Abstract
Pediatric pulmonary hypertension (PH) is a severe, life‐threatening disease associated with diverse cardiac, pulmonary, and systemic disorders, which generally requires expertise from multiple disciplines for management. Unfortunately, expert centers are limited, often due to inadequate resources or unfamiliarity with needed components for success. The Pediatric Pulmonary Hypertension Network (PPHNet) includes expert centers in North America specifically dedicated to advancing the field of pediatric PH through research and excellent clinical care. PPHNet member sites were queried for valuable program components and these findings were discussed for consensus. Here we provide a collective overview of key elements of an optimal pediatric PH program: team composition, access to services, and commitment to education. It is our intention that this document will assist newer and/or smaller programs identify avenues and resources for growth and provide avenues for collaboration.
Keywords: program development, pulmonary vascular disease, regional care
INTRODUCTION
Pulmonary hypertension (PH) causes significant morbidity and mortality in children either as an isolated condition (idiopathic pulmonary arterial hypertension; IPAH) or in the setting of diverse diseases, including cardiac, pulmonary, hematologic, and other systemic disorders of childhood.1, 2 Over the past decade, the field of pediatric PH has evolved into a subspecialty, caring for very high‐risk, complex children. Pediatric PH programs grew throughout North America to meet this need but usually consisted of one physician who was relied upon heavily for expertise and continuous coverage. During this early era (1990–2010), these programs faced multiple challenges, including the lack of pediatric‐specific guidelines for medical decision‐making, minimal administrative support, and insufficient professional resources.
Today, a number of established pediatric PH programs in North America at varying levels of size and breadth have developed to meet the changing needs of the growing pediatric PH population. Although joint guidelines on the care of children with PH from the American Heart Association (AHA) and American Thoracic Society (ATS) recommend the development of multidisciplinary programs to optimize the management of neonates, infants, and children with PH, 3 details regarding the nature and composition of these programs are lacking. While some pediatric PH programs have developed and successfully overcome early hurdles, many other medical centers either lack PH programs or are still struggling to surmount these critical barriers to enhance care delivery, improve health outcomes and enhance program growth.
In 2011 the Pulmonary Hypertension Association (PHA) developed an accreditation program to define centers of comprehensive care (CCC), to highlight centers providing outstanding care, and to delineate a standard criterion‐based approach to diagnosis, evaluation, and management. The led to the development of the PHA‐Accredited PH Care Centers (PHCC) Initiative, which is a formalized accreditation process with specific requirements for CCC in both adults and children (https://phassociation.org/phcarecenters). This provides an excellent framework for defining and maintaining center excellence, as well as a method for patients, families, and medical personnel to identify highly qualified referral centers. 4 The criteria were chosen based on expert consensus by PHA clinician leadership and have standard requirements for Center Director, Center Coordinator, Program Staff and Support Services, Facility, and Research—plus elements that are required to demonstrate expertise in the care of Groups 1, 3, and 4 in the World Symposium for Pulmonary Hypertension (WSPH) classification schema.
The Pediatric Pulmonary Hypertension Network (PPHNet) was created as a network of established academic PH programs in North America to improve the health, quality of care, and outcomes of all children with PH by enhancing collaboration. 2 Each member site is led by a primary investigator or site director to support the mission of the PPHNet in clinical care, education, training, advocacy, and high‐quality research to push the field of pediatric PH forward. 2 In this way, the PPHNet differs from the PHA in its focus on NIH research for pediatrics, including the development of the largest single registry of pediatric patients with PH.4, 5, 6, 7, 8 Another focus includes collaborative authoring of practice guidelines, multicenter reviews, and consensus statements.
To address the lack of criteria for an optimal pediatric PH program, members of the PPHNet sought to determine similarities and differences between the PPHNet programs that may better define the favorable composition of a PH program. We approached this goal by surveying site directors at PPHNet sites and then engaging in extensive consensus discussions with PPHNet members. We further reviewed recommendations from the PHA accreditation process to provide important constructs for a successful pediatric PH program. In this paper, we provide a collective overview of key elements which were instrumental for the growth of established pediatric PH care programs, to advise and assist the development of new PH programs and to identify avenues and resources for growth and collaboration.
METHODS
To determine the constitution and design of the current PPHNet programs we administered a Qualtrics survey to every site director of participating centers and supported extensive discussion to develop a group consensus regarding the recommended composition of a dedicated program. The site directors were encouraged to confer with their respective teams for survey participation. Two consecutive surveys were administered to this group of site directors at each of the PPHNet sites to determine program composition at each center, outpatient care, inpatient service, research, education, and collaboration. The first survey, administered through Qualtrics (Provo, UT) consisted of 33 questions. The second survey was administered through web‐based survey platform Survey Monkey (SurveyMonkey Inc.; www.surveymonkey.com) and consisted of 14 follow‐up questions for more detailed characterization of the surveyed sites.
PPHNet members reviewed survey results, discussed the composition of each program and what would be optimal for the growth and maintenance of excellence. This discussion focused on three main aspects of an optimal pediatric PH program: (1) team composition; (2) treatments, services, and facilities offered for patient care; (3) scholarly activity: research and education. Consensus was derived from PPHNet member discussion.
RESULTS
Surveys were administered to all 13 PPHNet sites. Characteristics of each program are included in Table 1. As noted, PPHNet sites generally have large PH programs. To our knowledge, the sites in the PPHNet comprise the largest pediatric PH centers in the country. Eight of these programs are also accredited by the PHA as CCCs. All programs managed patients on continuous parenteral prostacyclin therapy, with the majority managing >11 patients at a time. All programs considered themselves PH referral centers or destinations for other smaller regional programs. It was noted that for referred patients, the PPHNet site assumed and maintained primary responsibility of the patient's PH care and saw the patient regularly in addition to communicating with external centers by phone or email. Additional survey results were tabulated, and trends noted (Figures 1 and 2).
Table 1. Pediatric pulmonary hypertension network sites.
| Name of PPHNet member site | Location | PHA accredited CCC? | Primary specialty of PH team | Number of pediatric PH patients currently receiving care at this site |
|---|---|---|---|---|
| Boston Children's Hospital | Boston, MA | No | Pediatric Cardiology | >301 |
| Children's Hospital of Philadelphia | Philadelphia, PA | No | Pediatric Cardiology | >301 |
| Children's Wisconsin | Milwaukee, WI | Yes | Pediatric Cardiology | 151–200 |
| Cincinnati Children's Hospital Medical Center | Cincinnati, OH | Yes | Pediatric Cardiology | >301 |
| New York‐Presbyterian Morgan Stanley Children's Hospital | New York, NY | Yes | Pediatric Cardiology | >301 |
| Children's Hospital Colorado | Denver, CO | Yes | Pediatric Cardiology | >301 |
| Johns Hopkins Children's Center | Baltimore, MD | No | Pediatric Cardiology | 151–200 |
| Monroe Carell Jr. Children's Hospital at Vanderbilt | Nashville, TN | Yes | Pediatric Pulmonology | 151–200 |
| Seattle Children's Hospital | Seattle, WA | Yes | Pediatric Cardiology | 201–250 |
| Lucile Packard Children's Hospital Stanford University | Palo Alto, CA | No | Pediatric Cardiology | >301 |
| Stollery Children's Hospital | Edmonton, AB, Canada | N/A | Pediatric Cardiac Critical Care | 151–200 |
| Texas Children's Hospital | Houston, TX | Yes | Pediatric Pulmonology | >301 |
| University of California San Francisco, Benioff Children's Hospital | San Francisco, CA | Yes | Pediatric Cardiology | 201–250 |
Abbreviations: CCC, centers of comprehensive care; PH, pulmonary hypertension; PHA, Pulmonary Hypertension Association; PPHNet, Pediatric Pulmonary Hypertension Network.
Figure 1.
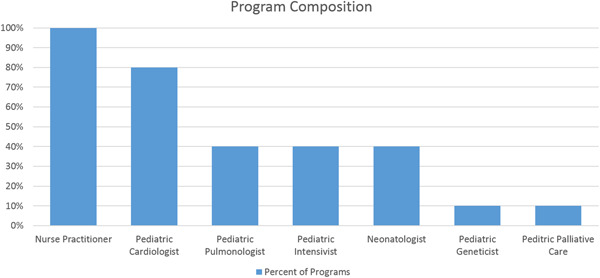
Composition of advanced practice provider and physician support in surveyed programs. Percentage indicated the numbers of programs with each type of provider
Figure 2.
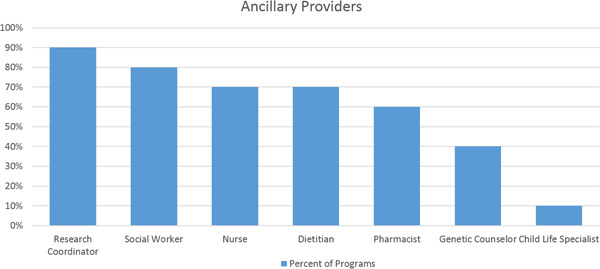
Composition of ancillary support in surveyed programs. Percentage indicated the numbers of programs with each type of provider
Team composition
Program sites reported at least one pediatric cardiologist (range: 1–3) in 80%, at least one pediatric pulmonologist (range: 1–4) in 40%, at least one pediatric critical care physician (range: 1–2) in 40%, and at least one neonatologist (range: 1–2) in 40% of the responding sites. One program also included a pediatric geneticist and pediatric palliative care physician. Of the sites surveyed, 50% included an MD from two or more disciplines on their PH team. All programs had dedicated nurse practitioners (range: 1–2). The majority (70%) of programs had a nurse (five dedicated, two shared), 90% had a research coordinator (six dedicated, three shared), 80% a social worker (one dedicated, seven shared), 60% a pharmacist (one dedicated, five shared), and 70% a dietician (one dedicated, six shared). In addition, 40% of programs had access to a shared genetics counselor and a shared child life specialist.
All programs indicated that they had a dedicated program coordinator. The majority of PH program coordinator positions were filled by a registered nurse (62%) or nurse practitioner (69%) with a smaller number of roles filled by research coordinators or respiratory therapists. The total full‐time equivalent (FTE) of the PH coordinator at these sites varied.
Pediatric lung transplant program access either at the same institution (40%) or through an established referral process to a lung transplant program at an external institution (60%) was available for all sites. All programs had an affiliated adult PH program, with varied processes for transition such as shared clinic with adult providers or a structured transition process beginning in adolescence, while others were still identifying a center‐specific protocol.
Clinical care
All sites had an outpatient PH clinic, ranging in frequency from 1 half‐day per week to 3 full days per week. Most providers saw between three and seven patients per half‐day session, although one site reported ≥8 patients per half‐day.
Inpatient care was a feature for the majority of the surveyed programs as well, providing consulting coverage in the various intensive care units (cardiac, pediatric, and neonatal intensive care units.) In addition to serving as intensive care consultants, 40% of survey respondents also had a primary admitting service, and 80% cared for children with PH on the acute care ward. All respondents cared for neonatal/infant Group 3 patients, with the majority in an interdisciplinary fashion with other pediatric subspecialists.
RESPONSES TO QUERIES ABOUT TESTING
Almost all responding sites reported ready access for echocardiograms, electrocardiograms, cardiopulmonary exercise tests, and pulmonary function tests within the PH clinic or institution. All sites performed 6‐min walk tests (6MWT), starting in preschool or school‐age. Genetic testing was performed for the majority of Group 1 patients but not routinely in Group 3 patients.
Sites were queried about timing for right heart catheterization (RHC) at diagnosis: 91% stated that they would seek this testing for Group 1 compared to 73% for Group 3 (n = 11) and were more likely to perform RHC routinely at regular intervals for Group 1 children. Repeated RHC in Group 3 population were typically done with changes in medical therapy or to inform clinical medical decision‐making.
Educational and scholarly activity
Programs provided hospital staff education, had a prostacyclin protocol(s), and participated in academic writing publications specific to PH. Almost 90% held regular teaching conferences for trainees. In addition to education, programs reported participation in PH‐specific research (n = 11), the majority of programs having two to four studies per site. Study types included industry‐sponsored trials, NIH/federally funded trials, local studies (prospective and retrospective), collaborative/multicenter studies, and registries. Most sites had a dedicated research coordinator for PH studies. Additional research support such as statistics, grant writing, and funding were shared across the institution.
CONSENSUS
Building on the survey results, consensus among PPHNet member sites, and taking the PHA recommendations for CCC into account, we identified required and recommended components to build a dedicated pediatric PH program. These are summarized in Table 2. For the team composition, the authors reviewed the multidisciplinary teams at PPHNet member sites necessary to provide comprehensive care for patients with all WSPH group patients. The following team components were determined to be essential either at the primary institution or available through referral to address all aspects of pediatric PH disease and to provide all possible treatment options for all WSPH groups, based on current PPHNet site composition.
Table 2.
Components of a dedicated pediatric PH program
| A. Team composition | ||
|---|---|---|
| Component | Required | Recommended (either at primary institution or through referral) |
|
Program director: The primary clinical focus is pediatric PH. May have protected PH research time |
⊗ | |
| PH team subspecialties: Multidisciplinary team for direct PH care | Pediatric cardiology and/or pediatric pulmonology |
Neonatology pediatric Intensive care Pediatric Pulmonology/cardiology |
|
Consulting services: Collaboration between PH team and consultant services |
Genetics Palliative care Rheumatology Gastroenterology Physical Medicine and rehabilitation Cardiac anesthesia Interventional cardiology Cardiac surgery |
Surgeons skilled in thromboendarterectomy (referral basis) Lung transplant specialists Developmentalist Palliative care Pediatric surgery |
|
Program coordinator (recommended RN or APP): Performs coordination of care between patient, specialty pharmacies, and insurance companies; first line of team contact for patient/caregiver |
⊗ | |
|
Nurse practitioner: Provides medical and research support through inpatient and outpatient care including transition, communication with family and prostacyclin management |
⊗ | |
|
Ancillary services: Diverse team to work with medical team for interdisciplinary care delivery of comprehensive services and assessment |
Social worker Pharmacist dietician Child life Administrative support |
Pain management Genetics counselor/genetics |
| B. Treatments, services, and facilities offered for patient care | ||
|---|---|---|
| Pediatric interventional cardiology/pediatric cardiothoracic surgery |
Acute vasodilator testing Atrial septostomy Ductus arteriosus stent |
Reverse Potts shunt Lung transplantation |
| Continuous patient care coverage: Daily service and physician call schedule for PH patients | ⊗ | |
|
Comprehensive offering of vasodilator therapies: Expertise with all available pulmonary vasodilator treatments (oral, inhaled, subcutaneous, intravenous) |
⊗ | |
|
Patient, caregiver, and staff education: Education on prostacyclin therapy inpatient and at home |
⊗ | |
|
Treatment expertise: All WSPH/Panama classification groups of PH |
Provide comprehensive care or consultation on neonatal/infant Group 3 patients | CTEPH program |
|
Transition pathway: Identified partner for continuing adult care |
⊗ | |
|
Referral destination: Center for regional and affiliate partners, able to provide care for Medicaid patients |
⊗ | |
| Full ancillary testing: Echocardiogram, catheterization, CT/MRI, V/Q, CPET, 6MWT, PFT | ⊗ | |
|
Institutional support: Recognized role of PH team within institution, support for expansion |
⊗ | |
| C. Scholarly activity: research and education | ||
|---|---|---|
| Active participation in research: Industry‐sponsored, PI‐initiated, registries | ⊗ | |
| Research support | Research coordinator | Statistician |
|
Education of learners: Students, residents, fellows, colleagues |
⊗ | |
| Hospital staff education | ⊗ | |
|
Conference participation: Regional (as applicable), local and national conferences |
⊗ | |
|
Commitment to quality improvement: Regular projects for program self‐assessment and growth |
⊗ | |
DISCUSSION
Pediatric PH has evolved into a complex subspecialty requiring multidisciplinary care, program expertise paired with national collaboration for research, efforts to train the next generation, and the establishment of clinical practice guidelines. Initially, care teams in a few programs across the nation focused primarily on children with Group 1 pulmonary arterial hypertension (PAH), according to the classification by the sixth WSPH and treatment with pulmonary vasodilators followed adult treatment guidelines. Improvements in diagnosis, treatment, and recognition of PH associated with other systemic childhood diseases, such as congenital heart disease and chronic lung disease, have now expanded the field and the need for pediatric PH specialty programs with pediatric‐specific guidelines.
Pediatric PH programs now include care for a large number of children with PH associated with lung disease (WSPH Group 3), which represents nearly 50% of subjects enrolled in the PPHNet Registry. 5 This finding underscores the advances in neonatal care allowing survival of premature infants with bronchopulmonary dysplasia and congenital diaphragmatic hernia, as well as a growing awareness of rare developmental lung diseases. This also highlights the need for developing multidisciplinary teams, including cardiologists, neonatologists, pulmonologists, intensivists, and others, to optimize outcomes.2, 3, 5, 9 In addition, a team approach is necessary to enhance the continuity of inpatient and outpatient care with a seamless transition to chronic ambulatory care with pediatric subspecialty providers and then ultimately for transition on to adult PH providers.1, 2, 10
Addressing the need for standardization of diagnostic evaluation and treatment of pediatric PH, PPHNet leaders along with the AHA and ATS created a working group to establish practice guidelines. 2 Acknowledging the guidelines were created from both best practice evidence as well as expert consensus, recommendations were made regarding initial diagnostic testing, treatment algorithms, and follow‐up care.
Throughout these joint guidelines, care is recommended at “an experienced center” or in “comprehensive, multidisciplinary clinics at specialized pediatric centers.” The recognition of the importance of expert, specialized care is evident by the multidisciplinary recommendations for all types of PH including patients with hereditary PAH or other genetic syndromes, as well as those related to other systemic diseases. Interestingly, despite the obvious need to conduct care in this setting, published patient care guidelines do not define the composition of such an established center nor what constitutes experience.
As a service to both collaborating physicians as well as families seeking specialized care, the PHA, an independent private nonprofit organization, created an accreditation framework for the designation of CCCs based on expert recommendations. 4 These criteria provide a construct for both the identification of necessary resources and the determination of the appropriate structure and experience of a comprehensive program. However, since the establishment of the PPHNet and PHA accreditation of CCCs, there has not been an overview of the makeup, clinical practice patterns, programmatic support structure, or infrastructure of these expert pediatric centers nor detailed recommendations. Now that it has been 10 years since the PHCC initiative was first undertaken, assessment of these centers in addition to PPHNet sites shows that expert pediatric PH care is often tailored for institutional and regional needs, resulting in significant heterogeneity. In reviewing program make‐up and proposed criteria, we acknowledge the overlap with the PHA criteria for a comprehensive pediatric center and aim to jointly determine and work toward the definition of an optimized, dedicated program for pediatric care.
However, for smaller or early‐stage programs, the importance of identifying necessary components for a successful program cannot be overstated. There is no guiding document on how to build a dedicated pediatric PH program and so this project was created to meet that need. Querying several successful programs in the country, we have identified the resources that are necessary to successfully deliver complete care to children with PH. Therefore, we present consensus recommendations with specific recommendations for the program “must‐haves” and “should‐haves.”
The clear need for multidisciplinary support from various team members—physicians, advanced practice providers, ancillary staff—was evident throughout this discussion. However, we submit that a multidisciplinary program is not enough; but rather interdisciplinary, highlighting a collaborative and integrated approach to care. The ability to provide continuous inpatient and outpatient care, as well as the infrastructure to support the stringent requirements for not only enrollment for medication administration, but maintenance of continuous prostacyclin therapy, can only be accomplished with this team structure. In addition, for the utmost safety of these patients when receiving care, an institution must have experienced pediatric providers of cardiac anesthesia and cardiac surgery.
A consistent finding across the surveyed PPHNet sites was the active role of a program coordinator. Generally, the coordinator provides the expertise necessary to bridge gaps between the patient, payer, and the dispensing pharmacy to ensure efficient delivery of therapies; engages the patient and caregiver as the first point of contact on the medical team for education and support; and contributes to program growth by participating in protocol development. A coordinator is a crucial component of a dedicated PH program, to consistently ensure a high level of comprehensive care for children with PH across various WSPH groups. In fact, most PPHNet sites had more than one program coordinator, commensurate with patient volume and complexity.
One distinct difference between PHA criteria and PPHNet recommendations is the strong recommendation in the latter for a dedicated advanced practice provider on the team. Through the administered survey and in our collective experience, the advanced practice provider truly completes the medical team by bridging the physician and nursing workforce, as well as providing transitional care, research management, and both nursing and administrative training through their advanced degree. An advanced practice provider also plays a key role in the successful and safe administration of continuous prostacyclin therapy, providing a necessary bridge for the inpatient and outpatient settings given the complexity and high‐risk nature of this population.
A second distinguishing feature of this consensus statement is the strong recommendation for participation in research and quality initiatives (Table 2C). This requirement highlights the responsibility that dedicated pediatric PH programs have to contribute to advancing the field and participate in regular interval assessments to ensure the quality of care delivery. Participation in research is necessary at a local and national levels and in collaboration with other programs to ensure that children with all types of PH are adequately represented in clinical trials, which is fostered by PPHNet membership and through national conferences to engage smaller programs who would join in efforts to conduct clinical trials, retrospective registry studies, and translational research.
Unfortunately, supporting the education of learners cannot be named as a program requirement, due to variable access to trainees among sites. We also recognize that the ability to support a dedicated PH fellowship is limited, even among established centers. However, as part of these consensus recommendations, we urge pediatric PH program directors to invest in training learners at each institution as familiarity with pediatric PH is generally low in communities.
With improvements in the long‐term outcomes and consideration for palliative treatment before lung transplant, it is also important to have the ability to provide evaluation for atrial septostomy, reverse Potts shunt creation, pulmonary thromboendarterectomy, and/or lung transplant either at the primary institution or with an established referral partner. Similarly, programs must have a process for the transition of patients to adult providers capable of caring for PH.
It is important to note that this consensus discussion was developed out of the experience gained by each of the PPHNet member sites, as they grew from clinics of one to two people to teams of multiple members with unique and complementary roles. We acknowledge that this expertise is North American specific based on the membership sites, and appreciate that treatment and training recommendations outside of North America may differ. Although each site is considered to be a complete, expert center, there remains significant heterogeneity among the development and growth of these sites and our approach to patient care. Therefore, this discussion was undertaken to learn from each other and create a common knowledge for pediatric expertise. What resulted from that discussion, is a set of recommendations to help build dedicated pediatric PH programs. Truly, each program has its unique growth story but ultimately creating and sustaining a care model for pediatric PH patients requires the presence of many of the same components for success. The PPHNet hopes that sharing these learned experiences with the larger community will encourage medical centers to invest in the creation of much‐needed PH programs, to expand services for children across North America. It is our wish that these recommendations will support the growth of dedicated pediatric PH programs, encourage a shared evolution in care practices and positively impact care delivery for children across the spectrum of WSPH groups.
CONFLICT OF INTERESTS
All authors contributed to the development of the survey, compilation and analysis of results, expert consensus discussion to frame guidelines and preparation and review of the manuscript.
ETHICS STATEMENT
Ethics statement is not applicable to this study.
Handler SS, Varghese NP, Rosenzweig EB, Yung D, Krishnan U, Whalen E, Bates A, Avitabile CM, Jackson EO, Hirsch R, Fineman J, Abman SH, For The Pediatric Pulmonary Hypertension Network (PPHNet) . Building a dedicated pediatric pulmonary hypertension program: a consensus statement from the pediatric pulmonary hypertension network. Pulm Circ. 2022;12:e12031. 10.1002/pul2.12031
Stephanie S. Handler and Nidhy P. Varghese are co‐first authors.
REFERENCES
- 1. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, Ivy DD, Berger R. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abman SH, Raj U. Towards improving the care of children with pulmonary hypertension: rationale for developing a pediatric pulmonary hypertension network. Prog Pediatr Cardiol. 2009;27:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society .Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132(32):2037–99. [DOI] [PubMed] [Google Scholar]
- 4. Sahay S, Melendres‐Groves L, Pawar L, Cajigas HR. Pulmonary hypertension care center network: improving care and outcomes in pulmonary hypertension. Chest. 2017;151:749–54. [DOI] [PubMed] [Google Scholar]
- 5. Abman SH, Mullen M, Sleeper L, Austin ED, Rosenzweig EB, Kinsella JP, Ivy D, Hopper RK, Raj JU, Fineman J, Keller RL, Bates A, Krishnan US, Avitabile CM, Davidson A, Natter MD, Mandl KD, Pediatric Pulmonary Hypertension Network . Characterization of pediatric pulmonary hypertensive vascular disorders from the pediatric pulmonary hypertension network registry. Eur Respir J. 2021;59:2003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whalen E, Ely E, Brown A. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatr. 2017;188:24–34. [DOI] [PubMed] [Google Scholar]
- 7. Sahay S, Melendres‐Groves L, Pawar L, Cajigas HR. The left ventricle in congenital diaphragmatic hernia: implications for the management of pulmonary hypertension. J Pediatr. 2018;197:17–22. [DOI] [PubMed] [Google Scholar]
- 8. Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, Hanna B, Romer L, Keller RL, Fineman J, Steinhorn R, Kinsella JP, Ivy DD, Rosenzweig EB, Raj U, Humpl T, Abman SH, Coulson J, Collaco M, Grenolds A. EXPRESS: acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an international survey on current practice. Pulm Circ. 2019;9:4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varghese N, Tilman R, Keller R. Pulmonary arterial hypertension is an important co‐morbidity in infant lung diseases: bronchopulmonary dysplasia and congenital diaphragmatic hernia. Pediatr Pulmonol. 2021;56:670–7. [DOI] [PubMed] [Google Scholar]
- 10. Whalen E, Ely E, Brown A. The role of the multidisciplinary team in a pediatric pulmonary hypertension center. Pediatr Pulmonol. 2021;630–5. [DOI] [PubMed] [Google Scholar]


