Abstract
The second hit hypothesis in pulmonary hypertension refers to the development of pulmonary vascular disease in individuals at risk, after an additional exposure or “hit” to factors with potential injury to the pulmonary circulation, such as drugs or toxins. We here present a case of severe pulmonary hypertension diagnosed during the third trimester of pregnancy, in a patient with familial history of pulmonary hypertension, found to have a heterozygous mutation in the BMPR2 gene, who also had chronic exposure to prescription amphetamines. We hypothesize that exposure to prescription amphetamines could act as a second hit of pulmonary vascular injury in individuals at risk of pulmonary vascular disease.
Keywords: BMPR2 mutation, pregnancy, prescription amphetamines, pulmonary arterial hypertension, second hit hypothesis
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by proliferation, narrowing, vasoconstriction, and remodeling of the pulmonary arterial tree, leading to abnormal right ventricular mechanics, and if left untreated, right heart failure, and death. 1 Second hit exposure such as certain drugs or toxins could contribute to the development of clinical pulmonary vascular disease in susceptible individuals. Here, we present a case of severe PAH diagnosed during the third trimester of pregnancy, in a patient with familial history of PAH, bone morphogenetic protein receptor type II (BMPR2) gene sequence mutation, and chronic exposure to prescription amphetamines.
CASE REPORT
A 30‐year‐old female at 30 weeks of pregnancy was referred for evaluation of rapidly progressive dyspnea. Her symptoms had started 10 weeks before presentation with dyspnea on exertion and intermittent palpitations. She denied any chest pain, edema, hemoptysis, orthopnea, paroxysmal nocturnal dyspnea, or syncope. Medical history was pertinent for attention‐deficit/hyperactivity disorder, treated with dextroamphetamine/amphetamine (Adderall) and lisdexamfetamine (Vyvanse) for several years. Family history was significant for PAH in her mother, who died at the age of 30. There was no history of connective tissue disease, venous thromboembolism, or exposure to illicit drugs.
On examination, she appeared euvolemic with no visible jugular vein distention or peripheral edema. Cardiac auscultation revealed loud P2, with a holosystolic murmur at the lower left sternal border. Her labs revealed mild thrombocytopenia but otherwise within normal limits. Urine toxicology was unrevealing. Transthoracic echocardiogram showed normal left ventricular function but a markedly dilated right ventricle with moderately reduced systolic function estimated right ventricular systolic pressure was 69 mmHg (Figure 1a,b). Ventilation–perfusion scan was negative for perfusion defects and computed tomography angiogram of the chest was negative for pulmonary embolism (Figure 1c).
Figure 1.
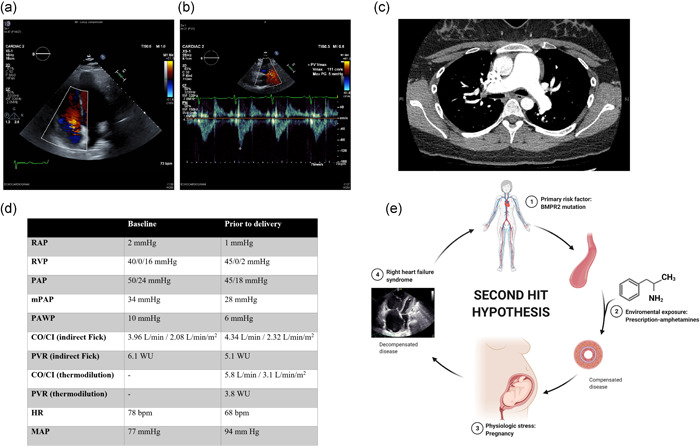
Imaging studies, hemodynamic values, and second hit hypothesis. (a) A two‐dimensional echocardiogram reveals right ventricular dilation and moderate tricuspid regurgitation. (b) Echocardiogram also reveals “notching” of the RVOT tract flow velocity doppler envelope, suggesting increased pulmonary vascular resistance. (c) CT angiogram of the chest reveals enlargement of the pulmonary artery, but no evidence of vascular filling defects. (d) Hemodynamic values at diagnosis (baseline) and after IV treprostinil titration (before delivery). (e) “Second hit” hypothesis. Susceptible individuals for pulmonary vascular disease, such as BMPR2 mutation carriers, could develop pulmonary arterial hypertension if exposed to an additional injury or second hit, such as exposure to amphetamine products like anorexigens or methamphetamines. Pregnancy imposes further physiological stress, with the potential worsening of the disease. We wonder if prescription amphetamines could be considered a second hit in individuals at increased risk. Abbreviations: BMPR2, bone morphogenetic protein receptor type II; CI, cardiac index; CO, cardiac output; CT, computed tomography; HR, heart rate; MAP, mean arterial pressure; mPAP, mean pulmonary arterial pressure; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVP, right ventricular pressure; RVOT, right ventricular outflow tract. Created with BioRender.com
Right heart catheterization confirmed severe PAH with decreased cardiac output (Figure 1d). She was started on intravenous treprostinil which was quickly up titrated to a dose of 40 ng kg−1 min−1. A multidisciplinary discussion with high‐risk obstetrics, neonatology, cardiac anesthesia, cardiothoracic surgery, and our PAH team recommended surgical delivery at 32 weeks of gestation under spinal anesthesia, with pre‐emptive cannulation for venoarterial extracorporeal membrane oxygenation (ECMO), if needed (ECMO on stand‐by approach).
A pulmonary artery catheter was placed predelivery, which showed improvement in her hemodynamic profile (Figure 1d). She had an uneventful delivery and postop course ultimately discharged home on triple therapy with sildenafil, ambrisentan, and treprostinil. Genetic testing was positive for a heterozygous mutation in the BMPR2 gene. At follow‐up, she is functional class II, 6‐minute walk distance is 530 m and brain natriuretic peptide levels are 17 ρg/ml. Cardiac magnetic resonance imaging revealed a right ventricular end‐systolic volume index of 42 ml and a right ventricular ejection fraction of 44%.
DISCUSSION
Familial PAH is a rare disorder characterized by sporadic or inherited germline mutations in the allele‐specific for BMPR2 gene sequence. However, sporadic BMPR2 mutations have been reported in 11%–40% of idiopathic PAH cases with no family history of PAH, indicating the occurrence of de novo mutations or incomplete penetrance. 2 Furthermore, asymptomatic BMPR2 mutation carriers were shown to have a 2.3% annual risk of developing PAH, with a marked female predominance. Second hits like drug exposure could play a role in disease development in such carriers. 3
PAH in pregnancy poses a high risk of maternal and fetal mortality. 4 Volume shifts in the peripartum period and physiological stress place these women at high risk of right ventricular failure. Therefore, it is recommended to manage these patients in specialized centers as PAH therapy needs to be optimized with a multidisciplinary care approach.
The association between PAH and exposure to drugs and toxins such as methamphetamines and anorexigens is well‐known. 5 , 6 Amphetamine‐analogs interact with serotonin transporters in the pulmonary vasculature, increasing concentrations of serotonin, which stimulates smooth muscle cell mitogenesis and growth. 7 However, it remains unknown if exposure to other amphetamine products such as prescription amphetamines could be associated with the development of PAH.
The second hit hypothesis refers to the observation that individuals at risk of pulmonary vascular disease (i.e., BMPR2 mutation) could develop the overt disease if influenced by another exposure (second hit) such as drugs, stimulants, and inflammation‐producing pathways known to injure the pulmonary vasculature. 8 , 9 It remains unknown if exposure to other amphetamine products such as prescription stimulants could be associated with the development of PAH. For instance, there is a previous report of a case of pulmonary hypertension associated with exposure to methylphenidate, a prescription stimulant; in that particular case, there was an improvement on estimated pulmonary pressures by echocardiogram after discontinuation of methylphenidate, although no right heart catheterization was performed. 10 To the best of our knowledge, no prior reports on the link between prescription amphetamines and the development of PAH have been reported.
The case we are describing could very well be just a case of hereditary PAH unmasked during pregnancy. However, the safety profile of amphetamine products in individuals at increased risk deserves further discussion. We wonder if exposure to prescription‐amphetamines such as dextroamphetamine/amphetamine and lisdexamfetamine could have provided an additional insult for the development of PAH, in addition to the underlying disease state (BMPR2 mutation carrier) and the physiological stress of pregnancy (Figure 1e). Given the widespread use of these medications, the pulmonary and cardiovascular complications associated with prescription stimulants deserve a more in‐depth discussion, and formal epidemiological studies are needed to determine the association between prescription amphetamines and PAH.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was published after obtaining informed consent from the patient.
AUTHOR CONTRIBUTIONS
All authors reviewed and revised the manuscript. Huzaifa A. Jaliawala: Initial draft of the manuscript and revisions. Miloni Parmar: Manuscript writing and revisions. Katherine Summers: Manuscript writing and revisions. Roberto J. Bernardo: Guarantor and clinician managing this patient. Involved in patient management, revisions of the manuscript.
Jaliawala HA, Parmar M, Summers K, Bernardo RJ. A second hit? Pulmonary arterial hypertension, BMPR2 mutation, and exposure to prescription amphetamines. Pulm Circ. 2022;12:e12053. 10.1002/pul2.12053
REFERENCES
- 1. Bernardo RJ, Haddad F, Couture EJ, Hansmann G, de Jesus Perez VA, Denault AY, de Man FS, Amsallem M. Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc Diagn Ther. 2020;10:1580–603. 10.21037/cdt-20-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC, Loyd JE. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801899. 10.1183/13993003.01899-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montani D, Girerd B, Jaïs X, Laveneziana P, Lau E, Bouchachi A, Hascoët S, Günther S, Godinas L, Parent F, Guignabert C, Beurnier A, Chemla D, Hervé P, Eyries M, Soubrier F, Simonneau G, Sitbon O, Savale L, Humbert M. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J. 2021;58:2004229. 10.1183/13993003.04229-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hemnes AR, Kiely DG, Cockrill BA, Safdar Z, Wilson VJ, Hazmi MA, Preston IR, MacLean MR, Lahm T. Statement on pregnancy in pulmonary hypertension from the Pulmonary Vascular Research Institute. Pulm Circ. 2015;5:435–65. 10.1086/682230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montani D, Seferian A, Savale L, Simonneau G, Humbert M. Drug‐induced pulmonary arterial hypertension: a recent outbreak. Eur Respir Rev. 2013;22:244–50. 10.1183/09059180.00003313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, Kudelko K, Liu J, Hsi A, Rupp A, Sweatt AJ, Tuder R, Berry GJ, Rabinovitch M, Doyle RL, de Jesus Perez V, Kawut SM. Features and outcomes of methamphetamine‐associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. 10.1164/rccm.201705-0943OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J Pharmacol Exp Ther. 2006;318:604–10. 10.1124/jpet.106.101618 [DOI] [PubMed] [Google Scholar]
- 8. Mizuno S, Farkas L, Al Husseini A, Farkas D, Gomez‐Arroyo J, Kraskauskas D, Nicolls MR, Cool CD, Bogaard HJ, Voelkel NF. Severe pulmonary arterial hypertension induced by SU5416 and ovalbumin immunization. Am J Respir Cell Mol Biol. 2012;47:679–87. 10.1165/rcmb.2012-0077OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian W, Jiang X, Sung YK, Shuffle E, Wu TH, Kao PN, Tu AB, Dorfmüller P, Cao A, Wang L, Peng G, Kim Y, Zhang P, Chappell J, Pasupneti S, Dahms P, Maguire P, Chaib H, Zamanian R, Peters‐Golden M, Snyder MP, Voelkel NF, Humbert M, Rabinovitch M, Nicolls MR. Phenotypically silent bone morphogenetic protein receptor 2 mutations predispose rats to inflammation‐induced pulmonary arterial hypertension by enhancing the risk for neointimal transformation. Circulation. 2019;140:1409–25. 10.1161/circulationaha.119.040629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karaman MG, Atalay F, Tufan AE, Erdogan A. Pulmonary arterial hypertension in an adolescent treated with methylphenidate. J Child Adolesc Psychopharmacol. 2010;20:229–31. 10.1089/cap.2009.0095 [DOI] [PubMed] [Google Scholar]


