Abstract
There was no structured method for safely transition from parenteral prostanoids to oral medication. We enrolled 37 idiopathic/hereditary pulmonary arterial hypertension patients receiving triple combination therapy including parenteral prostanoids into structured transition program to oral selexipag. Four (10.8%) patients successfully transitioned under the protocol, and all of them presented long‐term safety.
Keywords: prostanoids, pulmonary arterial hypertension, transition therapy
Abbreviations
- I/H PAH
idiopathic/hereditary pulmonary arterial hypertension
- ERA
endothelin‐receptor antagonists
- PAH
pulmonary arterial hypertension
- PAP
pulmonary arterial pressure
- PDE‐5
phosphodiesterase‐5
- PVR
pulmonary vascular resistance
- WHO
World Health Organization
INTRODUCTION
Upfront triple combination therapy that includes parenteral prostanoids provides drastic improvements in the hemodynamics and prognosis of patients with idiopathic/hereditary pulmonary arterial hypertension (I/H PAH). 1 Some of these patients eventually wish to transition from parenteral prostanoids to oral medications, however, the transition is associated with a risk for clinical deterioration, 2 particularly hemodynamic compromise. 3 Moreover, there are no clear indicators for when the transition can be done safely. 2 , 4 In this study, we assessed the long‐term safety of a structured transition protocol from parenteral prostanoids to oral selexipag among patients with I/H PAH whose pulmonary arterial pressure (PAP) improved significantly with combination therapy.
METHODS
We enrolled 37 I/H PAH patients who were receiving triple combination therapy that included parenteral prostanoids in our local cohort (Figure 1a) from April 2009 to August 2021. Patients became candidates for transition to oral selexipag if they demonstrated sufficient improvement (right heart catheterization demonstrated a mean PAP < 30 mmHg and the WHO functional class was I or II) while on upfront triple combination therapy, and parenteral epoprostenol or treprostinil was slowly reduced (Panel b). Frequent catheterization was performed during the reduction, and the process was continued only if the favorable clinical status mentioned above was maintained. Patients were transitioned to oral selexipag (3.2 mg/day) when they reached their reduction goals (shown in Panel b). After the switching, the patient received hemodynamic follow‐up evaluations after 3 months and 1 year, and yearly thereafter. Patients who discontinued parenteral prostanoids due to complications or those who wished to be excluded by personal wish were not included in the analysis. Descriptive statistics were expressed as mean ± standard deviation. Continuous variables were analyzed with the Wilcoxon signed‐ranked test. The procedures followed were in accordance with the “Declaration of Helsinki” and the ethical standards of the local responsible committee on human experimentation. The analysis of the results in this study was performed retrospectively. And informed consent was obtained by allowing patients to opt‐out of the study on the website.
Figure 1.
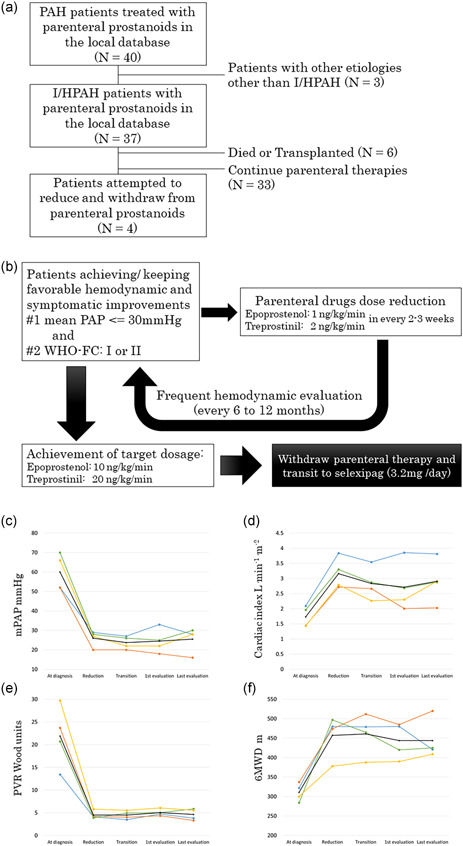
(a) Consort diagrams for this analysis. (b) Transition protocol from parental prostanoid to selexipag. (c–f) Hemodynamic and exercise tolerance measurements among patients. (c) Mean pulmonary arterial pressure (PAP) (mmHg); (d) cardiac index (L/min/m2); (e) pulmonary vascular resistance (PVR) (Wood units); (f) 6‐min walking distance (m). Measurements at “diagnosis” were performed without any pulmonary arterial hypertension (PAH) treatment, measurements at “reduction” were performed with starting reduction of parental prostanoid dose and measurements at “transition” in patients achieving reduction goal dose of parental prostanoids for transition to selexipag. The black lines indicate mean values
RESULTS
In the cohort, four (10.8%) patients with I/H PAH successfully transitioned from parenteral prostanoids to oral medication under the protocol (Panel a). These patients were all female; had severe PAH at the time of diagnosis; and were treated with a combination of parenteral prostanoids, endothelin‐receptor antagonists (ERA), and phosphodiesterase‐5 (PDE‐5) inhibitors. One patient received subcutaneous treprostinil, whereas the other three patients received intravenous epoprostenol. The mean maximum prostanoid dose was 43.3 ± 2.4 ng/kg/min. The mean PAP and pulmonary vascular resistance (PVR) at the time of diagnosis and transition showed significant hemodynamic improvement, from 60.0 ± 4.7 to 23.8 ± 1.7 (mmHg, p < 0.05) and 21.9 ± 3.4 to 4.5 ± 0.5 (Wood units, p < 0.05), respectively. The mean time from diagnosis to transition was 2400 ± 747 days, which suggested that the patients were stabilized by long‐term prostanoid therapy. All patients were initially evaluated in about 3 months after the transitions (mean period: 110 ± 10 days), and they were observed for more than 12 months after their transition, with a mean observation period of 628 ± 247 days. The exercise tolerance and hemodynamics of all four patients showed long‐term stability (Panels c–f) after the transitions. Patients continued to receive an ERA and a PDE‐5 inhibitor with selexipag in the follow‐up period.
DISCUSSION
This study was the first to examine the long‐term safety profile of transitioning from parenteral prostanoids to oral selexipag among patients with I/H PAH. Previous reports only examined the short‐term success of parenteral or inhaled prostanoid transition programs. 5 , 6 , 7 In addition, most of the studies only documented successful cases. As such, it was unclear how many patients attempted the transition but failed. A French study 3 examined eight patients who were transitioned from epoprostenol to selexipag. The long‐term follow‐up data from this study demonstrated deterioration in the mPAP and PVR values of some patients, although exercise tolerance remained stable. In contrast, our study demonstrated long‐term exercise tolerance and hemodynamic stability. The difference in the results may be due to different mPAP and PVR levels at the time of transition.
It is important to note that we also documented limited success in our study because only approximately 10% of the patients with I/H PAH were able to meet the criteria for a successful transition. While the efficacy of upfront triple combination therapy with parenteral prostanoids has been reported, 1 , 8 “super‐responders” with marked improvement in hemodynamics and prognosis remain limited. Transitioning from parenteral prostanoids to oral medication should only be considered in specialized centers. Patients must show subjective and objective improvements while on combination therapy to increase the likelihood of a safe transition.
This study demonstrated the long‐term safety of transitioning from parenteral prostanoids to oral selexipag among patients with I/H PAH who demonstrated improvement with upfront triple combination therapy. Our study examined a small number of patients; further validation with a larger study population or through a multicentre study may provide better data.
CONFLICTS OF INTEREST
Yuichi Tamura receives grants and personal fees from Bayer HealthCare, Nippon Shinyaku Co. Ltd., Daiichi Sankyo Co. Ltd., and Janssen Pharmaceuticals outside of this submitted work. The remaining authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the International University of Health and Welfare Ethics Review Board (No. 5‐16‐30). Informed consent was obtained by allowing patients to opt‐out of the study on the website.
AUTHOR CONTRIBUTIONS
Yuichi Tamura takes responsibility for the content of the manuscript, including the data and analysis. Asuka Furukawa conceived and designed the study. Akio Kawamura participated in data analysis and interpretation. Yudai Tamura, Kenta Yamada, and Hirohisa Taniguchi contributed to sample collection. Keiichi Fukuda, Akio Kawamura, and Toru Satoh supervised the manuscript preparation process. All authors reviewed the content of the draft, approved the final manuscript, and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors wish to acknowledge Rika Takeyasu and Hiromi Takada who helped coordinate the clinical care and data collection during transitions. This study was supported by AMED under Grant Number JP21ek0109567h0001.
Tamura Y, Furukawa A, Tamura Y, Yamada K, Taniguchi H, Fukuda K, Kawamura A, Satoh T. Long‐term safety of a structured transition protocol from parenteral prostanoids to selexipag in pulmonary arterial hypertension. Pulmonary Circulation. 2022;12:e12058. 10.1002/pul2.12058
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tamura Y, Kumamaru H, Satoh T, Miyata H, Ogawa A, Tanabe N, Hatano M, Yao A, Abe K, Tsujino I, Fukuda K, Kimura H, Kuwana M, Matsubara H, Tatsumi K, Japan PH Registry (JAPHR) Network . Effectiveness and outcome of pulmonary arterial hypertension‐specific therapy in Japanese patients with pulmonary arterial hypertension. Circ J. 2017;82:275–82. [DOI] [PubMed] [Google Scholar]
- 2. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yanaka K, Guillien A, Soumagne T, Benet J, Piliero N, Picard F, Pison C, Sitbon O, Bouvaist H, Degano B. Transition from intravenous epoprostenol to selexipag in pulmonary arterial hypertension: a word of caution. Eur Respir J. 2020;55:1902418. [DOI] [PubMed] [Google Scholar]
- 4. Pan IZ, Carey JR, Jacobs JA, Dechand J, Sessions JJ, Sorensen T, Penn BA, Mayeux JD, Hatton ND, Ryan JJ. Transitioning between prostanoid therapies in pulmonary arterial hypertension. Front Med (Lausanne). 2020;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frost A, Janmohamed M, Fritz JS, McConnell JW, Poch D, Fortin TA, Miller CE, Chin KM, Fisher M, Eggert M, McEvoy C, Benza RL, Farber HW, Kim NH, Pfister T, Shiraga Y, McLaughlin V. Safety and tolerability of transition from inhaled treprostinil to oral selexipag in pulmonary arterial hypertension: results from the TRANSIT‐1 study. J Heart Lung Transplant. 2019;38:43–50. [DOI] [PubMed] [Google Scholar]
- 6. Fanous SM, Janmohamed M. Transition from treprostinil to selexipag in patients with pulmonary arterial hypertension: case series. Am J Health Syst Pharm. 2018;75:1877–81. [DOI] [PubMed] [Google Scholar]
- 7. Holthaus N, Prins K, Rose L, Prisco S, Pritzker M, Thenappan T. EXPRESS: transition from parental prostacyclin to selexipag: a case series of five pulmonary arterial hypertension patients. Pulm Circ. 2019;9:2045894019862167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sitbon O, Jaïs X, Savale L, Cottin V, Bergot E, Macari EA, Bouvaist H, Dauphin C, Picard F, Bulifon S, Montani D, Humbert M, Simonneau G. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014;43:1691–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


