Abstract
Recently, balloon pulmonary angioplasty (BPA) for chronic thromboembolic pulmonary hypertension (CTEPH) and chronic thromboembolic pulmonary disease (CTEPD) has become an established procedure with stable results. The number of elderly CTEPH/CTEPD patients has also increased due to the widespread recognition of the disease concept. However, the reports of BPA in the elderly are limited. The aim of this study was to evaluate the efficacy and safety of BPA in elderly patients (>80 years). From April 2016 to December 2020, 344 BPA sessions (74 patients) were performed. The safety and efficacy of the BPA procedures were compared in the younger group (<80 years; 278 sessions) and the elderly group (≥80 years; 66 sessions). The hemodynamic parameters were significantly improved in both groups (mean pulmonary arterial pressure: 34.4 ± 9.9 → 21.2 ± 6.2 mmHg, p < 0.001 and 33.2 ± 9.6 → 21.8 ± 8.5 mmHg, p < 0.001; pulmonary vascular resistance: 474.5 ± 248.6 → 201.3 ± 108.7 dyne sec cm−5, p < 0.001 and 496.4 ± 290.9 → 260.5 ± 120.2 dyne sec cm−5, p = 0.002, in younger and elderly group, respectively). The rate of death within 30 days of BPA (0.3% vs. 0%, p = 1.000) and use of positive pressure ventilation (1.4% vs. 3.0%, p = 0.600) were not different between the groups (younger vs. elderly, respectively). BPA significantly improved the hemodynamic parameters of elderly CTEPH/CTEPD patients, and the safety is comparable to that of younger patients.
Keywords: BPA, CTEPD, CTEPH, elderly
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is caused by organized thrombi in pulmonary arteries, which lead to stenosis and occlusion of pulmonary arteries and result in increased pulmonary vascular resistance and pulmonary hypertension. 1 , 2 Pulmonary endarterectomy (PEA) is considered the standard and curative treatment strategy for CTEPH and has been reported to improve the prognosis of patients with proximal CTEPH. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 However, PEA is an invasive procedure that may not be suitable for some patients, such as elderly or highly frail individuals, and maintaining the safety of the PEA procedure requires experienced surgeons and institutions. 7 , 8 , 9 Inoperable cases, such as patients with a distal type of CTEPH or patients with comorbidities have been indicated for balloon pulmonary angioplasty (BPA), and the results are acceptable. 10 , 11 , 12 , 13 , 14 Recently, BPA has not only improved life prognosis but also achieved high quality of life (QOL) of CTEPH patients. 11 , 12 , 13 , 15 , 16 In addition, BPA is now being performed on symptomatic patients with chronic thromboembolic pulmonary disease (CTEPD) without pulmonary hypertension at rest. 16
With the aging of society, the number of elderly CTEPH/CTEPD patients has also increased due to the widespread recognition of the disease concept. However, the efficacy of BPA in elderly patients remains unclear. To the best of our knowledge, there is only one report showing the results of BPA in slightly older (65 years and more) CTEPH patients. 17 The aim of this study was to assess the efficacy and safety of BPA for 80‐years‐old and more CTEPH/CTEPD patients.
METHODS
Study population
From April 2016 to December 2020, 344 BPA treatment sessions (74 patients) were performed at our institution. All 344 treatment sessions were analyzed for safety endpoints. Of the 74 patients, 69 patients completed all the scheduled BPA treatments (307 sessions) and were assessed for efficacy endpoints. The patients were divided into two groups according to age: younger group (<80 years) and elderly group (≥80 years). CTEPH/CTEPD was diagnosed using the results of right heart catheterization (RHC), perfusion lung scintigraphy, pulmonary artery angiography, and contrast‐enhanced lung computed tomography and the exclusion of other diseases that cause pulmonary hypertension. The specific details of the BPA treatment for each case were at the discretion of the operators. Procedural and clinical data from these BPA sessions were collected from the patients' medical records and retrospectively analyzed.
Procedure
The catheter access sites were mainly the femoral or right internal jugular veins, and 8.2‐Fr sheaths were inserted. From the sheath, a 6‐Fr, 70‐cm‐long guiding sheath (ParentPlus60Ⓡ; Medikit) was inserted into the main pulmonary arteries. We advanced a 6‐Fr guiding catheter (e.g., Judkins right or left, Amplatz left, Ikari left, multipurpose, or Cobra) to the target lesions of the segmental pulmonary arteries through the guiding sheath. After visualizing the target lesions by injecting a contrast medium, we advanced 0.014‐in. wires through the lesions. The target lesions were dilated with an appropriately sized balloon, mainly judged by angiography. The activated clotting time during the procedures was maintained within 180–250 s. Usually, multiple sessions (3–10 sessions) of BPA are required to achieve optimal results and the number of sessions depends on the discretion of the operators considering the severity of CTEPH or lesion morphology. The endpoint of each session was determined by considering the amount of contrast medium or radiation time. The strategy of BPA at our institution is to treat all lesions that can be accessed by catheters.
Evaluation of efficacy and safety
RHC was performed pre‐ and post‐BPA sessions for measurement of right ventricular pressure, pulmonary arterial pressure (PAP), pulmonary arterial wedge pressure (PAWP), and cardiac index (CI). CI was measured by the thermodilution method. Pulmonary vascular resistance (PVR) was calculated as mean (PAP‐PAWP)/cardiac output. The efficacy endpoints were improvements of hemodynamics, such as mean PAP, PVR, and CI. Six‐min walking distance (6MWD) was evaluated pre‐ and post‐BPA. The safety endpoints were the occurrence of complications with BPA, including death within 30 days after BPA, the use of positive pressure ventilation (e.g., ventilator or noninvasive positive pressure ventilation), appearance of bloody sputum, contrast‐induced nephropathy, and other complications requiring treatment.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation, and categorical variables are expressed as counts and proportions. The χ 2 test and t‐test were used to analyze continuous and categorical variables, respectively. Paired serial comparisons of the hemodynamic parameters were analyzed with paired Student's t test as necessary. Statistical significance was defined as a p < 0.05. The SPSS (IBM Japan) software package (ver. 23) was used for the analyses.
RESULTS
Patient characteristics
The patient and lesion characteristics are summarized in Table 1. The pre‐BPA data of mean PAP, PVR, CI, and 6MWD were not different between the younger group and elderly group (mean PAP: 34.4 ± 9.9 vs. 33.2 ± 9.6 mmHg, p = 0.698; PVR: 474.5 ± 248.6 vs. 496.4 ± 290.9 dyne sec cm−5, p = 0.777; CI: 2.7 ± 0.6 vs. 2.7 ± 0.7 L min−1 m2, p = 0.800; 6MWD: 323.8 ± 159.7 vs. 248.5 ± 154.7 m, p = 0.137). Although the post‐BPA 6MWD of the elderly group was significantly lower than that of the younger group, the other parameters after the BPA sessions showed no significant differences between the two groups (mean PAP: 21.2 ± 6.2 vs. 21.8 ± 8.5 mmHg, p = 0.763, PVR: 201.3 ± 108.7 vs. 260.5 ± 120.2 dyne sec cm−5, p = 0.080; CI: 3.0 ± 0.9 vs. 2.7 ± 0.7 L min−1 m2, p = 0.292; 6MWD: 463.2 ± 140.1 vs. 251.5 ± 146.6 m, p < 0.001; younger group vs. elderly group, respectively). The medical treatment remained essentially unchanged before and after treatment.
Table 1.
Patient characteristics (307 sessions/69 patients)
| All patients (n = 69) | Younger group (n = 55) | Elderly group (n = 14) | p Value | |
|---|---|---|---|---|
| Age (years) | 68.8 ± 13.7 | 64.9 ± 12.6 | 84.0 ± 3.6 | <0.001 |
| Female, n (%) | 47 (68) | 36 (65) | 11 (79) | 0.523 |
| History of VTE, n (%) | 54 (78) | 45 (82) | 9 (64) | 0.156 |
| CTEPD, n (%) | 10 (14) | 8 (15) | 2 (14) | 0.980 |
| Reasons for inoperability, n (%) | ||||
| Technically inoperable | 24 (35) | 21 (38) | 3 (21) | |
| Unfavorable risk/benefit ratio | 26 (38) | 18 (33) | 8 (57) | |
| Patient choice | 19 (28) | 16 (29) | 3 (21) | |
| Anticoagulant agents, n (%) | 69 (100) | 55 (100) | 14 (100) | 1.000 |
| VKA, n (%) | 31 (45) | 26 (47) | 5 (36) | 0.438 |
| Use of pulmonary vasodilators, n (%) | 31 (45) | 26 (47) | 5 (36) | 0.438 |
| ERA, n (%) | 8 (12) | 8 (15) | 0 (0) | |
| PDE5 inhibitor, n (%) | 2 (3) | 1 (2) | 1 (7) | |
| Oral PGI2, n (%) | 10 (14) | 7 (13) | 3 (21) | |
| sGC stimulator, n (%) | 23 (33) | 21 (38) | 2 (14) | |
| NYHA functional classification, n (%) | ||||
| Ⅰ | 0 (0) | 0 (0) | 0 (0) | |
| Ⅱ | 43 (62) | 37 (67) | 6 (43) | |
| Ⅲ | 18 (26) | 12 (22) | 6 (43) | |
| Ⅳ | 8 (12) | 6 (11) | 2 (14) | |
| Mean number of procedures, n | 4.5 ± 2.5 | 4.6 ± 2.5 | 4.0 ± 2.7 | 0.463 |
| <pre> | ||||
| Mean PAP (mmHg) | 34.1 ± 9.8 | 34.4 ± 9.9 | 33.2 ± 9.6 | 0.698 |
| PVR (dyne sec cm−5) | 479.0 ± 255.7 | 474.5 ± 248.6 | 496.4 ± 290.9 | 0.777 |
| CI (L min−1 m2) | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.7 | 0.800 |
| 6MWD (m) | 307.0 ± 160.4 (n = 58) | 323.8 ± 159.7 (n = 45) | 248.5 ± 154.7 (n = 13) | 0.137 |
| <post> | ||||
| Mean PAP (mmHg) | 21.3 ± 6.6 | 21.2 ± 6.2 | 21.8 ± 8.5 | 0.763 |
| PVR (dyne sec cm−5) | 213.5 ± 112.8 | 201.3 ± 108.7 | 260.5 ± 120.2 | 0.080 |
| CI (L min−1 m2) | 3.0 ± 0.9 | 3.0 ± 0.9 | 2.7 ± 0.7 | 0.292 |
| 6MWD (m) | 418.4 ± 165.0 (n = 52) | 463.2 ± 140.1 (n = 41) | 251.5 ± 146.6 (n = 11) | <0.001 |
Abbreviations: 6MWD, 6‐min walking distance; CI, cardiac index; CTEPD, chronic thromboembolic pulmonary disease; ERA, endothelin receptor antagonists; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PDE5, phosphodiesterase type 5; PGI2, prostacyclin; PVR, pulmonary vascular resistance; sGC, soluble guanylate cyclase; VKA, vitamin‐K antagonist; VTE, venous thromboembolism.
Efficacy endpoints
The mean PAP, PVR, and 6MWD in each group (overall cohort, younger group, and elderly group) significantly improved after all the BPA sessions (mean PAP: 34.1 ± 9.8 → 21.3 ± 6.6 mmHg, p < 0.001, 34.4 ± 9.9 → 21.2 ± 6.2 mmHg, p < 0.001, and 33.2 ± 9.6 → 21.8 ± 8.5 mmHg, p < 0.001, Figure 1; PVR: 479.0 ± 255.7 → 213.5 ± 112.8 dyne sec cm−5, p < 0.001, 474.5 ± 248.6 → 201.3 ± 108.7 dyne sec cm−5, p < 0.001, and 496.4 ± 290.9 → 260.5 ± 120.2 dyne sec cm−5, p = 0.002, Figure 2; 6MWD: 306.9 ± 148.8 → 420.8 ± 165.7 m, p < 0.001, 334.2 ± 141.0 → 463.2 ± 140.1 m, p < 0.001, and 195.1 ± 131.9 → 247.2 ± 153.8 m, p = 0.017, Figure 3). CI in overall cohort and the younger group was significantly improved after all the BPA sessions; however, CI in the elderly group did not significantly change (2.7 ± 0.6 → 3.0 ± 0.9 L min−1 m2, p = 0.039, 2.7 ± 0.6 → 3.0 ± 0.9 L min−1 m2, p = 0.035, 2.7 ± 0.7 → 2.7 ± 0.7 L min−1 m2, p = 0.856, Figure 4). The 6MWD results from the efficacy endpoints were analyzed only in cases who could be measured and compared before and after BPA (overall cohort: n = 51, younger group: n = 41, elderly group: n = 10).
Figure 1.
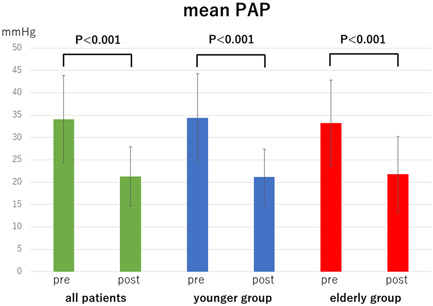
Comparison of mean pulmonary arterial pressure (PAP) pre‐ and post‐balloon pulmonary angioplasty in all patients, younger group (<80 years) and oldest‐old group (≥80 years)
Figure 2.
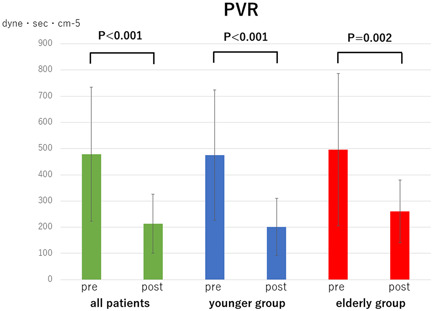
Comparison of pulmonary vascular resistance (PVR) pre‐ and post‐balloon pulmonary angioplasty in all patients, younger group (<80 years) and oldest‐old group (≥80 years)
Figure 3.
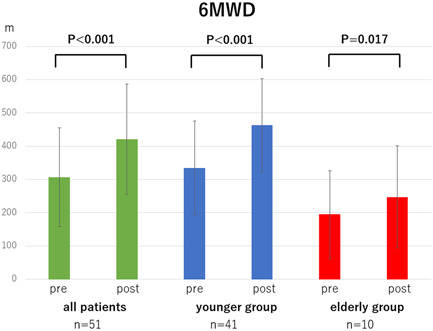
Comparison of 6‐min walking distance (6MWD) pre‐ and post‐balloon pulmonary angioplasty in all patients, younger group (<80 years) and oldest‐old group (≥80 years)
Figure 4.
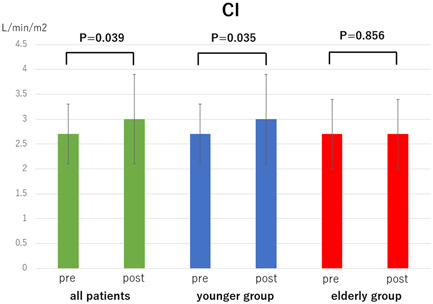
Comparison of cardiac index (CI) pre‐ and post‐balloon pulmonary angioplasty in all patients, younger group (<80 years), and oldest‐old group (≥80 years)
Safety endpoints
The safety endpoints are summarized in Table 2. There was no difference about the occurrence of procedure‐related complications between the two groups (younger group; 12.9% vs. elderly group; 19.7%, p = 0.171). The appearance of bloody sputum was the most common complication in both groups (11.5% in the younger group vs. 19.7% in the elderly group, p = 0.102). Although one patient in the younger group, unfortunately, died within 30 days of BPA because of procedure‐related complications, the results demonstrated that there were no significant differences of each safety endpoint between the two groups. Moreover, major complications (total deaths within 30 days of BPA and use of positive pressure ventilation) were not different between the two groups (1.4% in the younger group vs. 3.0% in the elderly group, p = 0.600). Other complications that occurred in the younger group were perforation of the right atrium, hematoma of the puncture site, pneumonia that occurred 2 days after the procedure.
Table 2.
Safety endpoints (344 sessions in 74 cases)
| All patients (n = 344 sessions) | Younger group (n = 278 sessions) | Elderly group (n = 66 sessions) | p Value | |
|---|---|---|---|---|
| Total of complications, n (%) | 49 (14.2) | 36 (12.9) | 13 (19.7) | 0.171 |
| Death within 30 days of balloon pulmonary angioplasty, n (%) | 1 (0.3) | 1 (0.4) | 0 (0) | 1.000 |
| Use of positive pressure ventilation, n (%) | 6 (1.7) | 4 (1.4) | 2 (3.0) | 0.600 |
| Appearance of bloody sputum, n (%) | 45 (13.1) | 32 (11.5) | 13 (19.7) | 0.102 |
| Contrast induced nephropathy, n (%) | 1 (0.3) | 1 (0.4) | 0 (0) | 1.000 |
| Other complications requiring treatment, n (%) | 3 (8.7) | 3 (1.1) | 0 (0) | 0.622 |
DISCUSSION
The main finding of this study was that BPA was safe and significantly improved hemodynamic parameters even in the elderly patients diagnosed with CTEPH/CTEPD. There were significant improvements in the efficacy endpoints in both groups. The results of the efficacy endpoints in the elderly patients (≥80 years), such as mean PAP, PVR, CI, and 6MWD, were acceptable and comparable with those in the younger patients (<80 years). 6MWD post‐BPA in the elderly group was significantly lower than that in the younger group. This may be because of the poor physical, orthopedic condition, or comorbidities of the elderly patients at baseline. Importantly, the efficacy endpoints were obtained similarly even though the hemodynamic severity of CTEPH at baseline was comparable between the two groups. Moreover, the present study indicated the safety of BPA even in elderly patients. The total number of complications, including death within 30 days of BPA, use of positive pressure ventilation, appearance of bloody sputum, and contrast‐induced nephropathy, after each session were comparable between the two groups. There was a low rate of occurrence of contrast‐induced nephropathy in both groups, especially in the elderly group (0.4% in the younger group vs. 0% in the elderly group, p = 1.000). The results may suggest that BPA has a low risk of contrast‐induced nephropathy. Also, there was a possibility that procedures in the elderly group were performed with a reduced amount of contrast medium suppressed at the operator's discretion because the amount of contrast medium of each session in the elderly group was significantly low in the present study (187.9 ± 55.2 ml in the younger group vs. 171.4 ± 45.0 in the elderly group, p = 0.026). Although there was, unfortunately, one case of death within 30 days of BPA in the younger group, there were no cases in the elderly group (p = 1.000). Because we counted the occurrence of bloody sputum even when it was only slightly bloody, the rate of bloody sputum appearance was relatively high (11.5% in the younger group vs. 19.7% in the elderly group, p = 0.102). However, considering that the use of positive pressure ventilation was relatively low in both groups (1.4% in the younger group vs. 3.0% in the elderly group, p = 0.600), patients with bloody sputum rarely experienced severe clinical problems. In clinical treatments, major complications have the greatest effect on prognosis, and the frequency of major complications was low and was not different between the two groups in the present study. Japanese multicenter registry data published in 2017 reported the following: procedure‐related complications (36.3%), pulmonary injury (17.8%), bloody sputum (14.0%), and intubation with mechanical ventilation (5.5%). 11 Even compared with these data, the results of the present study indicate that BPA can be safely performed even in elderly patients.
While promising outcomes of BPA have been shown, PEA is the first line, curative treatment for CTEPH. 7 , 8 , 9 Some studies have reported that the mortality of PEA in the early phase is 2.2%–9.8%, and the average age of patients who underwent PEA is 51–60 years. 4 , 7 , 8 , 9 , 18 , 19 PEA is generally less frequently used to treat elderly patients than younger patients and it has also been reported that an age of more than 60 years is a risk factor for hospital mortality following PEA. 20 The mortality of both the younger and elderly groups following BPA in the present study was low, demonstrating that BPA could be performed at the same or safer level as PEA, even in elderly patients. Although PEA should be considered first for the treatment of CTEPH, some patient groups are not indicated for PEA because of their frailty or comorbidities. 1 , 2 , 8 , 9 For such patients, BPA, which is a less‐invasive procedure than PEA, must be seen as an effective and safe strategy, especially in elderly patients.
Yanagisawa reported that BPA in elderly patients (≥65 years) could be performed safely and its outcome was effectively equivalent to that in younger patients (<65 years). 17 However, due to the aging of the population and the widespread recognition of the disease concept of CTEPH/CTEPD, patients over 65 years of age are not uncommon. The mean age of all patients in our study was 68.8 years. It is extremely important to examine the results of BPA in very elderly patients who are not good candidates for PEA.
Ikeda et al. 21 and Ogawa et al. 11 reported that abnormal shadows observed on computed tomography after BPA may be caused by wire perforation or vessel injury. These results indicate that an accurate procedure performed without wire perforation or vessel injury could prevent the occurrence of complications such as the use of positive pressure ventilation and the safety of BPA could be ensured, even in elderly patients. In the present study, the acceptable low rate of using positive pressure ventilation may indicate the accuracy of the procedure.
Recently, the BPA procedure has been rapidly improved, and the aim is to achieve a normal mPAP to maintain high QOL. 13 , 14 , 15 , 16 , 22 , 23 , 24 , 25 This advancement in BPA has expanded the candidates for BPA to include not only CTEPH but also CTEPD patients. 16 Considering that the life expectancy of the very elderly is not very long, it is important not only to improve their life prognosis but also to improve their symptoms. In this sense, the demand for BPA for CTEPD as well as CTEPH is increasing. The results of this study will contribute to the further spread of BPA in the future.
Accurate and appropriate BPA procedures are required to achieve sufficient results, especially in elderly patients; however, the strategies used to perform BPA are still different at each institute, and standardization is required. Further investigation and long‐term follow‐up data are required to discuss the endpoint of the BPA procedure for improving prognosis and symptoms of elderly patients.
Study limitations
There are several limitations in the present observational study. First, this was a single‐center, nonrandomized, retrospective study with a relatively small sample size. Second, the variety and dose of oral medications were at the discretion of each physician. In addition, the details of the procedure, such as the balloon size and the number of sessions, were also at the operator's discretion. Third, the pre‐BPA hemodynamics are relatively mild, due to the inclusion of patients with CTEPD as well as CTEPH, and the inclusion of patients who have been started on pulmonary vasodilators before BPA.
CONCLUSIONS
The efficacy and safety of BPA for CTEPH/CTEPD in elderly patients (≥80 years) were not different from those in younger patients (<80 years). BPA has the potential to be the standard strategy for CTEPH/CTEPD in elderly patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study was performed in accordance with the Code of Federal Regulations and the Declaration of Helsinki. The present study was approved by the Ethics Committee of our institution (approval number: H20001). A comprehensive agreement was obtained from all patients and an opt‐out form on the website of our university provided the target patients with the opportunity to refuse participation in the present study.
AUTHOR CONTRIBUTIONS
Taito Nagai: manuscript writing; Nobutaka Ikeda: study design and manuscript writing; Raisuke Iijima: study design and statistical analysis; Hidehiko Hara: study design; Masato Nakamura: final approval.
Nagai T, Ikeda N, Iijima R, Hara H, Nakamura M. Impact and safety of balloon pulmonary angioplasty for elderly patients. Pulm Circ. 2022;12:e12009. 10.1002/pul2.12009
REFERENCES
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 2. Fukuda K, Date H, Doi S, Fukumoto Y, Fukushima N, Hatano M, Ito H, Kuwana M, Matsubara H, Momomura SI, Nishimura M, Ogino H, Satoh T, Shimokawa H, Yamauchi‐Takihara K, Tatsumi K, Ishibashi‐Ueda H, Yamada N, Yoshida S, Abe K, Ogawa A, Ogo T, Kasai T, Kataoka M, Kawakami T, Kogaki S, Nakamura M, Nakayama T, Nishizaki M, Sugimura K, Tanabe N, Tsujino I, Yao A, Akasaka T, Ando M, Kimura T, Kuriyama T, Nakanishi N, Nakanishi T, Tsutsui H, Japanese Circulation Society and the Japanese Pulmonary Circulation and Pulmonary Hypertension Society Joint Working Group . Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017). Circ J. 2019;83:842–945. 10.1253/circj.CJ-66-0158 [DOI] [PubMed] [Google Scholar]
- 3. Pepke‐Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D'Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barberà JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez‐Sanchez MA, Kovacs G, Hamid AM, Jaïs X, Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–81. 10.1161/CIRCULATIONAHA.110.015008 [DOI] [PubMed] [Google Scholar]
- 4. Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke‐Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–10. 10.1016/j.jtcvs.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 5. Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, Pepke‐Zaba J, Jenkins DP. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(383):383–7. 10.1016/j.jtcvs.2009.12.056 [DOI] [PubMed] [Google Scholar]
- 6. Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, Treacy C, Ponnaberanam A, Condliffe R, Sheares K, Taboada D, Dunning J, Tsui S, Ng C, Gopalan D, Screaton N, Elliot C, Gibbs S, Howard L, Corris P, Lordan J, Johnson M, Peacock A, MacKenzie‐Ross R, Schreiber B, Coghlan G, Dimopoulos K, Wort SJ, Gaine S, Moledina S, Jenkins DP, Pepke‐Zaba J. Dynamic risk stratification of patient long‐term outcome after pulmonary endarterectomy: Results From the United Kingdom National Cohort. Circulation. 2016;133:1761–71. 10.1161/CIRCULATIONAHA.115.019470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. 10.1016/j.athoracsur.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 8. Madani MM. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016;12:213–8. 10.14797/mdcj-12-4-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenkins D, Madani M, Fadel E, D'Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:160111. 10.1183/16000617.0111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5:748–55. 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 11. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, Yamada N, Yao A, Ando M, Ogino H, Tanabe N, Tsujino I, Hanaoka M, Minatoya K, Ito H, Matsubara H. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10:e004029. 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 12. Aoki T, Sugimura K, Tatebe S, Miura M, Yamamoto S, Yaoita N, Suzuki H, Sato H, Kozu K, Konno R, Miyata S, Nochioka K, Satoh K, Shimokawa H. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo‐embolic pulmonary hypertension: long‐term effects and procedure‐related complications. Eur Heart J. 2017;38:3152–9. 10.1093/eurheartj/ehx530 [DOI] [PubMed] [Google Scholar]
- 13. Kataoka M, Inami T, Kawakami T, Fukuda K, Satoh T. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. JACC Cardiovasc Interv. 2019;12(1382):1382–8. 10.1016/j.jcin.2019.01.237 [DOI] [PubMed] [Google Scholar]
- 14. Ikeda N. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther. 2020;35:130–41. 10.1007/s12928-019-00637-2 [DOI] [PubMed] [Google Scholar]
- 15. Ikeda N, Hatano M, Nagamatsu T, Nakamura M. Successful right heart remodelling and subsequent pregnancy in a patient with chronic thromboembolic pulmonary hypertension undergoing balloon pulmonary angioplasty: a case report. Eur Heart J Case Rep. 2019;3:ytz06. 10.1093/ehjcr/ytz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inami T, Kataoka M, Kikuchi H, Goda A, Satoh T. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int J Cardiol. 2019;289(116):116–8. 10.1016/j.ijcard.2019.04.080 [DOI] [PubMed] [Google Scholar]
- 17. Yanagisawa R, Kataoka M, Inami T, Shimura N, Ishiguro H, Fukuda K, Yoshino H, Satoh T. Safety and efficacy of percutaneous transluminal pulmonary angioplasty in elderly patients. Int J Cardiol. 2014;175:285–9. 10.1016/j.ijcard.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 18. Jenkins D, Mayer E, Screaton N, Madani M. State‐of‐the‐art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev. 2012;21:32–9. 10.1183/09059180.00009211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Committee for Scientific Affairs, The Japanese Association for Thoracic S, Masuda M, Okumura M, Doki Y, Endo S, Hirata Y, Kobayashi J, Kuwano H, Motomura N, Nishida H, Saiki Y, Saito A, Shimizu H, Tanaka F, Tanemoto K, Toh Y, Tsukihara H, Wakui S, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2014: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016;64:665–97. 10.1007/s11748-016-0695-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogino H, Ando M, Matsuda H, Minatoya K, Sasaki H, Nakanishi N, Kyotani S, Imanaka H, Kitamura S. Japanese single‐center experience of surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82:630–6. 10.1016/j.athoracsur.2006.03.121 [DOI] [PubMed] [Google Scholar]
- 21. Ikeda N, Kubota S, Okazaki T, Iijima R, Hara H, Hiroi Y, Nakamura M. The predictors of complications in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Catheter Cardiovasc Interv. 2019;93:E349–56. 10.1002/ccd.28133 [DOI] [PubMed] [Google Scholar]
- 22. Kawakami T, Kataoka M, Arai T, Yanagisawa R, Maekawa Y, Fukuda K. Retrograde approach in balloon pulmonary angioplasty: useful novel strategy for chronic total occlusion lesions in pulmonary arteries. JACC Cardiovasc Interv. 2016;9:19–20. 10.1016/j.jcin.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 23. Saito S, Ikeda N, Toi S, Nakamura M. Gadolinium contrast balloon pulmonary angioplasty for a patient with chronic thromboembolic pulmonary hypertension and severe iodine allergy. Catheter Cardiovasc Interv. 2020;97:525. 10.1002/ccd.29004 [DOI] [PubMed] [Google Scholar]
- 24. Nagayoshi S, Fujii S, Nakajima T, Muto M. Intravenous ultrasound‐guided balloon pulmonary angioplasty in the treatment of totally occluded chronic thromboembolic pulmonary hypertension. EuroIntervention. 2018;14:234–5. 10.4244/EIJ-D-17-00770 [DOI] [PubMed] [Google Scholar]
- 25. Takano T, Ozaki K, Hoyano M, Yanagawa T, Kashimura T, Minamino T. Angioscopic findings during balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther. 2019;35:421–2. 10.1007/s12928-019-00635-4 [DOI] [PubMed] [Google Scholar]


