotherapy for infants with KMT2A-r.8 Infants with KMT2A-g were treated on a chemotherapy only arm, with a modified Interfant-99 based induction3,9 followed by modified COG P94079 therapy, with continuation therapy extending to 2 years from diagnosis (as compared to 46 weeks in the predecessor P9407 trial). The trial enrolled 64 infants with KMT2A-g and resulted in survival outcomes superior to those reported for infants with KMT2A-g in all prior COG and Interfant trials.
Infants less than 366 days of age with a new diagnosis of B- or T-cell lineage ALL, acute undifferentiated leukemia (AUL), or mixed phenotype acute leukemia (MPAL) with predominantly lymphoid morphology and immunophenotype were eligible to enroll. Neonates less than 4 weeks old and at least 36 weeks gestational age at the time of diagnosis were eligible. Patients with Down syndrome, mature B-cell leukemia, acute myeloid leukemia, or who had received prior anti-leukemic therapy (with the exception of limited corticosteroids or intrathecal chemotherapy) were ineligible. All patients had karyotypes and fluorescence in situ hybridization (FISH) to determine KMT2A status performed in COGapproved laboratories, with central review of results (AJC and NAH). Informative KMT2A FISH data were required to continue on AALL0631 post-induction therapy.
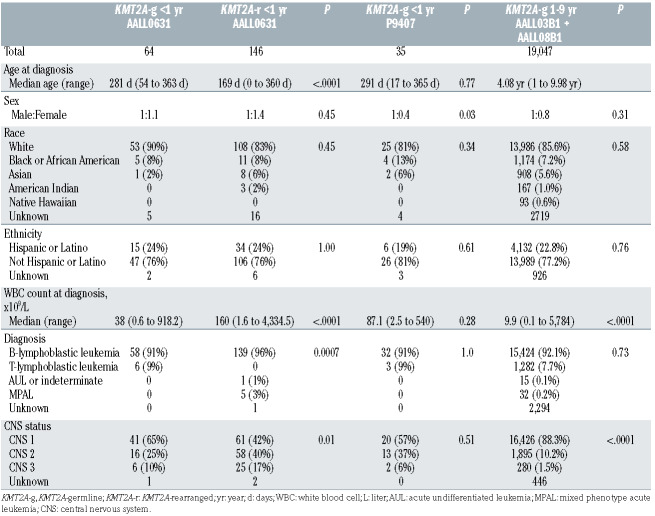
Table 1.
Patient characteristics.
AALL0631 opened to accrual in January 2008 and the original COG P9407-based induction regimen (cohort 1) resulted in excessive toxic mortality due to infections (4 of 26 patients, 15.4%).9 The study was temporarily closed to accrual in November 2008 and amended to substitute an Interfant-99 based induction3 with additional supportive care guidelines (cohort 2). This led to significantly less induction mortality and maintained complete remission rates.9 Post-induction, infants with KMT2A-g were non-randomly assigned to the Standard Risk (SR) arm (Online Supplementary Table S1). The study met accrual goals and closed to enrollment in June 2014.
Data as of March 31, 2019, are included in this report. Median follow-up was 6.3 years. EFS was defined as the time from study entry to first event (treatment failure, relapse, second malignant neoplasm [SMN], or death) or censored at date of last contact. OS was defined as the time from study entry to death or censored at last contact. Estimates of EFS and OS were calculated using the Kaplan-Meier method with standard errors (SE) using Peto’s formula.10,11 Two-sided log-rank tests were used to compare survival between curves. Fisher’s exact tests were used to compare proportions and Wilcoxon ranksum tests were used to compare distributions of continuous measures. Statistical significance was defined as Pvalue less than 0.05.
AALL0631 enrolled 210 eligible patients, including 64 (30.5%) with KMT2A-g (4 in cohort 1 and 60 in cohort 2). Patient characteristics were compared to infants with KMT2A-r in AALL0631, infants with KMT2A-g in P9407, and children age 1-9 years with KMT2A-g ALL and without Down Syndrome or Philadelphia chromosome-positive ALL, enrolled on the COG ALL classification studies AALL03B1 and AALL08B1 from March 1, 2004 to July 20, 2018, using frozen data from December 31, 2020 (Table 1). Notable differences in comparison to infants with KMT2A-r include older age and lower WBC count at diagnosis. Among infants with KMT2A-g, the proportion of females was higher in AALL0631 than in P9407. Central nervous system (CNS) leukemia was more common in infants than in children age 1-9 years with KMT2A-g, but less frequent than in infants with KMT2Ar (Table 2). The cytogenetic findings for infants with KMT2A-g are listed in the Online Supplementary Table S2.
Among 64 infants with KMT2A-g, 62 were evaluable for morphologic remission at the end of induction (week 6). One patient was removed from protocol prior to postinduction evaluation of remission due to withdrawal of consent and one did not have bone marrow morphology evaluated due to an administrative error. Of the 62 patients with marrow assessed, 55 (89%) achieved remission and seven (11%) did not achieve remission (all had ≥5% marrow blasts). Four patients went off protocol prior to post-induction therapy, one each for: withdrawal of consent, physician preference, family preference, and severe adverse event (cerebral edema). All remaining 60 patients (100%) achieved remission by the end of induction intensification (week 10). There were no treatment failure events.
The 5-year EFS (±SE) was 87.3 ± 4.7% and 5-year OS (±SE) was 93.6 ± 3.5% for infants with KMT2A-g. There were no deaths as first events. Eight infants relapsed (5 bone marrow and 3 isolated CNS). All relapses occurred within the first 3 years after diagnosis; five during continuation chemotherapy and three within 12 months after completion of continuation therapy. One infant developed a SMN (mucoepidermoid carcinoma) during the 5- year follow-up period after the completion of chemotherapy. The relapse pattern was similar to that of P9407, which recorded five relapses, two marrow and three isolated extramedullary (1 subcutaneous, 1 CNS, 1 testicular), in 35 infants with KMT2A-g.
Table 2.
Univariate analysis of prognostic factors in infants with KMT2A-g leukemia.
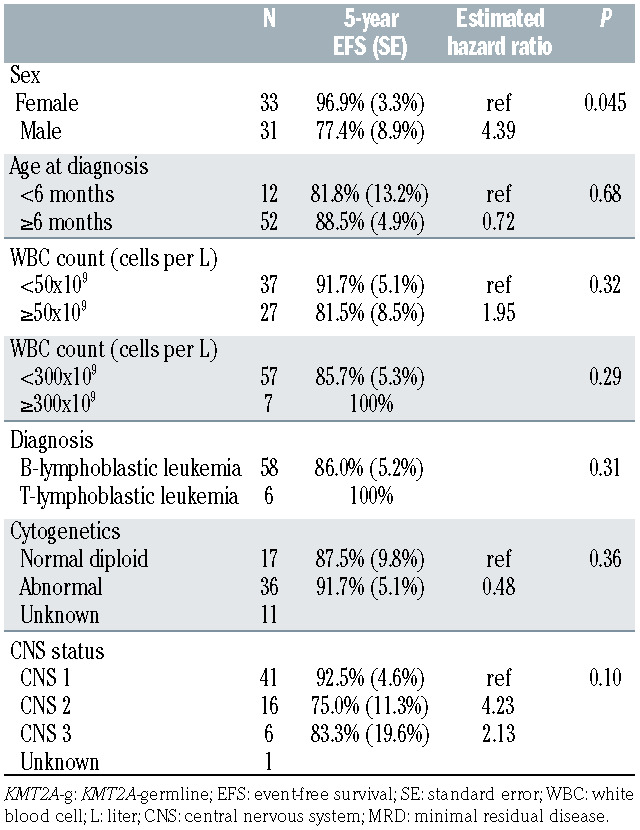
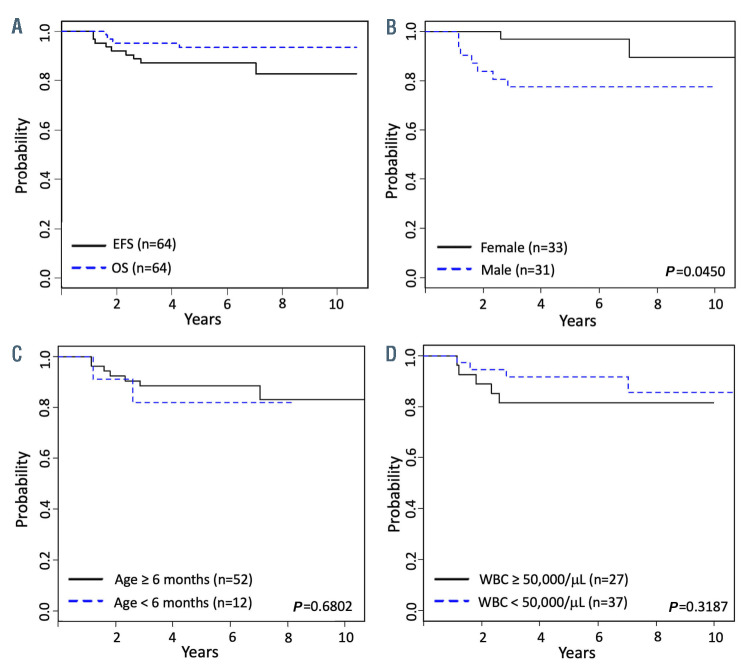
The Kaplan-Meier survival curves for OS and EFS for the overall cohort and EFS curves for subgroups by sex, age < or ≥6 months at diagnosis, and WBC count < or ≥50,000 cells/μL at diagnosis are shown in Figure 1. The 5-year EFS among girls was superior to that of boys (96.9 ± 3.3% vs. 77.4 ± 8.9%, P=0.045; estimated hazard ratio: 4.4). In univariate analyses, age <6 months, WBC count ≥50,000 cells/μL, WBC count ≥300,000 cells/μL, B-cell vs. T-cell phenotype, normal diploid vs. abnormal cytogenetics, and CNS classification were not prognostic of 5-year EFS (Table 2).
In cases with karyotypic data (n=53), the most frequent recurrent cytogenetic abnormalities involved chromosome 9p (10 patients, 19%) and t(1;19)(q23;p13.3) or 19p13.3 variant (5 patients, 9%). The recurrent chromosome abnormality dic(9;20)(p13.2;q11) was identified in five of 53 cases (9%). Translocation of chromosome 5p15 with chromosome 15 was observed in two cases. Abnormalities of chromosome bands 15q11-15 have previously been identified in cases of infant ALL, occur in 1% of pediatric ALL overall, and may indicate a favorable prognosis, if NUTM1 fusion is involved.12-14 Molecular studies for PAX5 and NUTM1 rearrangements were not performed in AALL0631, and the prognostic significance of the cytogenetic findings in KMT2A-g cases could not be determined, given the sample size and rarity of events.
Figure 1.
Kaplan-Meier survival curves for infants with KMT2A-germline leukemia. (A) Event-free survival (EFS) and overall survival (OS) for the standard risk group, KMT2A-germline. (B) EFS was lower for males vs. females. (C) There were no differences in EFS for infants < vs. ≥6 months at diagnosis or (D) with white blood cell (WBC) count < vs. ≥50,000/μL at diagnosis.
The most common reported toxicities were infectious, gastrointestinal, metabolism/nutrition disorders and neutropenia. Grade 3 or 4 infections were reported for 20% or greater of infants during each chemotherapy course and were observed in approximately 40% of infants during each of the continuation phases (Online Supplementary Table S3). Gastrointestinal toxicities were reported most often in the induction intensification and consolidation phases, the two phases containing high-dose methotrexate. Neurologic, respiratory, skin, cardiac, and vascular toxicities were less commonly reported. The toxicities were comparable to those observed in prior infant ALL trials, with notably fewer toxic deaths than P9407, which resulted in five deaths as first events.1,3,5
The high dose intensity of AALL0631, similar to that of P9407, and the extended duration of AALL0631 therapy may both have contributed to the observed excellent outcomes for infants with KMT2A-g. AALL0631, in comparison to the standard arm of the contemporary Interfant- 06 trial, gave considerably higher doses of chemotherapy. Considering age-based dose reductions in Interfant-06, the differences in cumulative chemotherapy doses were greatest in the youngest infants. Though well tolerated, the chemotherapy intensity of AALL0631 is also higher than that given to older children with KMT2A-g ALL on COG trials. The optimal therapy that will minimize toxicity risks and achieve superior survival for this very rare subset of pediatric ALL patients has yet to be defined. Future trials could consider the incorporation of targeted immunotherapy agents and prioritize the identification of prognostic factors that will enable some infants with KMT2A-g ALL to be treated less intensively.
Supplementary Material
Acknowledgments
LG is the Ergen Family Chair in Pediatric Oncology at Children’s Hospital Colorado. MLL is the Benioff Chair of Children’s Health and the Deborah and Arthur Ablin Endowed Chair for Pediatric Molecular Oncology at Benioff Children’s Hospital. EAR is a KiDS of NYU Foundation Professor at NYU Langone Health. SPH is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at The Children’s Hospital of Philadelphia.
Funding Statement
Funding: this study was supported by NIH grants U10 CA98543 (COG Chair’s Grant), U10 CA180886 (NCTN Operations Center Grant), U10 CA98413 and U10 CA180899 (COG Statistics and Data Center Grants), and St. Baldrick’s Foundation.
References
- 1.Dreyer ZE, Hilden JM, Jones TL, et al. Intensified chemotherapy without SCT in infant ALL: results from COG P9407 (Cohort 3). Pediatr Blood Cancer. 2015;62(3):419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieters R, De Lorenzo P, Ancliffe P, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: results from an international phase III randomized study. J Clin Oncol. 2019;37(25):2246-2256. [DOI] [PubMed] [Google Scholar]
- 3.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240-250. [DOI] [PubMed] [Google Scholar]
- 4.Hilden JM, Dinndorf PA, Meerbaum SO, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children's Oncology Group. Blood. 2006;108(2):441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomizawa D, Miyamura T, Imamura T, et al. A risk-stratified therapy for infants with acute lymphoblastic leukemia: a report from the JPLSG MLL-10 trial. Blood. 2020;136(16):1813-1823. [DOI] [PubMed] [Google Scholar]
- 6.Tomizawa D, Koh K, Sato T, et al. Outcome of risk-based therapy for infant acute lymphoblastic leukemia with or without an MLL gene rearrangement, with emphasis on late effects: a final report of two consecutive studies, MLL96 and MLL98, of the Japan Infant Leukemia Study Group. Leukemia. 2007;21(11):2258-2263. [DOI] [PubMed] [Google Scholar]
- 7.Nagayama J, Tomizawa D, Koh K, et al. Infants with acute lymphoblastic leukemia and a germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group. Blood. 2006;107(12):4663-4665. [DOI] [PubMed] [Google Scholar]
- 8.Brown PA, Kairalla JA, Hilden JM, et al. FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631. Leukemia. 2021;35(5):1279-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salzer WL, Jones TL, Devidas M, et al. Decreased induction morbidity and mortality following modification to induction therapy in infants with acute lymphoblastic leukemia enrolled on AALL0631: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2015;62(3):414-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [Google Scholar]
- 11.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazio G, Bardini M, De Lorenzo P, et al. Recurrent genetic fusions redefine MLL germ line acute lymphoblastic leukemia in infants. Blood. 2021;137(14):1980-1984. [DOI] [PubMed] [Google Scholar]
- 13.Boer JM, Valsecchi MG, Hormann FM, et al. Favorable outcome of NUTM1-rearranged infant and pediatric B cell precursor acute lymphoblastic leukemia in a collaborative international study. Leukemia. 2021;35(10):2978-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Lorenzo P, Moorman AV, Pieters R, et al. Cytogenetics and outcome of infants with acute lymphoblastic leukemia and absence of MLL rearrangements. Leukemia. 2014;28(2):428-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.