Abstract
Context.
More than 80 countries fortify flour, yet the public health impact of this intervention on iron and anemia outcomes has not been reviewed.
Objective.
The objective of this systematic review was to review published and gray literature pertaining to the impact of flour fortification on iron and anemia.
Data Sources.
A systematic review was conducted by searching 17 databases and appealing for unpublished reports, yielding 1881 documents.
Study Selection.
Only studies of government-supported, widely implemented fortification programs in which anemia or iron status was measured prior to and ≥12 months after initiation of fortification were included.
Data Extraction.
Details about the design, coverage, compliance with national standards, and evaluation (e.g., anemia prevalence before and after fortification) of flour fortification programs were extracted from the reports.
Data Synthesis.
Thirteen studies describing 26 subgroups (n = 14 for children ≤15 y, n = 12 for women of reproductive age) were included. During the period from pre- to postfortification (and as difference-in-difference for those studies that included a control group), there were statistically significant decreases in the prevalence of anemia in 4 of 13 subgroups of children and in 4 of 12 subgroups of women of reproductive age as well as significant decreases in the prevalence of low ferritin in 1 of 6 subgroups of children and in 3 of 3 subgroups of women of reproductive age.
Conclusions.
Evidence of the effectiveness of flour fortification for reducing the prevalence of anemia is limited; however, evidence of effectiveness for reducing the prevalence of low ferritin in women is more consistent.
Keywords: enrichment, ferritin, hemoglobin, maize flour, wheat flour
INTRODUCTION
Food fortification is one of the leading public health interventions recommended to prevent and control micronutrient deficiencies.1 Staple foods and condiments are among the foods most commonly fortified with vitamins and minerals. Wheat flour was the first cereal-grain product to be widely fortified, and the first cereal-grain recommendations issued by the World Health Organization (WHO) pertained to maize and wheat flour.2 As of 2015, 83 countries have mandated wheat-flour fortification; 14 of these have simultaneously mandated maize-flour fortification.3 Most of these countries mandate fortification of wheat and maize flour with at least iron and folic acid, with a few exceptions: Australia does not require flour fortification with iron, and Congo, Nigeria, the Philippines, the United Kingdom, and Venezuela do not require fortification of flour with folic acid.
The public health impact of fortification of wheat flour with iron as implemented in government programs has been incompletely documented. A review of efficacy and effectiveness trials found that fortification of staple foods with iron increased hemoglobin levels and serum ferritin levels and decreased anemia prevalence in children, but not in women4; however, the effect of specific staples could not be determined. A review of efficacy trials showed that iron fortification of food increased hemoglobin levels in children less than 10 years of age,5 but the effect of specific staples could not be determined. Per another systematic review of efficacy trials conducted with “apparently healthy (nondiseased) individuals, families or communities,” iron-fortified wheat flour and rice increased hemoglobin levels.6 A desk review of flour fortification programs concluded that most programs that fortify with iron use nonrecommended forms of iron that have low bioavailability (n = 50 of 78 programs) and hence would be “expected to have little impact on iron status at the national level.”7 However, no systematic reviews have been completed on the post hoc effectiveness of flour fortification on iron status or anemia.
The objectives of this systematic review were to answer the following questions about flour fortification (wheat flour alone or combined with maize flour) (Table 18): 1) In effectiveness settings, does flour fortification improve iron status and anemia?; 2) Is there a difference, by age group, in the impact of flour fortification on iron status and anemia?; 3) Are there differences in iron status and anemia if WHO recommended iron compounds are used for flour fortification?; and 4) If programs are well implemented, does flour fortification improve iron status and anemia?
Table 1.
PICOS criteria8 used to define the four research questions
| Question 1 | Question 2 | Question 3 | Question 4 | |
|---|---|---|---|---|
|
| ||||
| Participants | General population older than 2 y (including pregnant women) from any country | |||
| Intervention | Wheat flour (alone or in combination with maize flour) fortified with iron | |||
| Comparators | Prefortification period vs postfortification period | Children 2–15 y vs women of reproductive age | WHO-recommended iron compounds are used (yes vs no) | Well-implemented programs (yes vs no) |
| Outcomes | Iron status and prevalence of anemia | |||
| Study design | Nonrandomized controlled trials and observational studies | |||
METHODS
The PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines for systematic reviews were followed8 (see Table S1 in the Supporting Information online). The study design drew heavily from the Cochrane protocol published for fortification of maize flour,9 although no review protocol was developed for the current study. The following types of studies were included: nonrandomized controlled trials; prospective observational studies that had a control group, such as cohort studies (prospective and retrospective) and controlled before-and-after studies; and prospective observational studies that did not have a control group, including before-and-after studies. Studies were included if there was a biochemical measurement of the primary outcomes (listed below) before fortification began (prefortification) and a measurement at least 12 months after fortification began (postfortification). The following documents were excluded: review articles, conference abstracts, letters to the editor, and presentations. If there were multiple documents from the same country’s experience, only the most recent and comprehensive paper was included. The participants included in the review consisted of the general population older than 2 years (including pregnant women) from any country.
The following interventions were included: wheat flour (alone or in combination with maize flour) fortified with iron; flour to which micronutrients were added during production of the flour; interventions that were part of a government program; and interventions that included any of the primary outcomes (listed below). The following interventions or studies were excluded: interventions that were provided only in an experimental capacity; interventions in which fortification took place at the point of use (e.g., micronutrient powders); studies of biofortified crops (biofortification); and in vitro, animal, or human bioavailability studies. Studies of interventions targeting participants with a critical illness or severe comorbidities were also excluded.
The primary outcomes of interest were as follows: hemoglobin concentration, anemia as defined by studies (e.g., hemoglobin below a cutoff and adjusted for altitude or other factors), iron status using any biomarker (e.g., ferritin, transferrin saturation, soluble transferrin receptor, soluble transferrin receptor-ferritin index, total iron-binding capacity, serum iron), and iron deficiency as defined by studies (e.g., biomarker below or above a cutoff and adjusted for inflammation). Because ferritin is not normally distributed in populations in which significant iron deficiency is present, geometric means were reported, if available; however, if only arithmetic were published, these were assessed. The secondary outcomes of interest were the biomarkers of any other nutrients added to flour (e.g., serum or red blood cell folate for folic acid) and the deficiency of nutrients as defined by studies (e.g., nutrient level below or above a cutoff).
The systematic review had two components: a search in electronic databases and an e-mail appeal. For the electronic searches, no date or language restrictions were imposed. Using the terms “wheat fortification” in English and in Spanish (for the IBECS [Índice Bibliográfico Español en Ciencias de la Salud]10 and SciELO [Scientific Electronic Library Online]11 databases), the following databases were searched on May 31, 2013: Cochrane Central Register of Controlled Trials,12 MEDLINE,13 Embase,14 Web of Science,15 CINAHL (Cumulative Index to Nursing and Allied Health Literature),16 POPLINE,17 AGRICOLA,18 BIOSIS Previews,19 OpenGrey,20 Bibliomap,21 TRoPHI (Trials Register of Promoting Health Interventions),22 IBECS,10,11 Global Health Library,23 Indian Medical Journals,24 Native Health Research Database,25 and ProQuest Dissertation and Theses.26 Email appeals for unpublished fortification evaluations were sent on June 4, 2013, and August 12, 2013, to approximately 2500 individuals in a listserv managed by the Food Fortification Initiative and representing public, private, and civic organizations.
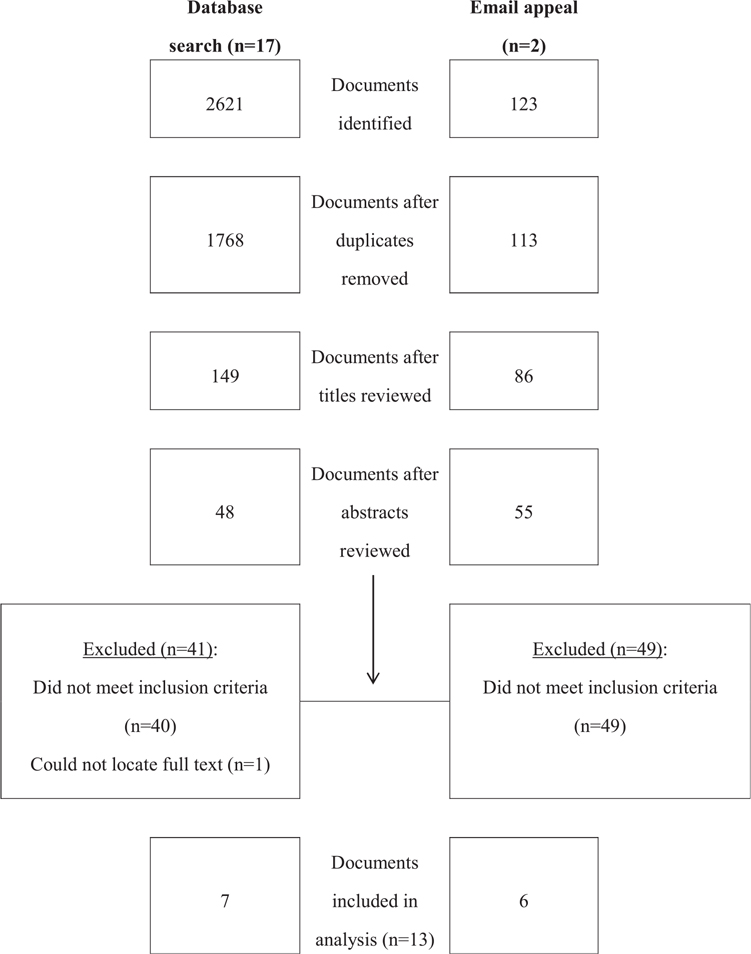
Two reviewers independently screened the titles of the documents retrieved from the database search and e-mail appeal to discard those that were irrelevant (Figure 1). Two reviewers then independently screened the abstracts of potentially relevant documents to discard those that were irrelevant. The full text of each potentially relevant document was read independently by two reviewers. From relevant documents, the reviewers independently extracted approximately 20 pieces of information related to study location, study design, design and implementation of the fortification program, and outcomes assessed (see Table S2 in the Supporting Information online).
Figure 1.
Flow chart of the search and selection process.
The extracted information was analyzed qualitatively. Specifically, the statistical significance of changes in outcomes of interest as reported in the studies was noted (i.e., reduction in anemia prevalence or no reduction in anemia prevalence). Additionally, the fortification program from each study was categorized on the basis of whether it met WHO recommendations2 for flour fortification with regard to type and amount of iron. The nutrition outcomes from the studies that did and did not meet the WHO recommendations were compared.
RESULTS
The database search yielded 2621 documents; when duplicates were eliminated, 1768 documents remained (Figure 1). Titles were reviewed independently, and 149 documents were selected for abstract review. Of these, 48 full-text documents were retrieved and reviewed. Of these, 7 were included in the analysis; 1 Portuguese-language document27 was reviewed by only 1 author (H.P.). The e-mail request yielded 123 documents. After deletion of duplicate documents, 113 documents remained. Titles were reviewed independently, and 86 documents were selected for abstract review. Of these, 55 full-text documents were reviewed. Of these, 6 were included in the analysis.
Description of documents
The documents selected for in-depth review presented evaluations of flour fortification programs in 13 countries: Azerbaijan, Brazil, China, Fiji, India, Iran, Kazakhstan, Mongolia, Nepal, Sri Lanka, Tajikistan, Uzbekistan, and Venezuela (Table 227–39 and Table S2 in the Supporting Information online). Most reported fortification of wheat flour only (n = 10), of wheat and maize flour (n = 2), and of wheat, maize, and millet flour (n = 1). Nine documents were published and 4 were available in the gray literature. The documents described 13 studies. Most studies (n = 9) were designed as pre–post independent cross-sectional surveys with or without a comparison group; 4 were pre–post cohort studies with or without a comparison group. Four of the studies employed a comparison group.
Table 2.
Description of documents (n = 13) included in the review
| Reference | Country (geographic level) | Study design (publication status) | Study population | No. and type of analytic subgroups |
|---|---|---|---|---|
|
| ||||
| Huo et al.28 | China (subnational, village) | Pre–post cohort study with comparison group (published) | Nonpregnant WRA, 20–60 y | 1: WRA |
| Huo et al.29 | China (subnational, village) | Pre–post cohort study with comparison group (published) | WRA, 20–60 y | 1: WRA |
| Tazhibayev et al.30 | Azerbaijan, Kazakhstan, Mongolia, Tajikistan, Uzbekistan (for all countries: subnational, pilot area) | Pre–post cohort study without comparison group (published) | Children, 2–15 y | 5: Azerbaijan children, Kazakhstan children, Mongolian children, Tajikistan children, Uzbekistan children |
| Assuncao et al.31 | Brazil (subnational, city) | Pre–post independent cross-sectional surveys without comparison group (published) | Children, <6 y | 1: children |
| Sadighi et al.32 | Iran (subnational, province) | Pre–post independent cross-sectional surveys without comparison group (published) | WRA, 15–49 y | 2: WRA from Bushehr Province, WRA from Golestan Province |
| Costa et al.27 | Brazil (subnational, municipality) | Pre–post independent cross-sectional surveys without comparison group (published) | Children, 2–5 y | 1: children |
| Layrisse et al.33 | Venezuela (subnational, municipality) | Pre–post independent cross-sectional surveys without comparison group (published) | Schoolchildren, 7 y, 11 y, and 15 y | 1: children |
| Northrop-Clewes et al.34 | Uzbekistan (national) | Pre–post independent cross-sectional surveys without comparison group (unpublished) | WRA | 1: WRA |
| National Food & Nutrition Centre35 | Fiji (national) | Pre–post independent cross-sectional surveys without comparison group (unpublished) | Nonpregnant WRA, 15–49 y | 1: WRA |
| Nestel et al.36 | Sri Lanka (subnational, tea estates) | Pre–post cohort study with comparison group (published) | Preschool children, 9–71 mo; schoolchildren, 6–11 y; nonpregnant WRA | 6: preschool children consuming flour fortified with electrolytic iron; preschool children consuming flour fortified with reduced iron; schoolchildren consuming flour fortified with electrolytic iron; schoolchildren consuming flour fortified with reduced iron; WRA consuming flour fortified with electrolytic iron; WRA consuming flour fortified with reduced iron |
| Nepali Technical Assistance Group37 | Nepal (subnational, district) | Pre–post independent cross-sectional surveys without comparison group (unpublished) | WRA, 15–45 y | 1: WRA |
| Fujimori et al.38 | Brazil (subnational, regional) | Pre–post independent cross-sectional surveys without comparison group (published) | Pregnant WRA | 1: WRA |
| Micronutrient Initiative et al.39 | India (subnational, district) | Pre–post independent cross-sectional surveys with comparison group (unpublished) | Preschool children and schoolchildren, 5–11 y; adolescent girls, 12–19 y (WRA); pregnant and lactating WRA | 4: preschool children, schoolchildren, adolescent girls, pregnant and lactating women |
Abbreviation: WRA, women of reproductive age.
Most studies presented data gathered at the subnational level; the only national-level data reported were for Fiji35 and Uzbekistan34 (Table 2). Four studies presented results for multiple subgroups (i.e., age, region, or type of iron); in total, 26 subgroups were reported. For example, Tazhibayev et al.30 reported 5 subgroups: children 2 to 15 years in Azerbaijan, Kazakhstan, Mongolia, Tajikistan, and Uzbekistan. Nestel et al.36 published reports for 6 subgroups: preschool children 9 to 71 months who consumed wheat flour fortified with electrolytic iron, preschool children 9 to 71 months who consumed wheat flour fortified with reduced iron, schoolchildren 6 to 11 years who consumed wheat flour fortified with electrolytic iron, schoolchildren 6 to 11 years who consumed wheat flour fortified with reduced iron, nonpregnant women who consumed wheat flour fortified with electrolytic iron, and nonpregnant women who consumed wheat flour fortified with reduced iron.
The 2 population groups represented by the studies were children ≤15 years (n = 14 subgroups) and women of reproductive age (n = 12 subgroups) (Table 2 and Table S2 in the Supporting Information online). The prefortification measurement was taken as early as 1992 in Venezuela33 and as late as 2009 in Nepal37; most studies gathered baseline data between 2000 and 2009 (Table 2). The sample sizes in the studies ranged from a low of 80 children aged 2 to 15 years30 to a high of 9189 children aged 2 to 5 years27 (Table 3).
Table 3.
Results for primary outcomes: ferritin level, low ferritin status, hemoglobin level, and prevalence of anemia in 26 subgroups
| Reference | Country | Study population (subgroup) | Sample size | Pre- and postfortification marker(s) of iron status | Definition of iron deficiency | Pre- and postfortification prevalence of iron deficiency (%) | Pre- and postfortification hemoglobin levels | Definition of anemia | Pre- and postfortification prevalence of anemia (%) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Huo et al.28 | China | Nonpregnant WRA, 20–60 y | Intervention, prefortification: 268; postfortification: 213. Control, prefortification: 277; postfortification: 235 |
Mean (SD): FEP: Control: 46.5 (10.0) μg/dL → 46.1 (10.7) μg/dL. Intervention: 46.4 (10.9) μg/dL → 43.2 (8.9) μg/dL, SSDa. Serum iron: Control: 0.80 (0.14) mg/L → 0.81 (0.19) mg/L. Intervention: 0.80 (0.16) mg/L → 0.85 (0.15) mg/L, SSDa |
NR | NR | Mean (SD): Control: 129.7 (12.2) g/L → 129.8 (13.2) g/L, SNR. Intervention: 129.3 (14.9) g/L → 132.0 (14.3) g/L, SSDb | Hb <120 g/L. No reported adjustments | Control: 30.2% → 29.8%. Intervention: 29.5 → 27.2%, SNR |
| Huo et al.29 | China | WRA, 20–60 y | Intervention, prefortification: 308; postfortification: 269 Control, prefortification: 298; postfortification: 247 |
Mean (SD): FEP: Control: 44.5 μg/dL → 46.5 (13.4) μg/dL. Intervention: 42.6 (13.7) μg/dL → 38.9 (7.5) μg/dL, SSDa. Serum iron: Control: 0.76 (0.28) mg/L → 0.76 (0.26) mg/L. Intervention: 0.75 (0.27) mg/L → 0.86 (0.16) mg/L, SSDa |
NR | NR | Mean (SD): Control: 131.9 g/L (13.3) → 131.58 (13.0) g/L. Intervention: 132.2 (13.3) g/L → 135.7 (14.3) g/L, SSDa | NR | Control: 13.1% → 14.2%. Intervention: 15.1% → 10.8%, SNR |
| Tazhibayev et al.30 | Azerbaijan | Children, 2–15 y | Prefortification: 80; postfortification: NR | Mean (SE): Ferritin: 29.4 (2.0) μg/dL → 36.7 (2.6) μg/dL, NS | Children <5 y: ferritin <12 μg/dL; children >5 y: ferritin <15 μg/dL. No reported adjustments | 15.2% → 16.3%, SSD | Mean (SE): 120 (10) g/L → 123 (1) g/L, SSD | 6–59 mo: Hb <110 g/L; 5–11 y: Hb <115 g/L; ≥ 12 y: Hb <120 g/L. No reported adiustments | 20.9% → 6.3%, SSD |
| Tazhibayev et al.30 | Kazakhstan | Children, 2–15 y | Prefortification: 80; postfortification: NR | Mean (SE): Ferritin: 17.6 (1.5) μg/dL → 32.5 (3.8) μg/dL, SSD | Children <5 y: ferritin <12 μg/dL; children ≥5 y: ferritin <15 μg/dL. No reported adjustments | 43.8% → 22.9%, SSD | Mean (SE): 114 (2) g/L → 123 (1) g/L, SSD | 6–59 mo: Hb <110 g/L; 5–11 y: Hb <115 g/L; ≥12 y: Hb <120 g/L. No reported adjustments | 50% → 32.4%, SSD |
| Tazhibayev et al.30 | Mongolia | Children, 2–15 y | Prefortification: 80; postfortification: NR | Mean (SE): Ferritin: 38.0 (2.2) μg/dL → 41.8 (4.1) μg/dL, NS | Children <5 y: ferritin <12 μg/dL; children ≥5 y: ferritin <15 μg/dL. No reported adjustments | 5% → 16.4%, NS | Mean (SE): 126 (1) g/L → 129 (2) g/L, NS | 6–59 mo: Hb <110 g/L; 5–11 y: Hb <115 g/L; ≥12 y: Hb <120 g/L. No reported adjustments | 12.5% → 20.3%, NS |
| Tazhibayev et al.30 | Tajikistan | Children, 2–15 y | Prefortification: 80; postfortification: NR | Mean (SE): Ferritin: 32.8 (2.0) μg/dL → 48.4 (4.8) μg/dL, SSD | Children <5 y: ferritin <12 μg/dL; children ≥5 y: ferritin <15 μg/dL. No reported adjustments | 8.7% → 12.9%, NS | Mean (SE): 107 (2) g/L → 128 (2) g/L, SSD | 6–59 mo: Hb <110 g/L; 5–11 y: Hb <115 g/L; ≥12 y: Hb <120 g/L. No reported adjustments | 70% → 20.3%, SSD |
| Tazhibayev et al.30 | Uzbekistan | Children, 2–15 y | Prefortification: 80; postfortification: NR | Mean (SE): Ferritin: 31.2 (1.6) μg/dL → 25.7 (1.6) μg/dL, NS | Children <5 y: ferritin <12 μg/dL; children ≥5 y: ferritin <15 μg/dL. No reported adjustments | 8.1% → 22.6%, NS | Mean (SE): 119 (1) g/L → 132 (2) g/L, SSD | 6–59 mo: Hb <110 g/L; 5–11 y: Hb <115 g/L; ≥12 y: Hb <120 g/L. No reported adjustments | 31.4% → 16.7%, SSD |
| Assuncao et al.31 | Brazil | Children, <6 y | Prefortification (2004): 507; postfortification (2008): 799 | NR | Ferritin <12 ng/mL or transferrin saturation <20% | NR | Mean (SE): 113 (1.3) g/L → 111 (0.9) g/L, SSDc | Hb <110 g/L. No reported adjustments | 30.2% → 42.6%, SSDd |
| Sadighi et al.32 | Iran (Bushehr Province) | WRA, 15–49 y | Prefortification: 593; postfortification: 600 | Mean (SD): Ferritin: 32.8 (48.4) ng/mL → 41.9 (44.3) ng/mL, SSD | Ferritin <10ng/mL. No reported adjustments | 22.2% → 15.7%, SSD | Mean (SD): 136 (16) g/L → 129 (14) g/L, SSD | Nonpregnant women: Hb <120 g/L; pregnant women: Hb <110 g/L. No reported adjustments | 12.1% → 20.8%, SSD |
| Sadighi et al.32 | Iran (Golestan Province) | WRA, 15–49 y | Prefortification: 579; postfortification: 652 | Mean (SD): Ferritin: 31.5 (42.9) ng/mL → 47.7 (46.3) ng/mL, SSD | Ferritin <10 ng/mL. No reported adjustments | 26.7% → 14.6%, SSD | Mean (SD): 129 (13) g/L → 125 (11) g/L, SSD | Nonpregnant women: Hb <120 g/L; pregnant women: Hb <110 g/L. No reported adjustments | 19.3% → 25.6%, SSD |
| Costa et al.27 | Brazil | Children, 2–5 y | Prefortification: 9189; postfortification: 329e | NR | NR | NR | Mean (SD): NR → 119.9 (14.3) g/L | Hb <110 g/L. No reported adjustments | 30.2–68.8% → 20.9%, SNR |
| Layrisse et al.33 | Venezuela | School children, 7 y, 11 y, and 15 y | Prefortification (1992): 282; postfortification (1999): 545 | Geometric mean: Ferritin: 13.46 μg/dL → 24.1 μg/dL, SSD | Girls & boys, 7–11 y: ferritin <10 μg/dL; girls & boys, 15 y: ferritin <12 μg/dL. No reported adjustments | 37.2% → 15.5%, NS | NR | 7 y girls & boys: Hb <115 g/L; 11 –15 y girls: Hb <120 g/L; 11 y boys: Hb <125 g/L; 15 y boys: Hb <130 g/L. No reported adjustments | 18.1% → 17.1%, NS |
| Northrop-Clewes et al.34 | Uzbekistan | WRA | Prefortification: 4333; postfortification: 2584 | Geometric mean: Ferritin: NR → 17.1 μg/dLf, SNR | Ferritin <12 μg/dL. Ferritin adjusted for CRP | NR → 47.5% (95%CI: 45.1–49.9), SNR | Geometric mean (95%CI): NR → 121.6 (120.4–122.7) g/L | 1996 and 2008: Hb <120 g/L. In 2008, Hb adjusted for altitude and smoking | 60.4% → 34.4% (95%CI: 32.0–36.7), SNR |
| National Food & Nutrition Centre35 | Fiji | Nonpregnant WRA, 15–45 y | Prefortification: NR; postfortification: 869 | Mean (SD): Ferritin: 51.7 (NR) μg/dL → 76.7 (41.63) μg/dL, SSD | Ferritin <15 μg/dL. No reported adjustments | 22.9% → 7.9% (95%CI: 6.3–10.0), SSD | Mean (SD): 122 (NR) g/L → 124.2 (13.7) g/L, SSD | Hb <120 g/L. No reported adjustments | 40.3% → 27.6% (95%CI: 24.7–30.7)9, SSD |
| Nestel et al.36 | Sri Lanka | Preschool children, 9–71 mo, electrolytic iron | Intervention, prefortification: 247; postfortification: 146 Control, prefortification: 267; postfortification: 131 |
NR | NR | NR | Mean (SD): Electrolytic: 122.2 (12.2) g/L → 126.3 (10.7) g/L, NSh | Children <60 mo: Hb <110 g/L; children 5–11.9 y: Hb <115 g/L. No reported adjustments | Electrolytic: 16.6% → NR. Control: 18.4% → NR |
| Nestel et al.36 | Sri Lanka | Preschool children, 9–71 mo, reduced iron | Intervention, prefortification: 231; postfortification: 81 Control, prefortification: 267; postfortification: 131 |
NR | NR | NR | Mean (SD): Reduced: 119.9 (15.3) g/L → 125.5 (11.3) g/L, NSh | Children <60 mo: Hb <110 g/L; children 5–11.9 y: Hb <115 g/L. No reported adjustments | Reduced: 19.5% → NR. Control: 18.4% → NR |
| Nestel et al.36 | Sri Lanka | School children, 6–11 y, electrolytic iron | Intervention, prefortification: 278; postfortification: 180 Control, prefortification: 306; postfortification: 180 |
NR | NR | NR | Mean (SD): Electrolytic: 129.6 (10.7) g/L → 128.4 (11.6) g/L, NSh | Children 5–11.9 y: Hb <115 g/L; children ≥12 y: Hb <120 g/L. No reported adjustments | Electrolytic: 6.1 % → NR. Control: 8.5% → NR |
| Nestel et al.36 | Sri Lanka | School children 6–11 y, reduced iron | Intervention, prefortification: 326; postfortification: 152 Control, prefortification: 306; postfortification: 180 |
NR | NR | NR | Mean (SD): Reduced: 129.2 (10.8) g/L → 129.9 (10.0) g/L, NSh | Children 5–11.9 y: Hb <115 g/L; children ≥12 y Hb <120 g/L. No reported adjustments | Reduced: 7.4% → NR. Control: 8.5% → NR |
| Nestel et al.36 | Sri Lanka | Nonpregnant WRA, electrolytic iron | Intervention, prefortification: 486; postfortification: 187 Control, prefortification: 541; postfortification: 198 |
NR | NR | NR | Mean (SD): Electrolytic: 126.2 (17.5) g/L → 125.9 (14.9) g/L, NSh | Hb <120 g/L. No reported adjustments | Electrolytic: 22.8% → NR. Control: 29.0% → NR |
| Nestel et al.36 | Sri Lanka | Nonpregnant WRA, reduced iron | Intervention, prefortification: 546; postfortification: 180 Control, prefortification: 541; postfortification: 198 |
NR | NR | NR | Mean (SD): Reduced: 120.7 (18.6) g/L → 122.4 (18.4) g/L, NSh | Hb <120 g/L. No reported adjustments | Reduced: 34.4% → NR. Control: 29.0% → NR |
| Nepali Technical Assistance Group37 | Nepal | WRA, 15–45 y | Prefortification: 270; postfortification: 570 | NR | NR | NR | Mean (SD): 125 (18.8) g/L → 131 (13.5) g/L, SSD | Hb <120 g/L. Hb adjusted for altitude. No reported adjustments | 33% → 18%. SNR |
| Fujimori et al.38 | Brazil | Pregnant WRA | Prefortification: 6062; postfortification: 6057 | NR | NR | NR | Mean (SD): 118 (13) g/L → 119 (12) g/L, SSD | Hb <110 g/L. No reported adjustments | 25.5% → 20.2%, SSD |
| Micronutrient Initiative et al.39 | India | Pregnant and lactating WRA | Intervention, prefortification: 376; postfortification: 372 Control, prefortification: 378; postfortification: 386 |
NR | NR | NR | Mean (SD): Intervention: 117.3 (17.1) g/L → 120.4 (17.1) g/L, SSDb,i. Control: 115.2 (16.6) g/L → 112.3 (16.3) g/L, SSDb,i | Hb cut-off NR. Hb adjusted for altitude | Intervention: 52.1% → 44.7%, SSDb,i. Control: 57.3%j → 63.2%, SNRi |
| Micronutrient Initiative et al.39 | India | School children, 5–11 y | Intervention, prefortification: 282; postfortification: 368 Control, prefortification: 343; postfortification: 367 |
NR | NR | NR | Mean (SD): Intervention: 120.6 (13.5) g/L → 125.2 (12.4) g/L, SSDb,i. Control: 115.9 (12.1) g/L → 122.8 (9.8) g/L, NSb,i | Children 5–11 y: Hb <115 g/L; children 12–14 y: Hb <120 g/L; nonpregnant females: Hb <120g/L. Hb adjusted for altitude | Intervention: 59.9% → 45.9%, SSDb,i. Control: 64.4% → 56.9%, SSDb,i |
| Micronutrient Initiative et al.39 | India | Adolescent WRA, 12–19 y | Intervention, prefortification: 296; postfortification: 359 Control, prefortification: 328; postfortification: 343 |
NR | NR | NR | Mean (SD): Intervention: 120.6 (16.5) g/L → 127.4 (14.1) g/L, SSDb,i. Control: 116.4 (16.9) g/L → 120.9 (13.4) g/L, SSDb,i | Children 12–14 y: Hb <120 g/L; nonpregnant females: Hb <120g/L. Hb adjusted for altitude | Intervention: 70.3% → 54.3%, SSDb,i. Control: 79.9% → 75.5%, NSb,i |
| Micronutrient Initiative et al.39 | India | Preschool children | Intervention, prefortification: 376; postfortification: 354 Control, prefortification: 364; postfortification: 370 |
NR | NR | NR | NR | NR | NR |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; FEP, free erythrocyte protoporphyrin; Hb, hemoglobin; NR, not reported; NS, not significantly different; SD, standard deviation; SE, standard error; SNR, statistics not reported; SSD, statistically significantly different; WRA, women of reproductive age.
Comparing the postfortification value between intervention and control.
Comparing the postfortification value with the prefortification value.
Hemoglobin across 4 surveys showed a statistically significant linear trend.
Elemoglobin across 4 surveys was statistically heterogeneous.
The sample size is sometimes noted as 459 children (e.g., Table 1) or 329 children (Abstract and Methods section) in the document.27
This represents ferritin adjusted for inflammation; geometric mean ferritin unadjusted for inflammation was 17.3 μg/dL.
The 24.7 value is reported in Table 3.5.2 of the document and as 27.7 in the narrative of the same document.35
Comparing mean change from pre- to postfortification in the intervention group with the mean change from pre- to postfortification in the control group.
The “difference of the difference” was not statistically tested.
The 57.3 value seems to be erroneously reported as 37.3% in Figure 3 of the document.39
All 13 programs added iron to flour (Table 4 and Table S2 in the Supporting Information online). In 2 countries, fortification legislation allowed 2 or more iron compounds to be used in the programs that were evaluated. Brazil31 permitted ferrous sulfate, ferrous fumarate, reduced iron 325 Tyler mesh, electrolytic iron 325 Tyler mesh, NaFeEDTA (ferric sodium ethylenediaminetetraacetate), and iron bisglycinate chelate for wheat and maize flour, and Venezuela33 allowed ferrous fumarate and electrolytic iron for maize flour.
Table 4.
Comparison of the iron compound and concentration used in fortification programs with WHO recommendations for flour2 in 13 countries
| Country | Reference | Flour | Flour extraction rate | Iron added | WHO recommendations followeda,b |
|
|---|---|---|---|---|---|---|
| Iron compound | Iron concentration | |||||
|
| ||||||
| Azerbaijan | Tazhibayev et al.30 | Wheat | First grade flour: 61%–72%. Premium grade flour: 55%–60% | First grade: 40 mg/kg as electrolytic iron. Premium grade: 50 mg/kg as electrolytic iron | Yes | Yes |
| Brazil | Assuncao et al.,31 Costa et al.,27 Fujimori et al.38 | Wheat, maize | Not reported | 42 mg/kg as ferrous sulfate, ferrous fumarate, reduced iron 325 Tyler mesh, electrolytic iron 325 Tyler mesh, NaFeEDTA, or iron bisglycinate chelate | Cannot determine | Cannot determine |
| China | Huo et al.,28 Huo et al.29 | Wheat | Not reported, but wheat flour was grade 2 flour | 20 mg/kg as electrolytic iron (Huo et al.28). 20 mg/kg as NaFeEDTAb (Huo et al.29) |
Yes | Noc |
| Fiji | National Food & Nutrition Centre35 | Wheat | Not reported | 60 mg/kg as hydrogen-reduced electrolytic iron | Yes | Yes |
| India | Micronutrient Initiative et al.39 | Atta wheat | Atta flour: 80%–85% | 60 mg/kg (compound not specified) | Cannot determine | Cannot determine |
| Iran | Sadighi et al.32 | Wheat | Not reported | 30 mg/kg as ferrous sulfate | Yes | Cannot determine |
| Kazakhstan | Tazhibayev et al.30 | Wheat | First grade flour: 61%–72%. Premium grade flour: 55%–60% | First grade: 40 mg/kg as electrolytic iron. Premium grade: 50 mg/kg as electrolytic iron | Yes | No |
| Mongolia | Tazhibayev et al.30 | Wheat | First grade flour: 61%–72%. Premium grade flour: 55%–60% | First grade: 40 mg/kg as electrolytic iron. Premium grade: 50 mg/kg as electrolytic iron | Yes | No |
| Nepal | Nepali Technical Assistance Group37 | Maize, wheat, millet | Not reported | 25 mg/kgd (compound not specified) | Cannot determine | Cannot determine |
| Sri Lanka | Nestel et al.36 | Wheat | Not reported | 66 mg/kg as electrolytic iron or reduced iron | No | No |
| Tajikistan | Tazhibayev et al.30 | Wheat | First grade flour: 61%–72%. Premium grade flour: 55%–60% | First grade: 40 mg/kg as electrolytic iron. Premium grade: 50 mg/kg as electrolytic iron | Cannot determine | Cannot determine |
| Uzbekistan | Tazhibayev et al.,30 Northrop-Clewes et al.34 | Wheat | 61%–72% | 40 mg/kg as electrolytic iron | Yes | No |
| Venezuela | Layrisse et al.33 | Maize, wheat | Not reported, but wheat flour was “white” | Wheat: 20 mg/kg as ferrous fumarate. Maize: 50 mg/kg as ferrous fumarate until 1994; since then, 30 mg/kg as ferrous fumarate and 20 mg/kg as electrolytic iron | Yes | Cannot determine |
The WHO recommendations for wheat and maize flour specify iron compounds and levels of iron, zinc, vitamin A, folic acid, and vitamin B12 to be added to flour.2 Recommendations depend on per capita intake of flour and the extraction level of flour. For iron levels, “yes” indicates that at least the WHO-recommended iron level was added to the flour. “No” indicates that less than the WHO-recommended iron level was added to the flour.
Without information on per capita flour intake or iron compound, it cannot be determined whether WHO recommendations are followed.
The compound specified in the Huo et al.29 document is electrolytic iron; however, the first author confirmed that the iron compound used in the study was NaFeEDTA (Junsheng Huo, China Center for Disease Control and Prevention, personal communication).
The concentration for iron added in the Nepali Technical Assistance Group study was obtained from Felix Brooks-Church (Project Healthy Children, personal communication).
Alignment of programs with WHO recommendations for flour fortification
All of the fortification programs included in this review began before WHO recommendations for wheat and maize flour fortification were issued in 2009.2 The recommendations for iron fortification depend on the per capita intake of the flour to be fortified and the extraction level of flour (i.e., whether it is refined or whole grain). The iron compound used in fortification was in line with WHO recommendations for 8 of 13 countries (Table 4). For the concentration of iron, 2 of the 13 countries reported adding at least the WHO-recommended levels to the flour.
Primary outcomes and age groups
The ferritin level (mean/median), prevalence of low ferritin, hemoglobin level (mean), and prevalence of anemia, stratified by subgroup, are shown in Table 3. Table 540 summarizes these primary outcomes. For children 15 years of age and younger, there were statistically significant increases in ferritin and hemoglobin for 330,33 of 6 and 530,39 of 12 subgroups, respectively, from the pre- to the postfortification periods. For children, there were statistically significant decreases in the prevalence of low ferritin and anemia for 130 of 6 and 430 of 13 subgroups, respectively. For women of reproductive age, there were statistically significant increases in ferritin and hemoglobin for 528,29,32,35 of 5 and 628,29,35,37–39 of 11 subgroups, respectively. There were statistically significant decreases in the prevalence of low ferritin, anemia, and iron-deficiency anemia for 332,35 of 3, 435,38,39 of 12, and 0 of 2, subgroups, respectively, in women of reproductive age.
Table 5.
Statistically significant changes in biological markers of iron status and anemia from flour fortification effectiveness trials, stratified by target population (n = 26 subgroups)
| Biological marker | Children ≤15 y (n = 14 subgroups) |
Women of reproductive age (n = 12 subgroups) |
||||
|---|---|---|---|---|---|---|
| Yes | No | Not assesseda | Yes | No | Not assesseda | |
|
| ||||||
| Increased ferritinb | 3 | 3 | 8 | 5 | 0 | 7 |
| Decreased prevalence of low ferritin | 1 | 5 | 8 | 3 | 0 | 9 |
| Increased hemoglobin | 5 | 7 | 2 | 6 | 5 | 1 |
| Decreased prevalence of anemia | 4 | 9 | 1 | 4 | 8 | 0 |
| Decreased prevalence of IDA | 0 | 0 | 14 | 0 | 2 | 10 |
Abbreviation: IDA, iron-deficiency anemia.
This outcome was not assessed.
For 2 subgroups in women, the markers of iron status were free erythrocyte protoporphyrin and serum iron, instead of ferritin.28,29 An improvement in iron status is marked by a decrease in free erythrocyte protoporphyrin and an increase in serum iron40; both of these improved and were classified as “yes” for “increased ferritin.” Only 2 documents specified that ferritin was expressed as a geometric mean.33,34
Primary outcomes and WHO flour fortification recommendations
The relationship between adding the WHO-recommended iron compounds and at least the WHO-recommended iron levels and having the expected outcomes was assessed. Only 2 subgroups30,35 met both WHO recommendations (iron type and concentration). Anemia prevalence decreased in both subgroups and ferritin improved in 1, but not the other.
Program implementation information
Most documents (n = 9) provided some evidence on the implementation of the flour fortification program under study: evidence of either compliance with standards or coverage of fortification (Table 6). The compliance and coverage information was not reported in a standard manner across studies, nor are there guidelines to classify compliance and coverage (e.g., as inadequate or adequate) information. Thus, it was not possible to link the compliance and coverage information with outcomes.
Table 6.
Implementation evidence reported on flour fortification programs
| Reference | Country | Fortification compliance | Fortification coverage |
|---|---|---|---|
|
| |||
| Huo et al.28 | China | None reported | Intervention group at baseline: of all floura consumed, 51.2% was fortified. Intervention group at last follow-up at 36 mo: of all flour consumed, 62.9% was fortified |
| Huo et al.29 | China | None reported | Intervention group at baseline: of all floura consumed, 62.9% was fortified. Intervention group at last follow-up at 36 mo: of all flour consumed, 53.7% was fortified |
| Tazhibayev et al.30 | Azerbaijan | At postfortification measurement (round 3), 83%–100% of wheat flour in home was fortified according to iron spot test | At postfortification measurement (round 3), 25% of study women reported using fortified wheat flour products in their homes |
| Tazhibayev et al.30 | Kazakhstan | At postfortification measurement (round 3), 15%–20% of wheat flour consumed annually nationwide was fortified and 83%–100% of wheat flour in home was fortified per iron spot test | At postfortification measurement (round 3), 100% of study women reported using fortified wheat flour products in their homes |
| Tazhibayev et al.30 | Mongolia | At postfortification measurement (round 3), 15%–20% of wheat flour consumed annually nationwide was fortified and 83%–100% of wheat flour in home was fortified per iron spot test | At postfortification measurement (round 3), 88% of study women reported using fortified wheat flour products in their homes |
| Tazhibayev et al.30 | Tajikistan | At postfortification measurement (round 3), 15%–20% of wheat flour consumed annually nationwide was fortified and 83%–100% of wheat flour in home was fortified per iron spot test | At postfortification measurement (round 3), 49% of study women reported using fortified wheat flour products in their homes |
| Tazhibayev et al.30 | Uzbekistan | At postfortification measurement (round 3), 35%–40% of wheat flour consumed annually nationwide was fortified and 83%–100% of wheat flour in home was fortified per iron spot test | At postfortification measurement (round 3), 87% of study women reported using fortified wheat flour products in their homes |
| Assuncao et al.31 | Brazil | None reported | None reported |
| Sadighi et al.32 | Iran | In 2009, flour samples taken from 3 factories in Bushehr Province contained 50–55 mg iron/kg. In 2009,119 flour samples were obtained from bakeries in Golestan Province; 94.1% of samples contained iron | One bread sample was taken per cluster of women in Bushehr Province in 2009; 98.7% of the samples were fortified with iron |
| Costa et al.27 | Brazil | None reported | None reported |
| Layrisse et al.33 | Venezuela | During the period 1993–1999, analysis of random samples of commercial flour showed 80%–120% contained the expected level of iron | None reported |
| Northrop-Clewes et al.34 | Uzbekistan | Among 2503 households where any wheat flour was available, 41.6% (95%CI: 39.2–43.9) of all flour tested was fortified per iron spot test | The overall weighted average coverage of UDM first grade flour in households at the time of the survey was 59.8% |
| National Food & Nutrition Centre35 | Fiji | Percent of iron standard met by flour samples tested ranged from 91.7% to 123.3% | None reported |
| Nestel et al.36 | Sri Lanka | Mean iron content of flour: 78 ± 15.2b mg/kg (electrolytic), 76 ± 6.6 mg/kg (reduced), and 15 ± 1.7 mg/kg (control); 100% and 95% of electrolytic and reduced iron flour was fortified to acceptable levels | None reported |
| Nepali Technical Assistance Group37 | Nepal | Baseline: iron content in maize flour from 30 household samples was 36.6 mg/kg. Endline II: iron content in maize flour from 60 household samples was 45.3 mg/kg; 81% of 535 flour samples contained added iron per iron spot test | 69.7% of women reported consuming fortified flour at endline II |
| Fujimori et al.38 | Brazil | None reported | None reported |
| Micronutrient Initiative et al.39 | India | None reported | None reported |
Abbreviations: CI, confidence interval; UDM, Uzdonmakhsulot, a state joint stock company association for wheat flour.34
At baseline, this refers to compensation flour that was to be fortified as part of the program.
Report does not specify whether error is standard deviation or another measure.
DISCUSSION
This was the first review of effectiveness studies describing the public health impact of implementing the fortification of wheat flour only, or the fortification of both wheat flour and maize flour, on iron status and anemia. Given the large number of fortification programs, few documents were identified from the published and gray literature. That is, few effectiveness evaluations of flour fortification programs have been completed for iron status and anemia. Subgroup analyses indicate that flour fortification is associated with consistent reductions in low ferritin prevalence in women, but not in children. Further, a reduction in anemia prevalence was observed in only one-third of the subgroups of women and children studied. There is insufficient evidence to evaluate whether programs that followed WHO iron recommendations for flour fortification had better outcomes. While most studies reported on some degree of compliance with national standards or coverage of fortification programs in the study area, this information could not be used to assess whether programs were well implemented, nor was it possible to link program implementation information with outcomes achieved.
In public health practice, the design of a program can affect its impact. All of the studies reviewed initiated fortification before global recommendations for flour fortification were issued.2 As part of the deliberations to establish those recommendations, Hurrell et al.7 assessed the design of flour fortification programs worldwide with respect to iron compounds and iron levels. They concluded that, of 78 countries with wheat-flour fortification programs at the time (e.g., mandatory, voluntary, World Food Program, and planned), only 9 were using recommended compounds or levels. Thus, few of the programs would be expected to have a positive impact on iron status. In the current analysis, 8 of 13 countries used the recommended iron compound, while only 2 of the 13 countries reported adding minimum iron levels. In those countries that used recommended iron compounds and levels, 1 of 2 subgroups showed reductions in the prevalence of low ferritin, and 2 of 2 subgroups had reductions in anemia prevalence. Taken together, these results suggest that more countries should be encouraged to design their fortification programs in line with WHO recommendations and to evaluate their fortification programs and adjust them as necessary.
Program implementation is another factor that contributes to program impact. Most studies reported information on program implementation, but the results for compliance and coverage varied widely. For example, in Venezuela, the iron level in commercial flour samples contained 80%–120% of expected levels, suggesting strong compliance.33 In contrast, in Azerbaijan, 25% of study women reported using fortified wheat-flour products in their homes, suggesting poor coverage.30 A case study from Fiji offers potential insights into the importance of program implementation.35 In Fiji, high compliance (flour samples met 91.7%–123.3% of the standard for iron) was reported, and WHO recommendations for iron compounds and levels were followed; this combination may have led to positive outcomes (reduction in the prevalence of low ferritin and anemia among women of childbearing age). These results suggest that two factors are important for countries with flour fortification programs: 1) compliance with and coverage of the program, in addition to the design of the program, should be documented; and 2) information on compliance and coverage should be used to determine when to evaluate the potential health impact of a program (i.e., when compliance and coverage are consistently high, and when the program is optimally designed in relation to international recommendations).
Most studies assessed hemoglobin levels or the prevalence of anemia; 5 used these biomarkers alone to evaluate the success of the fortification program.27,31,36–38 Biomarkers specific to iron or folate status, for example, were assessed infrequently (n = 7 for iron28–30,32–35 and n = 4 for folate28,30,34,35). Researchers who have assessed multiple nutrition indicators as well as hemoglobin have found that anemia may not be principally due to iron deficiency (e.g., <10% of anemia among preschool children and nonpregnant, nonlactating women in Bangladesh41 was caused principally by iron deficiency) or other nutrient deficiencies (e.g., anemia may be caused by bacteremia, malaria, hookworm infestation, HIV infection, or the G6PD−202/−376 genetic disorder42). In these cases, flour fortified with iron, folic acid, and other nutrients will have a limited impact on hemoglobin levels and anemia prevalence. For these reasons, the sole use of anemia biomarkers to assess the impact of flour fortification should be discouraged, and the use of biomarkers specific to the nutrients added to flour should be encouraged.
Study design influences both the ability to detect improvements in nutritional outcomes and the ability to attribute results to an intervention implemented on a large scale. Most studies were pre–post independent cross-sectional surveys, and most did not have a comparison group. Both of these factors preclude any changes observed in health outcomes from being unequivocally attributed to an intervention. When a study’s design limits the ability to attribute results to the intervention, confidence in assigning some contribution of the intervention to the results can be strengthened if there is adequate information about program implementation.43 Researchers have developed program-implementation pathways to express their a priori expectations of how a program will lead to improvements in measurable health outcomes.44 Then, they measure indicators along the implementation pathway to conclude whether it is more or less likely that the program contributed to the outcomes observed.
Some of the studies in this review included data on iron compounds and levels as well as information on program compliance and coverage, which are important elements along the program-implementation pathway for flour fortification.45 For example, Fijian investigators reported the addition to flour of the recommended iron compound at the recommended level, evidence that flour was adequately fortified (based on measurement of samples from mills, retail outlets, or households), and the intake of flour-containing foods in sufficient quantities to fill nutrient gaps in the diet.35 This study reported reductions in the prevalence of both low ferritin and anemia. This example suggests that program evaluations should be designed to measure inputs and outputs of the fortification program (as elucidated through a program-implementation pathway) in addition to health impacts. Such information can add plausibility to findings about the impact of fortification on health outcomes if the study design precludes attributing causality to fortification.
This review has several limitations. The number of documents obtained was small and included no African countries, thus compromising the generalizability of the results. The design of most studies precludes attributing improvements in health outcomes to flour fortification. Moreover, the lack of information on program implementation adds to this problem in some cases. The presentation of findings by subgroup resulted in some studies contributing many more data points to the analysis than others, potentially biasing the findings. Furthermore, subgroup analyses resulted in small sample sizes that limited the ability to detect meaningful effects.
In turn, this review has several strengths. This was the first attempt to assess the effectiveness of flour fortification on iron and anemia outcomes. A rigorous search strategy of both the published and the gray literature was followed, which permitted inclusion of several unpublished studies. Program-specific information that could influence health outcomes, such as the iron compounds used, the levels of iron added, compliance with national standards, and coverage of the program, was systematically assessed.
CONCLUSION
This is the first systematic review of the effectiveness of flour fortification on iron and anemia outcomes. There is limited evidence of the effectiveness of flour fortification in reducing the prevalence of anemia; however, evidence of effectiveness in reducing the prevalence of low ferritin in women is more consistent. There is also limited evidence that relates health outcomes with compliance with international fortification recommendations. This review highlights the challenges of evaluating the impact of fortification when implemented on a wide scale. These challenges, along with those related to the design and execution of fortification programs, represent opportunities to strengthen both existing fortification programs and those yet to be implemented.
Supplementary Material
Table S1 PRISMA 2009 checklist
Table S2 Extracted information for the 13 documents included in the review
ACKNOWLEDGMENTS
Funding. H.P.’s time was supported by an appointment to the Research Participation Program at the US Centers for Disease Control and Prevention (CDC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC. R.S.’s time was supported by the Global Alliance for Improved Nutrition, and Z.M. and M.K.S.’s time was supported by the CDC.
Footnotes
Declaration of interest. The authors have no relevant interests to declare.
Disclaimer. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Contributor Information
Helena Pachón, Food Fortification Initiative, Atlanta, Georgia, USA, and the Hubert Department of Global Health, Emory University, Atlanta, Georgia, USA..
Rebecca Spohrer, Global Alliance for Improved Nutrition, Geneva, Switzerland..
Zuguo Mei, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA..
Mary K. Serdula, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
REFERENCES
- 1.World Health Organization, Food and Agriculture Organization of the United Nations. Guidelines on food fortification with micronutrients. Geneva: World Health Organization; 2006. [Google Scholar]
- 2.World Health Organization, Food and Agriculture Organization of the United Nations, United Nations Children’s Fund, Global Alliance for Improved Nutrition, the Micronutrient Initiative, and Flour Fortification Initiative. Recommendations on Wheat and Maize Flour Fortification. Meeting Report: Interim Consensus Statement. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 3.Food Fortification Initiative. FFI website. http://www.ffinetwork.org/global_progress/index.php. Accessed August 31, 2015.
- 4.Das JK, Salam RA, Kumar R, et al. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Syst Rev. 2013;2:67. doi: 10.1186/2046-4053-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Athe R, Rao MVV, Nair KM. Impact of iron-fortified foods on Hb concentration in children (<10 years): a systematic review and meta-analysis of randomized controlled trials. Public Health Nutr. 2013;17:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gera T, Sachdev HS, Boy E. Effect of iron-fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials. Am J Clin Nutr. 2012;96:309–324. [DOI] [PubMed] [Google Scholar]
- 7.Hurrell R, Ranum P, de Pee S, et al. Revised recommendation for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull. 2010;31(1 suppl):S7–S21. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasricha SR, De-Regil LM, Garcia-Casal MN, et al. Fortification of maize flour with iron for preventing anaemia and iron deficiency in populations. Cochrane Database Syst Rev. 2012;(11):CD010187. doi: 10.1002/14651858.CD010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Índice Bibliográfico Español en Ciencias de la Salud (IBECS). IBECS database. Madrid, Spain: Biblioteca Nacional de Ciencias de la Salud del Instituto de Salud Carlos III del Ministerio de Sanidad y Consumo de España. http://ibecs.isciii.es/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=IBECS&lang=e. Accessed August 22, 2014. [Google Scholar]
- 11.Scientific Electronic Library Online (SciELO). SciELO database. Sao Paulo, Brazil: FAPESP, CNPq, BIREME/OPS/OMS, FapUnifesp. http://www.scielo.org/php/index.php?lang=es. Accessed August 22, 2014. [Google Scholar]
- 12.Cochrane Central Register of Controlled Trials [Internet]. Cochrane Library. Hoboken, NJ, USA: John Wiley & Son, Inc. http://onlinelibrary.wiley.com/cochranelibrary/search. Accessed August 22, 2014. [Google Scholar]
- 13.MEDLINE. PubMed database. Bethesda, MD, USA: US National Library of Medicine. http://www.ncbi.nlm.nih.gov/pubmed. Accessed August 22, 2014. [Google Scholar]
- 14.Embase database. London, UK: Reed Elsevier. http://www.embase.com/home#/search:quickSearch/. Accessed August 22, 2014. [Google Scholar]
- 15.Web of Science database. Philadelphia,, PA, USA: Thomson Reuters. http://wokinfo.com/. Accessed August 22, 2014. [Google Scholar]
- 16.Cumulative Index to Nursing and Allied Health Literature. CINAHL database. Ipswich, MA, USA: EBSCO Nursing Resources. http://www.ebscohost.com/nursing/products/cinahl-databases/cinahl-complete. Accessed August 22, 2014. [Google Scholar]
- 17.POPLINE database. Washington, DC, USA: United States Agency for International Development. http://www.popline.org/. Accessed August 22, 2014. [Google Scholar]
- 18.AGRICOLA database. Washington, DC, USA: US Department of Agriculture, National Agricultural Library. http://agricola.nal.usda.gov/. Accessed August 22, 2014. [Google Scholar]
- 19.BIOSIS Previews database. Philadelphia, PA, USA: Thomson Reuters. http://thomsonreuters.com/biosis-previews/. Accessed August 22, 2014. [Google Scholar]
- 20.OpenGrey database. Paris, France: Institut de l’Information Scientifique et Technique- Laboratoire CNRS, Centre national de la recherche scientifique. http://www.opengrey.eu/. Accessed August 22, 2014. [Google Scholar]
- 21.Bibliomap. EPPI-Centre database of health promotion research. London, UK: EPPI-Centre. http://eppi.ioe.ac.uk/webdatabases/Intro.aspx?ID=7. Accessed August 22, 2014. [Google Scholar]
- 22.Trials Register of Promoting Health Interventions. TRoPHI database. London, UK: EPPI-Centre. http://eppi.ioe.ac.uk/webdatabases/Intro.aspx?ID=5. Accessed August 22, 2014. [Google Scholar]
- 23.Global Health Library database. Geneva, Switzerland: World Health Organization. http://www.globalhealthlibrary.net/php/index.php. Accessed August 22, 2014. [Google Scholar]
- 24.Indian Medical Journals. IndMED database. New Delhi, India: National Informatics Centre. http://indmed.nic.in/. Accessed August 22, 2014. [Google Scholar]
- 25.University of New Mexico Health Sciences Library and Informatics Center. Native Health Database. Albuquerque, NM, USA: University of New Mexico. https://hscssl.unm.edu/nhd/. Accessed August 22, 2014. [Google Scholar]
- 26.ProQuest Dissertation and Theses. Dissertation and Theses database. Ann Arbor, MI, USA: ProQuest. http://www.proquest.com/products-services/dissertations/. Accessed August 22, 2014. [Google Scholar]
- 27.Costa CA, Machado EH, Colli C, et al. Anemia in pre-school children attending day care centers of São Paulo: perspectives of the wheat and maize flour fortification [in Portuguese]. Nutrire: Rev Soc Bras Alim Nutr. 2009;34:59–74. [Google Scholar]
- 28.Huo J, Sun J, Huang J, et al. Effectiveness of fortified flour for enhancement of vitamin and mineral intakes and nutrition status in northwest Chinese villages. Food Nutr Bull. 2012;33:161–168. [DOI] [PubMed] [Google Scholar]
- 29.Huo J, Sun J, Huang J, et al. The effectiveness of fortified flour on micronutrient status in rural female adults in China. Asia Pac J Clin Nutr. 2011;20:118–124. [PubMed] [Google Scholar]
- 30.Tazhibayev S, Dolmatova O, Ganiyeva G, et al. Evaluation of the potential effectiveness of wheat flour and salt fortification programs in five Central Asian countries and Mongolia, 2002–2007. Food Nutr Bull. 2008;29: 255–265. [DOI] [PubMed] [Google Scholar]
- 31.Assuncao MCG, Santos IS, Barros AJD, et al. Flour fortification with iron has no impact on anaemia in urban Brazilian children. Public Health Nutr. 2012;15: 1796–1801. [DOI] [PubMed] [Google Scholar]
- 32.Sadighi J, Mohammad K, Sheikholeslam R, et al. Anaemia control: lessons from the flour fortification programme. Public Health. 2009;123:794–799. [DOI] [PubMed] [Google Scholar]
- 33.Layrisse M, Garcáa-Casal MN, Méndez-Castellano H, et al. Impact of fortification of flours with iron to reduce the prevalence of anemia and iron deficiency among school children in Caracas, Venezuela: a follow-up. Food Nutr Bull. 2002;23:384–389. [DOI] [PubMed] [Google Scholar]
- 34.Northrop-Clewes C, Hund L, Valadez J, et al. LC-LQAS Survey Report: Ministry of Health, Uzbekistan. National Flour Fortification Program. Tashkent, Uzbekistan: Ministry of Health; 2013. [Google Scholar]
- 35.National Food and Nutrition Centre. Impact of Iron Fortified Flour in Child Bearing Age (CBA) Women in Fiji, 2010 Report. Suva, Fiji: National Food and Nutrition Centre; 2012. [Google Scholar]
- 36.Nestel P, Nalubola R, Sivakeneshan R, et al. The use of iron-fortified wheat flour to reduce anemia among the estate population in Sri Lanka. Int J Vitam Nutr Res. 2004;74:35–51. [DOI] [PubMed] [Google Scholar]
- 37.Nepali Technical Assistance Group. An Impact Study on Small Scale Fortification Project in Lalitpur District Survey Period: February 2009–April 2011. Kathmandu, Nepal: Nepali Technical Assistance Group; 2011. [Google Scholar]
- 38.Fujimori E, Sato APS, Szarfarc SC, et al. Anemia in Brazilian pregnant women before and after flour fortification with iron. Rev Saúde Publica. 2011;45:1027–1035. [DOI] [PubMed] [Google Scholar]
- 39.Initiative Micronutrient, Child in Need Institute, Department of Social Welfare in West Bengal State. Wheat flour fortification: a pilot project. Kolkata, India: Child in Need Institute; 2008. [Google Scholar]
- 40.World Health Organization, Centers for Disease Control and Prevention. Assessing the Iron Status of Populations, Including Literature Reviews: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. 2nd ed. Geneva: World Health Organization; 2007. [Google Scholar]
- 41.icddr,b. United Nations Children’s Fund, Global Alliance for Improved Nutrition, Institute of Public Health and Nutrition. National Micronutrients Status Survey 2011–12. Dhaka, Bangladesh: icddr,b; 2013. [Google Scholar]
- 42.Calis JCJ, Phiri KS, Faragher EB, et al. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–899. [DOI] [PubMed] [Google Scholar]
- 43.Habicht J-P, Victora CG, Vaughan JP. Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. Int J Epidemiol. 1999;28:10–18. [DOI] [PubMed] [Google Scholar]
- 44.Olney DJ, Talukder A, Iannotti LL, et al. Assessing impact and impact pathways of a homestead food production program on household and child nutrition in Cambodia. Food Nutr Bull. 2009;30:355–369. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization, Centers for Disease Control and Prevention. Logic Model for Micronutrient Interventions in Public Health. Vitamin and Mineral Nutrition Information System. Ref. no. WHO/NMH/NHD/MNM/11.5. Geneva: World Health Organization; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 PRISMA 2009 checklist
Table S2 Extracted information for the 13 documents included in the review