Abstract
Background
Sex has consistently been shown to affect COVID-19 mortality, but it remains unclear how each sex’s clinical outcome may be distinctively shaped by risk factors.
Methods
We studied a primary cohort of 4930 patients hospitalized with COVID-19 in a single healthcare system in New York City from the start of the pandemic till August 5, 2020, and a validation cohort of 1645 patients hospitalized with COVID-19 in the same healthcare system from August 5, 2020, to January 13, 2021.
Results
Here we show that male sex was independently associated with in-hospital mortality, intubation, and ICU care after adjusting for demographics and comorbidities. Using interaction analysis and sex-stratified models, we found that hypoxia interacted with sex to preferentially increase women’s mortality risk while obesity interacted with sex to preferentially increase women’s risk of intubation and intensive care in our primary cohort. In the validation cohort, we observed that male sex remained an independent risk factor for mortality, but sex-specific interactions were not replicated.
Conclusions
We conducted a comprehensive sex-stratified analysis of a large cohort of hospitalized COVID-19 patients, highlighting clinical factors that may contribute to sex differences in the outcome of COVID-19.
Subject terms: Viral infection, Public health, Epidemiology
Plain Language Summary
Men are at higher risk of death from COVID-19 than women, but the underlying reasons are not fully understood. We examined the medical data of men and women hospitalized with COVID-19 in New York City to determine whether there were factors which raised the risk of death or requiring intensive care more for one sex rather than the other. We observed that men hospitalized with COVID-19 had a higher risk of death than women when other factors taken into account. Some conditions, like low oxygen levels and obesity, appeared to be associated with worse outcomes in women compared to men early in the pandemic but further studies will be necessary for confirmation. These findings highlight groups of men and women who may be at increased risk of severe COVID-19.
Jun et al. evaluate sex-stratified clinical outcomes in two cohorts of patients hospitalized with COVID-19 in New York. While male sex risk is a risk factor for poor outcome in both cohorts – one from earlier and one from later on in the pandemic – some of the sex-specific risk factors observed initially are not observed later on.
Introduction
Reports of the COVID-19 pandemic from around the world have described more severe disease and worse outcomes among men1–7. Men with COVID-19 appear to preferentially require hospitalization and intensive care2,8,9. For example, one large Italian case series reported that 82% of COVID-19 patients requiring intensive care were men10. Case fatality rates are also higher among men; a nationwide analysis from China reported a case fatality rate of 2.8% for men, compared to 1.7% for women4. Male sex has been identified as a risk factor for mortality in several studies of hospitalized COVID-19 patients11–15.
These observations have been variously attributed to underlying comorbidities among men, hormonal factors, or immune differences between men and women16–19. Some comorbidities associated with worse COVID-19 outcomes may be more common among men, though published studies have not provided sex-disaggregated data2,20,21. An Italian study observed that among prostate cancer patients, those on androgen deprivation therapy had better outcomes than those who were not, suggesting a sex hormonal contribution to COVID-19 mortality22. In addition, there are sex-specific differences in expression of the ACE2 and TMPRSS2 proteins, which facilitate the entry of the SARS-CoV-2 virion into cells23,24. Understanding how risk factors of severe disease differ between sexes can improve clinical risk assessment and shed new biological insights into disease etiology.
Given the sex difference in COVID-19 outcomes, we hypothesized that clinical risk factors for mortality might show sex-specific effects, which remain largely uncharacterized. Although multiple prior studies have included multivariable regression models adjusting for sex, they have not explored the possibility of interactions between sex and other predictors, nor have they examined differences in clinical course between men and women admitted with COVID-19.
To fill this knowledge gap, we conduct a sex-specific analysis of clinical data from 4930 COVID-19 patients hospitalized in New York City from the start of the pandemic to August 5, 2020. We confirm the association between male sex and adverse COVID-19 outcomes in this primary cohort and in a validation cohort of 1645 COVID-19 patients hospitalized from August 5, 2020, to January 13, 2021. We also explore sex-specific risk factors of in-hospital mortality, intubation, and intensive care.
Methods
Study setting
Mount Sinai Health System is a large hospital system in the New York City area, comprising 8 hospitals and more than 410 ambulatory practice locations. Our analysis focused on patients who were admitted to five hospitals: The Mount Sinai Hospital (1134 beds), Mount Sinai West (514 beds), and Mount Sinai Morningside (495 beds) in Manhattan; Mount Sinai Brooklyn (212 beds) in Brooklyn; and Mount Sinai Queens (235 beds) in Queens.
Data sources
Data were derived from clinical records from Mount Sinai facilities using the Epic electronic health record (Epic Systems, Verona, WI). Data were directly extracted from Epic’s Clarity and Caboodle servers. In the setting of the COVID-19 pandemic, the Mount Sinai Data Warehouse (MSDW) developed and released a de-identified dataset encompassing all COVID-19 related patient encounters within the Mount Sinai system, accompanied by selected demographics, comorbidities, vital signs, medications, and lab values. As part of de-identification, all patients over the age of 89 had their age set to 90. Updated versions of the dataset have been released on a weekly schedule. For this study, we used the dataset released on August 5, 2020.
Patient population and definitions
The MSDW dataset captured 224,018 inpatient and outpatient encounters involving 144,518 distinct patients at a Mount Sinai facility with any of the following: a COVID-19 related encounter diagnosis, a COVID-19 related visit type, a SARS-CoV-2 lab order, a SARS-CoV-2 lab result, or a SARS-CoV-2 lab test result from the New York State Department of Health’s Wadsworth laboratory up to August 5, 2020. Of these, 75,996 patients had at least one COVID-19 test and 12,347 tested positive. COVID-19 positivity was defined as a positive or presumptive positive result from a nucleic acid-based test to detect SARS-CoV-2 in nasopharyngeal or oropharyngeal swab specimens.
We limited our analysis to 4930 adult patients (at least 18 years old) who were admitted to the hospital and had a positive or presumptive positive SARS-CoV-2 test within 2 days of admission. Race and ethnicity were self-reported. Comorbidity status was assessed cross-sectionally using each patient’s problem list in the electronic medical record as of their hospital encounter. The problem list consists of diagnoses linked to International Classification of Disease codes; it is updated by medical providers during clinical encounters (inpatient and outpatient) and reflects ongoing medical issues. Obesity was based on body mass index (BMI) ≥ 30 kg/m2 or a pre-existing diagnosis in the medical record if BMI was not available.
The dataset captured inpatient medication exposures for a pre-selected list of drugs, including COVID-19 therapeutics such as remdesivir, steroids, and dexamethasone. Of note, medications administered in the context of clinical trials were not captured in this dataset.
Initial vital signs were the first vital signs documented for the encounter. Fever was defined as temperature ≥100.4 °F; tachycardia was defined as heart rate >100 beats per minute; tachypnea was defined as respiratory rate >25 breaths per minute; hypotension was defined as systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg; hypoxia was defined as oxygen saturation <92%.
We defined initial labs as the first lab value within 24 h of the start of the encounter. A subset of patients had baseline serum interleukin-1-beta (IL-1B), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-a) values obtained as part of a study, which enrolled COVID-19 patients hospitalized between March 21 and April 28, 202025,26.
SARS-CoV-2 viral loads from nasopharyngeal swabs were measured in a randomly selected cohort of 1146 MSHS patients hospitalized between March 13 and May 4, 2020, using quantitative RT-PCR27. Direct overlap with our de-identified dataset was not feasible, but this random sample was drawn from the same population as our cohort and is likely representative.
Outcomes
The primary outcome was death from any cause during admission. The primary outcome was known for 99.5% of patients; only 23 patients had missing data. Secondary outcomes were mechanical ventilation during admission and intensive care unit (ICU)-level care (defined as admission to an ICU or mechanical ventilation) during admission.
Logistic regression analysis
Univariable and multivariable logistic regression were used to identify factors associated with each outcome. To identify sex-specific effects of risk factors, subgroup analysis was performed using a multivariable model including an interaction term between sex and the subgroup variable. This was performed for each subgroup variable to identify significant sex interactions. We further used sex-stratified multivariable models to estimate the magnitude of effect of each covariate within each sex cohort.
Predictors analyzed included demographic factors, comorbidity status, initial vital signs, treatment facility location (Manhattan vs. Brooklyn/Queens), and time period (prior to April 13, April 14 to June 2, June 2 to August 5). There was minimal clustering of the primary outcome by treatment site (ICC (ρ) = 0.021). Covariates were chosen a priori based on prior reports and low missingness. The covariates included in the final models were age (as a categorical variable), sex, race/ethnicity, treatment location, treatment time period, baseline hypoxia, and the following comorbidities: hypertension, diabetes, coronary artery disease, heart failure, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease (COPD) or asthma, obesity, cancer, chronic liver disease. We report the odds ratios derived from the coefficients of each model, along with the Wald-type confidence interval and p values.
Statistics
Patient characteristics and baseline vitals and labs were described using medians and ranges for continuous variables, and proportions for categorical variables. Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using Fisher’s exact test or the chi-squared test, as appropriate. All statistical analyses and data visualizations were carried out using R 4.0.0 (The R foundation, Vienna, Austria), along with the tidyverse, ggpubr, forestplot, and Hmisc packages. Statistical significance was defined as a two-tailed p < 0.05 unless otherwise specified.
Study approval
The MSDW project (GCO# 12-0361) was reviewed and approved by the Institutional Review Board (IRB) of the Mount Sinai School of Medicine. The MSDW serves as a repository for data from the electronic medical record which can be used for research. The IRB has determined that the MSDW research involves no greater than minimal risk and approved the waiver for informed consent. A de-identified COVID-19 dataset was generated by the MSDW team for use by the Mount Sinai research community. The present study used this de-identified dataset for the rest of the analyses.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
The study cohort comprised 4930 COVID-19 patients hospitalized within the Mount Sinai Health System in New York City, of whom 2757 (55.9%) were men and 2173 (44.1%) were women. The majority (65.4%) of patients were treated in Manhattan facilities, which had larger bed capacities than facilities in Brooklyn or Queens. Most of the patients in the cohort (65.3%) were admitted before April 13, 2020, which corresponded to the peak of COVID-19 admissions within the hospital system.
Demographics and comorbidities
Women in the cohort were older overall. A greater proportion of women were 75 or older (36.6% vs. 26.5%, Fisher’s exact p = 2.8 × 10−14) and the median age among women was 68 (IQR 55–80) compared to 65 (IQR 53–75, Wilcoxon p = 1.1 × 10−10) among men. Women in the cohort were more likely to have hypertension (38% vs. 34.9%, %, Fisher’s exact p = 0.03), COPD/asthma (10.9% vs. 7.2%, %, Fisher’s exact p = 6.6 × 10−6), and to be obese (38.9% vs. 27.9%, %, Fisher’s exact p = 3.9 × 10−16). Men in the cohort were more likely to be a current or former smoker (31.1% vs. 20.6%, %, Fisher’s exact p = 4.2 × 10−24) and to have chronic kidney disease (13.1% vs. 11%, %, Fisher’s exact p = 0.03), cancer (8.1% vs. 6.6%, %, Fisher’s exact p = 0.04), chronic liver disease (3.9% vs. 2.6%, %, Fisher’s exact p = 0.02), and HIV (2.4% vs. 0.9%, %, Fisher’s exact p = 4.6 × 10−5). Additional clinical features are presented in Table 1.
Table 1.
Baseline clinical characteristics, by sex.
| Variable | Male (N = 2757) | Female (N = 2173) | p value | Overall (N = 4930) |
|---|---|---|---|---|
| Age (yrs) | 65 (53–75) | 68 (55–80) | <0.001 | 66 (54–78) |
| Age < 55 | 758 (27.5%) | 527 (24.3%) | 0.01 | 1285 (26.1%) |
| Age 55–64 | 617 (22.4%) | 359 (16.5%) | <0.001 | 976 (19.8%) |
| Age 65-74 | 651 (23.6%) | 491 (22.6%) | 0.4 | 1142 (23.2%) |
| Age ≥ 75 | 731 (26.5%) | 796 (36.6%) | <0.001 | 1527 (31%) |
| Asian | 137 (5%) | 89 (4.1%) | 0.2 | 226 (4.6%) |
| Hispanic | 780 (28.3%) | 613 (28.2%) | 0.9 | 1393 (28.3%) |
| Non-Hispanic Black | 674 (24.4%) | 630 (29%) | <0.001 | 1304 (26.5%) |
| Non-Hispanic White | 637 (23.1%) | 534 (24.6%) | 0.3 | 1171 (23.8%) |
| Other race/ethnicity | 443 (16.1%) | 247 (11.4%) | <0.001 | 690 (14%) |
| Manhattan facility | 1741 (63.1%) | 1481 (68.2%) | <0.001 | 3222 (65.4%) |
| Before April 13 | 1860 (67.5%) | 1358 (62.5%) | <0.001 | 3218 (65.3%) |
| April 13 to June 2 | 782 (28.4%) | 717 (33%) | <0.001 | 1499 (30.4%) |
| June 2 to August 5 | 115 (4.2%) | 98 (4.5%) | 0.6 | 213 (4.3%) |
| Current/former smoker | 857 (31.1%) | 448 (20.6%) | <0.001 | 1305 (26.5%) |
| Body mass index (kg/m2) | 27.0 (23.7–31.1) | 28.4 (24.3–33.9) | <0.001 | 27.5 (23.9–32.3) |
| Hypertension | 961 (34.9%) | 825 (38%) | 0.03 | 1786 (36.2%) |
| Diabetes | 653 (23.7%) | 543 (25%) | 0.3 | 1196 (24.3%) |
| Coronary artery disease | 403 (14.6%) | 282 (13%) | 0.1 | 685 (13.9%) |
| Heart failure | 220 (8%) | 164 (7.5%) | 0.6 | 384 (7.8%) |
| Atrial fibrillation | 222 (8.1%) | 148 (6.8%) | 0.1 | 370 (7.5%) |
| Chronic kidney disease | 360 (13.1%) | 239 (11%) | 0.03 | 599 (12.2%) |
| COPD/asthma | 199 (7.2%) | 237 (10.9%) | <0.001 | 436 (8.8%) |
| Obesity | 770 (27.9%) | 846 (38.9%) | <0.001 | 1616 (32.8%) |
| Cancer | 224 (8.1%) | 143 (6.6%) | 0.04 | 367 (7.4%) |
| Chronic liver disease | 107 (3.9%) | 57 (2.6%) | 0.02 | 164 (3.3%) |
| HIV | 67 (2.4%) | 20 (0.9%) | <0.001 | 87 (1.8%) |
| Initial vital signs | ||||
| Temperature (°F) | 98.7 (98–100) | 98.6 (98–99.8) | 0.001 | 98.6 (98–99.9) |
| Fever | 586 (21.3%) | 391 (18%) | 0.005 | 977 (19.8%) |
| Heart rate (bpm) | 97 (84–111) | 93 (82–108) | <0.001 | 96 (83–110) |
| Tachycardia | 1187 (43.1%) | 787 (36.2%) | <0.001 | 1974 (40%) |
| Systolic blood pressure (mmHg) | 130 (116–147) | 127 (113–143) | <0.001 | 129 (115–145) |
| Hypotension | 92 (3.3%) | 84 (3.9%) | 0.4 | 176 (3.6%) |
| Respiratory rate (bpm) | 20 (18–22) | 20 (18–22) | <0.001 | 20 (18–22) |
| Tachypnea | 422 (15.3%) | 300 (13.8%) | 0.1 | 722 (14.6%) |
| Oxygen saturation (%) | 95 (92–98) | 96 (93–98) | <0.001 | 95 (92–98) |
| Oxygen sat. <92% | 670 (24.3%) | 440 (20.2%) | <0.001 | 1110 (22.5%) |
Fever: temperature ≥100.4 °F; tachycardia: heart rate >100 beats/min; tachypnea: respiratory rate >25 breaths/min; hypotension: systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg.
Vital signs and laboratory values at presentation
At presentation, men were more likely to be febrile (21.3% vs. 18%, Fisher’s exact p = 0.005), tachycardic (43.1% vs. 36.2%, Fisher’s exact p = 1.3 × 10−6), and hypoxic (24.3% vs. 20.2%, Fisher’s exact p = 8.6 × 10−4). Men and women had similar white blood cell counts (7.9 vs. 7.6 K/μL, Wilcoxon p = 0.1), but men had lower lymphocyte counts (0.87 vs. 1.05 K/μL, Wilcoxon p = 1.4 × 10−26). Among inflammatory markers, C-reactive protein was higher in men than women at baseline (125.3 vs. 88.4 mg/L, Wilcoxon p = 3.4 × 10−21), whereas ferritin was more elevated (relative to the upper limit of normal [ULN] for each sex) in women than in men (3.06 vs. 2.35 xULN, Wilcoxon p = 5.4 × 10−8).
A subset of patients had IL-1B, IL-6, IL-8, and TNF-a measured from serum at baseline. Of these cytokines, only IL-6 was significantly different between men and women (78.7 vs. 57.9 pg/mL, Wilcoxon p = 7.2 × 10−5). Additional laboratory values are presented in Supplemental Table 1.
We examined SARS-CoV-2 viral load by sex using a randomly selected cohort of 1146 MSHS patients hospitalized between March 13 and May 4, 2020, previously described by Pujadas et al.27. This cohort consisted of 494 (43.1%) female and 651 (56.9%) male patients, with average log10 viral loads of 5.61 and 5.51 copies/mL, respectively (Welch t-test p = 0.53) (Supplemental Fig. 1).
Treatment and outcomes
The most common treatments administered in the cohort were as follows: hydroxychloroquine (N = 3156, 64%), azithromycin (N = 3070, 62.3%), and steroids (N = 1343, 27.2%) including methylprednisolone (N = 1067, 21.6%), prednisone (N = 386, 7.8%), dexamethasone (N = 164, 3.3%), and hydrocortisone (N = 15, 0.3%) (Supplemental Tables 2 and 3). Overall, men were more likely to receive hydroxychloroquine (67.5% vs. 59.5%, Fisher’s exact p = 7.8 × 10−9), azithromycin (65.5% vs. 58.2%, Fisher’s exact p = 1.9 × 10−7), and steroids (29.1% vs. 24.9%, Fisher’s exact p = 0.001). However, there were no significant differences in the use of medications between men and women requiring ICU-level care (Supplemental Tables 2 and 3).
Treatment patterns in the cohort changed over time. The proportion of patients receiving remdesivir and dexamethasone increased over the time periods of pre-April, April–June, and June–August (remdesivir: 1.6% vs. 4.9% vs. 6.1%, chi-squared p = 1.4 × 10−11; dexamethasone: 2.8% vs. 3.1% vs. 12.7%; chi-squared p = 6.0 × 10−14), whereas the receipt of hydroxychloroquine and azithromycin decreased over the same period (hydroxychloroquine: 78.6% vs. 41.8% vs. 0%; azithromycin: 72.8% vs. 46.4% vs. 15%; chi-squared p < 2.2 × 10−16 for both).
There were 1198 in-hospital deaths (24.3%). The rate of in-hospital mortality was 25.2% in men and 23.1% in women (Fisher’s exact p = 0.09). Among the patients who died, the median time from admission to death was 7.1 days (IQR 3.2–13). There was no significant difference in the median length of time from admission to death between men (7.3 days, IQR 3.3–12.4) and women (6.2 days, IQR 2.9–13.2; Wilcoxon p = 0.24). Among the patients who did not die, the median length of admission was 6 days (IQR 3–11) and men spent longer in the hospital (median 6.2 days, IQR 3.3–10.8) than women (median 5.7 days, IQR 2.8–10.2; Wilcoxon p = 0.004).
1176 patients (23.9%) received ICU-level care during their admissions; 737 patients (14.9%) received invasive ventilation. More men than women received ICU-level care (26.7% vs. 20.3%, Fisher’s exact p = 1.8 × 10−7) and invasive ventilation (16.4% vs. 13.2%, Fisher’s exact p = 0.002). The mortality rate among ICU patients was 55.7%; among intubated patients, it was 68.5%. There were no differences in mortality between men and women among ICU (56.6% vs. 54.1%, Fisher’s exact p = 0.43) or intubated (70.1% vs. 66.1%, Fisher’s exact p = 0.25) patients. Among those who were intubated, the median number of days from admission to intubation was 1.3 (IQR −0.3 to 5.4). There was no significant difference in the median time to intubation between men and women (1.4 vs. 1.3 days, Wilcoxon p = 0.69).
There was no significant difference in the proportion of men and women developing acute kidney injury (7% vs 6%, Fisher’s exact p = 0.2) or venous thromboembolism (1% vs. 0.7%, Fisher’s exact p = 0.3) during their hospitalizations.
Sex-specific predictors of hospitalization outcomes
In multivariable logistic regression models adjusting for demographics, comorbidities, admission time period, and baseline oxygen saturation, male sex was an independent predictor of in-hospital mortality (OR 1.24, 95% CI 1.06–1.44), intubation (OR 1.22, 95% CI 1.03–1.46), and ICU care (OR 1.37, 95% CI 1.19–1.59) (Supplemental Fig. 2 and Supplemental Table 4). Other predictors of mortality included obesity (OR 1.38, 95% CI 1.17–1.62), hypertension (OR 0.81, 95% CI 0.68–0.97), chronic kidney disease (OR 1.16, 95% CI 1.08–1.72), and older age (age ≥ 74 vs. 55–64 OR 3.35, 95% CI 2.71–41.5). Interestingly, while mortality increased with age, the youngest and oldest age groups were associated with less intubation (age < 55 OR 0.61, 95% CI 0.47–0.79; age ≥ 75 OR 1.22, 95% CI 1.03–1.46) and less ICU-level care (age < 55 OR 0.76, 95% CI 0.61–0.94; age ≥ 75 OR 0.65, 95% CI 0.52–0.8), compared to those aged 55–64 (Supplemental Fig. 2).
To compare predictors of mortality, intubation, and ICU care between men and women, we first performed subgroup analysis to identify significant interactions between sex and other covariates. Then, we used sex-stratified regression models to estimate the effect sizes of the individual predictors in each sex.
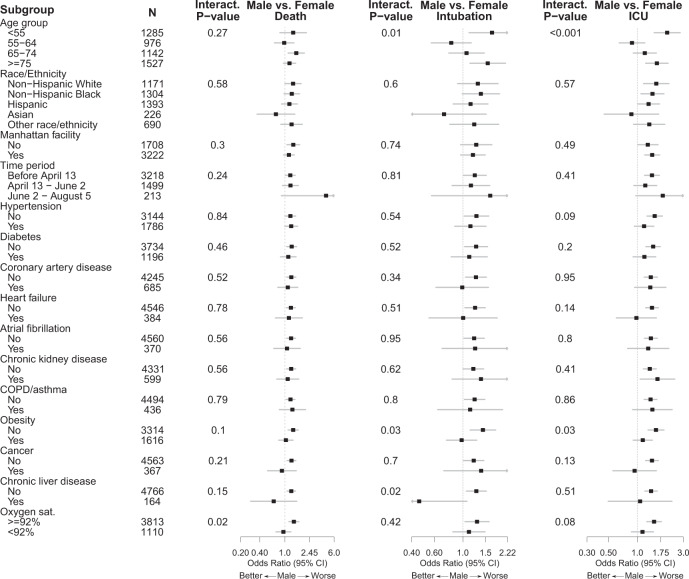
Subgroup analysis for predictors of mortality identified a significant interaction between sex and baseline hypoxia (interaction p = 0.02) (Fig. 1 and Supplemental Table 5). Among predictors of intubation, there were significant sex interactions for age (interaction p = 0.01), obesity (interaction p = 0.03), and chronic liver disease (interaction p = 0.02). Among predictors of ICU-level care, there were significant sex interactions for age (interaction p < 0.001) and obesity (interaction p = 0.02); there were also suggestive but non-significant interactions with hypertension (interaction p = 0.09) and hypoxia (interaction p = 0.08).
Fig. 1. Subgroup analysis for male sex as a predictor of mortality, intubation, or ICU care.
The forest plot depicts the odds ratio associated with male sex (versus female sex) within the subgroup specified in a multivariable logistic regression model, including an interaction term between the subgroup variable and sex and adjusting for the other variables listed. The interaction p value for sex and each subgroup variable is shown.
In sex-stratified models, we observed that while hypoxia was associated with increased mortality in both sexes, the effect size was larger among women (OR 3.67, 95% CI 2.85–4.73) than men (OR 2.44, 95% CI 1.98–3.01) (Fig. 2 and Supplemental Table 6). Similarly, obesity was associated with increased risk of intubation and ICU-level care in both sexes, but had a greater effect on the risk among women (Intubation OR 1.92, 95% CI 1.45–2.54; ICU OR 1.95, 95% CI 1.52–2.49) than men (Intubation OR 1.28, 95% CI 1.02–1.63; ICU OR 1.32, 95% CI 1.08–1.6). Chronic liver disease was associated with a greater risk of intubation in women (OR 2.26, 95% CI 1.15–4.44) than men (OR 0.84, 95% CI 0.47–1.51). Within the youngest (<55) and oldest (≥75) age groups, women had a lower risk of intubation and ICU care than men.
Fig. 2. Forest plots depicting sex-stratified multivariable logistic regression models predicting mortality (Male N = 2657; Female N = 2099), intubation (Male N = 2669; Female N = 2108), or ICU care (Male N = 2669; Female N = 2108).
The odds ratio associated with each variable is depicted in blue for the male-specific models and in red for the female-specific models.
Validation cohort
To assess the stability of our findings, we assembled a validation cohort consisting of 1645 patients admitted with COVID-19 from August 5, 2020, to January 13, 2021. The validation cohort had a similar age (median 66 vs. 66, Fisher’s exact p = 0.3) and sex (56% vs. 55.9% male, Fisher’s exact p = 1) distribution as the primary cohort, but had more White patients (29.4% vs. 24.5%, Fisher’s exact p = 7.3 × 10−29) and had lower rates of comorbidities such as hypertension (30.2% vs. 36.9%, Fisher’s exact p = 6.3 × 10−7), diabetes (19.6% vs. 24.6%, Fisher’s exact p = 2.5 × 10−5), and chronic kidney disease (8.9% vs. 12.6%, Fisher’s exact p = 4.3 × 10−5) (Supplemental Table 7). One notable exception was obesity, which was more common (36.2% vs 32.9%, Fisher’s exact p = 0.02) in the validation cohort.
Validation patients were less likely to be febrile (17.6% vs. 19.8%, Fisher’s exact p = 0.05), tachycardic (31.8% vs. 40.1%, Fisher’s exact p = 9.9 × 10−14), hypotensive (2.4% vs. 3.6%, Fisher’s exact p = 0.03), or hypoxic (13.5% vs. 22.6%, Fisher’s exact p < 0.001) on presentation. Treatment with remdesivir (35% vs. 2.8%, Fisher’s exact p = 4.0 × 10−246) and dexamethasone (65.8% vs. 3.3%, Fisher’s exact p < 0.001) were significantly more common in the validation cohort. Unadjusted outcomes were also better in validation cohort: mortality (10.2% vs. 24.1%, Fisher’s exact p = 1.8 × 10−34), intensive care (19% vs. 23.4%, Fisher’s exact p = 1.7 × 10−4), and intubation (9.4% vs. 15%, Fisher’s exact p = 3.2 × 10−9) (Supplemental Table 7).
Multivariable logistic regression in the validation cohort confirmed male sex as an independent risk factor for in-hospital mortality (OR 1.56, 95% CI 1.05–2.32), intensive care (OR 1.65, 95% CI 1.24–2.2), and intubation (OR 1.72, 95% CI 1.17-2.53) (Supplemental Table 8). Older age (age ≥ 74 vs. 55–64 OR 8.5, 95% CI 4.25–16.9), hypoxia (OR 3.9, 95% CI 2.49–6.1), and chronic kidney disease (OR 2.04, 95% CI 1.13–3.67) were also replicated as independent predictors of mortality. There was no significant interaction between sex and hypoxia with regards to mortality (interaction p = 0.94), but there was an interaction between sex and obesity (interaction p = 0.03) (Supplemental Fig. 3). Obesity had a greater impact on men’s risk of death (OR 1.82, 95% CI 1.04–3.19) than women’s (OR 0.74, 95% CI 0.39–1.42) (Supplemental Table 9).
Discussion
Sex differences in prevalence, pathogenesis, and outcomes have been observed across a variety of infectious diseases27. In general, men are more susceptible to infection while women mount more vigorous immune responses28,29. However, this does not necessarily translate into worse outcomes for men; a 2010 WHO study concluded that influenza outcomes are often worse for women, even though they consistently produce higher antibody titers in response to vaccination30,31. Sex differences in immunity may be related to the “dose” of immune-related genes located on the X chromosome, or sex hormonal effects on immune cells32.
Observational cohort studies have consistently identified a difference in mortality outcomes between men and women infected with SARS-CoV-21–6. An emerging body of literature has characterized differences in cytokines between men and women with COVID-1926,32–34. However, these findings have yet to be translated into effective therapies, indicating that our mechanistic understanding of COVID-19 pathogenesis and sex differences remains limited. To enhance our understanding of the effect of sex on COVID-19 outcomes, we characterized the COVID-19 disease course of hospitalized men and women in a large health-system cohort and used stratified and interaction analyses to identify interactions between sex and other COVID-19 risk factors.
Men in our primary cohort were younger than the women and were less likely to have common comorbidities such as obesity and hypertension, which have been linked to adverse COVID-19 outcomes20,35. Despite this apparently favorable clinical profile, men were more likely to present with signs of severe disease, such as hypoxia and lymphopenia10,36, and were more likely to be intubated (16.4% vs. 13.2%, Fisher’s exact p = 0.002) or receive intensive care (26.7% vs. 20.3%, Fisher’s exact p = 1.8 × 10−7). After adjusting for demographics and comorbidities, male sex was an independent risk factor for mortality, intubation, and intensive care, in accordance with findings of studies from the USA2,5, the UK6, Italy1, and China11. Male sex remained an independent risk factor for adverse outcomes in a validation cohort comprised of patients admitted to our health system at a later phase of the pandemic.
Given this persistent male–female disparity, we reasoned that there might be clinical factors, which preferentially affected men’s or women’s outcomes. By testing interaction terms and using sex-stratified models, we identified hypoxia and obesity as risk factors, which preferentially increased women’s risk of mortality and intubation/intensive care, respectively, in the primary cohort. However, in the validation cohort, the sex specificity of hypoxia did not replicate, while the sex specificity of obesity was reversed.
In the primary cohort, men were more likely to present with hypoxia (24.3% vs. 20.2%, Fisher’s exact p = 8.6 × 10−4), but the presence of hypoxia only increased men’s risk of death by 2.44 times, compared to an increase of 3.67 times for women (interaction p = 0.02). In the validation cohort, hypoxia remained an independent predictor of mortality (OR 3.9, 95% CI 2.49–6.1), but there was no significant interaction between sex and hypoxia (interaction p = 0.94).
In a recent study of 6916 patients in Southern California, Tartof et al. reported an interaction between BMI and sex; increasing BMI had a greater impact on men’s mortality risk than women’s35. By contrast, we initially found that obesity preferentially increased women’s risk of intubation and intensive care while there was no interaction between sex and obesity with regards to in-hospital mortality. However, the validation cohort showed a significant interaction between sex and obesity (interaction p = 0.03) with higher mortality for obese men, in line with the findings of Tartof et al.
There were also significant interactions in the primary analysis between chronic liver disease and sex with regards to intubation, and between age and sex for both intubation and intensive care. Liver disease had a greater impact on women’s risk of intubation than men; however, the number of patients with chronic liver disease in the primary cohort was small (N = 164), and this finding requires further evaluation. The interaction analysis for sex and age revealed that men in the youngest and oldest age groups were more likely than women to be intubated or receive intensive care (Fig. 1). These interactions were not significant in the validation cohort, but power was limited due to the reduced rate of intensive care intubation during the validation period.
Differences in the conditions of the pandemic and patient population may account for the differences in sex-specific risk factors between our primary and validation cohorts. The primary cohort consisted of patients admitted during the initial pandemic surge; there were no proven COVID-19 therapies at that time, and hospital capacity was severely taxed. The validation cohort reflects a later period of the pandemic, after the establishment of evidence-based treatment protocols and without the same strains on bed capacity37–40. The use of treatments such as remdesivir and dexamethasone was significantly more common in the validation cohort, and outcomes were correspondingly better. The comorbidity profile of patients in the validation cohort was also different. Consequently, it is challenging to compare results from these two cohorts. Nevertheless, the persistent association of male sex with worse outcomes is notable. Sex-specific interactions noted in either cohort should be taken in context.
The strengths of our study include its size, the diversity of the patient population, and two cohorts encompassing the evolution of the pandemic in New York City from initial surge to implementation of evidence-based treatments. Although larger, national-level patient cohorts have been published6,41, this Mount Sinai Health System cohort is on par with other health-system and hospital-network-based cohorts from the US and Italy1–3,5,8. The detailed clinical information in this cohort empowers us to explicitly address sex differences in mortality risk factors using a rigorous combination of stratification and interaction models. To our knowledge, we are the first to report comprehensive sex-stratified data on clinical features, risk factors, and their association with outcomes.
This study has several limitations. Our dataset did not capture socioeconomic factors nor pre-admission clinical factors such as duration of symptoms; we cannot rule out the possibility that sex differences in seeking or accessing care may have confounded the outcomes. Comorbidity data were extracted from the electronic medical record and were not manually reviewed; their completeness and accuracy depend on data entered by medical providers during routine clinical care. In addition, due to limited exchange of medical data between institutions, comorbidity data may be incomplete for some patients who receive care from multiple health systems. Our results should be considered hypothesis-generating and require confirmation in other large cohorts as well as causal studies to establish mechanisms.
In conclusion, this study provides a detailed characterization of the clinical features and outcomes of men and women hospitalized with COVID-19. It highlights interactions between sex, obesity, and hypoxia with regards to mortality, intubation, and intensive care. These interactions nominate patient subgroups for further study and provide insights that may help explain the sex differences in outcomes of this disease.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We dedicate this work to the frontline health workers and staff of the Mount Sinai Healthcare System. This work was supported in part through the Mount Sinai Data Warehouse (MSDW) resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Author contributions
T.J. and K.H. conceived the idea and designed the study. S.N., P.K., E.P., and C.C.C. collected the data. T.J., K.H., T.W., N.S., and E.P. conducted the statistical analysis. All authors interpreted the results. T.J. drafted the initial manuscript and K.H. edited the manuscript. All authors revised and approved the manuscript.
Data availability
Data underlying Figs. 1 and 2 are available in Supplementary Data 1 and 2. Other de-identified data is available in accordance with Mount Sinai policy. Requests will be assessed by the Icahn School of Medicine Institutional Data Access Committee (contact via Joy.Dicker@mssm.edu).
Code availability
R code associated with these analyses is available at https://github.com/Huang-lab/SParCS/tree/master/MSSM_COVID/R.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-021-00006-2.
References
- 1.Grasselli, G. et al. Risk factors associated with mortality among patients with COVID-19 in Intensive care units in Lombardy, Italy. JAMA Intern. Med.10.1001/jamainternmed.2020.3539 (2020). [DOI] [PMC free article] [PubMed]
- 2.Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ369. 10.1136/bmj.m1966 (2020). [DOI] [PMC free article] [PubMed]
- 3.Gupta, S. et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med.10.1001/jamainternmed.2020.3596 (2020). [DOI] [PMC free article] [PubMed]
- 4.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. CCDCW. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 5.Lewnard, J. A. et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ369. 10.1136/bmj.m1923 (2020). [DOI] [PMC free article] [PubMed]
- 6.Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peckham H, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 10.1001/jama.2020.6775 (2020). [DOI] [PMC free article] [PubMed]
- 9.O’Brien J, Du KY, Peng C. Incidence, clinical features, and outcomes of COVID-19 in Canada: impact of sex and age. J. Ovarian Res. 2020;13:137. doi: 10.1186/s13048-020-00734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli, G. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 10.1001/jama.2020.5394 (2020). [DOI] [PMC free article] [PubMed]
- 11.Li, X. et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 10.1016/j.jaci.2020.04.006 (2020). [DOI] [PMC free article] [PubMed]
- 12.Galbadage, T. et al. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. https://www.medrxiv.org/content/10.1101/2020.05.11.20098673v1 (2020). [DOI] [PMC free article] [PubMed]
- 13.Wang D, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Critical Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palaiodimos L, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metab. Clin. Exp. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Vignera, S. et al. Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int. J. Mol. Sci. 21. 10.3390/ijms21082948 (2020). [DOI] [PMC free article] [PubMed]
- 18.Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID-19 pandemic. JACC Case Rep. 2020;2:1407–1410. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maleki Dana P, et al. An insight into the sex differences in COVID-19 patients: what are the possible causes? Prehosp. Disaster Med. 2020;35:438–441. doi: 10.1017/S1049023X20000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan, W.-J. et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J.10.1183/13993003.00547-2020 (2020). [DOI] [PMC free article] [PubMed]
- 21.Du, R.-H. et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J.10.1183/13993003.00524-2020 (2020). [DOI] [PMC free article] [PubMed]
- 22.Montopoli, M. et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n = 4532). Ann. Oncol.0. 10.1016/j.annonc.2020.04.479 (2020). [DOI] [PMC free article] [PubMed]
- 23.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11:1–13. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charney AW, et al. Sampling the host response to SARS-CoV-2 in hospitals under siege. Nat. Med. 2020;26:1157–1158. doi: 10.1038/s41591-020-1004-3. [DOI] [PubMed] [Google Scholar]
- 26.Del Valle DM, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pujadas E, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020;8:e70. doi: 10.1016/S2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steeg LGvom, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathogens. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 30.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J. Leukoc. Biol. 2012;92:67–73. doi: 10.1189/jlb.0811427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. WHO|Sex, gender and influenza. WHO. https://www.who.int/gender-equity-rights/knowledge/9789241500111/en/ (2021).
- 33.Takahashi T, Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science, 370, 10.1126/science.abd4585 (2020). [DOI] [PMC free article] [PubMed]
- 36.Tartof, S. Y. et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 10.7326/M20-3742 (2020). [DOI] [PMC free article] [PubMed]
- 37.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beigel JH, et al. Remdesivir for the treatment of covid-19—preliminary report. N. Engl. J. Med. 2020;0:null. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 39.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19 — preliminary report. N. Engl. J. Med. 2020;0:null. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karaca-Mandic, P., Sen, S., Georgiou, A., Zhu, Y., Basu, A. Association of COVID-19-related hospital use and overall COVID-19 mortality in the USA. J. Gen. Intern. Med. 10.1007/s11606-020-06084-7 (2020). [DOI] [PMC free article] [PubMed]
- 41.Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ369, 10.1136/bmj.m1985 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Data underlying Figs. 1 and 2 are available in Supplementary Data 1 and 2. Other de-identified data is available in accordance with Mount Sinai policy. Requests will be assessed by the Icahn School of Medicine Institutional Data Access Committee (contact via Joy.Dicker@mssm.edu).
R code associated with these analyses is available at https://github.com/Huang-lab/SParCS/tree/master/MSSM_COVID/R.