Abstract
Mortality in pulmonary arterial hypertension (PAH) remains high and referral to palliative or supportive care (P/SC) specialist services is recommended when appropriate. However, access to P/SC is frequently a challenge for patients with a noncancer diagnosis and few patients living with PAH report P/SC involvement in their care. A modified Delphi process of three questionnaires completed by a multidisciplinary panel (N = 15) was used to develop expert consensus statements regarding the use of P/SC to support patients with PAH. Panelists rated their agreement with each statement on a Likert scale. There was a strong consensus that patients should be referred to P/SC when disease symptoms become unmanageable or for end‐of‐life care. Services that achieved consensus were pain management techniques, end‐of‐life care, and psychosocial recommendations. Palliative or supportive care should be discussed with patients, preferably in‐person, when disease symptoms become unmanageable, when starting treatment, when treatment‐related adverse events occur or become refractory to initial intervention. Care partners and patient support groups were considered important in improving a patient's overall health outcomes, treatment adherence, and perception of care. Most patients with PAH experience cognitive and/or psychosocial changes and those who receive psychosocial management have better persistence and/or compliance with their treatment. These consensus statements provide guidance to healthcare providers on the “who and when” of referral to palliative care services, as well as the importance of focusing on the psychosocial aspects of patient care and quality of life.
Keywords: palliative care, psychosocial support, pulmonary arterial hypertension, quality of life, supportive care
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare, progressive pulmonary vascular disease with a prevalence of 15–50 cases per million individuals in the United States and a median survival of 7 years. 1 It is associated with a range of nonspecific symptoms (e.g., fatigue, dyspnea, chest pain, presyncope, and syncope) that can often result in delayed diagnosis. This potentially leads to substantial morbidity, poor prognosis, and high healthcare resource utilization. 2 , 3 , 4 , 5 , 6 , 7 The clinical course of PAH is one of progressive deterioration interspersed with episodes of acute decompensation. 8 Current management is based on the severity of the newly diagnosed PAH patient as assessed by a multiparametric risk stratification approach, and includes agents targeting the nitric oxide, endothelin‐1, and prostacyclin pathways, administered as monotherapy and often in combination, per current guidelines. 8 , 9 While these medications have been shown to increase functionality as measured by 6‐min walking distance (6MWD) as well as to delay clinical worsening, they are also associated with significant adverse effects which may negatively impact patients' quality of life (QOL). 8 , 9 , 10 , 11
Due to the life‐limiting nature of this condition, proactive referral to specialist palliative or supportive care (P/SC) services is recommended for advanced care planning and interventions targeted at improving QOL. 8 Palliative care improves QOL for patients and their families through the mitigation of suffering, which involves early identification, comprehensive assessment, and treatment of pain and other physical, psychosocial, and spiritual problems. 12 Although P/SC has been associated with end‐of‐life and hospice care, the World Health Organization (WHO) emphasized it should be utilized early in the course of an illness, in conjunction with other therapies that are intended to prolong life. 12 In the United States, P/SC is one of the fastest‐growing fields as payers, providers, and policymakers have recognized its potential to improve QOL and reduce costs. 13 Despite this growth, health disparity impacts access to P/SC and is influenced by factors such as geographic location and access to resources related to hospital characteristics such as size, funding source, and tax status. 14 Further, there is a well‐recognized equity gap in the utilization of P/SC services between people with cancer versus noncancer primary diagnosis, and those with a noncancer diagnosis are less functional and more likely to be hospitalized at the time of referral compared with those living with cancer. 15 , 16 Despite guideline recommendations regarding the utilization of P/SC in people with PAH, few patients report the involvement of such services in their care and a majority to suffer with a high symptom burden. 17 , 18 , 19 Furthermore, when P/SC services are engaged, it is most frequently to address goals of care at end of life. 18
An expert consensus may provide useful guidance for PAH healthcare providers (HCPs) in the appropriate engagement of P/SC to identify strategies that are clinically meaningful and improve patient outcomes. The objective of this Delphi study was to develop consensus statements based on expert HCP insights regarding access, patient engagement, and appropriate palliative care services for those living with PAH (PH classification: Group 1). 20 , 21
METHODS
A modified Delphi process was used to develop expert consensus statements regarding the use of P/SC to support people living with PAH. The Delphi process, originally described by Delbecq and colleagues, is a proven methodology to reach consensus on a topic of interest. 22 , 23 , 24 , 25 A multidisciplinary cohort of approximately 20 US‐based physicians, pharmacists, advanced practice providers, and registered nurses, selected based on their experience in the management of PAH or palliative care, were invited to participate in the study. Those panelists who actively participated in the Delphi study (defined as completing at least two of the three questionnaires, including the final questionnaire, and reviewing the draft and final manuscripts) are included as authors.
The modified Delphi procedure deployed was as follows (Figure 1):
-
1.
The moderators developed an initial open‐ended survey pertaining to the use of palliative care in patients with PAH. Panelists provided responses for each statement independently via an online survey platform (SurveyGizmo.com) and added additional relevant statements at their discretion.
-
2.
Panelists' aggregated responses were summarized by the moderators to generate the second survey, distributed through the same online platform, so panelists could rate each statement using a Likert scale ranging from –5 (strongly disagree with statement) to +5 (strongly agree with the statement) to establish preliminary consensus. Consensus was defined prospectively as a mean panelist rating of ≤ –2.5 or ≥ +2.5 (Figure 2), with a standard deviation (SD) that did not cross zero. Again, panelists were given the opportunity to add clarifying comments.
-
3.
The moderators reviewed the aggregated responses and further refined the statements, if needed, to generate the final survey. This was distributed to each panelist with a summary of their responses to the second survey and the panel's aggregated results (mean and SD of Likert scale scores), to promote consensus by making panelists aware of the group's opinions and allowing them the opportunity to validate or modify their responses accordingly.
-
4.
The aggregated final results were circulated to panelists for review and endorsement.
Figure 1.
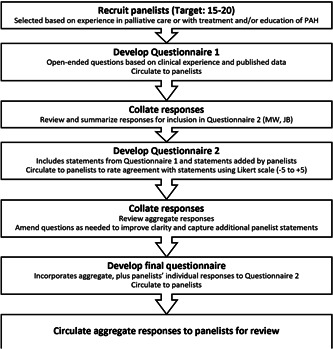
The Delphi process used in the study
Figure 2.
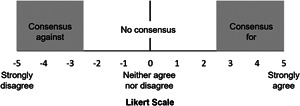
The Likert scale and definitions of consensus used throughout the Delphi process. Panelists rated their agreement on a Likert scale from −5 (complete disagreement) to +5 (complete agreement). Consensus was predefined as a mean Likert scale score of ≤ −2.5 or ≥ +2.5 with a standard deviation (SD) not crossing zero
As an essential component of the Delphi methodology, panelists’ anonymity was ensured throughout the process and all opinions were weighed equally, thereby minimizing risk for confirmation bias and helping ensure all panelists were comfortable offering their opinions freely. Panelists were encouraged to provide feedback on the validity, specificity, and content of the items under consideration. All comments were incorporated verbatim at each round.
The Delphi method was implemented using electronic communications to gather and distribute information, allowing panelists to complete the surveys without stringent time restrictions or the need for travel.
RESULTS
The Delphi panel consisted of 15 healthcare professionals, including the moderators, who accepted the invitation to participate and comprised physicians, pharmacists, advanced practice providers, and registered nurses (Table 1). All geographic regions in the US except the southwest were represented. All panelists reported experience in treating persons with PAH for at least 6 years.
Table 1.
Characteristics of the Delphi panelists
| Characteristic | Panelists (N = 15) |
|---|---|
| Specialty | |
| Physician | 2 |
| Pharmacist | 4 |
| Nurse practitioner | 5 |
| Physician assistant | 1 |
| Registered nurse | 3 |
| Region | |
| West | 1 |
| Mid West | 7 |
| Southwest | 0 |
| Northeast | 3 |
| Southeast | 4 |
| Years treating PAH (median [range]) | 10 (6–18) |
| Number of patients with PAH treated in the past month (median [range]) | 35 (3–200) |
| PAH treatment setting | |
| Outpatient only | 5 |
| Both | 8 |
Note: Treatment setting was not provided for two panelists.
Abbreviations: PAH, pulmonary arterial hypertension, P/SC, palliative and supportive care.
The final questionnaire included 86 statements. Consensus in the agreement was achieved for 45 statements, and there was a consensus to disagree with one statement (Supporting information).
Role of palliative or supportive care in the management of PAH
There was strong consensus for the statement that for most PAH HCPs, P/SC encompasses more than hospice care (mean [SD] score: 4.83 [0.39], Figure 3). Moreover, the panel reached consensus that P/SC services include psychosocial recommendations (4.33 [0.39], pain management techniques (4.83 [0.58]), and end‐of‐life care (4.92 [0.29]). However, no consensus was reached regarding complementary and alternative medicine services (0.75 [2.73]) or nutritional recommendations (2.17 [2.25]). The panel recommended referral to a P/SC specialist when disease symptoms become unmanageable (4.00 [1.21]) and when patients would benefit from it for end‐of‐life care (5.00 [0.00]). Significantly, the panel reached consensus to disagree with the statement “My patients are not referred at all” (−4.08 [1.93]).
Figure 3.

Consensus statements for the role of palliative or supportive care in the management of pulmonary arterial hypertension
Factors affecting access to palliative or supportive care
The greatest levels of variance in panelist responses were in response to two statements describing access to P/SC services: “It is difficult for my patients to access P/SC specialists within” (−0.92 [3.80]), and “outside” (−0.25 [3.82]) “my practice/hospital system” (Figure 4). Factors that the panel reached consensus as being important in providing P/SC options to patients with PAH included disease severity (4.25 [1.54]), availability of service centers (3.75 [1.48]), geographic location (3.42 [1.88]), and HCP and/or patient understanding of P/SC services (3.25 [2.99]).
Figure 4.

Consensus statements regarding access to palliative and supportive care for people with pulmonary arterial hypertension
Patient identification and communication
Consensus was achieved that patients who would benefit from P/SC services (Figure 5) were those in New York Heart Association (NYHA)/WHO functional Class III (4.17 [1.19]) or IV (4.67 [1.15]), with the panel advising to discuss P/SC options with patients when disease symptoms become unmanageable (4.83 [0.58]), when starting treatment (2.50 [1.88]), and when treatment‐related adverse events (AEs) occur (3.83 [1.47]), or when treatment‐related AEs become refractory to initial intervention (4.67 [0.78]).
Figure 5.

Consensus statements for identifying and communicating with people with pulmonary arterial hypertension who may benefit from palliative or supportive care
The panel identified in‐person discussion with the HCP as helpful for patients’ decision‐making in choosing P/SC options (4.50 [1.00]) and acknowledged the importance of a care partner (e.g., spouse, family member, or friend) in improving overall health outcomes, treatment adherence, and perception of care (3.83 [0.94]). The panel reached consensus that most patients with PAH have at least one partner who is integral to their treatment plan (3.58 [1.44]).
The positive role of patient support groups was also recognized by the panel, with consensus that participation can help to normalize one's experience of living with PAH (4.75 [0.45]), informs patients of available resources and raises disease awareness (4.58 [0.90]), offers reassurance and a sense of community (4.42 [0.79]), helps them learn how to manage their disease (4.17 [0.83]), and devise coping mechanisms (4.08 [1.08]). There was a strong consensus that patients are provided with information on support groups along with opportunities to access them (4.67 [0.65]).
Psychosocial support
As the Delphi process progressed, psychosocial support emerged as an area of focus within the spectrum of P/SC services (Figure 6). The panel acknowledged that most patients with PAH experience cognitive and/or psychosocial changes (e.g., depression and/or anxiety) during the course of their disease (4.67 [0.49]), and that those who receive psychosocial treatment have better persistence and/or compliance with their PAH treatment (2.75 [1.71]). There was, however, a clear lack of consensus regarding whether that translated into improved clinical outcomes (1.42 [2.31]). The panel agreed that patients should receive psychosocial treatment from a healthcare professional (3.17 [1.11]), typically a licensed mental health professional (3.92 [1.00]). Reasons for referral to a licensed mental health professional that achieved consensus were developing cognitive or psychosocial changes (3.50 [2.20]), comorbid conditions, and other relevant medical histories (3.33 [1.56]), as well as issues with their support system (3.00 [2.22]). The factors deemed by the panel to be important when conducting a psychosocial assessment or referring to a mental health professional were when the patient requested such help (5.00 [0.00]), issues with mood and temperament (4.00 [1.71]), QOL changes (3.92 [1.08]), a high disease burden from PAH (3.92 [1.38]), the presence of a support system (3.00 [2.41]), and a new diagnosis of PAH (2.92 [2.50]).
Figure 6.
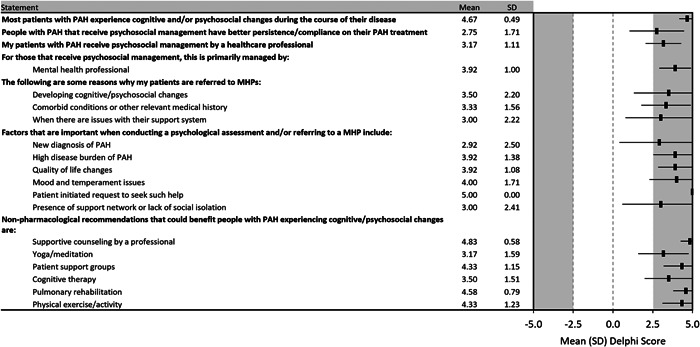
Consensus statements regarding the utility and delivery of psychosocial support for people with pulmonary arterial hypertension
Psychosocial support also emerged as the one area where the panel agreed nonpharmacological management strategies could be of benefit to their patients, with professional counseling (4.83 [0.58]), pulmonary rehabilitation (4.58 [0.79]), patient support groups (4.33 [1.15]), physical activity (4.33 [1.23]), cognitive therapy (3.50 [1.51]), and yoga or meditation (3.17 [1.59]).
DISCUSSION
This Delphi panel study was initiated to develop an expert consensus regarding access, patient engagement, and appropriate P/SC services for those living with PAH. In line with the multidisciplinary nature of P/SC, the Delphi panelists included physicians, pharmacists, advanced practice providers, and registered nurses with experience treating persons with PAH. The panel also included experts whose primary area of expertise was P/SC (including the provision of such services to people with PAH), as well as experts in the field of PAH. As would be expected, their detailed knowledge of P/SC was counterbalanced by seeing fewer patients with PAH.
There was almost universal support among panelists that for most PAH HCPs, P/SC encompasses more than hospice care, which may be indicative of an evolution in attitudes; in 2014, a survey of physicians treating PAH reported the most common reasons for obtaining a P/SC consultation were in the setting of the end of life or active dying (59%) or for hospice referral (46%). 26 Corroborating this, the panel's strong disagreement with the statement “my patients are not referred at all,” contrasts with the results from a survey of people with PAH that reported only 1.4% of patients had a P/SC specialist involved in their care (although it should be noted there is a difference between referral and specialist involvement). 19 Guidelines for the management of PAH, and other literature, suggest referral to specialist P/SC services when appropriate and this is reflected in the consensus that referral should be when symptoms become unmanageable or for end‐of‐life care. 8 , 17 , 27 , 28 Although there has been a marked expansion in P/SC capacity in the United States over recent years, the factors affecting access are broadly aligned with those identified in the Center to Advance Palliative Care report card: geography, availability of services (smaller hospitals in rural areas are less likely to have palliative care programs), and HCP and/or patient understanding of P/SC services. 14 Of note, a survey of people with PAH reported that approximately one‐third of respondents considered an alternative term, such as “supportive care,” more favorably than “palliative care.” 29 The 2014 survey of physician attitudes to palliative care in PAH corroborated this with 17% of respondents agreeing that the term “palliative” has a negative connotation. In the aforementioned survey, the most frequently cited barriers to referral were nonapproval by the patient or family, and a view that palliative care is “giving up hope.” 26 Evidence suggests, however, that early palliative care intervention can result in improved patient‐centered and clinical outcomes in patients with advanced cancer and end‐organ disease including patient and caregiver satisfaction, reduced psychological symptoms, reduced symptom burden, reduced hospital readmission, more advanced care planning, more days alive outside of the hospital, and even increased survival. 30 , 31 , 32 , 33 Interestingly, a significant correlation was observed in people with PAH between cardiopulmonary symptom intensity and the negative emotional reaction towards palliative care, something presumably that could be addressed by effective symptom control from earlier utilization of such resources. 29
There was consensus that P/SC services should be discussed with patients when starting treatment, when treatment‐related AEs first manifest or when AEs become refractory to initial treatment, and that patients in NYHA/WHO functional Class III or IV may benefit from P/SC. In addition to end‐of‐life care, P/SC services should be discussed, and patients should be referred to a P/SC specialist when disease symptoms become unmanageable. While acknowledging the lack of data from randomized controlled trials to support a benefit of such strategies, the consensus that patients starting treatment would benefit from P/SC services aligns with the growing opinion across chronic diseases that P/SC should be initiated at the time of diagnosis and provided concomitantly with disease‐directed or curative treatments. 27 , 34 This again appears consistent with evolution in this area from 2014, when only 40% of PAH physicians surveyed considered palliative care an appropriate adjunct to traditional PAH therapies, 26 and is significant, given the panelists’ endorsement of in‐person discussions with an HCP for helping people with PAH make informed choices when considering P/SC services. Treatment guidelines for PAH recommend incorporating P/SC in the management of PAH patients. The CHEST guidelines acknowledge that the addition of palliative care to assist in the management of disease burden and symptoms can often be beneficial to improving QOL in patients. 35 The ESC/ERS guidelines suggest that as part of the treatment strategy in patients with PAH, the initial approach should include general measures, such as psychosocial support. 8 In addition, there should be proactive advanced care planning with referral to specialist palliative care services when appropriate.
Discussions about palliative care should be guided by the patient's articulated goals, expressed preferences and questions. 17 Unpredictability of disease course has been identified as a barrier to referral for P/SC in patients with progressive lung disease and heart failure, 36 , 37 and introducing discussions about palliative care support and end‐of‐life issues earlier allows patients to learn about additional options to help manage their disease and its effects. 17
Differences in which statements achieved consensus regarding the timing of when to discuss P/SC and when to refer to specialist P/SC services may reflect a distinction between symptom and treatment‐related adverse effect management provided by the PAH treatment team as part of the standard of care, and referral to a specialist care team in response to a temporary, acute exacerbation of symptoms, or a need for specialist end‐of‐life care. 17 The appropriate timing for introducing P/SC services should be tailored to the needs and circumstances of each patient, including consideration of how the patient will respond to such a suggestion. As mentioned earlier, patients with advanced disease and a higher symptom burden had a more negative emotional reaction to palliative care. 29 Initiating a conversation earlier in the patient journey may help mitigate any negative reactions and leverage a window when patients are more receptive to such conversations. Words are important: approximately one‐third of patients surveyed also viewed “supportive care” as a more favorable term than “palliative care.” 29
The panel acknowledged the positive influence and support of care partners for people with PAH. What was not addressed in this process was the potential burden of the condition on such care partners. A cross‐sectional survey reported 14% of caregivers for people with PAH resigned from their jobs or reduced working hours to provide care for their loved one, and that 14% of caregivers had signs and symptoms of clinical depression. 38 This is likely more challenging with the financial burden of living with PAH, reported to equal or exceed $1000 per month in out‐of‐pocket expenses for 31.1% of patients and considered burdensome by 37.9% of the overall cohort surveyed. 4 The positive role of patient support groups in helping people with PAH normalize their experience with PAH and devise coping mechanisms to manage their condition was strongly endorsed by the panelists and identifies a critical evidence gap supporting such recommendations despite it seeming intuitively correct.
In addition to pain management techniques and end‐of‐life care, the Delphi process identified psychosocial support as an area where palliative care services can benefit people with PAH. High rates of anxiety and depression have been reported in people with PAH, 4 , 17 , 19 , 28 , 39 and these are also among the most common symptoms encountered by treating physicians. 26 Profound impact on cognition, memory, emotional and social well‐being, intimacy, and relationships have also been reported, along with feelings of frustration, anger, low self‐esteem, and worthlessness. 2 , 19 , 28 , 39 One study reported an increase in the prevalence of psychological disorders in people with PAH with worsening functional class. 40
The lack of consensus regarding the use of complementary and alternative medicines, or nutritional recommendations may reflect a lack of availability, low evidence base or standard guidelines, institutional regulations, legal restrictions, or a lack of HCP experience in their use.
There are limitations inherent in the Delphi process. There are no standard criteria defining consensus in Delphi studies, the process is not statistically rigorous, and when consensus is reached there is no guarantee that it is generalizable or appropriate. Given that the Delphi process was designed to elicit guidance when no strong evidence is available, and the breadth of topics investigated, such standardization may not be possible. Panel selection and the development of the initial questionnaire may allow the introduction of bias. Anonymity, integral to the Delphi process, and equal weighting of panelist responses may result in outcomes based on insufficient consideration of or limited experience with the subject matter. Participants in the expert panel were limited to the United States and although over 20 individuals were invited to participate only 15 were accepted (only two of whom were physicians). This may have created selection bias, excluding perspectives present in a larger, multinational group of HCPs, although with the benefit of focusing recommendations on those relevant and feasible within that healthcare environment. The exclusion of patients, payers, practitioners of complementary and alternative therapies, and other potential stakeholders may have impacted the diversity of perspectives around appropriate symptom management strategies. Finally, the opportunity to be an author on any resultant communications such as this manuscript may have acted as an incentive to participate and introduced bias.
CONCLUSION
This modified Delphi study involving experts in the management of PAH facilitated the development of consensus statements regarding access, patient engagement, and appropriate palliative care services for those living with PAH. There are few studies exploring the appropriate assessment and timing of delivery for palliative care in this rare debilitating condition, and not surprisingly PAH HCPs report being most comfortable assessing and managing PAH‐specific, disease‐related symptoms. These consensus statements give guidance to HCPs on the “who and when” of referral to P/SC services, as well as the potential value of focusing on the psychosocial aspects of patient care and QOL.
CONFLICTS OF INTEREST
Rebekah H. Anguiano, Rana L. A. Awdish, Melissa Morrison, Frank Spexarth, Keith M. Swetz, Nathan J. Verlinden report no conflict of interest. Melisa Wilson reports receiving honoraria for Advisory Board participation (Bayer and United Therapeutics), consult services (Bayer), and speaker for United Therapeutics. James C. Coons reports research funding from United Therapeutics Corp. Sara Paulus reports receiving honoraria for Advisory Board participation (Bayer and United Therapeutics) and consult services (Bayer), and is currently a paid employee of Acceleron Pharma. Ann Schmit reports. Mary E. Whittenhall has served as a consultant and advisory board member for United Therapeutics. Amy Kimber, Margaret R. Sketch, Meredith Broderick are paid employees of United Therapeutics Corp. Jacqueline Brewer reports research grant, consultant, speaker, manuscript preparation from United Therapeutics Corp.
ETHICS STATEMENT
This work did not involve research on human subjects or animals and as such ethics approval was not required or sought.
AUTHOR CONTRIBUTIONS
Melisa Wilson, Jacqueline Brewer, Margaret R. Sketch, and Meredith Broderick conceived and designed the research. Melisa Wilson, Rebekah H. Anguiano, Rana L. A. Awdish, James C. Coons, Melissa Morrison, Sara Paulus, Ann Schmit, Frank Spexarth, Keith M Swetz, Nathan J. Verlinden, Mary E. Whittenhall, Amy Kimber, and Jacqueline Brewer participated in the study, contributed to the data acquisition, and interpretation of the results. All authors participated in drafting and critical revision of the manuscript, and have approved the final version.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank John Howell, PhD, for providing editorial support and for support with statistical analyses. This study was sponsored by United Therapeutics Corporation.
Wilson M, Anguiano RH, Awdish RLA, Coons JC, Kimber A, Morrison M, Paulus S, Schmit A, Spexarth F, Swetz KM, Verlinden NJ, Whittenhall ME, Sketch MR, Broderick M, Brewer J. An expert panel Delphi consensus statement on the use of palliative care in the management of patients with pulmonary arterial hypertension. Pulmonary Circulation. 2022;12:e12003. 10.1002/pul2.12003
Contributor Information
Melisa Wilson, Email: melisa.wilson@adventhealth.com.
Jacqueline Brewer, Email: jacqueline.brewer@beaumont.org.
REFERENCES
- 1. Beshay S, Sahay S, Humbert M. Evaluation and management of pulmonary arterial hypertension. Respir Med. 2020;171:106099. 10.1016/j.rmed.2020.106099 [DOI] [PubMed] [Google Scholar]
- 2. Delcroix M, Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev. 2015;24:621–9. 10.1183/16000617.0063-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Exposto F, Petrică N, Davies E, Beaudet A. Identification of a pulmonary arterial hypertension (PAH) patient cohort and study of its burden of illness in Programme de Medicalisation des Systemes d'information (PMSI). Int J Cardiol. 2020;306:175–80. 10.1016/j.ijcard.2020.02.034 [DOI] [PubMed] [Google Scholar]
- 4. Helgeson SA, Menon D, Helmi H, Vadlamudi C, Moss JE, Zeiger TK, Burger CD. Psychosocial and financial burden of therapy in USA patients with pulmonary arterial hypertension. Diseases. 2020;8:22. 10.3390/diseases8020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matura LA, McDonough A, Carroll DL. Symptom Prevalence, symptom severity, and health‐related quality of life among young, middle, and older adults with pulmonary arterial hypertension. Am J Hosp Palliat Care. 2016;33:214–21. 10.1177/1049909114554079 [DOI] [PubMed] [Google Scholar]
- 6. Matura LA, McDonough A, Hanlon AL, Carroll DL, Riegel B. Sleep disturbance, symptoms, psychological distress, and health‐related quality of life in pulmonary arterial hypertension. Eur J Cardiovasc Nurs. 2015;14:423–30. 10.1177/1474515114537951 [DOI] [PubMed] [Google Scholar]
- 7. Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW, Frost AE, Liou TG, Turner M, Feldkircher K, Miller DP, Elliott CG. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest. 2011;140:19–26. 10.1378/chest.10-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 9. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long‐term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–56. 10.1378/chest.11-1460 [DOI] [PubMed] [Google Scholar]
- 11. Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2‐year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest. 2013;144:1521–9. 10.1378/chest.12-3023 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Integrating palliative care and symptom relief into primary health care: a WHO guide for planners, implementers and managers; 2020. Geneva: World Health Organization.
- 13. Rogers M. Growth of Palliative Care in U.S. Hospitals: Snapshot (2000‐2016). https://www.capc.org/capc-reports-and-publications/(2018). Accessed 3 Feb 2021.
- 14.America's Care of Serious Illness: A State‐by‐State Report Card on Access to Palliative Care in Our Nation's Hospitals. 2019. New York, NY: Center to Advance Palliative Care and the National Palliative Care Research Center. [DOI] [PMC free article] [PubMed]
- 15.Palliative Care Referrals: 2018–2019 Snapshot. 2020. Center to Advance Palliative Care.
- 16. Bostwick D, Wolf S, Samsa G, Bull J, Taylor DH Jr., Johnson KS, Kamal AH. Comparing the palliative care needs of those with cancer to those with common non‐cancer serious illness. J Pain Symptom Manage. 2017;53:1079–84. 10.1016/j.jpainsymman.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 17. Gin‐Sing W. Palliative care in pulmonary arterial hypertension. Curr Opin Support Palliat Care. 2017;11:7–11. 10.1097/SPC.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 18. Grinnan DC, Swetz KM, Pinson J, Fairman P, Lyckholm LJ, Smith T. The end‐of‐life experience for a cohort of patients with pulmonary arterial hypertension. J Palliat Med. 2012;15:1065–70. 10.1089/jpm.2012.0085 [DOI] [PubMed] [Google Scholar]
- 19. Swetz KM, Shanafelt TD, Drozdowicz LB, Sloan JA, Novotny PJ, Durst LA, Frantz RP, McGoon MD. Symptom burden, quality of life, and attitudes toward palliative care in patients with pulmonary arterial hypertension: results from a cross‐sectional patient survey. J Heart Lung Transplant. 2012;31:1102–08. 10.1016/j.healun.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 20. Simonneau G, Galiè N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. 10.1016/j.jacc.2004.02.037 [DOI] [PubMed] [Google Scholar]
- 21. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. 10.1016/j.jacc.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 22. de Meyrick J. The Delphi method and health research. Health Educ. 2003;103:7–16. 10.1108/09654280310459112 [DOI] [Google Scholar]
- 23. Delbecq AL, Van de Ven AH, Gustafson DH. Group techniques for program planning: a guide to nominal groups and Delphi process. Middleton: Green Briar Press; 1975. [Google Scholar]
- 24. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- 25. Hsu C‐C, Sandford B. The Delphi technique: making sense of consensus. Pract Assess Res Evaluation. 2007;12:10. [Google Scholar]
- 26. Fenstad ER, Shanafelt TD, Sloan JA, Novotny PJ, Durst LA, Frantz RP, McGoon MD, Swetz KM. Physician attitudes toward palliative care for patients with pulmonary arterial hypertension: results of a cross‐sectional survey. Pulm Circ. 2014;4:504–10. 10.1086/677365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelley AS, Morrison RS. Palliative Care for the Seriously Ill. N Engl J Med. 2015;373:747–55. 10.1056/NEJMra1404684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khirfan G, Tonelli AR, Ramsey J, Sahay S. Palliative care in pulmonary arterial hypertension: an underutilised treatment. Eur Respir Rev. 2018;27. 10.1183/16000617.0069-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hrustanovic‐Kadic M, Ziegler C, El‐Kersh K. Palliative care perception in pulmonary arterial hypertension: evaluating the interaction of PPCI, PAH‐SYMPACT Questionnaire, and the REVEAL 2.0 Risk Score. Ann Am Thorac Soc. 2021;18:361–4. 10.1513/AnnalsATS.202005-552RL [DOI] [PubMed] [Google Scholar]
- 30. Shinall MC Jr., Karlekar, M , Martin S, Gatto CL, Misra S, Chung CY, Porayko MK, Scanga AE, Schneider NJ, Ely EW, Pulley JM, Jerome RN, Dear ML, Conway D, Buie R, Liu D, Lindsell CJ, Bernard GR. COMPASS: a pilot trial of an early palliative care intervention for patients with end‐stage liver disease. J Pain Symptom Manage. 2019;58:614–22. 10.1016/j.jpainsymman.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mah K, Swami N, O'Connor B, Hannon B, Rodin G, Zimmermann C. Early palliative intervention: effects on patient care satisfaction in advanced cancer. BMJ Support Palliat Care. 2021. 10.1136/bmjspcare-2020-002710 [DOI] [PubMed] [Google Scholar]
- 32. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18:134–42. 10.1089/jpm.2014.0192 [DOI] [PubMed] [Google Scholar]
- 33. Tassinari D, Drudi F, Monterubbianesi MC, Stocchi L, Ferioli I, Marzaloni A, Tamburini E, Sartori S. Early palliative care in advanced oncologic and non‐oncologic chronic diseases: a systematic review of literature. Rev Recent Clin Trials. 2016;11:63–71. 10.2174/1574887110666151014141650 [DOI] [PubMed] [Google Scholar]
- 34. Center to Advance Palliative Care Multi‐Payer Workgroup . Serious illness strategies for health plans and accountable care organizations. 2020. Center to Advance Palliative Care.
- 35. Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve‐Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155:565–86. 10.1016/j.chest.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 36. Lynn J, Harrell F Jr., Cohn, F , Wagner D, Connors AF Jr. Prognoses of seriously ill hospitalized patients on the days before death: implications for patient care and public policy. New Horiz. 1997;5:56–61. [PubMed] [Google Scholar]
- 37. Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, Dodson GC, O'Connor CM, Felker GM. Discordance between patient‐predicted and model‐predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299:2533–42. 10.1001/jama.299.21.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hwang B, Howie‐Esquivel J, Fleischmann KE, Stotts NA, Dracup K. Family caregiving in pulmonary arterial hypertension. Heart Lung. 2012;41:26–34. 10.1016/j.hrtlng.2011.03.002v [DOI] [PubMed] [Google Scholar]
- 39. Guillevin L, Armstrong I, Aldrighetti R, Howard LS, Ryftenius H, Fischer A, Lombardi S, Studer S, Ferrari P. Understanding the impact of pulmonary arterial hypertension on patients' and carers' lives. Eur Respir Rev. 2013;22:535–42. 10.1183/09059180.00005713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Löwe B, Gräfe K, Ufer C, Kroenke K, Grünig E, Herzog W, Borst MM. Anxiety and depression in patients with pulmonary hypertension. Psychosom Med. 2004;66:831–6. 10.1097/01.psy.0000145593.37594.39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


