Abstract
The microbicidal efficacies of two anionic surfactants, sodium lauryl sulfate (SLS) and n-lauroylsarcosine (LS), were evaluated in cultured cells and in a murine model of herpes simplex type 2 (HSV-2) intravaginal infection. In vitro studies showed that SLS and LS were potent inhibitors of the infectivity of HSV-2 strain 333. The concentrations of SLS which inhibit viral infectivity by 50% (50% inhibitory dose) and 90% (90% inhibitory dose) were 32.67 and 46.53 μM, respectively, whereas the corresponding values for LS were 141.76 and 225.30 μM. In addition, intravaginal pretreatment of mice with thermoreversible gel formulations containing 2.5% SLS or 2.5% LS prior to the inoculation of HSV-2 strain 333 completely prevented the development of genital herpetic lesions and the lethality associated with infection. Of prime interest, no infectious virus could be detected in mouse vaginal mucosa. Both formulations still provided significant protection when viral challenge was delayed until 1 h after pretreatment. Finally, intravaginal application of gel formulations containing 2.5% SLS or 2.5% LS once daily for 14 days to rabbits did not induce significant irritations to the genital mucosa, as demonstrated from macroscopic and histopathologic examinations. These results suggest that thermoreversible gel formulations containing SLS or LS could represent potent and safe topical microbicides for the prevention of HSV-2 and possibly other sexually transmitted pathogens, including human immunodeficiency virus.
The spread of infectious diseases caused by the sexual transmission of human immunodeficiency virus (HIV), herpesviruses, and other pathogens is growing dramatically. Each year, more than 12 million people in the United States are newly infected with pathogens causing sexually transmitted diseases (STDs) (8). The global incidence of STDs and the morbidity and mortality associated with STDs are very significant. Herpes simplex viruses (HSVs) type 1 (HSV-1) and type 2 (HSV-2) are the most common infective causes of genital ulceration in developed countries. One of five Americans over the age of 12 has been infected with HSV. Genital herpesvirus infection is lifelong and may result in painful and recurrent genital lesions, systemic complications, psychosocial morbidity, and also serious neonatal diseases following intrapartum transmission of the virus (5). On the other hand, recent statistics (as of the end of 2000) from the World Health Organization estimated that 36.1 million individuals are infected with HIV type 1 (HIV-1) worldwide. Of that number, 16.4 million (47.3%) women are living with HIV infection or AIDS. Globally, heterosexual transmission may account for as much as 85 to 90% of new cases of HIV infection. The consistent and careful use of latex condoms represents an effective barrier against sexually transmitted pathogens, but unfortunately, their use is not generalized. More attention is now given to female-controlled methods for the prevention of HIV-1 infection since many women are unable to negotiate condom use with their partners (10, 11, 19, 36, 38, 39). Therefore, it is important to develop topical microbicides that could be used as an alternative to condoms for women to control their own protection against STDs.
Nonoxynol-9, a nonionic detergent, is the most currently used active ingredient in available vaginal formulations. This product has been shown to be effective against numerous sexually transmitted pathogens in vitro, including HIV-1 (1, 3, 4, 12, 20, 21, 27, 32). However, the in vivo efficacy of nonoxynol-9 against HIV-1 has never been clearly established, and the results of clinical studies are controversial. A cohort study conducted among 273 female sex workers in Cameroon reported a lower rate of HIV infection among women using vaginal suppositories containing 100 mg of nonoxynol-9 (44). On the other hand, a randomized placebo-controlled trial with 138 HIV-seronegative female prostitutes in Kenya showed that a nonoxynol-9-impregnated sponge was not effective in reducing the risk of HIV infection (26). Another clinical study testing the efficacy of a vaginal film containing 70 mg of nonoxynol-9, conducted among 1,292 HIV-1-negative female sex workers in Cameroon, reported that the product did not reduce the rates of new cases of HIV-1 infection, gonorrhea, and chlamydia infection (35). More recently, results presented at the XIII International AIDS Conference in Durban, South Africa, revealed that a vaginal cream containing 52 mg of nonoxynol-9, marketed under the trade name of Advantage S, not only was ineffective against HIV but was also actually harmful as a microbicide (L. Van Damme, XIII Int. AIDS Conf.). The activity of nonoxynol-9 is nonspecific and is often associated with adverse effects including epithelial disruption, genital inflammation and ulceration, and reductions in the number of lactobacilli (23, 26, 28, 34, 35, 37), which may explain the lack of protection that nonoxynol-9 offers against HIV-1 and other pathogens causing STDs. Consequently, there is an urgent need to develop new and safe topical microbicides for women.
Sodium lauryl sulfate (SLS) is an anionic surfactant which denatures membrane proteins of pathogens. Ward and Ashley (41) were the first to demonstrate that SLS is a potent inactivator of rotavirus and poliovirus infectivities at quite low concentrations and under very mild conditions. Previous studies from our laboratory have demonstrated that SLS inhibits in vitro the infectivities of different enveloped viruses such as HSV-1, HSV-2, and HIV-1 (31) SLS was also reported to inhibit the infectivities of nonenveloped rabbit, bovine, and human papillomaviruses (17). This suggests that SLS could be a potential candidate for use as a microbicide in vaginal formulations to prevent the sexual transmission of pathogens causing STDs and HIV infection. In the present study, we have evaluated the efficacies of thermoreversible gel formulations containing SLS or n-lauroylsarcosine (LS), an anionic surfactant structurally closely related to SLS, to prevent herpes genitalis in a murine model of HSV-2 intravaginal infection. The tolerance and toxicity of the two gel formulations for the vaginal mucosa have also been evaluated in a rabbit model.
MATERIALS AND METHODS
Chemicals.
SLS and LS were obtained from Sigma Chemical Co. (St. Louis, Mo.).
Cell line.
Vero cells (African green monkey kidney cells; American Type Culture Collection, Manassas, Va.) were grown in Eagle's minimum essential medium (EMEM; Wisent, St. Bruno, Québec, Canada) supplemented with 5% heat-inactivated fetal bovine serum (FBS; Wisent), sodium bicarbonate (0.22%), penicillin-streptomycin (100 U/ml), and l-glutamine (2 mM) in a 5% CO2 atmosphere at 37°C.
Virus strain.
HSV-2 strain 333 was kindly provided by Lawrence R. Stanberry (Children's Hospital Medical Center, Cincinnati, Ohio). The virus was propagated in Vero cells by using EMEM supplemented with 2% FBS (EMEM–2% FBS) as maintenance medium at 37°C. At approximately 80% cell lysis, cells were scrapped off the dishes with a sterile cell scraper. The cellular suspension was centrifuged (1,450 × g for 10 min at 4°C), and the supernatant was retained. The pellet was frozen-thawed three times with liquid nitrogen and centrifuged again. Supernatants were pooled, filtered on a 0.45-μm-pore-size Durapore low-binding membrane (Millipore Co., Bedford, Mass.), and centrifuged (100,000 × g for 2 h and 40 min at 4°C). The supernatant was discarded, and the pellet was resuspended in 1 ml of EMEM–2% FBS overnight at 4°C and stored at −80°C until use. The viral titer determined on Vero cells was 109 PFU/ml.
Preparation of gel formulations.
Polymers composed of polyoxypropylene and polyoxyethylene are known to form thermoreversible gels when incorporated into aqueous solutions. These polymers have the ability to change from the liquid state to the gel state at temperatures close to body temperature, therefore allowing advantageous topical applications. The liquid state-to-gel phase transition is dependent on the polymer concentration and the ingredients incorporated into the solution. Therefore, the concentration of the polymer was adjusted in order to obtain a phase transition temperature of 28°C. Gel formulations were prepared by suspending an appropriate amount of the polymer in citrate buffer (50 mM, pH 4.0). The gel alone was suspended at a concentration of 17% (wt/wt) in citrate buffer, and the components were mixed under agitation overnight at 4°C to ensure complete dissolution. For the gel formulations containing SLS or LS, these surfactants were added to the polymer powder and dissolved in citrate buffer as described above. SLS was used at concentrations of 1% (34.67 mM) and 2.5% (86.69 mM) in formulations containing 21 and 30% (wt/wt) polymer, respectively. LS was used at concentrations of 1% (34.08 mM) and 2.5% (85.21 mM) in formulations containing 15 and 18% (wt/wt) polymer, respectively. We have also used a gel formulation composed of 30% (wt/wt) polymer in citrate buffer as a control in some experiments.
HSV-2 inactivation.
Vero cells were seeded in 24-well plates. Prior to infection, HSV-2 strain 333 was preincubated for 1 h at 37°C in phosphate-buffered saline (PBS; pH 7.4) or with different concentrations of SLS (6.25 to 50 μM) or LS (50 to 250 μM) in PBS. Confluent cells were infected with the virus (approximately 50 to 100 PFU/500 μl), and the plates were centrifuged (750 × g for 45 min at 20°C). Virus was removed by aspiration, and the cell sheets were overlaid with 500 μl of EMEM–2% FBS containing 0.6% SeaPlaque agarose (Mandel Scientific, St. Laurent, Québec, Canada). The plates were incubated for 48 h in a 5% CO2 atmosphere at 37°C. The cells were then fixed with 10% formaldehyde in PBS for 20 min, washed with demineralized water, and stained with 0.05% methylene blue. Virus infectivity was evaluated from the determination of the numbers of PFU.
Cellular viability.
Vero cells, seeded at midconfluency in 24-well plates, were incubated with EMEM–5% FBS (control) or with different concentrations of SLS or LS in EMEM–5% FBS for 24 h at 37°C in a 5% CO2 atmosphere. Afterward, the cell sheets were washed twice with EMEM–5% FBS. Cell viability was then monitored with a tetrazolium salt (MTS; Promega, Madison, Wis.) which, in the presence of phenazine methosulfate, is reduced by living cells to yield a formazan product that can be assayed colorimetrically (6).
HSV-2 intravaginal infection.
The murine model of HSV-2 intravaginal infection was adapted from previously described protocols (29, 42, 43). Female BALB/c mice (age, 4 weeks; Charles River Laboratories, Inc., St. Constant, Québec, Canada) were used for this study. On days 7 and 1 prior to infection, the mice were injected subcutaneously in the neck region with 2.5 mg of progesterone (Pharmacia and Upjohn, Don Mills, Ontario, Canada) diluted in sterile physiological water. Mice were anesthetized by intraperitoneal injection of 70 mg of ketamine and 11.5 mg of xylazine per kg of body weight. Vaginal secretions were removed with two calcium alginate swabs. Mice were pretreated by delivering intravaginally 15 μl of each gel formulation with a micropipette. At different times after pretreatment, 5 μl of 1.2 × 105 PFU of HSV-2 strain 333 was inoculated into the vagina while the micropipette was moved in and out five times to simulate coitus. The mice were returned to their cages and examined daily for a period of 14 days. The criteria used for the evaluation of herpetic genital infection were the degree of redness and swelling in the perineal region (ranked 1 for minimal, 2 for moderate, and 3 for marked), viral titers in the vaginal mucosa, and survival rates.
Viral titers in tissue samples.
The mice were pretreated intravaginally with 15 μl of each gel formulation applied 5 min prior to infection with HSV-2 strain 333 as described above. preliminary experiments showed that viral titers in the vaginal mucosa were maximal on day 3 postinfection (data not shown). The mice were killed on day 3 postinfection, and the vaginas were excised and maintained in Hank's balanced salt solution in an ice bath. The vaginas were weighed, diluted in 1 ml of EMEM–2% FBS, and submitted to three cyles of sonication for 10 s each with 5-s intervals. Debris was removed by low-speed centrifugation (1,100 × g for 15 min at 4°C). The supernatants were collected and stored in duplicate at −80°C until assayed for the presence of virus. Samples were diluted, as appropriate, and inoculated onto confluent monolayers of Vero cells seeded in 24-well plates as described previously for HSV-2 inactivation. Virus-induced cytopathic effect was evaluated from the determination of the numbers of PFU, and results were expressed as the number of PFU per gram of tissue.
In vivo tolerance and toxicity.
Female New Zealand rabbits (weight 2.5 to 3.5 kg; Charles River Laboratories Inc.) were used for this study. Five milliliters of the formulations was administered intravaginally to rabbits once daily for 14 days by using a catheter (Dover Catheter; Sherwood Medical, St. Louis, Mo.) connected to a syringe. Twenty four hours after the last application, the animals were killed. The vagina (cervical end, middle, and vulvar end), cervix (left and right), uterine horns (left and right), uterus (left and right), ovaries (left and right), and urinary bladder were excised for macroscopic and histologic examinations. The degree of irritation was determined as described previously (9). The four basic criteria used for macroscopic evaluations were vascularization, edema, ulceration, and necrosis. Similarly, the criteria used for histologic examinations were vascularization, edema, the presence of inflammatory cell infiltrates, and epithelial exfoliation. Optic microscopic observations were evaluated randomly by two different scientists (S.R. and P.G.). The scoring system was as follows: 0, none; 1, minimal; 2, slight; 3, moderate; and 4, marked. The scores for rabbits in each group were totaled and averaged. The total scoring system was as follows: 1 to 4, minimal; 5 to 8, mild; 9 to 12, moderate; and 13 to 16, marked. A total score of between 0 and 8 is considered acceptable, a total score of 9 or 10 is marginal, and a total score of over 11 is unacceptable (9).
Tissue preparation for optical microscopy.
Tissues were fixed with 10% formaldehyde in PBS, dehydrated, and embedded in paraffin (Paraplast X-TRA; Fisher Scientific, Montréal, Québec, Canada) by routine procedures. Paraffin sections were stained with hematoxylin-eosin and mounted with Permount (Fisher Scientific). The observations were made with a Nikon microscope (Labophot-2; Nikon, Québec, Québec, Canada), and pictures were taken with a Nikon camera (MicroFlex HFX-DX; Nikon).
Statistical analysis.
The areas under the curve (AUCs) of the mean lesion scores between days 4 and 9 were calculated for all animals including those that were asymptomatic. The AUC values for the different treatment groups and the mean scores for the histopathologic observations of the vaginal mucosa were compared by a one-way analysis of variance test, followed as appropriate by the t test with Fisher's corrections for multiple simultaneous comparisons. The significance of the differences (i) in the proportion of mice presenting with genital lesions and (ii) mortality rates between control infected mice and pretreated mice was evaluated by a chi-square test. All statistical analyses were performed with a computer package (Statview+SE Software; Abacus Concepts, Berkeley, Calif.). A P value of less than 0.05 was considered statistically significant.
RESULTS
Infectivity of HSV-2 pretreated with SLS or LS.
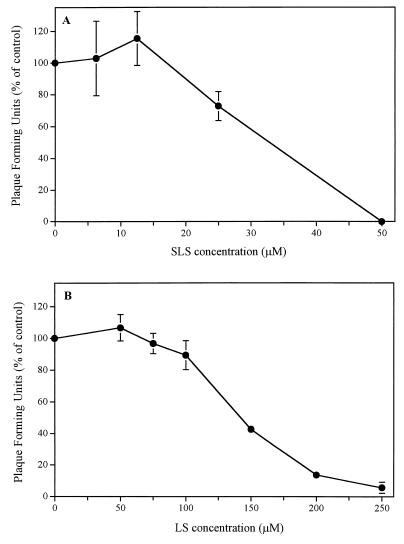
Figure 1 shows that pretreatment of HSV-2 strain 333 with SLS (Fig. 1A) or LS (Fig. 1B) for 1 h at 37°C decreased, in a dose-dependent manner, its infectivity for Vero cells. The concentrations of SLS which inhibit viral infectivity by 50% (50% inhibitory dose) and 90% (90% inhibitory dose) were 32.67 ± 2.18 and 46.53 ± 0.44 μM, respectively, whereas the corresponding values for LS were 141.76 ± 4.10 and 225.30 ± 12.79 μM. No signs of cytoxicity were observed in the range of concentrations of SLS or LS used (data not shown).
FIG. 1.
Infectivity of HSV-2 strain 333 to Vero cells following pretreatment of the virus with different concentrations of SLS (A) or LS (B) for 1 h at 37°C. Results are expressed as a percentage of the value for the control and are the mean ± standard deviation for three independent experiments.
Efficacies of gel formulations to prevent intravaginal HSV-2 infection.
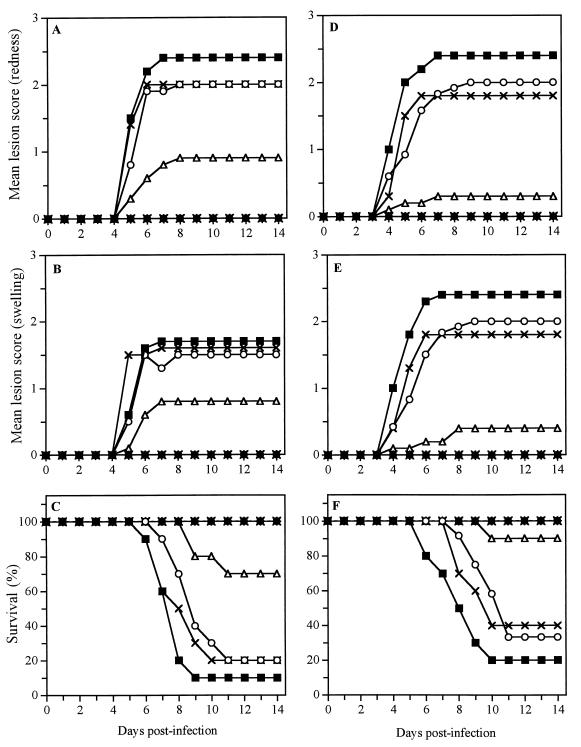
Figure 2 shows the time evolution of the mean lesion score associated with redness (Fig. 2A and D) and swelling (Fig. 2B and E) in the perineal region and the time evolution of survival rates (Fig. 2C and F) for untreated infected mice and mice pretreated 5 min prior to infection with citrate buffer, gel alone (17% [wt/wt]), SLS or LS (1 or 2.5% [wt/wt]) in buffer, and SLS or LS incorporated into the gel formulation. No signs of genital herpetic lesions were visible until day 4 postinfection. Marked signs of redness and swelling appeared in the perineal region of untreated infected mice between days 4 and 7 and were maintained up to day 14 after infection. Most animals died from encephalitis between days 6 and 10 postinfection. Only 20% of the animals pretreated with the buffer or the gel alone (17% [wt/wt]) survived the infection. Of prime interest, all mice pretreated with 1% SLS or 1% LS in buffer or with the gel containing 2.5% SLS or 2.5% LS survived the infection and did not develop any visible sign of herpetic lesions (see Table 1 for P values). Significant protection was also observed when mice were pretreated with gel formulations containing 1.0, 1.5, and 2.0% SLS or LS (data not shown). In contrast to pretreatment with the gel alone (17% [wt/wt]), pretreatment of the mice with a more concentrated gel formulation (30% [wt/wt]) completely prevented the development of herpetic lesions and death of the animals (data not shown).
FIG. 2.
Time evolutions of the mean lesion score associated with redness (A and D) and swelling (B and E) in the perineal region and of survival rates (C and F) of mice pretreated at 5 min prior to HSV-2 infection with citrate buffer (○), gel alone (17% [wt/wt]) (×), 1% SLS or 1% LS in buffer (●), or gel formulations containing 1% SLS or 1% LS (▵) or 2.5% SLS or 2.5% LS (✴). Untreated infected mice were used as controls (■). (A, B, and C) Data for SLS; (D, E, and F) data for LS. The results are the means for 10 mice per group.
TABLE 1.
AUC of the time evolution of the mean lesion score associated with redness and swelling for mice treated 5 min prior to infection with different formulations
| Treatment group and symptom | AUCa |
P (compared to the following group)b
|
No. of mice with lesions/total no. of mice | No. of dead mice/total no. of mice | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | ||||
| SLS | |||||||||
| Redness | |||||||||
| (a) Untreated | 12.10 ± 1.46 | NSc | NS | <0.01 | <0.01 | <0.01 | 9/10 | 9/10 | |
| (b) Citrate buffer | 9.60 ± 1.67 | NS | NS | <0.01 | <0.01 | <0.01 | 9/10 | 8/10 | |
| (c) Gel alone | 10.40 ± 1.81 | NS | NS | <0.01 | <0.01 | <0.01 | 8/10 | 8/10 | |
| (d) SLS 1% | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| (e) Gel + 1% SLS | 3.95 ± 2.04 | <0.01 | <0.01 | <0.01 | NS | NS | 3/10d | 3/10d | |
| (f) Gel + 2.5% SLS | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| Swelling | |||||||||
| (a) Untreated | 8.15 ± 1.70 | NS | NS | <0.01 | <0.05 | <0.01 | 8/10 | 9/10 | |
| (b) Citrate buffer | 7.05 ± 1.32 | NS | NS | <0.01 | NS | <0.01 | 8/10 | 8/10 | |
| (c) Gel alone | 8.60 ± 1.63 | NS | NS | <0.01 | <0.01 | <0.01 | 8/10 | 8/10 | |
| (d) SLS, 1% | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| (e) Gel + 1% SLS | 3.50 ± 1.83 | <0.05 | NS | <0.01 | NS | NS | 3/10d | 3/10d | |
| (f) Gel + 2.5% SLS | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| LS | |||||||||
| Redness | |||||||||
| (a) Untreated | 14.10 ± 2.41 | NS | NS | <0.01 | <0.01 | <0.01 | 8/10 | 8/10 | |
| (b) Citrate buffer | 10.13 ± 2.34 | NS | NS | <0.01 | <0.05 | <0.01 | 8/12 | 8/12 | |
| (c) Gel alone | 10.05 ± 2.75 | NS | NS | <0.01 | <0.01 | <0.01 | 6/10 | 6/10 | |
| (d) LS, 1% | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| (e) Gel + 1% LS | 1.60 ± 1.60 | <0.01 | <0.05 | <0.01 | NS | NS | 1/10d | 1/10d | |
| (f) Gel + 2.5% LS | 1.80 ± 1.34 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| Swelling | |||||||||
| (a) Untreated | 14.00 ± 2.42 | NS | NS | <0.01 | <0.01 | <0.01 | 8/10 | 8/10 | |
| (b) Citrate buffer | 9.71 ± 2.23 | NS | NS | <0.01 | <0.05 | <0.01 | 8/12 | 8/12 | |
| (c) Gel alone | 10.00 ± 2.73 | NS | NS | <0.01 | <0.01 | <0.01 | 6/10 | 6/10 | |
| (d) LS 1% | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
| (e) Gel + 1% LS | 1.65 ± 1.39 | <0.01 | <0.05 | <0.01 | NS | NS | 2/10d | 1/10d | |
| (f) Gel + 2.5% LS | 0.00 | <0.01 | <0.01 | <0.01 | NS | NS | 0/10d | 0/10d | |
Values are means ± standard errors of the means, calculated as [(score on day 4 + score on day 9)/2] + sum of all scores between days 4 and 9.
The letters correspond to the letters for each treatment in the column on the left.
NS, not significant (i.e., P > 0.05).
P < 0.01 compared with untreated mice.
Viral titers in vaginal mucosa.
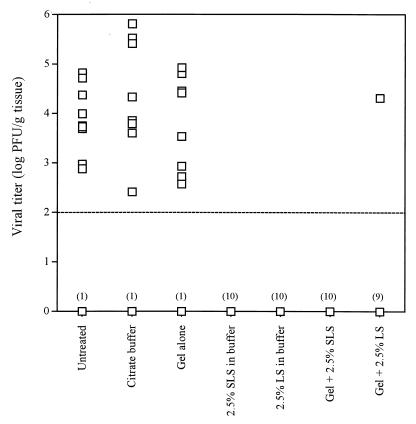
Figure 3 shows the viral titers measured in the vaginal mucosae of untreated infected mice and mice pretreated 5 min prior to infection with citrate buffer, gel alone (17% [wt/wt]), 2.5% SLS or 2.5% LS in the buffer, or 2.5% SLS or 2.5% LS incorporated into the gel formulation. The average viral titer found in the vaginal mucosa of untreated infected mice was 103.5 PFU/g of tissue. Pretreatment of mice with the buffer or the gel alone (both 17 and 30% [wt/wt]) did not affect the viral titers in the vaginal mucosae. Of prime interest, no infectious virus was detected by culture, under our assay conditions, in the vaginal mucosae of mice pretreated with 2.5% SLS in the buffer, 2.5% SLS incorporated into the gel formulation, or 2.5% LS in buffer. Infectious virus was detected in the vaginal mucosa of only one animal pretreated with the gel containing 2.5% LS.
FIG. 3.
Viral titers in the genital mucosae of mice pretreated intravaginally with citrate buffer, gel alone (17% [wt/wt]), 2.5% SLS or 2.5% LS in the buffer, or gel containing 2.5% SLS or 2.5% LS and infected 5 min later with HSV-2 strain 333. Untreated infected mice were used as controls. Viral titers are expressed as the log number of PFU per gram of tissue. The results are the means for 10 mice per group. The broken line shows the limit of detection of the assay. The numbers in parentheses represent the numbers of mice without infectious virus in the vaginal mucosa.
Time-dependent efficacies of gel formulations to prevent HSV-2 intravaginal infection.
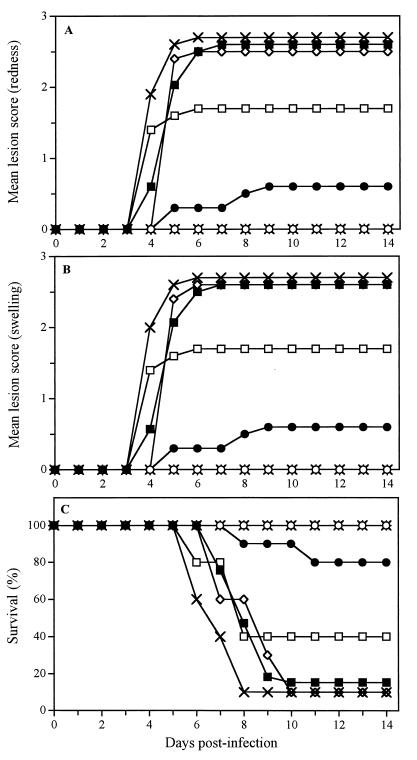
Figure 4 shows the time evolution of the mean lesion score associated with redness (Fig. 4A) and swelling (Fig. 4B) in the perineal region and the time evolution of survival rates (Fig. 4C) of untreated infected mice and mice pretreated with the gel containing 2.5% SLS at different times prior to HSV-2 intravaginal challenge. On day 4 postinfection, untreated infected mice developed genital herpetic lesions, and almost all animals died from encephalitis between days 7 and 10. Pretreatment of mice 5 or 30 min prior to infection completely protected animals against the development of herpetic lesions and lethality associated with infection. Pretreatment of mice 1 h prior to infection could still reduce the mortality rates for the animals (20 versus 90% for untreated infected mice). However, a lower level of protection was observed when mice were pretreated 2, 4, and 6 h prior to infection (see Table 2 for P values). The time-dependent efficacy of the gel formulation containing 2.5% LS was comparable to that of the gel formulation containing 2.5% SLS (data not shown). In fact, pretreatment of mice within 1 h before viral challenge completely prevented the development of herpetic lesions and lethality associated with infection.
FIG. 4.
Time evolutions of the mean lesion score associated with redness (A) and swelling (B) and of survival rates (C) of mice infected intravaginally with HSV-2 strain 333 5 min (○), 30 min (✴), 1 h (●), 2 h (□), 4 h (◊), and 6 h (×) after pretreatment with a gel formulation containing 2.5% SLS. Untreated infected mice were used as controls (■). The results are the means for 10 mice per group.
TABLE 2.
AUC of the time evolution of the mean lesion score associated with redness and swelling for mice treated at different times prior to infection with a gel formulation containing 2.5% SLS
| Symptom and time of initiation of pretreatment prior to infection | AUCa |
P (compared to the following group)b
|
No. of mice with lesions/total no. of mice | No. of dead mice/total no. of mice | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | ||||
| Redness | ||||||||||
| (a) Untreated | 14.56 ± 11.04 | <0.01 | <0.01 | <0.01 | NS | <0.05 | NS | 29/33 | 28/33 | |
| (b) 5 min | 0.00 | <0.01 | NS | NS | <0.01 | <0.01 | <0.01 | 0/10d | 0/10d | |
| (c) 30 min | 0.00 | <0.01 | NS | NS | <0.01 | <0.01 | <0.01 | 0/10d | 0/10d | |
| (d) 1h | 2.30 ± 1.70 | <0.01 | NS | NS | <0.01 | <0.01 | <0.01 | 2/10d | 2/10d | |
| (e) 2h | 11.35 ± 3.15 | NS | <0.01 | <0.01 | <0.01 | <0.05 | NS | 6/10 | 6/10 | |
| (f) 4h | 17.60 ± 1.96 | <0.05 | <0.01 | <0.01 | <0.01 | <0.05 | NS | 9/10 | 9/10 | |
| (g) 6h | 13.65 ± 1.77 | NS | <0.01 | <0.01 | <0.01 | NS | NS | 9/10 | 9/10 | |
| Swelling | ||||||||||
| (a) Untreated | 14.56 ± 1.07 | <0.01 | <0.01 | <0.01 | NS | <0.05 | NS | 29/33 | 28/33 | |
| (b) 5 min | 0.00 | <0.01 | NS | NS | <0.01 | <0.01 | <0.01 | 0/10d | 0/10d | |
| (c) 30 min | 0.00 | <0.01 | NS | NS | <0.01 | <0.01 | <0.01 | 0/10d | 0/10d | |
| (d) 1h | 2.30 ± 1.70 | <0.01 | NS | NS | <0.01 | <0.01 | <0.01 | 2/10d | 2/10d | |
| (e) 2h | 11.35 ± 3.15 | NS | <0.01 | <0.01 | <0.01 | <0.05 | NS | 6/10 | 6/10 | |
| (f) 4h | 17.75 ± 1.99 | <0.05 | <0.01 | <0.01 | <0.01 | <0.05 | NS | 9/10 | 9/10 | |
| (g) 6h | 14.10 ± 1.65 | NS | <0.01 | <0.01 | <0.01 | NS | NS | 9/10 | 9/10 | |
Values are means ± standard errors of the means, calculated as [(score on day 4 + score on day 9)/2] + sum of all scores between days 4 and 9.
The letters correspond to the letters for each time of initiation of pretreatment in the column on the left.
NS, not significant (i.e., P > 0.05).
P < 0.01 compared with untreated mice.
Tolerance and toxicity of SLS and LS.
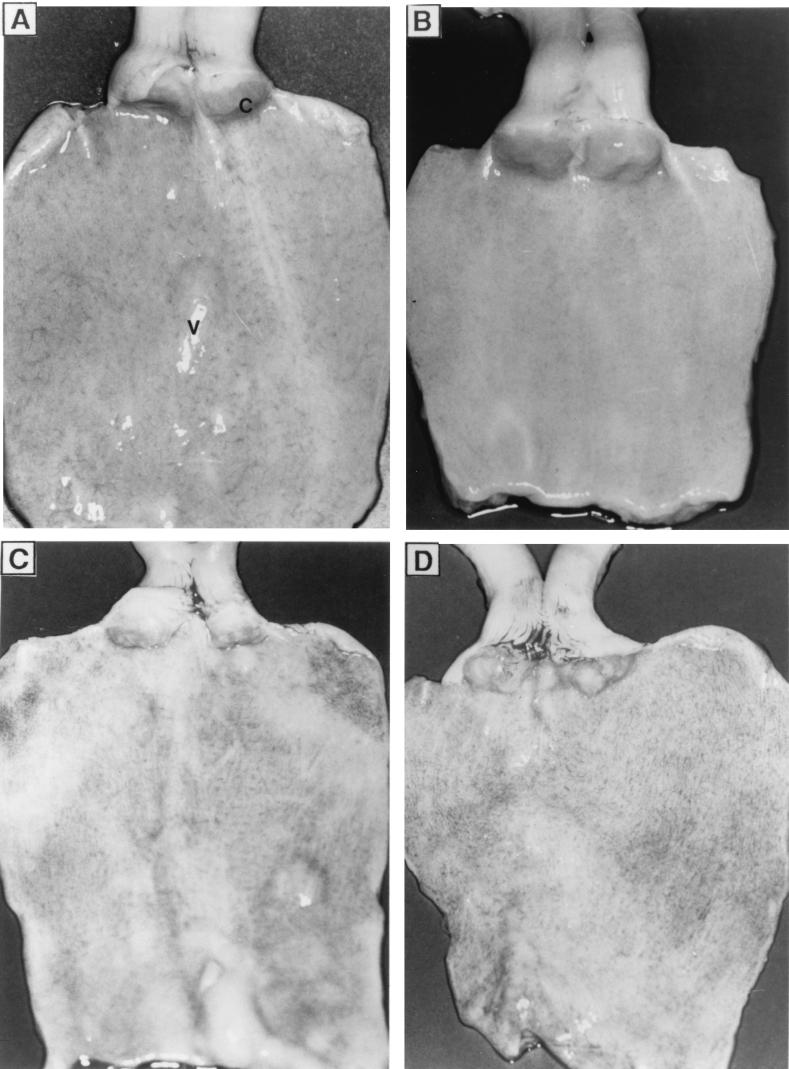
Figure 5 shows pictures of the genital mucosae of rabbits following intravaginal application of gel formulations (30% [wt/wt]) containing or not containing 2.5% SLS once daily for 14 days. The macroscopic appearances of the vaginal and cervival mucosae of rabbits which received the gel alone (Fig. 5B) were comparable to those of the mucosae of control animals treated with citrate buffer (Fig. 5A). A mild irritation was observed after treatment with SLS in buffer (Fig. 5C) or SLS incorporated into the gel formulation (Fig. 5D), but no ulcerations or necrosis of tissues was observed. After treatment with 2.5% LS in buffer or 2.5% LS incorporated into the gel formulation, the appearance of the vaginal and cervical mucosae was similar to that observed following intravaginal application of 2.5% SLS (data not shown).
FIG. 5.
Vaginal and cervical mucosae of New Zealand rabbits following intravaginal application of buffer (A), gel alone (30% [wt/wt]) (B), 2.5% SLS in buffer (C), or 2.5% SLS incorporated into the gel (D) once daily for 14 days. Photographs are representative of three animals per treatment. C, cervix; V, vagina.
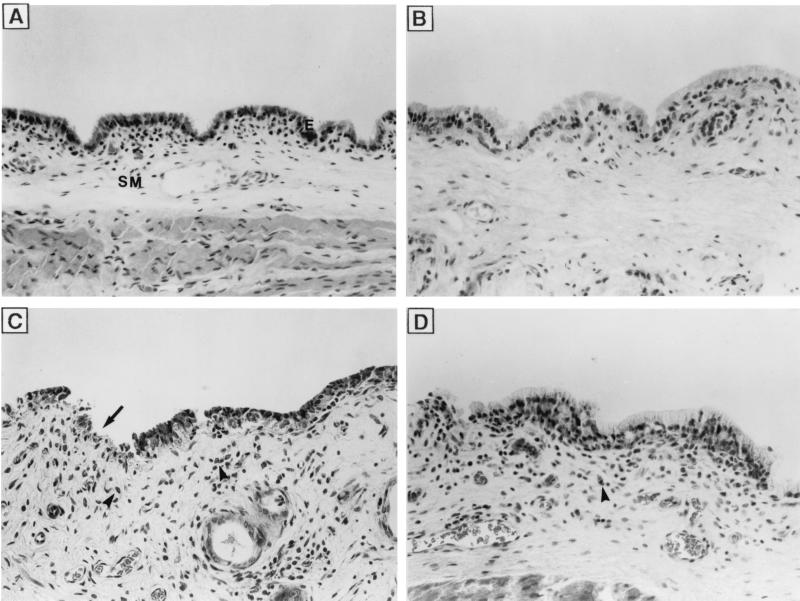
Figure 6 shows the histologic appearance of the corresponding vaginal mucosae described above. No major histologic changes were observed after treatment with citrate buffer (Fig. 6A) or gel alone (Fig. 6B). However, a slight loss of integrity of epithelial cells and accumulation of leukocytes and erythrocytes in the vaginal submucosae were observed in animals treated with 2.5% SLS in buffer (Fig. 6C) or 2.5% SLS incorporated into the gel formulation (Fig. 6D). Table 3 shows the mean and total composite scores for the cervical end, the middle, and the vulvar end of the vaginal mucosae. As expected, intravaginal application of citrate buffer and gel alone resulted in minimal toxicity (total scores, 1.93 and 0.84, respectively) for the vaginal mucosae. On the other hand, the intravaginal application of 2.5% SLS in buffer or incorporated into the gel formulation resulted in mild and minimal toxicities for the vaginal mucosae, respectively. Rabbits treated with 2.5% SLS in buffer showed increased levels of inflammatory cell infiltrates compared with those for rabbits treated with citrate buffer. However, the level of inflammatory cell infiltrate was significantly reduced when SLS was incorporated into the gel formulation. Similar observations were made for the cervixes of the animals (data not shown). Intravaginal application of 2.5% LS in buffer or 2.5% LS incorporated into the gel formulation also resulted in minimal toxicity for the vaginal and cervical mucosae of rabbits (data not shown). Finally, the application of citrate buffer, gel alone, 2.5% SLS or 2.5% LS in buffer, or 2.5% SLS or 2.5% LS incorporated into the gel was nontoxic to the uterine horns, uterus, ovaries, and urinary bladder (data not shown).
FIG. 6.
Vaginal mucosae of New Zealand rabbits following intravaginal application of citrate buffer (A), gel alone (30% [wt/wt]) (B), 2.5% SLS in buffer (C), or 2.5% SLS incorporated into the gel (D) once daily for 14 days Photographs are representative of three regions of the vagina (cervical end, middle, and vulvar end) and three animals per treatment. E, epithelium; SM, submucosa; ➞, epithelial exfoliation; ➤, inflammatory cell infiltrate. Magnification, ×100.
TABLE 3.
Mean and total composite scores after histopathologic observations of the vaginal mucosae of rabbits following intravaginal application of gel and/or SLS once daily for 14 days
| Parameter | Mean score (day 15)a |
|---|---|
| Citrate buffer | |
| Vascularization | 0.56 ± 0.16 |
| Epithelial exfoliation | 0.17 ± 0.12 |
| Inflammatory cell infiltrate | 0.94 ± 0.13 |
| Edema | 0.33 ± 0.12 |
| Total composite score | 2.00 |
| 30% (wt/wt) gel | |
| Vascularization | 0.28 ± 0.22 |
| Epithelial exfoliation | 0.28 ± 0.15 |
| Inflammatory cell infiltrate | 0.22 ± 0.15b |
| Edema | 0.06 ± 0.06 |
| Total composite score | 0.84 |
| 2.5% SLS in buffer | |
| Vascularization | 0.39 ± 0.14 |
| Epithelial exfoliation | 1.50 ± 0.54cd |
| Inflammatory cell infiltrate | 2.22 ± 0.28cd |
| Edema | 0.72 ± 0.24d |
| Total composite score | 4.83 |
| 30% gel + 2.5% SLS | |
| Vascularization | 1.50 ± 0.19cde |
| Epithelial exfoliation | 0.50 ± 0.22f |
| Inflammatory cell infiltrate | 1.50 ± 0.37df |
| Edema | 0.22 ± 0.15 |
| Total composite score | 3.72 |
Values are means ± standard errors of the means for three anatomical regions (cervical end, middle and vulvar end) for three rabbits per treatment.
P < 0.05 compared with the same parameter with citrate buffer.
P < 0.01 compared with the same parameter with citrate buffer.
P < 0.01 compared with the same parameter with gel alone.
P < 0.01 compared with the same parameter with 2.5% SLS.
P < 0.05 compared with the same parameter with 2.5% SLS.
DISCUSSION
Given the limited use of condoms by men and the difficulty for women to negotiate condom use with their partners, the development of safe and effective topical microbicides is an urgent need to reduce the sexual transmission of HIV-1, HSV, and other pathogens causing STDs. In the present study, we have evaluated the efficacies of two anionic surfactants, SLS and LS, incorporated into a thermoreversible gel formulation as potential microbicides against HSV-2. The results showed that both gel formulations were highly effective in preventing HSV-2 intravaginal infection in mice and were well tolerated by the vaginal mucosae of rabbits. Ward and Ashley (41) were the first to report that SLS inactivates the infectivities of rotavirus and poliovirus at quite low concentrations and under very mild conditions. Previous studies from our laboratory have demonstrated that SLS also inhibits the infectivities of different HSV strains as well as HIV-1 (31). HSV infectivity was completely inhibited following pretreatment with 50 μM SLS. Viral attachment was not prevented by SLS pretreatment, and virus entered the cells to produce capsid shells devoided of a DNA core in the nuclei. The amount of the glycoprotein D gene in these cells remained unchanged compared to the amounts in control cells, suggesting that SLS could interfere with the maturation of nucleocapsids by reducing their rates of maturation or by interfering with the encapsidation of DNA. In contrast, our recent studies showed that SLS decreased the infectivity of HIV-1 by inhibiting the attachment of the virus to the target cells. (J. Bestman-Smith, J. Piret, A. Désormeaux, R. F. Omar, M. J. Tremblay, and M. G. Bergeron, submitted for publication). Howett et al. (17) also showed that SLS is a potent inactivator of HSV-2 and HIV-1 infectivities and extended this observation to nonenveloped viruses such as rabbit, bovine, and human papillomaviruses. They suggested that SLS denatures the capsid proteins of nonenveloped viruses, whereas both envelope disruption and denaturation of structural proteins occurred simultaneously for enveloped viruses. In addition, Krebs et al. (25) also showed, using strains with different tropisms, that SLS inactivated the cell-associated infectivity of HIV-1.
Our present data show that SLS and LS are potent inhibitors of HSV-2 infectivity of HIV-1. Compared with SLS, the concentration of LS required to completely inactivate the infectivity of HSV-2 strain 333 was fivefold higher. SLS and LS are both anionic surfactants which solubilize and denature proteins. These chaotropic agents affect the higher-order (secondary and higher) structures of proteins mainly by disrupting the hydrogen bonds and through other interactions that maintain these higher-order structures (15). LS is a less effective solubilizing denaturant than SLS (7). In this respect, Ward and Ashley (40) have reported that pretreatment of rotavirus with 0.1% LS at 21°C for 1 h did not affect viral infectivity, whereas it completely inhibited viral infectivity following pretreatment at 45°C for 20 min. Naturally occurring anionic surface-active agents present in bile acids such as cholic acid derivatives were also shown to be effective against HIV-1 in vitro, but with a poor selectivity index (2, 33). In addition, taurolithocholic acid 3-sulfate, alone or in combination with glycocholic acid, was recently demonstrated to be highly effective against HSV-1, HSV-2, HIV-1, Neisseria gonorrhoeae, and Chlamydia trachomatis in vitro, with little or no cytotoxicity (16).
The active ingredients, SLS and LS, were incorporated into a polymer composed of polyoxypropylene and polyoxyethylene which forms a thermoreversible gel. Its transition temperature from the liquid state to the gel state can be modulated by varying the concentration of the polymer. Gel formulations were prepared in acidic buffer to maintain a normal vaginal flora and environment in women. In addition, acidic pHs irreversibly altered the conformations of virus components and inactivated herpesviruses, as demonstrated in vitro (18). The addition of surfactant and salts into the polymer affects its transition temperature (14). Therefore, the concentration of polymer was adjusted for each gel formulation to get a transition temperature close to 28°C. This transition temperature was selected to allow, when it is fluid at room temperature, an easier application of the gel formulation and, consequently, a better distribution in the irregularities of the vaginal and cervical mucosae. Thereafter, once gelified at body temperature, the gel will persist for a longer period of time in the vaginal cavity.
Our data showed that pretreatment of mice with citrate buffer or with the gel alone at a concentration of 17% (wt/wt) in citrate buffer had no effect on the development of herpetic lesions, viral titers in the vaginal mucosa, or the death of the animals from encephalitis. In vitro, treatment of HSV-2 strain 333 adsorbed to Vero cells with citrate buffer (pH 4.0) inactivated the virus to 40% of control values (unpublished data). This suggests that the neutral environment of the mouse vagina may alter the inactivating potency of the buffer, probably because it decreases its buffering capacity. In addition, if given at a sufficiently high concentration (30% [wt/wt]), the gel alone prevented the appearance of visible signs of infection and death of the animals but not the presence of infectious virus in the vaginal mucosa. This suggests that, under these conditions, the gel could partly hinder the attachment of the virus to cell surface receptors and therefore reduce the amount of virus which infects epithelial cells. The gel may thus act alone as a physical barrier, as we have previously demonstrated in an in vitro system. (J. Piret, N. Gagné, S. Perron, A. Désormeaux, M. J. Tremblay, P. Gourde, R. F. Omar, and M. G. Bergeron, in press).
Pretreatment of mice with 1% SLS in buffer alone has been shown to completely prevent lethal HSV-2 intravaginal infection (31). Incorporation of SLS into the gel formulation also protected mice against HSV-2, suggesting that SLS completely destroyed the virus prior to its entry into epithelial cells. This full protection lasted for at least 1 h. Similar observations were made with LS for all except one LS-treated mouse, in which virulent particles were still observed after treatment. These data suggest that whether it is used at a high or a low concentration, the gel does not hinder the activity of SLS or LS. The gel, which acts as a physical barrier, combined with either surfactant, which plays the role of a chemical barrier, could thus offer a double protection against STDs.
SLS has been shown to be minimally toxic to primary human vaginal keratinocytes (24) and cultured human skin fibroblasts (30). In the present study, we have evaluated the in vivo toxicities of the gel formulations after intravaginal application once daily for 14 days to rabbits. The rabbit is considered to be the most appropriate model with which to establish the tolerance of vaginal formulations because of the high sensitivity of its vaginal mucosa (9, 22). We have previously reported that the gel alone prepared in acetate buffer is well tolerated by the vaginal mucosae of rabbits when it is applied once daily for 14 days (13). The gel formulation prepared in citrate buffer, as used in the present study, was tolerated as well. When given alone or within the gel, both SLS and LS induced only a limited toxicity. Our previous studies showed that the toxicity of nonoxynol-9, which was extreme for the genital mucosa, was greatly reduced upon incorporation into the gel (13). Similarly, the present study has demonstrated that the incorporation of SLS into the gel significantly reduced the level of inflammatory cell infiltrates in the mucosae of the animals, indicating that the polymer has a protective effect on the toxicities of these surfactants. This characteristic is highly important, as it is well known that the presence of proinflammatory cells in the epithelium may increase the risk of acquiring infection.
In conclusion, pretreatment of mice with gel formulations containing 2.5% SLS or 2.5% LS prior to HSV-2 intravaginal infection prevents the colonization of the vaginal mucosa with infectious virus. These formulations, which combine physical and chemical barriers, induced only a mild irritancy to the vaginal mucosae of rabbits. Taken together, these results demonstrated that thermoreversible gel formulations containing SLS or LS could represent potent and safe topical microbicides for the prevention of infections with HIV-1, HSV, and other pathogens causing STDs.
ACKNOWLEDGMENTS
This study was supported by grants from the American Foundation for AIDS Research (AMFAR) and from the Canadian Foundation for AIDS Research (CANFAR) and Infectio Recherche Inc.
We thank Nathalie Gagné for constructive comments and helpful discussions.
REFERENCES
- 1.Asculai S S, Weis M T, Rancourt M W, Kupferberg A B. Inactivation of herpes simplex viruses by nonionic surfactants. Antimicrob Agents Chemother. 1978;13:686–690. doi: 10.1128/aac.13.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba M, Schols D, Nakashima H, Pauwels R, Parmentier G, Meijer D, Clercq E D. Selective activity of several cholic acid derivatives against human immunodeficiency virus replication in vitro. J Acquir Immune Defic Syndr. 1989;2:264–271. [PubMed] [Google Scholar]
- 3.Benes S, McCormack W M. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob Agents Chemother. 1985;27:724–726. doi: 10.1128/aac.27.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourinbaiar A S, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9 and gossypol. Contraception. 1994;49:131–137. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 5.Brugha R, Keersmaekers K, Renton A, Meheus A. Genital herpes infection: a review. Int J Epidemiol. 1997;26:698–709. doi: 10.1093/ije/26.4.698. [DOI] [PubMed] [Google Scholar]
- 6.Buttke T M, McCubrey J A, Owen T C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphocyte-dependent cell lines. J Immunol Methods. 1993;157:223–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- 7.Chambers J A A. Buffers, chelating agents and denaturants. In: Chambers J A A, Rickwood D, editors. Biochemistry. San Diego, Calif: Academic Press, Inc.; 1993. pp. 1–36. [Google Scholar]
- 8.Ebrahim S H, Peterman T A, Zaidi A A, Kamb M L. Mortality related to sexually transmitted diseases in US women, 1973 through 1992. Am J Public Health. 1997;87:938–944. doi: 10.2105/ajph.87.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein P, Jackson M C N, Millman N, Sobrero A J. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkey. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 10.Elias C J, Heise L L. Challenges for the development of female-controlled vaginal microbicides. AIDS. 1994;8:1–9. doi: 10.1097/00002030-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Feldblum P J, Morrison C S, Roddy R E, Cates W. The effectiveness of barrier methods of contraception in preventing the spread of HIV. AIDS. 1995;9(Suppl. A):S85–S93. [PubMed] [Google Scholar]
- 12.Feldblum P J, Weir S S. The protective effect of nonoxynol-9 against HIV infection. Am J Public Health. 1994;84:1032–1034. doi: 10.2105/ajph.84.6.1032-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagné N, Cormier H, Omar R F, Désormeaux A, Gourde P, Tremblay M J, Juhász J, Beauchamp D, Rioux J E, Bergeron M G. Protective effect of a thermoreversible gel against the toxicity of nonoxynol-9. Sex Transm Dis. 1999;26:177–183. doi: 10.1097/00007435-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Hecht E, Mortensen K, Gradzielski M, Hoffmann H. Interaction of ABA block copolymers with ionic surfactants: influence on micellization and gelation. J Phys Chem. 1995;99:4866–4874. [Google Scholar]
- 15.Helenius A, Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 16.Herold B, Kirkpatrick R, Marcellino D, Travelstead A, Pilipenko V, Krasa H, Breme J, Dong L, Cooper M. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob Agents Chemother. 1999;43:745–751. doi: 10.1128/aac.43.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howett M K, Neely E B, Christensen N D, Wigdahl B, Krebs F C, Malamud D, Patrick S D, Pickel M D, Welsh P A, Reed C A, Ward M G, Budgeon L R, Kreider J W. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother. 1999;43:314–321. doi: 10.1128/aac.43.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang A, Wagner R. Penetration of herpes simplex virus into human epidermoid cells. Proc Soc Exp Biol Med. 1964;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- 19.Irwin K, Scarlett M, Moseley R. Observations from the CDC. The urgent need for new HIV/STD prevention options for women. J Women's Health. 1998;7:1081–1086. doi: 10.1089/jwh.1998.7.1081. [DOI] [PubMed] [Google Scholar]
- 20.Jennings R, Clegg A. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in vitro. J Antimicrob Chemother. 1993;32:71–82. doi: 10.1093/jac/32.1.71. [DOI] [PubMed] [Google Scholar]
- 21.Judson F N, Ehret J M, Bodin G F, Levin M J, Rietmeijer C A. In vitro evaluations of condoms with and without nonoxynol-9 as physical and chemical barriers against Chlamydia trachomatis, herpes simplex virus type 2, and human immunodeficiency virus. Sex Transm Dis. 1989;16:51–56. doi: 10.1097/00007435-198904000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Kaminsky M, Szivos M M, Brown K R. Comparison of the sensitivity of the vaginal mucous membranes of the albino rabbit and laboratory rat to nonoxynol-9. Food Chem Toxicol. 1985;23:705–708. doi: 10.1016/0278-6915(85)90161-9. [DOI] [PubMed] [Google Scholar]
- 23.Klebanoff S J. Effects of the spermicidal agent nonoxynol-9 on vaginal microbial flora. J Infect Dis. 1992;165:19–25. doi: 10.1093/infdis/165.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Krebs F C, Miller S R, Catalone B J, Welsh P A, Malamud D, Howett M K, Wighdahl B. Sodium dodecyl sulfate and C31G as microbicidal alternatives to nonoxynol-9: comparative sensitivity of primary human vaginal keratinocytes. Antimicrob Agents Chemother. 2000;44:1954–1960. doi: 10.1128/aac.44.7.1954-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs F C, Miller S R, Malamud D, Howett M K, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir Res. 1999;43:157–173. doi: 10.1016/s0166-3542(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 26.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P L, Roberts P L, Ruminjo I, Sajabi R, Kimata J, Fleming T R, Anzala A, Holton D, Plummer F. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 27.Malkovsky M, Newell A, Dalgleish A G. Inactivation of HIV by nonoxynol-9. Lancet. 1988;i:645. doi: 10.1016/s0140-6736(88)91440-7. [DOI] [PubMed] [Google Scholar]
- 28.Niruthisard S, Roddy R E, Chutivongse S. The effect of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18:176–179. doi: 10.1097/00007435-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Parr M B, Kepple L, McDermott M R, Drew M D, Bozzola J J, Parr E L. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Investig. 1994;70:369–380. [PubMed] [Google Scholar]
- 30.Piret J, Désormeaux A, Cormier H, Lamontagne J, Gourde P, Juhász J, Bergeron M G. Sodium lauryl sulfate increases the efficacy of topical formulation of foscarnet against herpes simplex virus type 1 cutaneous lesions in mice. Antimicrob Agents Chemother. 2000;44:2263–2270. doi: 10.1128/aac.44.9.2263-2270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piret J, Lamontagne J, Bestman-Smith J, Roy S, Gourde P, Désormeaux A, Omar R F, Juhász J, Bergeron M G. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J Clin Microbiol. 2000;38:110–119. doi: 10.1128/jcm.38.1.110-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polsky B, Baron P A, Gold J W, Smith J L, Jensen R H, Armstrong D. In vitro inactivation of HIV-1 by contraceptive sponge containing nonoxynol-9. Lancet. 1988;i:1456. doi: 10.1016/s0140-6736(88)92261-1. [DOI] [PubMed] [Google Scholar]
- 33.Psychoyos A, Creatsas G, Hassan E, Georgoulias V, Gravanis A. Spermicidal and antiviral properties of cholic acid: contraceptive efficacy of a new vaginal sponge (Protectaid) containing sodium cholate. Hum Reprod. 1993;8:866–869. doi: 10.1093/oxfordjournals.humrep.a138156. [DOI] [PubMed] [Google Scholar]
- 34.Roddy R E, Cordero M, Cordero C, Fortney J A. A dosing study of nonoxynol-9 and genital irritation. Int J STDs AIDS. 1993;4:165–170. doi: 10.1177/095646249300400308. [DOI] [PubMed] [Google Scholar]
- 35.Roddy R E, Zekeng L, Ryan K A, Tamoufé U, Weir S S, Wong E L. A controlled trial of nonoxynol-9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 36.Short R V. Contraceptives of the future in the light of HIV infection. Aust NZ J Obstet Gynaecol. 1994;34:330–332. doi: 10.1111/j.1479-828x.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 37.Stafford M K, Ward H, Flanagan A, Rosenstein I J, Taylor-Robinson D, Smith J R, Weber J, Kitchen V S. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 38.Stein Z A. HIV prevention: the need for methods women can use. Am J Public Health. 1990;80:460–462. doi: 10.2105/ajph.80.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wainberg M A. The need for vaginal microbicides with antiviral specificity. AIDS. 1998;12:4–6. [Google Scholar]
- 40.Ward R L, Ashley C S. Comparative study on the mechanisms of rotavirus inactivation by sodium dodecyl sulfate and ethylediaminetetraacetate. Appl Environ Microbiol. 1980;39:1148–1153. doi: 10.1128/aem.39.6.1148-1153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward R L, Ashley C S. pH modification of the effects of detergents on the stability of enteric viruses. Appl Environ Microbiol. 1979;38:314–322. doi: 10.1128/aem.38.2.314-322.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whaley K J, Barratt R A, Zeitlin L, Hoen T E, Cone R A. Nonoxynol-9 protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1993;168:1009–1111. doi: 10.1093/infdis/168.4.1009. [DOI] [PubMed] [Google Scholar]
- 43.Whaley K J, Zeitlin L, Barratt R A, Hoen T E, Cone R A. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 44.Zekeng L, Feldblum P J, Oliver R M, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7:725–731. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]