Abstract
Mycobacterial granuloma formation involves significant stromal remodeling including the growth of leaky, granuloma-associated vasculature. These permeable blood vessels aid mycobacterial growth, as antiangiogenic or vascular normalizing therapies are beneficial host-directed therapies in preclinical models of tuberculosis across host-mycobacterial pairings. Using the zebrafish–Mycobacterium marinum infection model, we demonstrate that vascular normalization by inhibition of vascular endothelial protein tyrosine phosphatase (VE-PTP) decreases granuloma hypoxia, the opposite effect of hypoxia-inducing antiangiogenic therapy. Inhibition of VE-PTP decreased neutrophil recruitment to granulomas in adult and larval zebrafish, and decreased the proportion of neutrophils that extravasated distal to granulomas. Furthermore, VE-PTP inhibition increased the accumulation of T cells at M. marinum granulomas. Our study provides evidence that, similar to the effect in solid tumors, vascular normalization during mycobacterial infection increases the T cell:neutrophil ratio in lesions which may be correlates of protective immunity.
Keywords: zebrafish, mycobacteria, vascular permeability, T cell, neutrophil
Reducing the leakiness of blood vessels improves the immune response to mycobacterial infection by increasing T cell and decreasing neutrophil recruitment.
Introduction
Mycobacteria induce changes in host vasculature, including angiogenesis and vascular permeability, through the production of a modified form of trehalose dimycolate (Datta et al. 2015a, Harding et al. 2015,2019, Polena et al. 2016, Oehlers et al. 2017, Walton et al. 2018, Oehlers 2019, Hortle and Oehlers 2020). Trehalose dimycolate triggers the expression of vascular endothelial growth factor (VEGF) by host cells, including macrophages (Walton et al. 2018). The VEGF ligand signals through endothelial cell vascular endothelial growth factor receptor (VEGFR) to stimulate angiogenesis and increase vascular permeability (Datta et al. 2015b, Oehlers et al. 2015, Walton et al. 2018). These vascular pathologies can be targeted by either antiangiogenic therapies, which prevent the growth of blood vessels or cause the regression of ectopic vascular beds, or by vascular normalization therapies, which reduce vascular permeability. Antiangiogenic host-directed therapies control mycobacterial growth by preventing the delivery of oxygen to granulomas and increasing granuloma hypoxia (Oehlers et al. 2015, Oehlers 2019). The Angiopoietin/Tie2 signaling pathway is a second vasoactive pathway that controls mycobacterial infection-induced vascular permeability (Oehlers et al. 2017). Expression of the Angiopoietin 2 (ANG-2) ligand is upregulated in mycobacterial infection, allowing it to bind to and activate the TIE2 receptor. Increased ANG-2/TIE2 signaling antagonizes the stabilizing effects of ANG-1/TIE2 binding through Vascular endothelial protein tyrosine phosphatase (VE-PTP)-mediated deactivation of TIE2, resulting in increased vascular permeability (Goel et al. 2013, Shen et al. 2014, Gurnik et al. 2016). Pharmacological inhibition of VE-PTP to reduce infection-induced vascular permeability results in reduced mycobacterial infection in the zebrafish model (Oehlers et al. 2017). However, the mechanism connecting vascular integrity and the outcome of mycobacterial infection is unknown.
The reduction in permeability of ectopic or lesion-associated vasculature has been mainly studied in relation to the normalization of tumor vasculature in the chemotherapy and immuno-oncology fields. Experiments in murine cancer models have shown that normalization of blood vessel structure and function within solid tumors is associated with improved antitumor immunity (Hamzah et al. 2008, Huang et al. 2012, Zhao et al. 2017). Vascular normalization increases the ratio of T cell to neutrophil infiltration into tumors, which is indicative of an improved prognosis and results in favorable responses to immunotherapy. Due to the morphological similarities of mycobacterial granulomas and tumors, most importantly their tendency to develop hypoxia, we hypothesize that a similar mechanism of action may explain the protective effect of vascular normalization during mycobacterial infection (Datta et al. 2015a, Oehlers et al. 2015, 2017, Oehlers 2019, Hortle and Oehlers 2020).
The zebrafish–Mycobacteriummarinum infection system is an ideal model to investigate the relationship between changes to the granuloma stroma and the host immune response; granulomas reminiscent of human–Mycobacterium tuberculosis granulomas readily form in a system, i.e. both genetically and pharmacologically tractable for in vivo studies (Ramakrishnan 2020). The adult zebrafish allows the study of innate and adaptive immune cells, while the live imaging capability of zebrafish embryos and larvae allows visualization of innate immune cell behavior in intact animals. The zebrafish has been particularly important in the study of granuloma-associated vascular pathologies that impact the outcome of infection including angiogenesis, vascular permeability, and hemostasis (Oehlers et al. 2015, 2017, Hortle et al. 2019, Hortle and Oehlers 2020).
In this study, the zebrafish–M. marinum infection model was used to interrogate the effect of vascular normalization on immunity to mycobacterial infections using the previously characterized VE-PTP inhibitor AKB-9785 to normalize the vasculature.
Methods
Zebrafish handling
Adult zebrafish were held at the Garvan Institute of Medical Research Biological Testing Facility (St Vincent's Hospital AEC Approval 1511), and the Centenary Institute (Sydney Local Health District AWC Approval 17–036). Experiments on adult zebrafish were carried out in accordance with Sydney Local Health District AWC Approval 16–037.
Transgenic strains used were: TgBAC(lck:gfp)vcc6 (Sugimoto et al. 2017), Tg(lyzC:dsred)nz50 and Tg(lyzC:gfp)nz117 (Hall et al. 2007), and Tg(kdrl:egfp)s843 (Jin et al. 2005).
Embryos were reared in E3 media at 28°C and supplemented with phenylthiourea (Sigma-Aldrich) to inhibit pigmentation from 1 day postfertilization (dpf).
Experimental infection of adults
Adult zebrafish aged older than 3 months post fertilization were infected with ∼200 CFU M. marinum M strain by intraperitoneal injection as previously described (Cheng et al. 2020). Briefly, adult fish were anaesthetized in tricaine and a 15 µl volume of bacterial inoculum was injected into the anaesthetized adult fish via intraperitoneal injection with a 28 G needle and syringe. Injected fish were recovered in 1 g/l salt water or aquarium system water. Infected fish were housed in a 28°C incubator with 14 h light:10 h dark cycle, and were fed and monitored daily. Adult infected fish were euthanized by tricaine overdose at timepoints indicated for the collection of histology specimens.
Drug treatment
Adult zebrafish were immersed in 50 µM AKB-9785 (Aerpio Therapeutics) dissolved in dimethyl sulfoxide (DMSO) or the equivalent volume of DMSO vehicle control at time points indicated to a final concentration of 0.1% DMSO. Embryos were immersed in 12.5 µM AKB-9785 to a final concentration of 0.025% DMSO. Drug was refreshed every second day for adults and embryos.
Hypoxyprobe staining
Hypoxyprobe staining was carried out as previously described (Oehlers et al. 2015, Cheng et al. 2020). Briefly, adult zebrafish were injected with 15 μl of a 10 mg/ml pimonidazole solution (HP7; Hypoxyprobe) every 2 days from 7 days postinfection (dpi) to 13 dpi and then euthanized for histology at 14 dpi.
Histology
Euthanized adult fish were fixed in 10% neutral buffered formaldehyde (NBF) for 1–2 days at 4°C. Fixed specimens were washed in PBS and processed for cryosectioning in OCT as previously described (Cheng et al. 2020). Frozen specimens were sectioned at 20 µm with a Leica Cryostat.
Hypoxyprobe was detected by staining with 4.3.1.3 mouse Dylight 549-MAb (HP7; Hypoxyprobe) or with unconjugated 4.3.1.3 mouse MAb and secondary detection with goat antimouse Alexa-Fluor 647 (A-21 235; Life Technologies). Immunofluorescence was used to boost GFP signal in TgBAC(lck:gfp)vcc6 specimens with a chicken anti-GFP primary (Abcam: ab13970) and goat anti-Chicken IgY (H + L), Alexa Fluor® 488 secondary (Abcam: ab150169).
Imaging
Histological sections of adult zebrafish were counterstained with DAPI and imaged with a Leica DM6000B. Static live imaging of zebrafish embryos was carried out on a Leica M205FA fluorescent stereo microscope. Time lapse imaging of zebrafish embryos was carried out using a Deltavision Elite High-resolution microscope.
Image analysis was carried out by fluorescent pixel count using ImageJ where appropriate (Matty et al. 2016, Cheng et al. 2020). Tracking of neutrophil extravasation and migration was carried out manually in ImageJ.
Infection of embryos
Microinjection was carried out as previously described (Takaki et al. 2013, Oehlers et al. 2015, 2017). Briefly, approximately 200 CFU of M. marinum M strain was microinjected into the neural tube or ‘trunk’ of 2 dpf embryos.
Tail wound neutrophil recruitment assay
Zebrafish embryos were anaesthetized, and tails were amputated using a surgical scalpel to induce a sterile wound. Embryos were immediately recovered into media containing AKB-9785 or DMSO and imaged at 6 h postwounding.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9. Statistical tests were carried out as indicated, the error bars represent standard deviation and P-values are supplied in the figures.
Results
Treatment with AKB-9785 reduces M. marinum burden as measured by histology
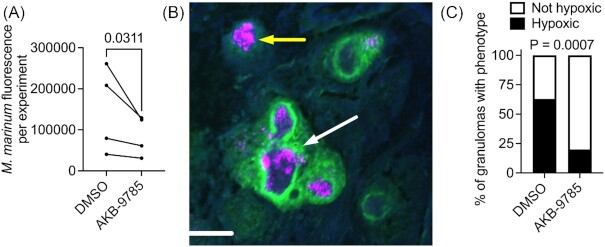
We first sought to validate our previously published report that AKB-9785 treatment of an existing M. marinum infection reduced the culturable bacterial burden using histological methods (Oehlers et al. 2017). We infected adult zebrafish with approximately 200 fluorescent M. marinum by intraperitoneal injection. Infection was allowed to proceed for 1 week before animals were randomly split into DMSO vehicle control and AKB-9785 treatment groups, where they were treated for another week before sacrifice. Consistent with our previously published CFU recovery assay data (Oehlers et al. 2017), analysis of total fluorescent pixel counts from census cryosections of adult zebrafish revealed reduced M. marinum burden (Fig. 1A).
Figure 1.
Vascular normalization reduces M. marinum granuloma hypoxia in adult zebrafish. (A) Quantification of average bacterial burden by histological fluorescent pixel count across four paired experiments with at least three animals. Each data point represents the average bacterial fluorescent pixel count for all animals within an experimental replicate. Statistical testing by ratio paired T-test. (B) Representative image of a cluster of hypoxyprobe-positive necrotic granulomas from the testes a 2 wpi M. marinum-infected zebrafish. M. marinum are magenta, hypoxyprobe staining is green, and DAPI staining is blue. White arrow indicates a hypoxyprobe positive granuloma and yellow arrow indicates a hypoxyprobe negative granuloma. Scale bar represents 100 µm. (C) Quantification of granuloma hypoxia by hypoxyprobe staining. Total granulomas analyzed DMSO = 27 and AKB-9785 = 35; total animals analyzed: DMSO = 3 and AKB-9785 = 3. Statistical testing by Fisher's exact test on raw counts.
Treatment with AKB-9785 reduces granuloma hypoxia
Vascular normalization by VEGF blockade has been demonstrated to reduce M. tuberculosis granuloma hypoxia in rabbits (Datta et al. 2015b). We performed hypoxyprobe staining of adult zebrafish that had been soaked in DMSO and AKB-9785 from 1 to 2 wpi to quantify the proportion of hypoxic granulomas in our system (Fig. 1B). Treatment with AKB-9785 reduced the proportion of hypoxic granulomas (Fig. 1C), confirming the fidelity of our model.
Treatment with AKB-9785 reduces neutrophil recruitment to M. marinum granulomas in adult zebrafish
As vascular normalization by modulation of endothelial junctional permeability had been reported to reduce the recruitment of neutrophils to solid tumors and neutrophil influx has been correlated with progressive histopathology in human TB (Hamzah et al. 2008, Huang et al. 2012, Zhao et al. 2017), we hypothesized that treatment with AKB-9785 would also reduce neutrophil recruitment to M. marinum granulomas in zebrafish.
Adult Tg(lyzC:dsred)nz50 zebrafish, where neutrophils are labeled by DsRed expression (Hall et al. 2007), were infected with M. marinum were treated with AKB-9785 from 1 to 2 wpi and cryosectioned to quantify the response of neutrophil recruitment to vascular normalization (Fig. 2A). Treatment with AKB-9785 increased the area of bacterial fluorescence per granuloma by a small but statistically significant margin (Fig. 2B), decreased neutrophil recruitment to granulomas by approximately 30% (Fig. 2C), resulting in a reduced neutrophil to bacteria ratio per granuloma (Fig. 2D).
Figure 2.
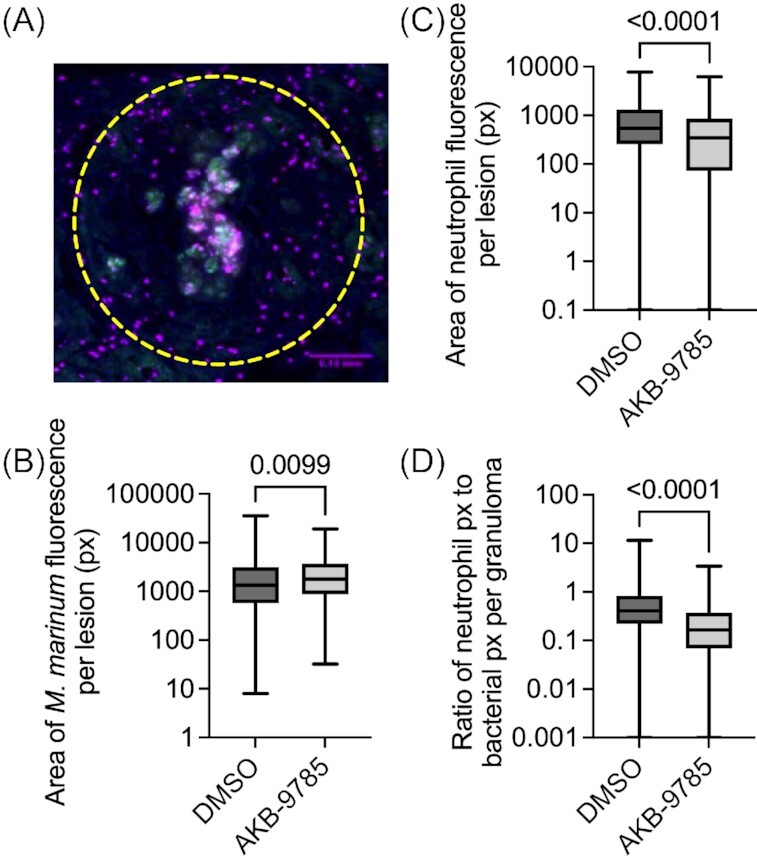
Vascular normalization reduces neutrophil recruitment to M. marinum infection in adult zebrafish. (A) Representative image of a DAPI-stained (blue) necrotic granuloma from a 2 wpi Tg(lyzC:dsred)nz50 (magenta) adult zebrafish infected with M. marinum-wasabi (green). Yellow dashed region indicates area measured for quantification. Scale bar represents 100 µm. (B) Quantification of granuloma bacterial load by fluorescent pixel count. Total granulomas analyzed DMSO = 324 and AKB-9785 = 187; total animals analyzed: DMSO = 4 and AKB-9785 = 4. (C) Quantification of neutrophil recruitment to granulomas by fluorescent pixel count. (D) Calculation of neutrophil to bacterial fluorescent pixel count ratio. Statistical tests for (B), (C), and (D) were performed using Mann–Whitney U-test. Data are representative of two experimental replicates with similar numbers of animals.
Treatment with AKB-9785 reduces neutrophil recruitment to M. marinum granulomas in zebrafish embryos
To further investigate the relationship between AKB-9785 treatment-induced vascular normalization and neutrophil recruitment we sought to image neutrophil extravasation live in optically transparent zebrafish embryos at 7 dpi (Fig. 3A). Treatment with AKB-9785 did not significantly affect the overall bacterial burden or neutrophil recruitment across all analyzed granulomas (Fig. 3B and C). However, AKB-9785 treatment did significantly reduce the neutrophil to bacteria ratio per granuloma, suggesting that vascular normalization reduced neutrophil recruitment to granulomas in zebrafish embryos (Fig. 3D).
Figure 3.
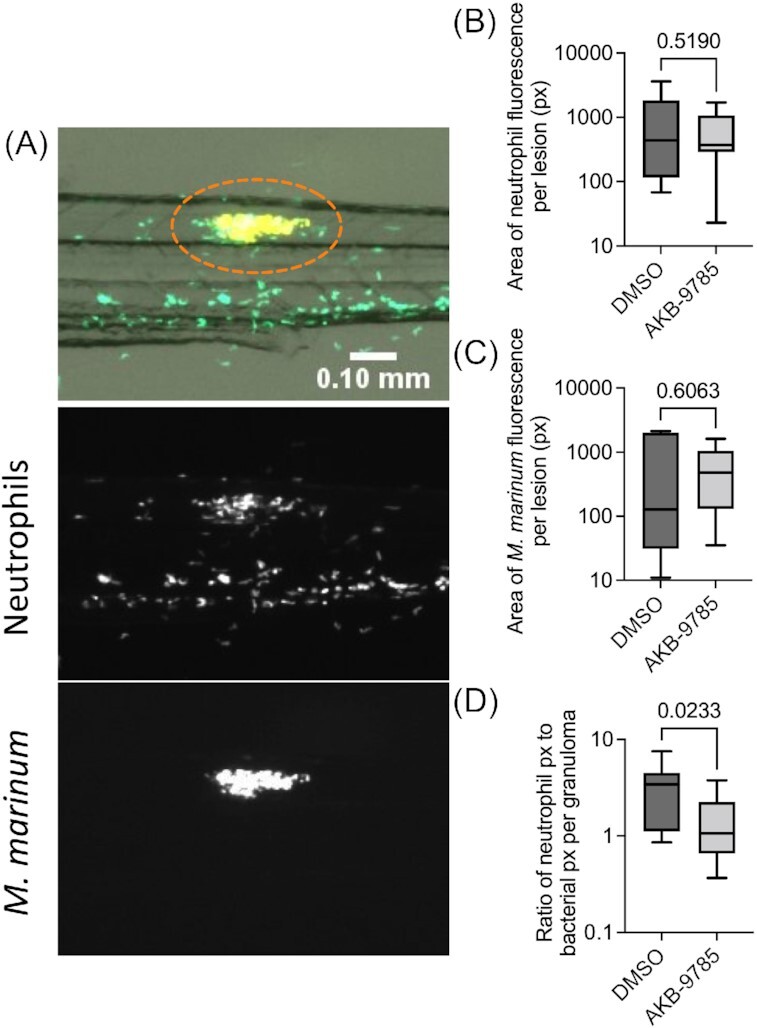
Vascular normalization reduces neutrophil recruitment to M. marinum infection in zebrafish embryos. (A) Representative image of 7 dpi M. marinum–TdTomato (appears yellow due in overlay) infected Tg(lyzC:gfp)nz117 zebrafish embryo with pseudo coloring of neutrophils green. Orange dotted lines in overlay image outline the area quantified. Scale bar represents 100 µm. (B) Quantification of granuloma bacterial load by fluorescent pixel count. Total granulomas analyzed DMSO = 12 and AKB-9785 = 13; total animals analyzed: DMSO = 5 and AKB-9785 = 5. (C) Quantification of neutrophil recruitment to granulomas by fluorescent pixel count. (D) Calculation of neutrophil to bacterial fluorescent pixel count ratio. Statistical tests for (B), (C), and (D) were performed using Mann–Whitney U-test. Data are representative of two experimental replicates with similar numbers of animals.
Treatment with AKB-9785 focuses the local extravasation of neutrophils
We next used live imaging to investigate if vascular normalization affected the extravasation of granuloma-associated neutrophils (Fig. 4A). Neutrophil extravasation was scored as either proximal, occurring within two intersegmental vessels of the granuloma, or distal, occurring more than two intersegmental vessels away from the granuloma. Treatment with AKB-9785 increased the proportion of neutrophils recruited from proximal blood vessels (Fig. 4B). Interestingly, AKB-9785 treatment reduced the point-to-point velocity of neutrophils that extravasated from proximal blood vessels, but no change was seen in neutrophils that extravasated from distal blood vessels (Fig. 4C).
Figure 4.
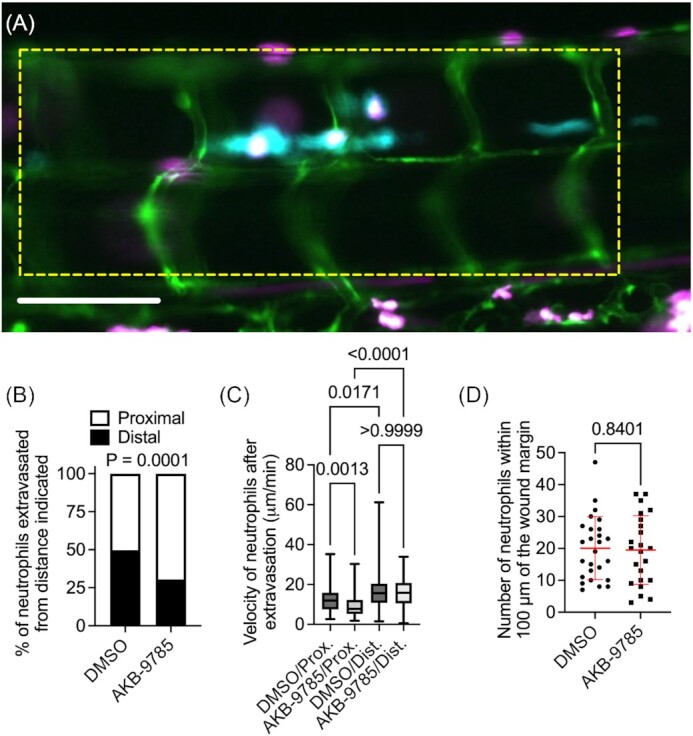
Vascular normalization focuses neutrophil extravasation to blood vessels near granulomas in zebrafish embryos. (A) Representative image of a granuloma from a 4 dpi M. marinum-Cerulean (blue) infected Tg(Tg(kdrl:egfp, lyzC:dsred) s843, nz50 zebrafish embryo with pseudo coloring of blood vessels green and neutrophils magenta. Yellow dotted box outlines the two intersegmental vessel bounds around the central granulomas used to define proximal extravasation events. Scale bar represents 100 µm. (B) Quantification of neutrophil extravasation event source for neutrophils recruited to M. marinum granulomas, proximal defined as within 2 intersegmental vessels. Total number of events measured: DMSO = 153, AKB-9785 = 262; total embryos imaged: DMSO = 8, AKB-9785 = 12. (C) Quantification of neutrophil straight-line velocity after extravasation until neutrophil reaches M. marinum granuloma. (D) Counts of neutrophils within 100 µm of the tail amputation margin at 6 h postwounding. Each data point represents a single animal. Statistical test for (B) was performed by Fisher's exact test on raw counts, statistical test for (C) was performed by ANOVA with Dunn's multiple comparisons test, and statistical test for (D) was performed using unpaired T-test. (D) data are representative of two experimental replicates with similar numbers of animals.
To investigate if treatment with AKB-9785 had a direct effect on neutrophil recruitment, we performed tail wound experiment to quantify neutrophil recruitment in a system with direct interstitial neutrophil recruitment. No significant differences were observed between DMSO and AKB-9785 soaked animals (Fig. 4D), suggesting that AKB-9785 acts to limit neutrophil recruitment only in the context of infection-induced vascular permeability.
Treatment with AKB-9785 increases T cell recruitment to M. marinum granulomas
The adult zebrafish contains an adaptive immune system with many similarities to that of mammals, making it an ideal animal model for the study of the adaptive immune response to natural mycobacterial infection. To study the effect of vascular normalization on T cell infiltration of M. marinum granulomas, we infected TgBAC(lck:gfp)vcc4 zebrafish, where T cells are labeled with GFP expression with M. marinum–TdTomato and treated with AKB-9785 from 1 to 2 wpi followed up cryosectioning to quantify the response of T cells to vascular normalization (Fig. 5A).
Figure 5.
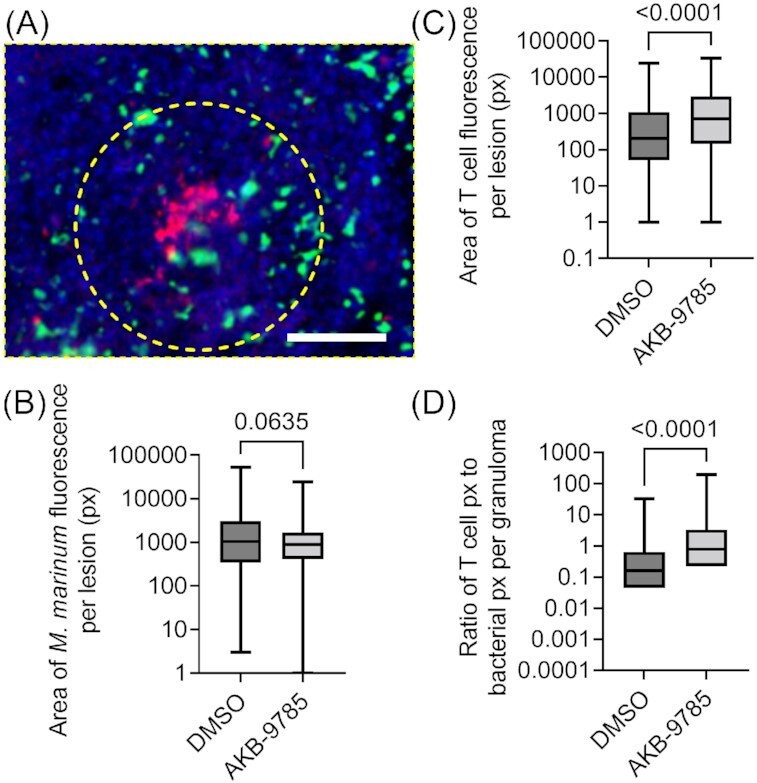
Vascular normalization increases T cell recruitment to M. marinum infection in adult zebrafish. (A) Representative image of a cellular granuloma from a 2 wpi TgBAC(lck:gfp)vcc6 adult zebrafish infected with M. marinum–Katushka. Yellow dashed region indicates area measured for quantification. Scale bar represents 100 µm. (B) Quantification of granuloma bacterial load by fluorescent pixel count. Total granulomas analyzed DMSO = 324 and AKB-9785 = 187; total animals analyzed: DMSO = 3 and AKB-9785 = 3. (C) Quantification of T cell recruitment to granulomas by fluorescent pixel count. (D) Calculation of T cell to bacterial fluorescent pixel count ratio. Statistical tests for (B), (C), and (D) were performed using Mann–Whitney U-test. Data are representative of three experimental replicates with similar numbers of animals.
Treatment with AKB-9785 did not affect the area of bacterial fluorescence per granuloma (Fig. 5B), but increased T cell recruitment to granulomas (Fig. 5C), resulting in an increased T cell to bacteria ratio per granuloma (Fig. 5D).
Discussion
Our studies demonstrate that vascular permeability alters neutrophil and T cell recruitment to mycobacterial granulomas, suggesting that infection-induced vascular permeability may facilitate unfavorable neutrophilic inflammation while preventing effective T cell recruitment. Furthermore, our observation that vascular normalization reduced granuloma hypoxia in our model fits with existing literature that neutrophils are retained in hypoxic lesions while T cells are repelled (Elks et al. 2011, 2013, McNamee et al. 2013, Oehlers 2019).
The importance of the zebrafish adaptive immune system has been previously established using rag knockout animals, which rapidly succumb to M. marinum infection (Swaim et al. 2006). Here, we have demonstrated the converse: that increasing T cell recruitment to M. marinum granulomas correlates with protection from infection. Our findings suggest that there is suboptimal T cell recruitment in natural M. marinum infection of zebrafish due to infection-induced vascular permeability and the induction of granuloma hypoxia.
While intensive infiltration of neutrophils is a negative prognostic marker in chronic TB, there is evidence from zebrafish models that neutrophils are beneficial early and when granuloma macrophage epithelization is blocked (Elks et al. 2013, Cronan et al. 2016). Thus, it is not clear why reducing the number of neutrophils recruited to granulomas is beneficial in the embryo, which lacks T cells to explain the responsiveness to vascular normalization therapy. While it is possible that the effect seen in embryos is due to another aspect of vascular normalization, we hypothesize that vascular normalization minimizes the interstitial tissue ‘commuting’ distance of extravasated neutrophils by reducing the distal extravasation of neutrophils, thereby reducing the amount of neutrophil-generated immunopathology caused by distally extravasated neutrophils migrating to granulomas (Lowe et al. 2012, Ong et al. 2018).
In addition to its effect on cellular immune responses, abnormal and dysfunctional vasculature in granuloma and tumor environments can also impair the delivery of antibiotics and this can greatly impair the effectiveness of antibiotic or anticancer therapy (Datta et al. 2015a). A study by Xu et al. (2018) demonstrated that inhibition of matrix metalloproteinase (MMP) increased pericyte coverage in blood vessels, stabilizing the integrity of M. tuberculosis infected mouse lung tissue, which in turn enhanced the in vivo potency of frontline tuberculosis drugs such as isoniazid and rifampicin. Vascular normalization is, thus more favorable as an adjunctive therapy for antibiotic-sensitive infection than antiangiogenic therapies, which may increase the exclusion of antibiotics from granulomas.
Neutrophil tail wound and migration velocity after extravasation from distal blood vessels were the same after AKB-9785 treatment, suggesting AKB-9785 does not act directly on neutrophil motility to limit their infiltration into M. marinum granulomas. These negative results remain consistent with a role for AKB-9785 acting at the gatekeeper step of reducing vascular permeability to limit the egress of neutrophils, as neutrophils mainly migrate to a tail wound via abluminal surfaces in zebrafish embryos and the infection-induced vascular permeability pathology is only induced proximal to granulomas (Oehlers et al. 2017). We did observe a decrease in the velocity of proximally extravasated neutrophils in AKB-9785 treated embryos suggesting a factor local to the site of granulomas could also have been altered and responsible for the reduced ‘urgency’ of neutrophil recruitment. This leads us to the hypothesis that AKB-9785 may reduce hypoxia within embryonic granulomas rendering them less ‘attractive’ to neutrophils who are activated by hypoxic conditions (Elks et al. 2011, 2013). While expression of the AKB-9785 target VE-PTP is considered endothelial-specific, the report that VEGF directly modulates the recruitment of immune cells in mouse mycobacterial infection models leaves the possibility that AKB-9785 may indirectly modulate immune cell recruitment through downstream cytokines (Harding et al. 2019).
We had previously reported AKB-9785 treatment reduced M. marinum CFU recovered from whole animal homogenates (Oehlers et al. 2017), and in this study we observed reduced overall bacterial fluorescence from our census histology estimation of bacterial burden. However, there was significant variability in the effect of AKB-9785 on the bacterial burden in individual granulomas across experiments in adult fish. We are confident that AKB-9785 treatment was responsible for the effects on leukocyte recruitment rather than the leukocyte recruitment differences being an artefact of increased or decreased M. marinum burden as AKB-9785 was able to uncouple the positive bacterial burden–leukocyte recruitment correlation (Cheng et al. 2020). This may have had the effect of increasing the magnitude of our leukocyte pixel per bacterial pixel ratio because the bacterial burden denominator was increased in the neutrophil experiment and decreased in the T cell experiment, however our raw leukocyte pixel counts were significantly different before transformation into the ratio.
Overall, this study provides evidence for the use of vascular normalization therapy to enhance a more effective immune response in mycobacterial infections. An increased ratio of T cell to neutrophil infiltration is frequently a favorable prognostic marker both in human infections, such as tuberculosis, and in cancer. Neutrophilic inflammation and lack of T cell recruitment is a particularly important component of the immunopathology in respiratory viral infections, such as influenza (Tang et al. 2019). We hypothesize that the use of vascular normalization strategies including VEGF or VEGFR blockade or inhibition (Huang et al. 2012), inhibition of STAT3 (Wang et al. 2021), targeted increase in VE-cadherin expression (Zhao et al. 2017), dual ANG2-blocking and TIE2-activating antibodies and the VE-PTP inhibition used in this study will be of benefit to a wide range of infectious diseases (Park et al. 2016).
Funding
This work was supported by the National Health and Medical Research Council (APP1099912 and APP1053407 to S.H.O); the Kenyon Family Foundation Inflammation Award (grant to S.H.O.); the University of Sydney (fellowship to S.H.O.); the US National Institutes of Health (AI125517 to D.M.T); the Duke University Center for AIDS Research, and an NIH-funded program (5P30 AI064518, small-project grant to D.M.T. and S.H.O.).
ACKNOWLEDGEMENTS
The authors acknowledge members of the Tuberculosis Research Program at the Centenary Institute for discussion of the manuscript, Dr Angela Fountaine of the BioImaging Facility, and Sydney Cytometry at the Centenary Institute for technical assistance with imaging, and Aerpio Therapeutics for AKB-9785.
Contributor Information
Julia Y Kam, Tuberculosis Research Program at the Centenary Institute, The University of Sydney, Camperdown, NSW 2050, Australia.
Tina Cheng, Tuberculosis Research Program at the Centenary Institute, The University of Sydney, Camperdown, NSW 2050, Australia.
Danielle C Garland, Tuberculosis Research Program at the Centenary Institute, The University of Sydney, Camperdown, NSW 2050, Australia.
Warwick J Britton, Tuberculosis Research Program at the Centenary Institute, The University of Sydney, Camperdown, NSW 2050, Australia; Department of Clinical Immunology, Royal Prince Alfred Hospital, Camperdown, NSW 2050, Australia.
David M Tobin, Department of Molecular Genetics and Microbiology, Duke University School of Medicine, Durham, NC 27710, USA.
Stefan H Oehlers, Tuberculosis Research Program at the Centenary Institute, The University of Sydney, Camperdown, NSW 2050, Australia; The University of Sydney, Sydney Institute for Infectious Diseases, Camperdown, NSW 2050, Australia; A*STAR Infectious Diseases Labs (A*STAR ID Labs), Agency for Science Technology and Research (A*STAR), Singapore, 138648, Singapore.
Conflict of interest statement
None declared.
References
- Cheng T, Kam JY, Johansen MDet al. . High content analysis of granuloma histology and neutrophilic inflammation in adult zebrafish infected with Mycobacterium marinum. Micron. 2020;129:102782. [DOI] [PubMed] [Google Scholar]
- Cronan MR, Beerman RW, Rosenberg AFet al. . Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity. 2016;45:861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta M, Via LE, Kamoun WSet al. . Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci. 2015a;112:1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta M, Via LE, Kamoun WSet al. . Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci USA. 2015b;112:1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks PM, Brizee S, van der Vaart Met al. . Hypoxia inducible factor signaling modulates susceptibility to mycobacterial infection via a nitric oxide dependent mechanism. PLoS Pathog. 2013;9:e1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks PM, van Eeden FJ, Dixon Get al. . Activation of Hif-1alpha delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–22. [DOI] [PubMed] [Google Scholar]
- Goel S, Gupta N, Walcott BPet al. . Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013;105:1188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnik S, Devraj K, Macas Jet al. . Angiopoietin-2-induced blood–brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of tie2 signaling. Acta Neuropathol. 2016;131:753–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm Tet al. . The zebrafish lysozyme c promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling Fet al. . Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–4. [DOI] [PubMed] [Google Scholar]
- Harding J, Ritter A, Rayasam Aet al. . Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol. 2015;185:432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JS, Herbath M, Chen Yet al. . VEGF-A from granuloma macrophages regulates granulomatous inflammation by a non-angiogenic pathway during mycobacterial infection. Cell Rep. 2019;27:2119–31. [DOI] [PubMed] [Google Scholar]
- Hortle E, Johnson KE, Johansen MDet al. . Thrombocyte inhibition restores protective immunity to mycobacterial infection in zebrafish. J Infect Dis. 2019;220:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortle E, Oehlers SH. Host-directed therapies targeting the tuberculosis granuloma stroma. Pathog Dis. 2020;78:ftaa015. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yuan J, Righi Eet al. . Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109:17561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell Tet al. . Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–209. [DOI] [PubMed] [Google Scholar]
- Lowe DM, Redford PS, Wilkinson RJet al. . Neutrophils in tuberculosis: friend or foe?. Trends Immunol. 2012;33:14–25. [DOI] [PubMed] [Google Scholar]
- Matty MA, Oehlers SH, Tobin DM, Live imaging of host–pathogen interactions in zebrafish larvae, In: Kawakami K, Patton EE, Orger M (eds). Zebrafish: Methods and Protocols. New York, NY: Springer, 2016, 207–23. [DOI] [PubMed] [Google Scholar]
- McNamee EN, Korns Johnson D, Homann Det al. . Hypoxia and hypoxia-inducible factors as regulators of t cell development, differentiation, and function. Immunol Res. 2013;55:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers S, Cronan M, Scott Net al. . Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, Cronan MR, Beerman RWet al. . Infection-induced vascular permeability aids mycobacterial growth. J Infect Dis. 2017;215:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH. Revisiting hypoxia therapies for tuberculosis. Clin Sci. 2019;133:1271–80. [DOI] [PubMed] [Google Scholar]
- Ong CWM, Fox K, Ettorre Aet al. . Hypoxia increases neutrophil-driven matrix destruction after exposure to Mycobacterium tuberculosis. Sci Rep. 2018;8:11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim IK, Han Set al. . Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell. 2016;30:953–67. [DOI] [PubMed] [Google Scholar]
- Polena H, Boudou F, Tilleul Set al. . Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination. Sci Rep. 2016;6:33162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L. Mycobacterium tuberculosis pathogenicity viewed through the lens of molecular Koch's postulates. Curr Opin Microbiol. 2020;54:103–10. [DOI] [PubMed] [Google Scholar]
- Shen J, Frye M, Lee BLet al. . Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest. 2014;124:4564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Hui SP, Sheng DZet al. . Zebrafish FOXP3 is required for the maintenance of immune tolerance. Dev Comp Immunol. 2017;73:156–62. [DOI] [PubMed] [Google Scholar]
- Swaim LE, Connolly LE, Volkman HEet al. . Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun. 2006;74:6108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki K, Davis JM, Winglee Ket al. . Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat Protoc. 2013;8:1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BM, Shojaei M, Teoh Set al. . Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat Commun. 2019;10:3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton EM, Cronan MR, Cambier CJet al. . Cyclopropane modification of trehalose dimycolate drives granuloma angiogenesis and mycobacterial growth through Vegf signaling. Cell Host Microbe. 2018;24:514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Astone M, Alam SKet al. . Suppressing STAT3 activity protects the endothelial barrier from VEGF-mediated vascular permeability. Dis Model Mech. 2021;14:33140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang L, Zimmerman MDet al. . Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog. 2018;14:e1006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ting KK, Li Jet al. . Targeting vascular endothelial-cadherin in tumor-associated blood vessels promotes T-cell-mediated immunotherapy. Cancer Res. 2017;77;4434–47. [DOI] [PubMed] [Google Scholar]