Abstract
Data regarding efficacy and toxicity of chimeric antigen receptor T (CAR-T) cell therapy in the elderly, geriatric population are insufficient. In 2019, tisagenlecleucel and axicabtagene-ciloleucel were commercially approved for relapsed/refractory diffuse large B-cell lymphoma. From May 2019 onwards, 47 relapsed/refractory diffuse large Bcell lymphoma patients, ≥70 years underwent lymphopharesis in three Israeli centers. Elderly (n=41, mean age 76.2 years) and young (n=41, mean age 55.4 years) patients were matched based on ECOG performance status and lactose dehydrogenase levels. There were no differences in CD4/CD8 ratio (P=0.94), %CD4 naive (P=0.92), %CD8 naive (P=0.44) and exhaustion markers (both HLA-DR and PD-1) between CAR-T cell products in both cohorts. Forty-one elderly patients (87%) received CAR-T cell infusion. There were no differences in the incidence of grade ≥3 cytokine-release-syndrome (P=0.29), grade≥3 neurotoxicity (P=0.54), and duration of hospitalization (P=0.55) between elderly and younger patients. There was no difference in median D7-CAR-T cell expansion (P=0.145). Response rates were similar between the two groups (complete response 46% and partial response 17% in the elderly group, P=0.337). Non-relapse mortality at 1 and 3 months was 0 in both groups. With a median follow-up of 7 months (range, 1.3-17.2 months), 6- and 12-months progression-free and overall survival in elderly patients were 39% and 32%, and 74% and 69%, respectively. EORTC QLQ-C30 questionnaires, obtained at 1 month, showed worsening of disability and cancer-related-symptoms in elderly versus younger patients. We conclude that outcomes of CAR-T cell therapy are comparable between elderly, geriatric and younger patients, indicating that age as per se should not preclude CAR-T cell administration. Longer rehabilitation therapy is essential to improve disabilities and long-term symptoms.
Introduction
The median age of diagnosis for diffuse large cell B-cell lymphoma (DLBCL), the most common subtype of aggressive lymphoma, is 66 years with approximately 40% of the patients above the age of 70 years (SEER cancer statistics). Advanced age is a major risk factor for relapse and death in patients with DLBCL.1 A recent study, evaluating the real-world outcome of DLBCL patients, reported a 52% complete remission rate in patients older than 65 years. However 22% of these complete responders subsequently experienced disease relapse, indicating that almost two thirds of elderly DLBCL patients will eventually require salvage therapy.2 Although for selected patients both allogeneic and autologous hematopoietic cell transplantations are curative therapeutic options, for elderly patients who are non-transplant eligible options are limited, and those who fail to respond/relapse following first line therapy, die of the disease.3,4
The introduction of chimeric antigen receptor T (CAR-T) cell therapy, providing long-term remission in 30-40% of patients,5,6 appears to be the most powerful, if not the only potentially curative therapy for these relapsed/refractory (R/R) elderly DLBCL patients.
Nevertheless, elderly patients (>65-70 years old) are often considered ineligible for CAR-T cell therapy; having a relatively poor performance status (PS) and concomitant comorbidities, making them more susceptible for treatment- related adverse events, particularly cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).7
A recent analysis, evaluating the outcome of young versus elderly patients (age >65 years, n=27), included in the ZUMA-1 trial after fulfilling the inclusion criteria (e.g., Eastern Cooperative Oncology Group performace status [ECOG PS]= 0-1, left ventricular ejection fraction [LVEF] >50%), reported similar rates of CRS, complete remission (CR) and long-term remissions in both age groups. However, the incidence of ICANS grade 3, was higher in elderly patients.8 In addition, encouraging response rates were also reported in several small retrospective studies, though toxicity profile remained debatable.9-11
Hence, we aimed to retrospectively analyze the realworld data of the efficacy and toxicity profile of a nonselective population of elderly DLBCL patients treated with CAR-T cell therapy, compared with matched younger patients. In addition, we focused on both clinical parameters and quality of life domains, as well as on T-cell subpopulations and fitness of elderly patients, compared to younger counterparts.
Methods
Since April 2019 tisagenlecleucel has been commercially available in Israel, while axicabtagene ciloleucel has been available since April 2020. The national infrastructure of eligibility for CART cell therapy does not require a centralized committee to approve the treatment. Each center has been approved by the relevant pharmaceutical companies, the MOH, and in some cases by JACIE for the infusion of CAR-T cells. Among all three accredited centers in Israel, we retrospectively searched the DLBCL CAR-T surveillance database for all patients referred for CD19-directed CAR-T. The study was performed in accordance with the Declaration of Helsinki and was approved by the institutional ethics committee.
For referral and eligibility, lymphopheresis, bridging therapy, and preparative regimen and supportive care sections see the Online Supplementary Appendix.
Definitions of endpoints
Microbiological and clinical documented infections (MDI and CDI, respectively) were defined according to the European Conference on Infections in Leukemia (ECIL) guidelines12 and organ dysfunction was defined as either congestive heart failure, acute kidney injury, or atrial fibrillation. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria Version 5.0.
Patients were monitored daily for the occurrence of CRS and ICANS. Grading and treatment followed the American Society for Transplantation and Cellular Therapy (ASTCT) and European Society for Blood and Marrow Transplantation (EBMT) recommendations.7,13 Briefly, tocilizumab was given in the context of fluid-resistant hypotension grade 2 CRS or low saturation and steroids were started in cases of tocilizumabrefractory CRS or ICANS grade 2 or higher. Disease status was evaluated by Positron emission tomography/computed tomography (PET-CT) scan, performed within 7 days prior to admission for CAR-T cell therapy, and on day 30 and 90 post CAR-T cell infusion. Following white blood cell count recovery, patients carried out a weekly full blood count, and monthly cytomegalo virus (CMV) and Kaposi's sarcoma-associated herpes virus 6 (HHV6), and immunoglobulins status for the first year. The cell therapy coordinator nurse assessed quality of life prior to, 30 days after and 90 days after infusion using the EORTC QLQC30 (version 3) questionnaires (including the following domains – disability assessment, cancer/toxicity-associated symptoms, emotional symptoms, overall health self-assessment, and overall quality of life self-assessment).
For evaluation of pretreatment T-cell compartment and assessment of CAR-T cell product and persistency see the Online Supplementary Appendix.
Statistical analysis
All consecutive patients ≥70 years (study cohort) were matched with patients younger than 70 years (control group). Patients were identified from a surveillance database of the participating centers. Matching was performed according to ECOG PS at screening (0-1 vs. 2-3) and lactose dehydrogenase (LDH) blood levels prior the infusion of CAR-T cell product (high vs. normal). Selection of these two parameters was based on previous real-word data, published by two different groups, confirming these parameters to predict patient's outcome.14,15
Continuous variables were described as the mean, median, standard deviation and range of number observations, as applicable. Categorical data were described with contingency tables including frequency and percent. Comparison between the different baseline domains of the study cohort and counterpart control cohort was performed using wither Pearson Chi-Square or non-parametric Student t-test, as appropriate. One-way ANOVA test with F calculation was performed to compare the quality-of-life questionnaire domains between base line, 30 days and 3 months values.
A linear regression was performed for the association between baseline characteristics and response to CAR-T cell infusion. Cox proportional-hazards model was performed to identify parameters associated with progression-free survival (PFS) or overall survival (OS). Status of disease at 1 month post CAR-T cell therapy was analyzed as a time-dependent covariate. A two-sided P-value of <0.05 was considered as statistically significant.
Results
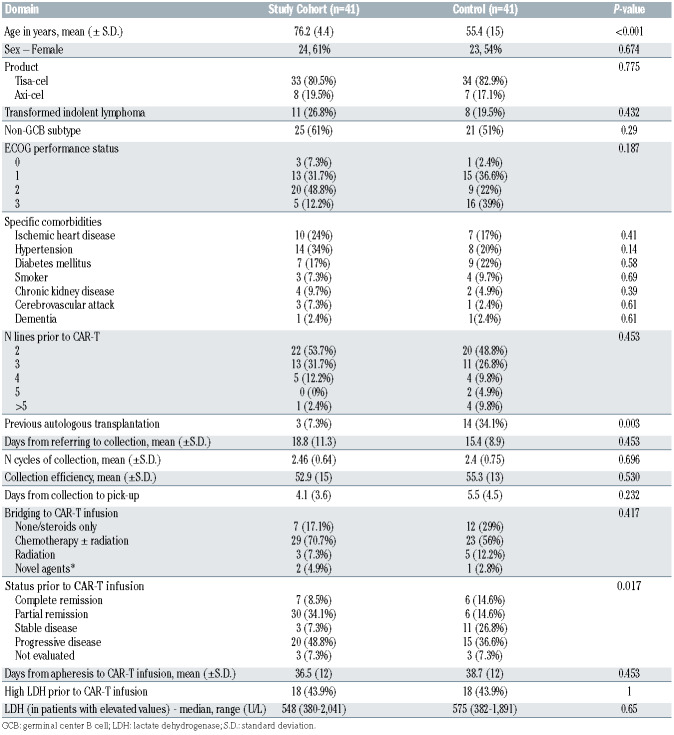
Between April 2019 and October 2020, 49 patients, ≥70 years of age were screened for eligibility in three CAR-T cell therapy centers in Israel. Two patients were ineligible for CAR-T cell collection (ECOG=4, n=1; active hepatitis B virus infection, n=1). Forty-seven patients (96%) were eligible and all underwent successful apheresis. Median time from referral to apheresis was 11 days (range, 1-71 days), with no difference in time to apheresis between elderly and younger cohorts (mean days from referral to apheresis 18.8 vs. 15.4, respectively, P=0.453). In six patients (12.7%), CAR-T manufactured cell product was not eventually infused (out of specification and termination, n=3; out of specification and progression of secondary malignancy, n=1; progression of DLBCL, n=2). One patient had an out of specification product due to low viability of CAR-T cells, however the product was infused.
Forty-one patients were given a commercial CAR-T cell product (87% out of all patients that underwent apheresis) and encompassed the study population. Forty-one matched patients aged <70 years were included in the control group. Table 1 depicts the characteristics of the two cohorts. The mean age of the study and the control cohorts were 76.2 (±4.4) and 55.4 (±15) years, respectively and the median follow-up of surviving patients was 7 months (range, 1.3-17.2 months) and 7 month (range, 1.3-16.7months), respectively. Similar percentage of patients in both cohorts had >2 lines of therapy prior to CAR-T cell therapy (47.3% and 51.2%, respectively). Percentage of patients that had a previous autologous hematopoietic cell transplantation (HCT) and CR state at CAR-T cell infusion was higher in the control group compared to the older aged group. While, overall, the percentage of patients with ECOG PS 2-3 was similar between the two groups (61%), there was a lower percentage of patients with ECOG PS 3 in the study cohort compared to the control group (12.2% vs. 39%), Table 1.
Table 1.
Characteristics of patients
Apheresis
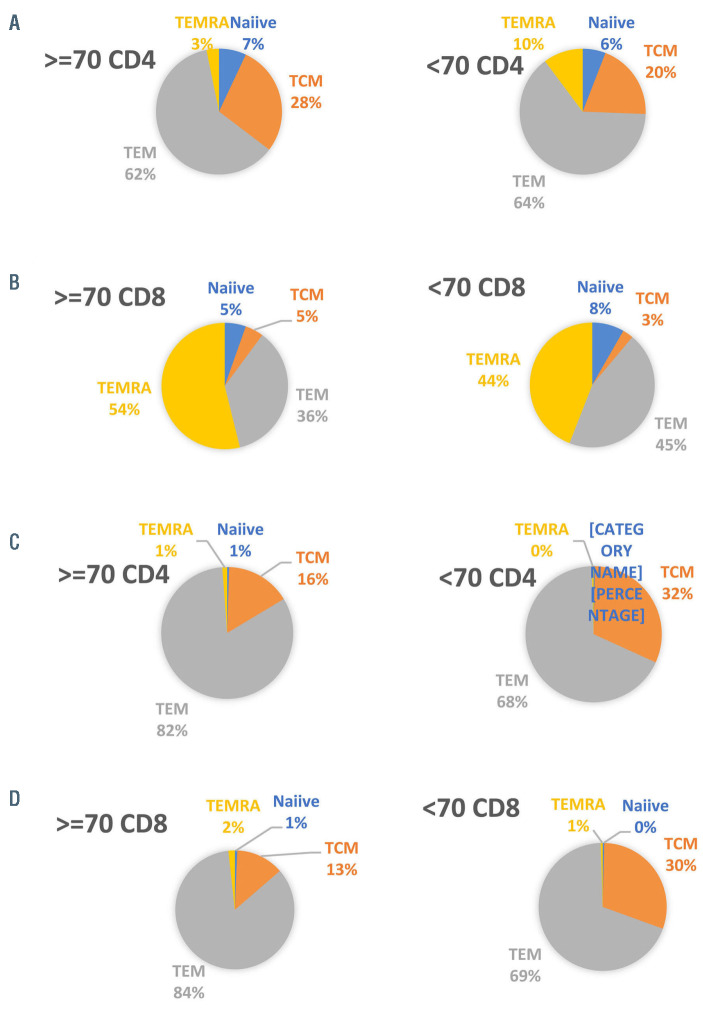
There was no difference in the mean number of collection cycles and in the collection efficiency between the two groups (2.46 vs. 2.4, P=0.696 and 52.9 vs. 55.3, P=0.53, respectively). Age (as a continuous variable), advanced ECOG PS, number of lines of therapy prior to lymphopheresis, and previous autologous HCT did not impact the required number of collection cycles (β=0.03, 95% confidence interval[CI]: -0.28 to 0.36, P=0.801; β=0.18, 95% CI: -0.06 to 0.54, P=0.114; β=-0.16, 95% CI: -0.02 to 0.36, P=0.172; and β=0.13, 95% CI: -0.2 to 0.64, P=0.305, respectively). Data of T-cell subpopulations were available in 19 (46%) patients and were compared to results obtained in younger patients (n=16, 39%), (Figure 1A to D; Online Supplementary Figure S1). There was no difference in the CD4/CD8 ratio between the two groups (P=0.94). There were no differences in percentages of naïve, TCM, TEM, and TEMRA-CD4 subpopulations in the apheresis product, between the two groups (P=0.92, P=0.35, P=0.45, and P=0.16, respectively). This was also true for the counterparts, CD8 subpopulations (P=0.44, P=0.35, P=0.33, and P=0.47, respectively), Figure 1A and B. There was also no difference in the expression of exhaustion markers by both CD4 and CD8 cells between the apheresis products of aged versus younger patients (P=0.172 for CD4-HLA-DR, P=0.244 for CD4-PD-1, P=0.06 for CD8-HLA-DR, and P=0.354 for CD8-PD-1).
Hospitalization and early toxicity
Mean days from apheresis to CAR-T cell infusion was 36.5 (standard deviation [SD] ±12), with no difference between the elderly and the control group (P=0.453), Table 2.
Analysis of the CAR-T cell product showed that there were no differences in the percentages of CD4 naive, TCM, TEM, and TEMRA subpopulations between the elderly and the younger-patients’ group (0.48 vs. 0.29, P=0.59; 15.9 vs. 31.5, P=0.39; 82.3 vs. 67.8, P=0.42; and 1.18 vs. 0.42, P=0.42, respectively). This was also true for the comparable CD8 subpopulations (0.67 vs. 0.39, P=0.37; 13 vs. 30.2, P=0.24; 84.7 vs. 70, P=0.28; and 1.75 vs. 0.49, P=0.32, respectively), Figure 1C and D. There was also no difference in the expression of exhaustion markers expressed by CD4 and CD8 cells (69.6 vs. 83.4, P=0.39 for CD4-HLA-DR, 81.1 vs. 90.6, P=0.22 for CD4-PD-1, 86.1 vs. 92.5, P=0.30 for CD8- HLA-DR, and 63.7 vs. 72.4, P=0.36 for CD8-PD-1).
Figure 1.
Subpopulations of T cells in the apheresis and in the CAR-T cell therapy product from a portion of the study group (n=19) and control group (n=16). (A) Subpopulations of CD4 T cells in apheresis product; (B) subpopulations of CD8 T cells in apheresis product; (C) subpopulations of CD4 T cells in the CAR-T cell therapy product; (D) subpopulations of CD8 T cells in the CAR-T cell therapy product.
Among the elderly group, there were six cases (15% of patients) of CDI (pneumonia, n=3; cellulitis, n=2, and lineassociated tunnel infection, n=1). There were four cases (10%) of MDI (gram negative bacteremia, n=2 and staphylococcus hominis bacteremia, n=2). Incidence of overall CDI and MDI infections were similar between the elderly and the control groups (26.8% and 19.5%, respectively, P=0.301). There were no cases of exacerbation of congestive heart failure. There were three cases of acute kidney injury (2 associated with preparative regimen and 1 associated with sepsis) and three cases of atrial fibrillation (all associated with ongoing CRS). Incidence of organ failure was also similar between the two groups (P=1).
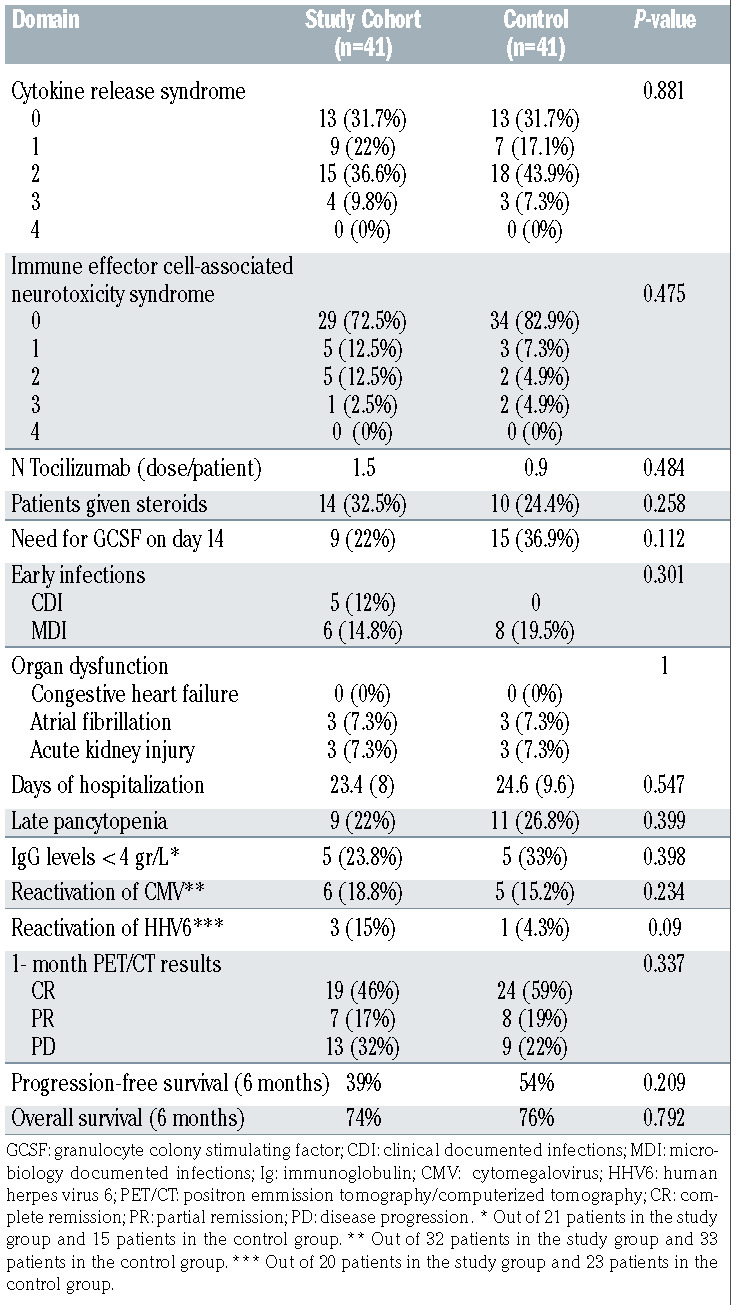
Median time to CRS was in the elderly cohort was 3 days (range, 0-6 days), similar to the control group (median 3 days [range, 1-6 days], P=0.65). Overall, there were 28 cases (68%) of CRS (grade 1, n=9; grade 2, n=15, and grade 3, n=4). There was no difference between the elderly and the control group in overall CRS (69.3% in both groups, P=0.88) and grade 3-4 CRS (9.8% vs. 7.3%, respectively, P=0.29). Median days to ICANS was 4 days (range, 2-8 days). Overall, there were 11 cases (27%) of ICANS (grade 1, n=5; grade 2, n=5, and grade 3, n=1). There was no difference between the elderly and the control group in overall ICANS (27.5% vs. 17.1%. respectively, P=0.48) and grade 3-4 ICANS (2.5% vs. 4.9%, respectively, P=0.54). History of vascular disease or dementia did not predict occurrence of ICANS in the elderly group (overall response [OR] 1.2, 95% CI: 0.78-1.81, P=0.45 and OR=1.4, 95% CI: 0.81-1.63, P=0.38, respectively). Mean doses of tocilizumab/patient was in the two groups (1.5 vs. 0.9, respectively, P=0.484). Similarly, the percentage of patients given steroids was similar (32.5% vs. 24.4%, respectively, P=0.596). Nine patients (22%) required granulocyte colony-stimulating factor (GCSF) on day 14 post CAR-T cell infusion due to delayed count recovery, no difference was found in with the control group (P=0.15). Mean days of admission was 23.4 (±8) days, compared to 24.6 (±9.6) in the control group (P=0.55).
Late toxicity
Late pancytopenia occurred in nine patients (absolute neutrophil count [ANC] only, n=2 [4.9%], platelet count only, n=2 [4.9%], and ≥ 2 cytopenia, n=5 [12%]). No difference was found in the incidence of late cytopenia between the two groups (P=0.399). There were five (15.2% of 32 patients with available data) patients with CMV reactivation, none had CMV disease. Two patients with continuous CMV viremia eventually received an anti-CMV therapy (1 valgancyclovir and 1 foscarnet) with no subsequent reappearance of CMV. There were three (15% of 20 patients with available data) patients that developed reactivation of HHV6. Of them, one patient had ongoing grade 3 ICANS. In this patient, HHV6-polymerase chain reaction (PCR) obtained from the cerebrospinal fluid was positive, however both electroencephalogram and magnetic resonance imaging were not suggestive of limbic encephalitis. The patient was treated with a 3-week course of foscarnet and steroids and subsequently recovered, however we were unable to conclude if this was a definite HHV6 encephalitis. The other two patients did not have clinical symptoms of HHV6 systemic infection or encephalitis, therefore, were only monitored until HHV6-PCR levels became negative.
Out of 21 patients with available immunoglobulin G (IgG) levels 1 month post CAR-T cell infusion, there were five cases (24%) with IgG levels below 400 mg/L, similar to the control group (P=0.398), Table 2.
Quality of life
In 23 patients (56%) EORTC QLQ-C30 questionnaires were available. Thirty-day questionnaire, compared to the baseline questionnaire, showed increased disability in four of five domains, increase in cancer/treatment-related symptoms in six of 11 domains and worsening of emotional symptoms in four of 12 domains, while there was no change in both overall health perception and overall quality of life. The 3-month questionnaire, when compared to the base-line questionnaire, showed improvement in disability in all five domains, improvement in all cancer/treatmentrelated symptoms, improvement of emotional symptoms in ten of 12 domains and improvement both overall health perception (mean baseline 3.83 vs. mean 3 months 5.6, F- 6.007, P=0.005) and overall quality of life (mean baseline 3.87 vs. mean 3 months 5.4, F-2.68, P=0.081).
Table 2.
Toxicity and response to CAR-T cell therapy
Efficacy
At date of analysis there were 31 patients (76%) alive and 16 patients (39%) in an ongoing CR state. Non-relapse mortality at 1 and 3 months was 0. Expansion of CAR-T cells on day 7 was available in 19 (46%) patients. There was no difference in the CAR-T cell blood levels between elderly and the control group (P=0.145).
PET-CT at 1 month post CAR-T cell infusion demonstrated CR, partial remission (PR) and progressive disease (PD) in 19 (46%), 7 (17 %), and 13 (32%) patients, respectively. In two patients the results of PET-CT are still pending. There was no difference in the overall response rate (ORR) between the elderly and the control group (63% vs. 78%, respectively, P=0.337). Multivariate binary logistic model identified that high LDH prior to admission for CAR-T cell infusion was associated with lower chances of achieving CR state at day 30 post CAR-T cell infusion (OR: -0.8, 95% CI: 0.48-0.97, P=0.048). Age, sex, ECOG PS, administration of bridging therapy and the occurrence of CRS had no impact on CR rate.
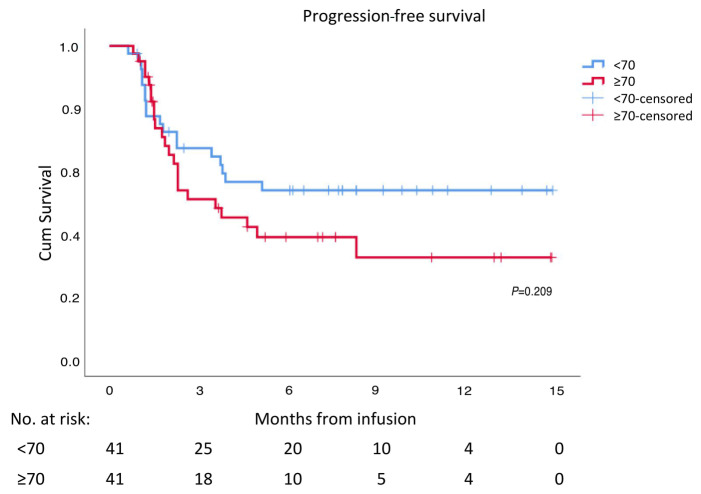
Figure 2.
Progression-free survival. Progressionfree survival after CAR-T infusion of elderly versus young patients with diffuse large cell B-cell lymphoma.
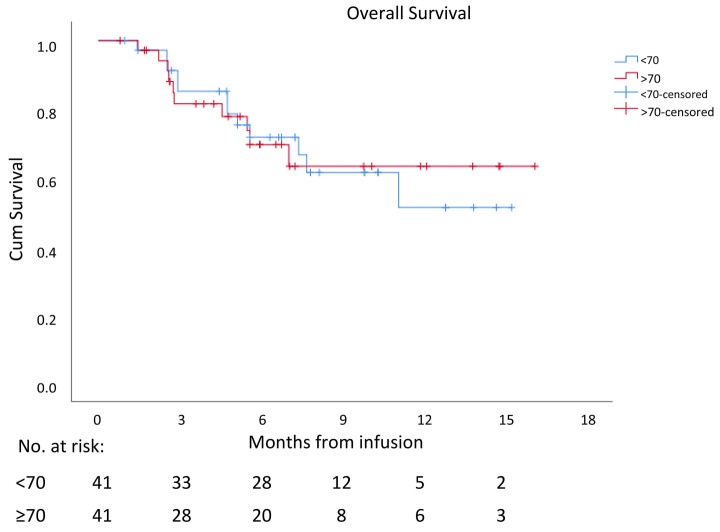
Figure 3.
Overall survival. Overall survival after CAR-T infusion of elderly versus young patients with diffuse large cell B-cell lymphoma.
At 3, 6, and 12 months, the projected PFS was 51%, 39%, and 32%, respectively in the elderly group, and approached 67%, 54%, and 54%, respectively in the younger group). Median PFS was 3.6 (95% CI: 1.6-5.6) months in the elderly patients, while not reached in the younger group of patients (P=0.209), Figure 2. Multivariate regression model identified prior autologous HCT (hazard ratio [HR]: 0.208, 95% CI: 0.05-0.87, P=0.032) and a CR state 1 month post CAR-T cell therapy (HR: -0.076, 95% CI: 0.025-0.23, P<0.001) to be associated with better PFS, while both high LDH prior infusion of CAR-T cells (HR: -2.7, 95% CI: 1.1-6.7, P=0.03) and non-germinal center B-cell immunophenotype (HR: 2.29, 95% CI: 0.98-5.4, P=0.057) were associated with shorter PFS. Age and ECOG PS had no impact on PFS.
At 3, 6, and 12 months, projected OS was 84%, 74%, and 69%, respectively in the elderly group, 87%, 76%, and 53% in the younger group. Median OS was not reached in both groups (P=0.792), Figure 3. Multivariate regression model for OS identified a CR state 1 month post CAR-T cell therapy (HR: 0.017, 95% CI: 0.003-0.11, P<0.001) to be associated with increased OS, while both receiving bridging therapy and high LDH prior to CAR-T cell nfusion (HR: 5.6, 95% CI: 0.99-32.3, P=0.05 and HR: 4.9, 95% CI: 1.5-16.1, P=0.008, respectively) were associated with shorter OS. Age and ECOG PS had no impact on OS.
Discussion
This study explored the characteristics and outcome of all consecutive patients, 70 years or older, referred for CAR-T cells during the study period, and matched these patients with younger individuals, based on PS and LDH prior to admission for infusion. We showed that both toxicity and efficacy are similar in elderly compared to the younger patients.
Despite the improvement in outcomes of elderly DLBCL patients over the last two decades, the prognosis remains disappointing, with 2-year event-free survival of 57%.16 Unfortunately, response rates achieved with second line salvage therapy in these non-transplant eligibility patients are generally dismal and most patients die of their disease in less than a year.17,18 Therefore, CAR-T cell therapy, providing the only curative option for these elderly patients, should be strongly considered.
At present, many countries employ very restrictive criteria for selecting patients to CAR-T cell therapy, considering CAR-T cell therapy as a "highly toxic" treatment.15,19,20 According to their policies, patients that present with significant comorbidities (i.e., <45% EF, PS>1, chronic renal disease etc.) are ineligible for CAR-T cell therapy. Thus, a substantial proportion of elderly patients, "fulfilling these criteria", would be ineligible for CAR-T cell therapy. Indeed, the French group excluded 41% of all referred DLBCL patients due to employment of these restrictive standards. As such, the median age of CAR-T cell therapy patients in some centers in Europe is lower than 60 years, indicating that the majority of elderly DLBCL patients that experienced disease relapse and required CAR-T cell therapy, were not considered for this life saving treatment.20,21
According to our data, almost all (96%) elderly patients that were referred to CAR-T cell therapy, were found to be eligible and underwent successful apheresis. although analyzed in only a portion of the patients in the elderly group," lymphocyte fitness", reflected by T-cell subsets and exhaustion markers, was non-inferior in elderly compared with younger patients, and translated into comparable CAR-T cell products with no increase in production failure and similar expansion of CAR-T cells. These results are analogous to those reported in the ZUMA-1 trial.8
Despite the fact that two thirds of our patients had a poor PS and almost half entered CAR-T cell therapy with increased LDH levels (reflecting highly proliferative disease), 84% were eventually transfused. This transfusion rate is not inferior to that reported in other real life series, including highly selective CAR-T cell programs, emphasizing the importance of logistical factors and decreasing the time from enrollment to infusion.14,20
Acute treatment related toxicities, including CRS and ICANS, were mainly grades 1-2, with no differences between the cohorts as previously proposed by others.10 The relatively low incidence of high grade ICANS in both young and elderly patients, might reflect the predominant employment of tisagenlecleucel in our cohort of patients. In contrast, studies focusing on early toxicity of axicabtagene, have generally reported higher rates of high grades ICANS, especially in older aged patients.8,22 Of note, prior cerebral vascular disease nor dementia were found to be associated with increased risk for ICANS in our cohort of patients.
Long-term toxicity was not higher in elderly versus younger patients. Interestingly, a substantial number of patients developed reactivation of CMV, and rarely, HHV6, with no need for active intervention in the majority, and with no infection-related sequels. This manifestation has not been reported yet and we continue to perform routine surveillance monitoring to further investigate post CAR-T cell therapy virus reactivation. The risk for significant hypogammaglobulinemia and persistent cytopenia were not higher in the aged patient and were in line with those reported in prior real-life series.6-8
An important finding of our study is the dual effect of CAR-T cell therapy on patients’ quality of life, during admission and after returning home. During hospitalization, patients reported on increase disability, aggravation in cancer/treatment-related symptoms and worsening emotional distress. Considering that patients are usually hospitalized for 3-4 weeks, direct intervention during that period, employing intensive physical and emotional rehabilitation programs may help alleviating symptoms. Thereafter, those patients who respond to CAR-T cell therapy may expect to gain a long-term clinically meaningful improvements in daily functioning.23 Indeed, in our cohort of patients, all symptoms improved after several months, and patients reported a significant progress in their quality of life.
Despite the poor features of our elderly cohort of patients (poor PS in 66%, high LDH in approximately 40%, progressive disease and ≥3 prior lines in almost half), the CR rate was 46.3%, similar to the CR rate reported in the Juliet study and in other real-world series.6,8,10 CR rate was affected by LDH level only, and was not adversely affected by age, supporting prior smaller unmatched studies, that reported similar overall and complete response rates in elderly versus younger patients.8 PFS in these elderly patients was also not statistically shorter than in their younger counterparts and was similar to that reported in the Juliet study.6 In line with previous reports, cell of origin (non-GCB vs. GCB ) and high LDH level, being surrogates for a more aggressive disease, were found to be associated with shorter PFS.14,15 In contrast, prior autograft (indicating the lack of primary refectory disease) and the achievement of CR at 30 days post CAR-T cell therapy predicted durable responses, supporting prior reports.14 The OS in our cohort of patients was also encouraging, approaching 69% at 12 months post CAR-T transfusion. In consensus with prior reports, administration of bridging therapy and high LDH levels were both found to be associated with shorter survival, whereas an achievement of CR at days 30 post CAR-T cell therapy predicted a longer survival.14,15
Our study has several limitations, mainly due to the retrospective nature and the relatively small number of patients. In several analyses, data on potential significant factors were missing for all or for some of the patients, e.g., molecular characteristics and T-cell subsets in apheresis bags. Moreover, there were other imbalances between the two groups (i.e., higher percentage of patients with ECOG PS of 3 in the control group and the number of lines prior to CAR-T cell therapy ), which might affect our results and make it difficult to conclude if elderly patients have a worse or a similar outcome to their younger counterparts. Although this cohort represent non-selected real-life elderly lymphoma patients, it is possible that presumably “ineligible” patients were not referred for CAR-T cell therapy and thus our population is still relatively “selective”. Nevertheless, in order to overcome the potential bias of age we performed matched control cohort analysis with younger counterparts and indeed showed that age in itself should not preclude elderly patients from undergoing CAR-T cell therapy. Ideally, a geriatric scoring assessment should be employed and help to establish eligibility criteria for CAR-T cell therapy in this aged group pf patients. Future studies should focus on improving the long-term efficacy of the product as well as earlier intervention in cases of progression and active rehabilitation to improve quality of life post therapy in this population.
Supplementary Material
Funding Statement
Funding: None of the authors received financial support for the research, authorship, and/or publication of this article.
References
- 1.Hedstrom G, Hagberg O, Jerkeman M, Enblad G. The impact of age on survival of diffuse large B-cell lymphoma - a populationbased study. Acta Oncol. 2015;54(6):916-923. [DOI] [PubMed] [Google Scholar]
- 2.Adiyaman SC, Alacacioglu A, Ersen Danyeli A, et al. Prognostic factors in elderly patients with diffuse large B-cell lymphoma and their treatment results. Turk J Haematol. 2019;36(2):81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarus HM, Carreras J, Boudreau C, et al. Influence of age and histology on outcome in adult non-Hodgkin lymphoma patients undergoing autologous hematopoietic cell transplantation (HCT): a report from the Center For International Blood & Marrow Transplant Research (CIBMTR). Biol Blood Marrow Transplant. 2008;14(12):1323-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NN, Ahn KW, Litovich C, et al. Outcomes of Medicare-age eligible NHL patients receiving RIC allogeneic transplantation: a CIBMTR analysis. Blood Adv. 2018;2(8):933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 7.Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica. 2020;105(2):297-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neelapu SS, Jacobson CA, Oluwole OO, et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2020;135(23):2106-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajra A, Zettler ME, Phillips EG, et al. Neurological adverse events following CAR T-cell therapy: a real-world analysis. Immunotherapy. 2020;12(14):1077-1082. [DOI] [PubMed] [Google Scholar]
- 10.Lin RJ, Lobaugh SM, Pennisi M, et al. Impact and safety of chimeric antigen receptor T cell therapy in older, vulnerable patients with relapsed/refractory large b-cell lymphoma. Haematologica. 2020;106(1):255-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zettler ME, Feinberg BA, Phillips EG, Klink AJ, Mehta S, Gajra A. Real-world adverse events associated with CAR T-cell therapy among adults age ≥65 years. J Geriatr Oncol. 2021;12(2):239-242. [DOI] [PubMed] [Google Scholar]
- 12.Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 update. Bone Marrow Transplant. 2011;46(5):709-718. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. [DOI] [PubMed] [Google Scholar]
- 17.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16): 1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. 2018;182(5):633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greinix HT, Attarbaschi A, Girschikofsky M, et al. Ensuring center quality, proper patient selection and fair access to chimeric antigen receptor T-cell therapy: position statement of the Austrian CAR-T Cell Network. memo. 2020;13(1):27-31. [Google Scholar]
- 20.Kuhnl A, Roddie C, Martinez-Cibrian N, et al. Real-world data of high-grade lymphoma patients treated with CD19 CAR-T in England. Blood. 2019;134(Suppl 1):S767. [Google Scholar]
- 21.Paillassa J, Di Blasi R, Chevret S, et al. CD19 CAR T-cell therapy in patients with relapse/refractory DLBCL: retrospective analysis of the eligibility criteria. Blood. 2019;134(Suppl 1):S2887. [Google Scholar]
- 22.Riedell PA, Walling C, Nastoupil LJ, et al. A multicenter retrospective analysis of clinical outcomes, toxicities, and patterns of use in institutions utilizing commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B-cell lymphomas. Blood. 2019;134(Suppl 1):s1599. [Google Scholar]
- 23.Maziarz RT, Waller EK, Jaeger U, et al. Patient-reported long-term quality of life after tisagenlecleucel in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(4):629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.