Abstract
Hepatoma‐derived growth factor (HDGF) was previously shown to be associated with increased mortality in a small study of idiopathic and connective tissue disease‐associated pulmonary arterial hypertension (PAH). In this study, we measured serum HDGF levels in a large multicenter cohort (total 2017 adult PAH‐Biobank enrollees), we analyzed the associations between HDGF levels and various clinical measures using linear or logistic regression models. Higher HDGF levels were found to be significantly associated with worse pulmonary hemodynamics, prostacyclin treatment; among PAH subtypes, higher HDGF levels were most associated with portopulmonary hypertension (beta = 0.469, p < 0.0001). Both Kaplan–Meier curve and Cox proportional hazard regression demonstrated that higher HDGF levels are associated with a higher risk of mortality (COX hazard ratio 1.31, p < 0.0001). Further, in the Sugen hypoxia (SuHx) rat model, the highest HDGF levels were post‐pulmonary circulation, and HDGF levels significantly increased with the development of PAH. In pulmonary arteries, immunohistochemistry staining showed that HDGF was highly expressed in pulmonary smooth muscle cells in both PAH patients and SuHx rats. In conclusion, we found that higher serum HDGF was linked with increased mortality, and associated with disease severity in a large multi‐center adult PAH cohort (n = 2017). In the SuHX PAH models, circulating HDGF levels are pulmonary in origin and increase with PAH progression. HDGF may be actively involved in vascular remodeling in PAH.
Keywords: biomarker, HDGF, PAH, vascular remodeling
Abbreviations
- 6MWD
6‐min walk distance
- APAH
PAH associated with other conditions
- BMP2
bone morphogenic protein 2
- CI
cardiac index
- CO
cardiac output
- FPAH
familial PAH
- HDGF
hepatoma‐derived growth factor
- IPAH
idiopathic PAH
- mPAP
mean pulmonary artery pressure
- mPCWP
mean pulmonary capillary wedge pressure
- mRAP
mean right atrial pressure
- NT‐proBNP
N‐terminal brain natriuretic peptide prohormone
- PAH
pulmonary arterial hypertension
- PDGF
platelet‐derived growth factor
- PoPH
portopulmonary hypertension
- PVR
pulmonary vascular resistance
- PVRi
pulmonary vascular resistance index
- REVEAL
Registry to Evaluate Early and Long‐Term PAH Disease Management
- SuHx
Sugen hypoxia
INTRODUCTION
Pulmonary hypertension (PAH) is a progressive, fatal disease characterized by abnormal elevation of pulmonary arterial pressure. 1 , 2 Sustained exposure of the right ventricle to high pressure leads to right ventricular failure and remains a predominant cause of death in PAH patients. The etiology of PAH is complex and incompletely understood, however, intense microvascular remodeling with an elevation of pulmonary vascular resistance plays a significant role in PAH development. 3 , 4
Several growth factors, including fibroblast growth factor 2, 5 , 6 , 7 platelet‐derived growth factor 8 , 9 and in particular bone morphogenic protein 2 (BMP2) and its receptor, BMPR2, are causally linked to the development of PAH. 10 , 11 , 12 , 13 In a previous single‐center pilot study, we found that hepatoma‐derived growth factor (HDGF), a novel circulating angiogenic factor and lung remodeling modulator, was shown to be significantly increased in idiopathic and connective tissue disease‐associated PAH patients, and more importantly, a high serum HDGF level was associated with worse functional class and increased mortality. 14
In the present study, we confirmed the association of serum HDGF with PAH severity, mortality, and expanded our analysis across PAH subtypes in a large multicenter PAH‐biobank cohort. In addition, we demonstrated that circulating HDGF had pulmonary vasculature origin, HDGF is associated with pulmonary vascular remodeling and increases with the development of PAH.
METHOD
Study cohort
National Biological Sample and Data Repository for Pulmonary Arterial Hypertension, or PAH Biobank is a National Heart, Lung, and Blood Institute (NHLBI) funded resource of WHO Group 1 PAH patient biological samples, genetic data, and clinical data enrolled from 38 US Centers (www.pahbiobank.org). 15 Under a PAH Biobank‐approved protocol, we analyzed the lab and clinical data from a total of 2017 PAH Biobank enrollees, collected during the 2012–2018 period. Their demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and characteristics of PAH‐Biobank cohort
| n | 2017 |
|---|---|
| Age (years), median (IQR) | 56 (45–66) |
| Female sex, n (%) | 1611 (80) |
| Race (Caucasian), n (%) | 1662 (82) |
| PAH subgroup, n (%) | |
| IPAH | 870 (43) |
| FPAH | 81 (4) |
| APAH | 961 (48) |
| APAH–CTD | 623 (31) |
| APAH–CHD | 171 (8) |
| PoPH | 111 (5.5) |
| APAH–HIV | 42 (2) |
| Other | 106 (5) |
| 6MWD (m), median (IQR) | 348 (258.5–426) |
| NYHA functional class, n (%) | |
| I | 90 (5) |
| II | 451 (22) |
| III | 789 (39) |
| IV | 118 (6) |
| Unknown | 569 (28) |
| Laboratory chemistry, median (IQR) | |
| HDGF (ng/ml) | 0.86 (0.51–1.37) |
| NT‐proBNP (pg/ml) | 672 (217–2164) |
| Hemodynamics, median (IQR) | |
| RAP (mmHg) | 8 (5–12) |
| mPAP (mmHg) | 49 (40–58) |
| mPCWP (mmHg) | 10 (7–13) |
| PVR (wood unit) | 8.61 (5.61–12.75) |
| CI (L/min/m2) | 2.52 (1.98–3.16) |
| REVEAL score‐category, n (%) | |
| 1 | 1251 (62.2) |
| 2 | 342 (17) |
| 3 | 217 (10.8) |
| 4 | 175 (8.7) |
| 5 | 25 (1.2) |
Abbreviations: 6MWD, six‐min walk distance; APAH, PAH associated with other conditions; CHD, congenital heart disease; CI, cardiac index; CTD, connective tissue disease; FPAH, familial PAH; HDGF, hepatoma‐derived growth factor; IPAH, idiopathic PAH; IQR, interquartile range; mPAP, mean pulmonary artery pressure; mPCWP, mean pulmonary capillary wedge pressure; mRAP, mean right atrial pressure; NT‐proBNP, N‐terminal brain natriuretic peptide prohormone; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PoPH; portopulmonary hypertension; PVR, pulmonary vascular resistance; REVEAL, Registry to Evaluate Early and Long‐Term PAH Disease Management.
Pulmonary Hypertension Breakthrough Initiative (PHBI) tissue core at the University of Alabama provided lung tissue sections from both control and PAH patients. PHBI was funded by the Cardiovascular Medical Research and Education Fund's (CMREF), the enrollees are a highly phenotyped cohort of patients with severe PAH; control lung samples were from discarded tissue prior to lung transplantation. The demographic and clinical data were described in Table S1.
All study cohorts were approved by the Institutional Review Board of each participating institution, with informed consent obtained from all patients.
Enzyme‐linked immunosorbent assay (ELISA)
HDGF ELISA was developed and described previously 14 with improvements as described below. We optimized the previous assay by robotically spotting the capture antibodies on the 96‐well plate format (Meso Scale Discovery [MSD]). A bacterial expressed full‐length human recombinant His‐HDGF calibrator was used at a concentration range of 40–0.029 ng/ml. A mixture of the mouse anti‐HDGF monoclonal antibody (Clone 2F12) and MSD SULFO‐TAG labeled anti‐mouse antibody (R32AC, MSD) were used as detection reagents. After incubation and washing, the plate was read in an MSD Sector Imager 2400. Inter‐assay reliability as measured by the percent coefficient of variation (CV) was 3.3% across 28 plates over a period of 4 months.
Statistical analysis
HDGF data were summarized by descriptive statistics. HDGF concentrations were logarithmically transformed for subsequent analyses due to nonnormal distribution. The association between serum HDGF and categorical variables, including subtypes of PAH, types of drug treatment received were analyzed using rank‐sum test without adjustment, or logistic regression adjusted for age and sex. Continuous variables, included invasive hemodynamics (cardiac index [CI], cardiac output [CO], pulmonary vascular resistance [PVR], pulmonary vascular resistance index [PVRi], mean pulmonary artery pressure [mPAP], mean right atrial pressure [mRAP], mean pulmonary capillary wedge pressure [mPCWP]), and 6‐min walk distance (6MWD); continuous variable associations with HDGF were analyzed by linear regression adjusted for age and sex.
Kaplan–Meier survival curve analysis was used to examine the association of PAH survival and HDGF levels dichotomized at the median. The association of HDGF level and mortality was also tested using Cox proportional hazard regression models adjusted for age and sex. Statistical analysis was performed using STATA (version 15; StataCorp LLC) and MedCalc statistical software version 18.11.3 (2019 version; MedCalc Software).
Sugen hypoxia PAH model (SuHx)
Male Wistar rats (250–275 g) were injected subcutaneously (sc) with SU5416 (20 mg/kg) (TOCRIS, 3037; Tocris Bioscience) on Day 1. Rats were then placed in a hypoxia chamber for 7, 14, and 21 days (n = 6 for each group). SU5416 was dissolved in a mixture of dimethyl sulfoxide and 0.5% (w/v) carboxymethylcellulose sodium, 0.9% (w/v) sodium chloride, 0.4% (v/v) polysorbate 80, 0.9% (v/v) benzyl alcohol in deionized water. The hypoxic chamber was continuously flushed with a mixture of room air and N2 (10% ± 0.5% O2) to maintain low CO2 concentrations (<0.5%). Chamber O2 and CO2 concentrations were continuously monitored (ProOx 110 oxygen analyzer; Biospherix and LB‐2 CO2 analyzer; Sensormedics). The rats were exposed to room air for 10 min based on protocol twice a week to clean the cages and refill food and water supplies. Sham control rats were injected subcutaneously. with only SU5416 vehicle on Day 1 and kept in room air next to the hypoxic chamber for 7, 14, and 21 days. Thus, all animals were exposed to the same light/dark cycle and ambient temperatures. On Days 7, 14, and 21 rats were anesthetized with an intraperitoneal (ip) injection of ketamine (50mg/ml/kg) and xylazine (Rompun) (5mg/ml/kg), ip and sacrificed. Invasive hemodynamics were assessed at 7, 14, and 21 days. Plasma samples from the right ventricle (prepulmonary) and left ventricle (postpulmonary) were collected at the end of each experimental time points, with lung tissue perfusion, dissection, and fixation performed at the end of the experiment.
Immunohistochemistry (IHC)
The IHC procedure and polyclonal HDGF antibody used for immunostaining were published before. 16 Briefing, lung sections from SuHx rat or human PAH patients were immunostained for HDGF using the ABC method (Cat# AK‐5001; Vector Laboratories Inc.) with the Vector red substrate (Cat# SK‐5100; Vector Laboratories Inc.) for fluorescent visualization. The sections were mounted with VECTASHIELD® Antifade Mounting Medium with DAPI (4′,6‐diamidino‐2‐phenylindole) (Cat# H‐1200‐10; Vector Laboratories Inc.) as a nuclear counterstain. Controls without primary HDGF antibody showed no background immunostaining.
RESULTS
Characteristics of PAH patients
The cohort in this validation study is different from the one in the original study, 14 it composed of a much larger collection of patients with more diverse etiologies. A total of 2017 patients were included in the analysis (Table 1). The majority of the cohort were white (n = 1662, 82%), women (n = 1611, 80%), with a median age of 56 years (IQR 45–66); 61% had NYHA FC II/III symptoms (n = 1240). The majority of the cohort was PAH associated with other conditions (APAH) (48%, n = 961), the second‐largest disease subtype was idiopathic PAH (IPAH, 43%, n = 870). The APAH subgroup was composed predominately of connective tissue disease (APAH‐CTD 31%, n = 623), APAH congenital heart disease (APAH‐CHD 8%, n = 171) and portopulmonary hypertension (PoPH 5.5%, n = 111). Overall, subjects had moderate‐to‐severe disease, with a median PAP of 49 (IQR 40–58) mmHg, median PVR of 8.61 (IQR 5.61–12.75) wood units, and median CI of 2.52 (IQR 1.98–3.16) L/min/m2. The majority of patients were treated with a phosphodiesterase‐5 inhibitor (n = 1546, 77%) or endothelin receptor antagonist (n = 1205, 60%). Subjects were followed for a median of 41 months (IQR 28–55 months) from the time of enrollment to the time of death or censor. Among 1984 subjects who had survival data, 324 died (16.3% mortality). The median HDGF and NT‐proBNP (N‐terminal brain natriuretic peptide prohormone) levels for all patients were 0.86 ng/ml (IQR 0.51–1.37) and 672 pg/ml (IQR 217–2164), respectively.
HDGF associated with clinical measures of PAH
Using linear or logistic regression models adjusted for age and sex, we evaluated the relationship between serum HDGF level and available clinical measures, including invasive hemodynamics, and 6MWD (Table 2). Worse hemodynamic measures were associated with higher serum HDGF levels, specifically PVRi (coefficient 0.559, p < 0.001), PVR (coefficient 0.399, p = 0.015), and mPAP (coefficient 0.881, p = 0.02), transpulmonary gradient (coefficient 0.958, p = 0.012) and diastolic gradient (coefficient 0.721, p = 0.018). In addition, there was a moderate association with dyspnea at rest (coefficient 0.214, p = 0.008). No significant relationship was found between 6MWD (coefficient −7.294, p = 0.177) and HDGF.
Table 2.
Hepatoma‐derived growth factor associated with clinical measures of pulmonary arterial hypertension
| Linear regressions adjusted for age and sex | |||||
|---|---|---|---|---|---|
| Coefficient | Standard error | 95% confidence interval | p Value | ||
| Continuous variables—Adult | |||||
| 6MWD (m) | −7.294 | 5.398 | −17.887 | 3.298 | 0.177 |
| Heart rate | 1.086 | 0.551 | 0.004 | 2.168 | 0.049 |
| Cardiac output | −0.011 | 0.049 | −0.108 | 0.085 | 0.817 |
| Cardiac index | 0.011 | 0.033 | −0.053 | 0.075 | 0.735 |
| PVR | 0.399 | 0.165 | 0.076 | 0.723 | 0.015 |
| PVRI | 0.559 | 0.13 | 0.305 | 0.813 | <0.001 |
| mPAP | 0.881 | 0.378 | 0.14 | 1.623 | 0.02 |
| mRAP | 0.285 | 0.152 | −0.014 | 0.583 | 0.062 |
| mPCWP | −0.172 | 0.115 | −0.398 | 0.053 | 0.135 |
| Shunt | −2.946 | 1.756 | −6.415 | 0.523 | 0.095 |
| Stroke volume | −0.001 | 0.001 | −0.003 | 0.001 | 0.392 |
| Pulse pressure | 0.481 | 0.412 | −0.326 | 1.288 | 0.243 |
| Pulmonary arterial compliance | −0.053 | 0.036 | −0.123 | 0.017 | 0.141 |
| Transpulmonary gradient | 0.958 | 0.379 | 0.215 | 1.702 | 0.012 |
| Diastolic gradient | 0.721 | 0.305 | 0.124 | 1.318 | 0.018 |
| Categorical variables | |||||
| Dyspnea at rest | 0.214 | 0.081 | 0.055 | 0.372 | 0.008 |
Note: Bold values denote statistical significance at the p < 0.05 level.
Abbreviations: 6MWD, 6‐min walk distance; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; mPCWP, mean pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index.
HDGF level and therapy
To determine if HDGF was associated with therapy, enrollees were grouped according to treatment drug class (Table 3). Enrollees with higher serum HDGF levels were more likely to receive prostacyclin treatment, without adjustment (N = 1546, rank‐sum test, p = 0.004), or after adjustment for age and sex (logistics regression coefficient 0.148, p = 0.009).
Table 3.
The association between level of hepatoma‐derived growth factor and the types of therapy
| Logistic regression adjusted for age and sex | Unadjusted rank‐sum test | ||||
|---|---|---|---|---|---|
| Coefficient | 95% confidence interval | p Value | p Value (rank‐sum) | ||
| PDE5 | 0.046 | −0.078 | 0.17 | 0.471 | 0.156 |
| ERA | −0.038 | −0.149 | 0.073 | 0.502 | 0.456 |
| PCA | 0.148 | 0.036 | 0.26 | 0.009 | 0.004 |
| CCB | −0.034 | −0.218 | 0.151 | 0.721 | 0.826 |
| cGMP | 0.027 | −0.441 | 0.496 | 0.909 | 0.78 |
Note: Bold values denote statistical significance at the p < 0.05 level.
Abbreviations: CCB, calcium channel blockers; cGMP, cyclic guanosine monophosphate; ERA, endothelin receptor antagonist; PCA, prostacyclin analogs; PDE5, phosphodiesterase‐5 inhibitor.
HDGF was associated with PAH‐associated PoPH
As shown in Table 1, the study cohort is composed of different PAH subtypes. HDGF levels were associated with both IPAH (p = 0.001) and APAH (p < 0.0001) subtypes by rank‐sum test, with the associations confirmed by logistics regression adjusted for age and sex (IPAH coefficient −0.181, p = 0.001; APAH coefficient 0.227, p < 0.0001) (Table 4). When we examined the APAH subtypes, only PoPH was associated with serum HDGF (coefficient 0.469, p < 0.0001). For each 1‐natural log unit higher HDGF level, the odds of having PoPH were 1.598‐fold (95% CI: 1.249–2.048, p < 0.001) than any other subtype of PAH.
Table 4.
The association between level of hepatoma‐derived growth factor and the types of pulmonary arterial hypertension (PAH)
| Logistic regression adjusted for age and sex | Unadjusted rank‐sum test | ||||
|---|---|---|---|---|---|
| Coefficient | 95% confidence interval | p Value | p Value (rank‐sum) | ||
| IPAH | −0.181 | −0.292 | −0.071 | 0.001 | 0.001 |
| FPAH | −0.15 | −0.421 | 0.121 | 0.277 | 0.631 |
| APAH | 0.227 | 0.116 | 0.337 | <0.0001 | <0.0001 |
| APAH‐CTD | 0.086 | −0.037 | 0.208 | 0.17 | 0.43 |
| APAH‐CHD | 0.187 | −0.043 | 0.416 | 0.111 | 0.27 |
| PoPH | 0.469 | 0.222 | 0.717 | <0.0001 | <0.0001 |
| APAH‐HIV | −0.102 | −0.476 | 0.273 | 0.595 | 0.854 |
| PAH‐DrugTox | −0.153 | −0.408 | 0.101 | 0.237 | 0.296 |
Note: Bold values denote statistical significance at the p < 0.05 level.
Abbreviations: APAH, PAH associated with other conditions; CHD, congenital heart disease; CI, cardiac index; CTD, connective tissue disease; FPAH, familial PAH; IPAH, idiopathic PAH; PoPH, portopulmonary hypertension.
HDGF predicting mortality in PAH
The REVEAL registry risk score is a well‐recognized mortality risk prediction score for PAH. 17 Using the REVEAL registry 2.0 algorithm, we calculated the REVEAL risk score for each subject, and further divided them into the five REVEAL risk categories. When HDGF levels were examined by REVEAL risk category (Figure 1), higher HDGF levels were associated with more severe REVEAL category (rank‐sum p < 0.0001).
Figure 1.
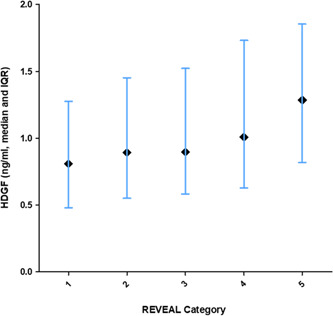
Plot of HDGF (log‐transformed) level versus REVEAL category scores. Error bars indicate 25%–75% IQR, black diamonds indicate median HDGF level. HDGF, hepatoma‐derived growth factor; IQR, interquartile range; REVEAL, Registry to Evaluate Early and Long‐Term PAH Disease Management
Kaplan–Meier analysis was used to assess the relationship between HDGF level and survivability; enrollees with higher than median serum HDGF levels had significantly increased risk of death (log‐rank p = 0.001, Figure 2). A Cox proportional hazard model was constructed to examine the relationship between HDGF and survival. After adjustment for age and sex, a higher HDGF level was still significantly associated with adverse outcomes (HR 1.31, 95% confidence interval: 1.14–1.50, p < 0.0001).
Figure 2.
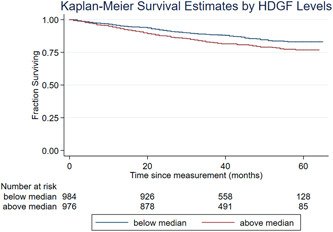
Kaplan–Meier survival curve for hepatoma‐derived growth factor (HDGF) in pulmonary arterial hypertension (PAH)‐Biobank cohort. The curve represents survival analysis of PAH‐Biobank cohort dichotomized by median serum HDGF (n = 2017, p = 0.001) levels
Circulating HDGF level was elevated post‐pulmonary circulation in PAH model
SuHx was established. As expected, the animals had increased right ventricle pressures and increased Fulton index assessed at 21 days (Figure S1). After hemodynamic measurements, prepulmonary (right ventricle) and postpulmonary (left ventricle) plasma samples were collected with HDGF levels measured by ELISA. As shown in Figure 3a, in sham animals at 7 days, circulating HDGF levels were below the low detection limit in the prepulmonary samples but were readily detectable in the postpulmonary samples (p = 0.04). As shown in Figure 3b, with the PAH progression over time in the SuHx rats, the post‐pulmonary HDGF levels significantly (p = 0.027) increased.
Figure 3.
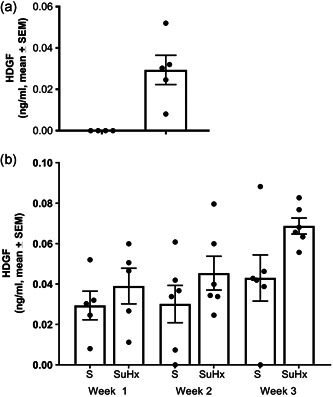
HDGF levels with development of pulmonary hypertension in the Sugen/hypoxia (SuHx) rat model. (a) Bar and scatter plot of HDGF levels from prepulmonary (right ventricle) versus post‐pulmonary circulation (left ventricle) blood samples in sham rats at 7 days (N = 4). (b) Bar and scatter plot of postpulmonary HDGF levels weekly in sham (S) and SuHx treated rats. Box graph is the mean and error bars of the SEM. ANOVA, analysis of variance; HDGF, hepatoma‐derived growth factor; SEM, scanning electron microscope. *p = 0.027, ANOVA SuHx group
HDGF highly expressed in hypertrophic smooth muscle cells in SuHx rats artery
SuHx rats usually exhibit extensive pulmonary artery remodeling, with small artery medial hypertrophy and lumen narrowing. 18 As shown in Figure 4, in a sham lung small pulmonary artery, HDGF expression was exclusively endothelial (arrows). In the SuHx lung at 3 weeks, the media of the representative small artery is hypertrophied and HDGF is now also highly expressed in the medial smooth muscle cells.
Figure 4.
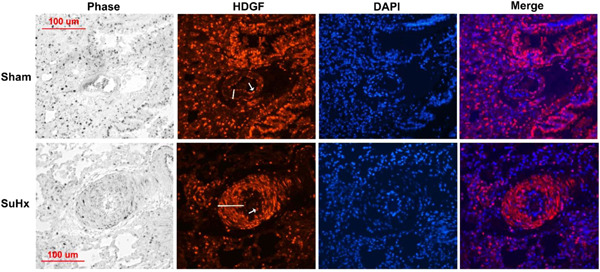
Hepatoma‐derived growth factor (HDGF) is highly expressed in Sugen/hypoxia (SuHx) rat pulmonary artery smooth muscle cells. The lung tissue architecture is shown in the phase panel and HDGF immunostaining (red florescent) with arrow indicating staining of the luminal endothelial layer and the bar the smooth muscle medial layer. Nuclei are stained blue (4′,6‐diamidino‐2‐phenylindole counterstaining) with merged images showing HDGF and nuclear colocalization. A 100 μM scale bar is included for reference
HDGF exhibit similar expression pattern in human PAH lung vasculature
We also performed HDGF immunohistochemistry using human lung sections from PAH subgroups and nontransplanted donor controls (four controls, three IPAH, three APAH‐shunt, and two APAH‐CTD; Table S1). As shown in the representative Figure 5, in the control lung, small arteries were abundant and arterial HDGF was expressed in the luminal endothelial cells, with little to no positive medial smooth muscle cells detected. In the representative lung sections from PAH patients, small arteries were practically obliterated. In the larger arteries shown, HDGF was highly expressed in the endothelial and medial smooth muscle layers of APAH‐shunt and APAH‐CTD lungs. Although not shown, neointima formation was observed in the PAH lungs with abundant HDGF expression.
Figure 5.
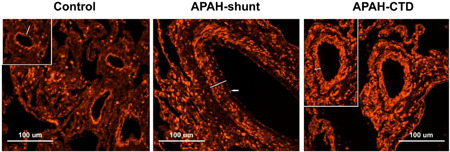
HDGF expression in human control and PAH pulmonary arteries. HDGF immunostaining is shown in representative non‐transplanted control and PAH (APAH‐Shunt and APAH‐CTD) lungs. HDGF positive immunostaining is red. White bar indicates the arterial smooth muscle medial layer and arrows the endothelial layer. Picture inserts highlight representative pulmonary arteries. A 100 μM scale bar is included for reference. APAH, PAH associated with other conditions; CTD, connective tissue disease; HDGF, hepatoma‐derived growth factor; PAH, pulmonary arterial hypertension
DISCUSSION
In the current study, we validated the association between HDGF and clinical severity and survival after adjustment for clinically relevant variables, using a large multicenter cohort (PAH‐Biobank, N = 2017). In this large, multicenter cohort, HDGF was found to be significantly associated with worsened invasive pulmonary hemodynamics, survival, and PoPH.
HDGF is a multifunctional protein functioning in either an autocrine or paracrine manner. The majority of the functional studies have focused on its growth‐promoting effects, 19 predominantly in tumorigenesis. Clinically, it has been correlated with poor prognosis for patients with a number of types of cancers. 20 , 21 However, HDGF is highly expressed in the early stage embryo lung, and re‐expressed in smooth muscle cells of the neointima after blood vessel injury indicating its role is not only limited to mitogenesis. 16 , 22 , 23 In addition, HDGF is highly expressed in idiopathic pulmonary fibrosis and in animal models involved in lung remodeling by stimulating the growth of bronchial and alveolar epithelial cells. 24 Previously we have shown increased HDGF expression in pulmonary microvascular endothelial cells in patients with PAH associated with congenital heart disease. 14 Recently, Lin et al. 21 demonstrated that in oral cancer cells, HDGF activated hypoxic (Akt/HIF‐α) and inflammatory pathways (NF‐κB) simultaneously, and induced VEGF expression through HIF‐α. In the present study, HDGF expression shifts from endothelial expression only to include a pattern of medial layer smooth muscle cell expression in small pulmonary arteries in SuHx rats and PAH patients. These animal and human expression results are in agreement with a previous study where HDGF was only re‐expressed in neointima smooth muscle cells after vessel injury. 16 Pulmonary vascular remodeling, inflammation, and hypoxic condition are all significant contributors to PAH pathogenesis. 3 , 4 Our data here indicates that HDGF is actively participating in pulmonary vascular remodeling, but it could also play a multifunctional role in PAH pathology.
In the SuHx model, we found that HDGF level significantly elevated compared to that in sham control; most importantly, postpulmonary HDGF levels from the left ventricle were higher than prepulmonary levels (right ventricle), indicating that a significant proportion of circulating HDGF is from the pulmonary vasculature. This is supported by the increased expression of HDGF in the arterial wall of both the SuHx rat model and in PAH patients. Compared to the current clinical marker NT‐proBNP, which is cardiac in origin, a biomarker secreted by the pulmonary vasculature may be an improved indicator for the disease progression and response to therapy.
Of interest, in this larger and pathologically more diversified cohort, we found HDGF was significantly associated with PoPH. Given HDGF is highly expressed in the liver and functions as an angiogenesis factor, 25 , 26 it is intriguing that HDGF may play a direct role in PoPH development and disease progression. Portal hypertension hepatic dysfunction is associated with an altered circulation of vasoreactive mediators which contribute to abnormal vascular remodeling and eventually lead to PAH. 27 , 28 , 29 , 30 A recent study of bone morphogenetic protein 9 (BMP9) which is highly associated with PoPH is a good representation of this model. 12 , 31 BMP9 is a TGF‐b ligand, predominantly produced in the liver, and selectively binds to bone morphogenetic protein receptor type 2 (BMPR2)/activin receptor‐like kinase 1(ALK1) complex, which is highly expressed in pulmonary endothelium. BMP9 is significantly reduced in PoPH, but not other forms of PAH, indicating an abnormal level of liver protein can cause pulmonary circulation dysfunction. Whether HDGF plays a role in BMP9 expression is presently unknown.
Although the sample size of the study cohort is very large, limitations of the study still exist. One, in particular, is that the registry, specimen collection, and clinical data collection are not contemporaneous. Another major hurdle is that the time and type of therapy the enrollees received, can not be controlled. As only a single blood sample was collected with enrollment, analysis of the therapeutic response was not possible.
In conclusion, HDGF is actively involved in pulmonary vascular remodeling, higher serum HDGF levels are associated with PAH clinical severity, and predict worse survival in PAH. Measurement of HDGF provides valuable clinical information for stratification of PAH in addition to current diagnostic standards and maybe particularly important for PoPH. How HDGF is involved in the pathophysiology of PAH and if it can be used for novel therapeutic development needs further investigation.
FUNDING INFORMATION
This study was supported by National Institutes of Health/National Heart, Lung, and Blood Institute awards R01HL135114 (Allen D. Everett, Jun Yang, Rachel Damico, Dhananjay Vaidya, William C. Nichols, Melanie Nies, Dunbar Ivy, and Eric D. Austin), PAH Biobank is support by NILBI R24 HL105333 (William C. Nichols, Michael W. Pauciulo, Melanie Nies, Dunbar Ivy, and Eric D. Austin). Serum/Tissue samples were provided by PHBI under the Pulmonary Hypertension Breakthrough Initiative (PHBI). PHBI is supported by NHLBI R24HL123767 and by the Cardiovascular Medical Research and Education Fund (CMREF). Megan Griffiths was supported by NIH K12‐HD000850. Melanie Nies was supported by The Matthew and Michael Wojciechowski Pulmonary Hypertension Pediatric Proof‐of‐Concept Grant (Dr. Robyn J. Barst Pediatric PH Research and Mentoring Fund Grant). Johns Hopkins Pulmonary Hypertension program was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute awards P50 HL084946/R01 and HL114910 (Paul M. Hassoun). Dunbar Ivy was supported by the Jayden de Luca Foundation.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee.
AUTHOR CONTRIBUTIONS
Jun Yang, Rachel Damico, and Allen D. Everett planned and designed the study; Jun Yang, Anjira S. Ambade, Megan Griffiths, Stephanie Brandal developed assays and conducted experiments, Melanie Nies, Michael W. Pauciulo, Katie A. Lutz, Anna W. Coleman, William C. Nichols, Eric D. Austin, Dunbar Ivy, and Paul M. Hassoun contributed biological specimens and clinical data; Jun Yang, Rachel Damico, Megan Griffiths, and Dhananjay Vaidya performed statistical analyses; Jun Yang furnished draft of the manuscript; all authors revised and approved the manuscript for important intellectual content; Jun Yang had full access to all of the data and takes full responsibility for the integrity of the manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We would like to thank contributors, including the Pulmonary Hypertension Centers who collected samples used in this study, as well as patients and their families, whose help and participation made this study possible.
Yang J, Ambade AS, Nies M, Griffiths M, Damico R, Vaidya D, Brandal S, Pauciulo MW, Lutz KA, Coleman AW, Nichols WC, Austin ED, Ivy D, Hassoun PM, Everett AD Hepatoma‐derived growth factor is associated with pulmonary vascular remodeling and PAH disease severity and survival. Pulm Circ. 2022;12:e12007. 10.1002/pul2.12007
Portions of this study were presented in the form of an abstract at the American Thoracic Society International Conference in Dallas, TX, May 2019.
REFERENCES
- 1. Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev. 2012;21:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin LJ. Pulmonary arterial hypertension. Proc Am Thorac Soc. 2006;3:111–15. [DOI] [PubMed] [Google Scholar]
- 3. Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(suppl 1):S10–S19. [DOI] [PubMed] [Google Scholar]
- 4. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–65. [DOI] [PubMed] [Google Scholar]
- 5. Tu L, Dewachter L, Gore B, Fadel E, Dartevelle P, Simonneau G, Humbert M, Eddahibi S, Guignabert C. Autocrine fibroblast growth factor‐2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, Raffestin B, Eddahibi S. Endothelial‐derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benisty JI, McLaughlin VV, Landzberg MJ, Rich JD, Newburger JW, Rich S, Folkman J. Elevated basic fibroblast growth factor levels in patients with pulmonary arterial hypertension. Chest. 2004;126(4):1255–61. [DOI] [PubMed] [Google Scholar]
- 8. Noskovičová N, Petřek M, Eickelberg O, Heinzelmann K. Platelet‐derived growth factor signaling in the lung. From lung development and disease to clinical studies. Am J Respir Cell Mol Biol. 2015;52(3):263–84. [DOI] [PubMed] [Google Scholar]
- 9. Perros F, Montani D, Dorfmüller P, Durand‐Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Hervé P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet‐derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(1):81–8. [DOI] [PubMed] [Google Scholar]
- 10. International PPH Consortium , Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips 3rd, JA , Loyd JE, Nichols WC, Trembath RC, Heterozygous germline mutations in BMPR2, encoding a TGF‐beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–4. [DOI] [PubMed] [Google Scholar]
- 11. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):13S–24S. [DOI] [PubMed] [Google Scholar]
- 12. Nikolic I, Yung LM, Yang P, Malhotra R, Paskin‐Flerlage SD, Dinter T, Bocobo GA, Tumelty KE, Faugno AJ, Troncone L, McNeil ME, Huang X, Coser KR, Lai CSC, Upton PD, Goumans MJ, Zamanian RT, Gregory Elliott C, Lee A, Zheng W, Berasi SP, Huard C, Morrell NW, Chung RT, Channick RW, Roberts KE, Yu PB. Bone morphogenetic protein 9 is a mechanistic biomarker of portopulmonary hypertension. Am J Respir Crit Care Med. 2019;199(7):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tu L, Desroches‐Castan A, Mallet C, Guyon L, Cumont A, Phan C, Robert F, Thuillet R, Bordenave J, Sekine A, Huertas A, Ritvos O, Savale L, Feige JJ, Humbert M, Bailly S, Guignabert C. Selective BMP‐9 inhibition partially protects against experimental pulmonary hypertension. Circ Res. 2019;124(6):846–55. [DOI] [PubMed] [Google Scholar]
- 14. Yang J, Nies MK, Fu Z, Damico R, Korley FK, Hassoun PM, Ivy DD, Austin ED, Everett AD. Hepatoma‐derived growth factor predicts disease severity and survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194(10):1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simpson CE, Chen JY, Damico RL, Hassoun PM, Martin LJ, Yang J, Nies M, Griffiths M, Vaidya RD, Brandal S, Pauciulo MW, Lutz KA, Coleman AW, Austin ED, Ivy DD, Nichols WC, Everett AD. Cellular sources of interleukin‐6 and associations with clinical phenotypes and outcomes in pulmonary arterial hypertension. Eur Respir J. 2020;55(4):1901761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Everett AD, Lobe DR, Matsumura ME, Nakamura H, McNamara CA. Hepatoma‐derived growth factor stimulates smooth muscle cell growth and is expressed in vascular development. J Clin Invest. 2000;105(5):567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benza RL, Gomberg‐Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, McGoon MD, Pasta DJ, Selej M, Burger CD, Frantz RP. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS‐based risk assessment strategies. Chest. 2019;156:323–37. [DOI] [PubMed] [Google Scholar]
- 18. Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121(25):2747–54. [DOI] [PubMed] [Google Scholar]
- 19. Everett AD, Stoops T, McNamara CA. Nuclear targeting is required for hepatoma‐derived growth factor‐stimulated mitogenesis in vascular smooth muscle cells. J Biol Chem. 2001;276:37564–68. [DOI] [PubMed] [Google Scholar]
- 20. Enomoto H, Nakamura H, Liu W, Nishiguchi S. Hepatoma‐derived growth factor: its possible involvement in the progression of hepatocellular carcinoma. Int J Mol Sci. 2015;16:14086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin YW, Huang ST, Wu JC, Chu TH, Huang SC, Lee CC, Tai MH. Novel HDGF/HIF‐1α/VEGF axis in oral cancer impacts disease prognosis. BMC Cancer. 2019;19:1083. 10.1186/s12885-019-6229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thirant C, Galan‐Moya EM, Dubois LG, Pinte S, Chafey P, Broussard C, Varlet P, Devaux B, Soncin F, Gavard J, Junier MP, Chneiweiss H. Differential proteomic analysis of human glioblastoma and neural stem cells reveals HDGF as a novel angiogenic secreted factor. Stem Cells. 2012;30:845–53. [DOI] [PubMed] [Google Scholar]
- 23. Okuda Y, Nakamura H, Yoshida K, Enomoto H, Uyama H, Hirotani T, Funamoto M, Ito H, Everett AD, Hada T, Kawase I. Hepatoma‐derived growth factor induces tumorigenesis in vivo through both direct angiogenic activity and induction of vascular endothelial growth factor. Cancer Sci. 2003;94:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori M, Morishita H, Nakamura H, Matsuoka H, Yoshida K, Kishima Y, Zhou Z, Kida H, Funakoshi T, Goya S, Yoshida M, Kumagai T, Tachibana I, Yamamoto Y, Kawase I, Hayashi S. Hepatoma‐derived growth factor is involved in lung remodeling by stimulating epithelial growth. Am J Respir Cell Mol Biol. 2004;30:459–69. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura H, Izumoto Y, Kambe H, Kuroda T, Mori T, Kawamura K, Yamamoto H, Kishimoto T. Molecular cloning of complementary DNA for a novel human hepatoma‐derived growth factor. Its homology with high mobility group‐1 protein. J Biol Chem. 1994;269:25143–49. [PubMed] [Google Scholar]
- 26. Everett AD, Narron JV, Stoops T, Nakamura H, Tucker A. Hepatoma‐derived growth factor is a pulmonary endothelial cell‐expressed angiogenic factor. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1194–L1201. [DOI] [PubMed] [Google Scholar]
- 27. Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver‐lung interactions. Clin Chest Med. 1996;17(01):151–69. [DOI] [PubMed] [Google Scholar]
- 28. Herve P, Le Pavec J, Sztrymf B, Decante B, Savale L, Sitbon O. Pulmonary vascular abnormalities in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(01):141–59. [DOI] [PubMed] [Google Scholar]
- 29. Mandell MS, Groves BM. Pulmonary hypertension in chronic liver disease. Clin Chest Med. 1996;17(01):17–33. [DOI] [PubMed] [Google Scholar]
- 30. Savale L, Watherald J, Sitbon O. Portopulmonary hypertension. Semin Respir Crit Care Med. 2017;38(5):651–61. [DOI] [PubMed] [Google Scholar]
- 31. Toshner M. BMP9 morphs into a potential player in portopulmonary hypertension. Am J Respir Crit Care Med. 2019;199(7):819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


