Abstract
While diabetes has profound effects on multiple organ systems, the loss of vision caused by diabetic retinopathy may be of one of the most impactful in a patient’s life. The retina is a highly metabolically active tissue that requires a complex interaction of cells spanning light sensing photoreceptors to neurons transferring the electrochemical signal to the brain with support by glia and vascular tissue. Neuronal function depends on a complex inter-dependency of retinal cells that includes the formation of a blood-retinal barrier (BRB). This dynamic system is negatively impacted by diabetes, which alters normal cell-cell interactions and leads to profound vascular abnormalities, loss of the blood-barriers and impaired neuronal function. Understanding the normal cell signaling interactions and how they are altered by diabetes has already led to novel therapies that have improved visual outcomes for many patients. Recent research highlighted in this review, has led to new understanding of retinal pathophysiology during diabetes and uncovered potential for new therapeutic avenues to treat this debilitating disease.
Introduction
Diabetic retinopathy (DR) is one of the most common complication of diabetes and remains a leading cause of visual loss and blindness globally1. Diabetes impacts many components of the eye, but the primary vision threatening pathology occurs in the retina. Research has revealed alterations to both neuronal and vascular cells of the retina in DR. While a complete understanding of disease etiology is needed, recent breakthroughs for treating DR that focus on targeting vascular endothelial growth factor A (VEGF-A) now provide effective treatment options in the clinic. However, anti-VEGF therapy is only effective in the late stages of DR, requires regular intravitreous injections and not all patients respond optimally. The increasing rate of diabetes globally, the need to prevent progression from the early stages of DR, patients that fail to respond to anti-VEGF therapy and patients with ischemic retinopathy for which anti-VEGF is inappropriate, collectively requires the need for the development of new therapeutic approaches for this disease.
This review focuses on the current understanding of the molecular and cellular pathology of DR with a primary focus on the cellular signaling between the neuronal and vascular retina that promote formation of the inner blood-retinal barrier (iBRB) of the retinal vasculature as an important point of intervention. Changes in visual function will be correlated with novel retinal biomarkers identified by clinical imaging modalities such as optical coherence tomography angiography and ultrawide field retinal imaging. New therapies under investigation that may complement current laser treatment and anti-VEGF therapy will be presented along with the mechanism of action. Finally, the translational potential of novel approaches such as the development of patient-derived cells and retinal organoids for experimental investigation and the potential of tissue restoration will be considered.
Classification of Disease Severity
Studies on pathogenesis and treatment of diabetic retinal disease rely on the use of accurate methods to classify DR that are reflective of its natural history. DR has been well described using the modified Airlie House classification scale as applied in the Early Treatment Diabetic Retinopathy Study (ETDRS)2 and recently detailed in a position statement from the American Diabetes Association3. Altered retinal blood flow and vascular permeability4, basement membrane thickening5, loss of pericytes and acellular capillary formation6 contribute to clinically visible nonproliferative DR (NPDR) lesions such as microaneurysms, venous beading and intraretinal microvascular abnormalities. As ischemia increases, patients may develop proliferative DR (PDR), which presents a substantial risk for visual loss due to neovascular complications such as vitreous hemorrhaging or retinal detachment as blood vessels grow into the vitreous7 (figure 1).
Figure 1. Diabetic Retinopathy (DR) Manifests with Multiple Pathologies.
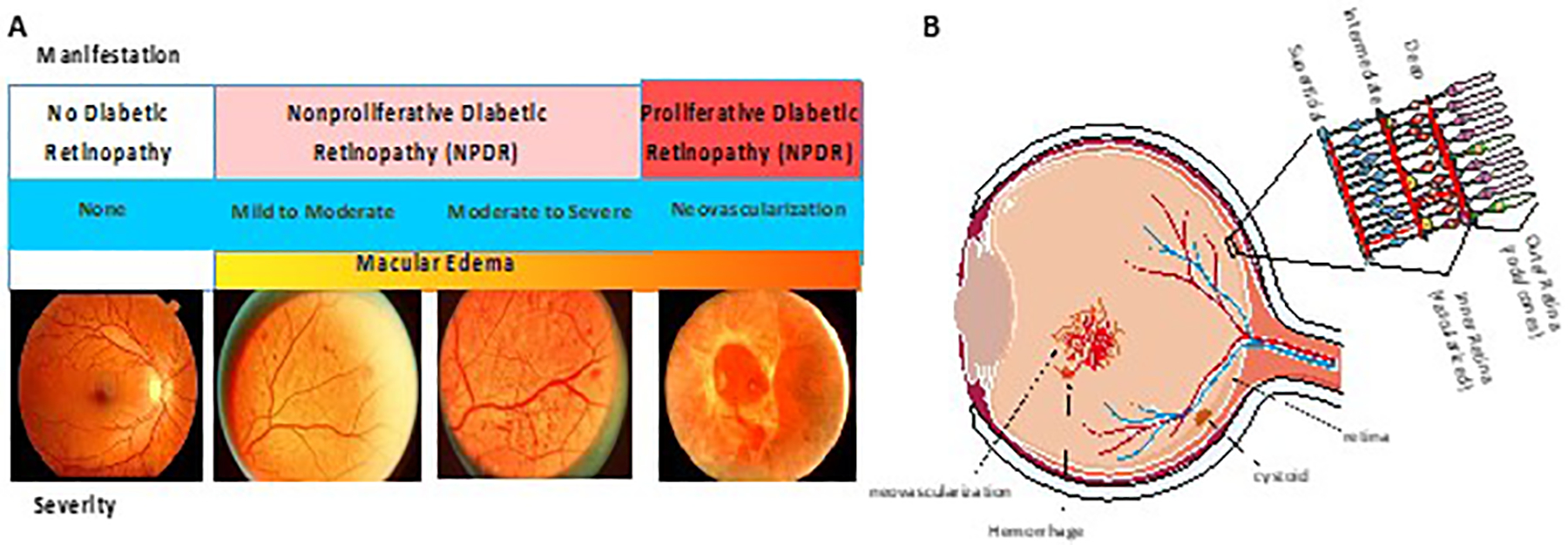
A. Patients with diabetes may have no readily observable alterations to the retina as observed by fundus photography. Alternatively, microvascular abnormalities, hemorrhages, microaneurysms and venous beading reveal evidence of disease process that may range from mild to severe and occur in patients with non-proliferative DR (NPDR). Patients with proliferative DR (PDR) have neovascularization in the retina that may lead to retinal detachment. Diabetic macular edema (DME) may occur in both NPDR or PDR. (Adapted from7) B. Schematic diagram of a cross section of the eye. Vessel leak, neovascularization and cystoid formation due to DME are indicated. Cross section of retina indicating organization of ganglion cells and bipolar cells in the inner retina versus rods and cones in the outer retina. Blood vessels in the inner retina make the inner blood-retinal barrier and the retinal pigment epithelium (RPE) makes the outer blood-retinal barrier.
Also linked to ischemia, diabetic macular edema (DME) develops as a result of abnormal permeability of retinal capillaries and from microaneurysms leading to the accumulation of extracellular fluid and thickening of the normally compact macular tissue. As the severity of DR increases, the risk of developing DME similarly increases8. Loss of vision from DME correlates with the location and extent of retinal thickening on optical coherence tomography (OCT) scans and, macular blood vessel permeability and perfusion as assessed by fluorescein angiography9,10. Data from the ETDRS evaluating eyes with DME have shown that thickening involving the center of the macula, termed center-involved DME, has a nearly ten-fold greater risk for developing moderate visual loss compared to eyes without center involvement11.
The retinal pigment epithelium (RPE) and the underlying choroid are also compromised during diabetes. The RPE provides a barrier controlling exchange of metabolites from the rods and cones with the underlying choroidal vessels and imaging focused on the RPE reveal evidence of permeability in patients with DME12 which may relate to breakdown of the outer BRB13 and activation of inflammation-linked pathways that drive pathology in the photoreceptors14. The RPE also shows impaired regulation of fluid outflow during diabetes that may be linked to dysfunction of the normal activity of Na/K ATPase pumps and aquaporin channels15. These outer retina changes occur concomitantly with what has been termed diabetic choroidopathy16 which manifests as progressive non-perfusion of the choriocapillaris.
ETDRS severity levels have been used to guide clinical practice recommendations for patient follow-up and treatment. In the ETDRS, a total of 13 eye and 26 patient levels of severity have been described and have been used extensively in research and clinical trials. The American Academy of Ophthalmology formed a consensus panel and created a simplified classification called the International Clinical DR and DME Disease Severity Scale17. This scale simplified descriptions of the categories of DR but is not a replacement for ETDRS levels of DR in large-scale clinical trials or studies in which precise DR classification is necessary. Despite advances in retinal imaging, the current DR classification scales have not incorporated new approaches such as ultrawide field imaging for the retinal periphery or optical coherence tomography for macular edema or neuroretinal changes. The current grading scales are still largely based on clinically visible retinal microvascular lesions and do not include neurodegenerative changes that may occur early and distinct from vascular changes18. The evolution of DR classifications are inevitable and should include measures that will better prognosticate and predict patient outcomes. But until then, the ETDRS severity levels should remain the standard for determining disease severity in both clinical and research settings.
While the pathological changes that occur during DR are often considered a progression, it remains possible that environmental or genetic factors promote a specific pathology. Epidemiological studies have quantified the risks for developing DR or DME and have shown significant differences between type 1 (T1DM) and 2 (T2DM) diabetes mellitus19. Both glycemic control and diabetes duration have been found to be significant risk factors in the development to DR and DME19. However, the 25 year rate of developing some degree of DR is over 95% in T1DM and only 60% in T2DM8. Furthermore, the 10 year rate of developing DME is 20% in T1DM, 25% in T2DM taking insulin and 14% in T2DM not taking insulin20. In general, T1DM patients tend to develop more DR and PDR while T2DM patients taking insulin are more at risk for developing DME. Future research on understanding what causes patients to present with DME, PDR or aspects of inflammation and whether these represent a progression or separate pathologies are greatly needed.
Multiple large-scale clinical studies have shown that glycemic control is essential to preventing progression of diabetic complications and DR (reviewed in21). A meta-analysis of multiple population-based studies of DR reveals glycosylated hemoglobin, blood pressure, and serum total cholesterol associate with the incidence and progression of retinopathy but only explain 9% of DR progression and 10% of PDR development22. Therefore, additional factors likely contribute to disease pathology. A recent study has implied very long chain (VLC) fatty acids that incorporate into VLC ceramides affect endothelial barrier properties23. Diabetes leads to loss of elongases including ELOV4 and alters the retinal lipid profile24. Depletion of ELOV4 can reduce endothelial barrier properties while overexpression promotes barrier properties and reduces diabetes effect on permeability in vivo23.
Markers for Disease Activity
The ETDRS standardized grading scale is based on 30° retinal images from 7 standard defined retinal fields and characterizes the extent of retinal lesions located in the posterior pole. However, ultrawide field imaging has demonstrated that retinal lesions can appear or develop outside of the ETDRS fields25–30. Predominantly peripheral lesions (PPL) describe eyes with DR lesions that are greater in extent or severity outside the ETDRS standard fields. Eyes with PPL were shown to have increased retinal nonperfusion compared to eyes without PPL. The cause of PPL is currently unknown and may involve loss of autoregulation in retinal arterioles or microvascular degeneration causing capillary nonperfusion and retinal ischemia31. PPL are present in ~50% of eyes with DR and identify a more severe level of DR in ~10% of eyes compared to standard ETDRS field imaging25,27. Moreover, the baseline presence of PPL in an eye suggests an increased risk of future DR worsening and the development of advanced, sight-threatening retinopathy over the subsequent 4 years by 3.2 and 4.7 fold, respectively27. These findings suggest that PPL may become a robust marker of DR progression. Another marker may be the presence of vitreous hyperreflective foci in OCT scans. In a study of 97 patients, these foci, presumed to represent inflammatory cells, were increased in patients with DME compared to control or diabetic patients without DME32. Future longitudinal analyses can reveal whether these scans provide true biomarkers for disease progression.
The advent of optical coherence tomography-angiography (OCT-A) is providing an unprecedented assessment of retinal vascular detail and may reveal important changes not previously observed by traditional methods available to ophthalmologists. OCT-A allows the noninvasive mapping of retinal vessels and blood flow allowing visualization of the retina and choroidal vasculature33,34. Both the superficial vessels and deep retinal vascular layers, can be readily differentiated with OCT-A enabling the identification of specific retinal capillary layers responsible for the underlying disease35. A deeper understanding of how capillaries change over the course of diabetes and in response to treatments for diabetic eye disease provided by OCT-A may provide novel insight into disease treatment approach. In addition, there has been recent interest in the use of metabolomics to identify biomarkers. Metabolomic analysis of vitreous and serum samples have identified dysregulation in pathways such as the pentose phosphate pathway, arginine to proline pathway, polyol pathway and ascorbic acidic pathways36,37. However, further research is necessary to establish causative and longitudinal associations with DR.
Diabetes Alters the Neural/Vascular Interaction in the Retina
The retinal neurovascular unit (NVU) refers to the inter-dependency of the vascular endothelial cells with pericytes, glia, neurons and retinal-resident immune cells. While the vasculature provides the required nutritional support for the neural tissue, the neural and glial cells along with pericytes, signal to the vascular endothelial cells creating the blood-retinal barrier (BRB) providing tight control of the neural environment (figure 2A). An early deficiency in the function of the NVU in diabetes is observed after short-term diabetes in animal models38 and patients39 and results in impaired neurovascular coupling, loss of autoregulation and control of blood flow as well as disruption of the iBRB. Diabetes also impacts Müller glia leading to mis-localized active transport mechanisms of inwardly rectifying channels at the capillary:Müller glial interface that contributes to swelling of Müller glia in diabetic retina40. Changes to Kir4.1 and aquaporins on Müller glia are consistent findings in diabetic animal models41 and these changes can be rectified by blocking the accumulation of lipoxidation end products42. Further, Müller glial response in diabetes may amplify inflammation by activating microglia through P2X7 purinergic receptors leading to neuroinflammation and vascular damage, including leakage43. Indeed, microglial activation has a significant impact on the retinal NVU and neuroinflammation-driven breakdown in the inner BRB in DR44.
Figure 2. The Neurovascular Unit (NVU) and Cytokine Signaling in Diabetic Retinopathy (DR).
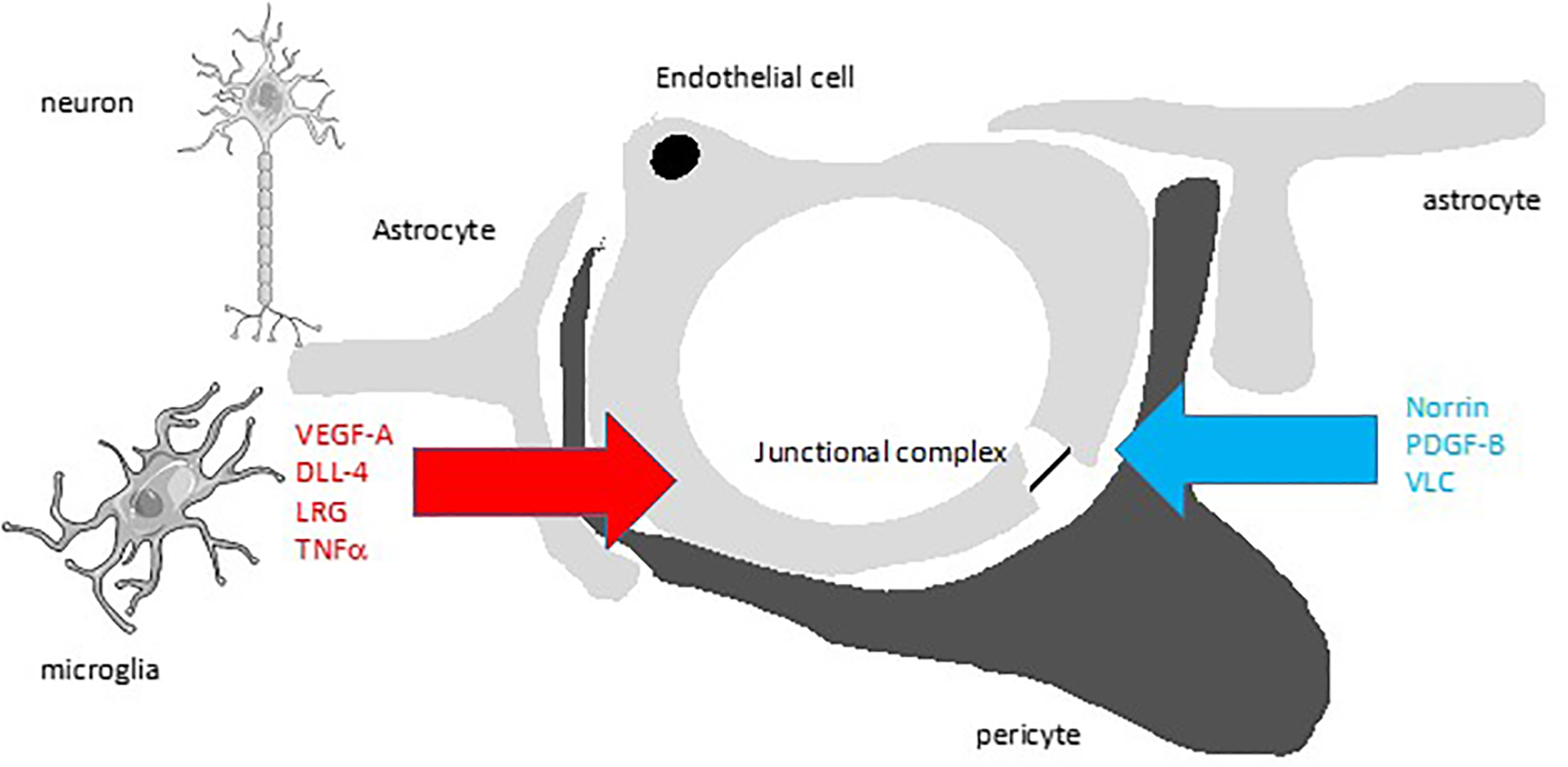
A) Proper retinal functions require an intimate relationship of the retinal blood vessels in the inner retina with neurons, glia (astrocytes and Müller cells), and pericytes. Glia provide norrin signaling required for BRB formation. Endothelial cells recruit pericytes by PDGF-B signaling and pericytes promote BRB by an unknown mechanism. B) In DR, glia have increased aquaporin and Kir4.1 channels contributing to swelling and now produce vasoactive substances such as VEGF-A and associated DLL-4, ANGPTL4 and LRG that promote permeability, angiogenesis or both. Loss of pericytes leads to hyper-responsiveness of endothelial cells to VEGF signaling. Further, inflammatory cytokines such as TNFα, IL1β and CCL2 among many others, are produced by microglia and other retinal cells as well as adherent inflammatory cells. In addition, hyperglycemia induces direct endothelial dysfunction through change in redox state (NAD(P)H and ROS). Not shown, RPE also undergo dysfunction with increased cytokine production. Collectively these changes disrupt the neurovascular unit and alter normal retinal function.
Vascular endothelial changes have so far, represented the only successful therapeutic target for diabetic retinopathy. Laser photocoagulation has long provided an effective means of controlling proliferation and edema in many patients45. More recent success in treating DR has evolved in the clinic by targeting factors that drive microvascular abnormalities. Vascular changes in DR have been attributed in part, to elevated VEGF-A that signals to retinal endothelial cells altering the blood vessel permeability and promoting neovascularization (reviewed in46,47). Multiple, multi-center clinical trials have demonstrated targeting VEGF-A with antibodies or trap can effectively reduce DME, prevent further vison loss and, in some patients, improve vision48–50. Among patients with PDR, anti-VEGF-A therapy has been shown to prevent or reverse neovascularization with 43% of treated patients demonstrating resolution of neovascularization after 2 years and only 27% worsening since the previous visit51. However, for PDR and DME52 clinical studies reveal that approximately half of patients receive benefit while others remain unresponsive to anti-VEGF-A therapy suggesting other factors may drive disease pathology in DR. Interestingly a recent study has shown significant correlations between inflammatory cytokines and VEGF and, in particular, that the iBRB is regulated by localization of the tight junction protein claudin-5 via rho-associated coiled-coil–containing protein kinase (ROCK) activation. Administration of ripasudil, a selective ROCK inhibitor, attenuated retinal inflammation and claudin-5 redistribution. When combined with an anti-VEGF agent, this ROCK inhibitor was synergistic in suppressing cytokine upregulation, monocyte/macrophage infiltration, macrophage/microglia activation, and claudin-5 redistribution, an effect that was demonstrated pre-clinically but also in patients resistant to anti-VEGF53. These data indicates that inflammation may be a key mechanism in the responsiveness to anti-VEGF therapy in DME.
Associated with VEGF-A, notch signaling may be altered in DR. In vascular angiogenesis during retinal development, VEGF-A signal stimulates an endothelial cell with the highest VEGFR2 response to become a tip cell that migrates toward the VEGF source and signal to neighboring cells to become the proliferating stalk cells of the angiogenic sprout. This cell to cell communication utilizes the notch signaling pathway with delta like canonical notch ligand 4 (DLL4) and notch receptor54. Recent studies suggest both DLL4 and the typical notch antagonist jagged-1, are increased in diabetic mouse models and in endothelial cells in a glucose dependent manner55. Intra-ocular injections of either ligand induced a modest increase in retinal permeability dependent on notch since conditional gene-deletion of notch prevented the permeability response. Further, a notch trap reduced permeability in a diabetic animal model55.
Diabetes alters the normal pericyte endothelial interaction in the retina. Studies using targeted genetic deletion of pericytes reveal that pericyte coverage of retinal vessels is required for proper formation of the BRB56. Platelet derived growth factor (PDGF)-B signaling to pericytes controls vessel stabilization as deletion of PDGF-B retention signal, that localizes the growth factor to the pericellular space, also causes deterioration of retinal vessels57 and PDGF receptor-β blocking antibody induces retinal hemorrhage and permeability in a FOX01 dependent manner58. Interestingly, this study also revealed that loss of retinal pericytes in adult mice using inducible, targeted diphtheria toxin expression, does not confer leaky retinal vessels as observed in other organs such as lung and skin. Instead, loss of pericytes make the retinal vasculature highly susceptible to VEGF-A signaling with a dramatic increase in hemorrhage and vascular permeability to dextran58. Pericytes control endothelial expression of angiopoietin 2 and VEGFR2 through transcription factor FOX01, with loss of pericytes dramatically promoting VEGF signaling. This heightened response of retinal vascular endothelial cells to VEGF-A after pericytes loss has stark implications for the well-established loss of retinal pericytes in diabetes. In addition, chronic hyperglycemia has been shown to reduce PDGF receptor tyrosine kinase signalling which promotes pericyte apoptosis and diabetic vasculopathy through activation of protein kinase C-δ (PKC-δ) and increased expression of the tyrosine phosphatase Src homology-2 domain–containing phosphatase-1 (SHP-1)59,60. These studies provide a mechanistic link to diabetes.
Glial cells provide Wnt signaling to retinal vascular endothelial cells required for formation of the BRB and may be a target for treating DR. The cytokine norrin is not a Wnt molecule but like Wnt, norrin signals through the frizzled 4 (FZD4) receptor complex61. Gene deletion studies of norrin, the receptor frizzled 4 or the co-receptors low density lipoprotein receptor-related protein 5/6 (LRP5/6), or tetraspanin (TSPAN)12 reveal that this signaling complex is required for both retinal angiogenesis62 and BRB formation63,64. Importantly, norrin and FZD4 knockout mice show high retinal vascular permeability that correlates with reduced endothelial cell border immunostaining of the TJ protein claudin 5, and increased expression of the transcytosis marker and plasmalemma vesicle associated protein. Further, this phenotype can be reversed by the expression of a stabilized form of β-catenin revealing the role of canonical Wnt pathway. Studies have begun to explore whether norrin signaling may be used to restore vascular function in animal models. Norrin treatment may reduce avascular area and inhibit neovascularization in oxygen-induced retinopathy models65 and transgenic expression of norrin may reduce vaso-obliteration and promote vascular growth66,67. Recent studies demonstrate that norrin can reverse VEGF induced permeability in cell culture and in animals after intravitreal injection of VEGF or in diabetes68. Interestingly, these studies reveal that VEGF actually promotes norrin signaling by increasing membrane content of the FZD4 co-receptor TSPAN12. The addition of norrin after VEGF then promotes barrier induction suggesting a potential novel approach to vascular restoration. It will be important to ascertain whether norrin expression, as well as other Wnt signaling mediators, changes during diabetes to determine whether neuronal changes in Wnt signaling alter BRB in diabetes.
A variety of studies suggest cell signaling through inflammatory factors may contribute to DR pathogenesis. Vitreous proteomic analyses have identified a host of altered inflammatory factors in the vitreous or aqueous humor at varying stages of diabetic retinopathy (reviewed in69 and70), many of which are highlighted here. Gene deletion and cytokine capture studies in animal models have provided strong evidence for a role of tumor necrosis factor-α (TNF-α)71,72 in DR and evidence of leukostasis with a role for intercellular adhesion molecule-1 or its binding partner CD1873. Human studies of vitreous fluid have found an association of elevated interleukin IL-1β and TNF-α in PDR patients74–76. IL-6, IL-8 and chemokine, C-C motif, ligand (CCL)-2 were also identified as elevated in patients with diabetic macular edema and PDR77. Conversely, antiangiogenic mediators such as pigment epithelium-derived factor (PEDF) have been reported to be in low patients with diabetes and in patients with active PDR78. Studies demonstrate targeting inflammation by inhibiting atypical protein kinase C (aPKC) may control vascular permeability in the retina. The aPKC isoforms contribute to endothelial permeability from a variety of inflammatory factors and growth factors including VEGF and also contribute to NFκB activation79,80. Reducing aPKC activation with a small molecule inhibitor or conditional expression of a dominant negative form of the kinase reduced permeability and monocyte and granulocytes recruitment in models of retinal inflammation81. Beyond broad-spectrum, anti-inflammatory approaches such as corticosteroids already in clinical use, targeting specific cytokines based on measures of patient vitreous or aqueous cytokine profiles remains an exciting possibility to improve therapeutic options82.
Given sufficient time the development of DR is nearly universal in patients with diabetes8 but the development of PDR plateaus at 60%, even after more than 50 years of diabetes8,83. Therefore, there may be protective mechanisms that delay or prevent the progression to PDR84. Proteomic analysis identified elevated concentrations of photoreceptor-secreted retinol binding protein 3 (RBP3) in the retina and vitreous of patients protected from advanced DR despite diabetes durations of over 50 years85, consistent with earlier findings that RBP3 was reduced in the general patient population with diabetic retinopathy86. Retinal cell based assays and rodent models have demonstrated that RBP3 can prevent diabetes induced vascular permeability and altered retinal function measured by electroretinogram (ERG)85. RBP3 may have a role in protection against the progression of DR by decreasing the expression and signalling of inflammatory cytokines and VEGF. Further this group provided evidence for RPB3 reducing glucose uptake into Müller cells by binding and inhibiting glucose transporter 1, thereby mitigating the effects of chronic hyperglycemia85. These studies require further exploration of the normal physiological role of RBP3 in mediating glucose uptake but provide novel insight into retinal metabolism and potential therapeutic approaches to treat DR.
Novel Pathogenic Pathways
Studies have identified a range of alternative neurovascular signaling pathways that lead to leakage and/or neovascularization in addition to VEGF. Amongst the most promising targets is the kinin–kallikrein system. Carbonic anhydrase I (CA-1) and activation of plasma kallikrein (PK) was identified in the vitreous of patients with advanced DR87. Subsequent studies established PK cleavage of kininogen generates bradykinin which acts through bradykinin receptors on the blood vessels to induce permeability. Inhibitors of PK can block or reduce retinal permeability in animal models of diabetes and in response to direct CA-1 and PK injection88 but not VEGF-A, suggesting a distinct pathway of vessel permeability. Currently, a range of PK inhibitors are being tested in clinical trials for patients with DME.
Recent experimental studies have implicated angiopoietin like 4 (ANGPTL4) in DR. ANGPTL4 was initially found elevated in aqueous fluid from the anterior chamber of patients with DME and the level of ANGPTL4 correlated with the ability of the aqueous fluid to induce permeability in an endothelial cell culture assay89. ANGPTL4 is downstream of hypoxia inducible factor regulated gene transcription and can induce endothelial permeability90. Interestingly ANGPTL4 was shown to bind to neuropilin and activates the small G-protein RhoA89. Neuropilin is a co-receptor for VEGFR2; however, the ability of ANGPTL4 to induce permeability was independent of VEGFR2, demonstrated in knockdown studies in cell culture. A soluble form of neuropilin was able to block ANGPTL4 and VEGF-induced permeability in cell culture and mice. It should be noted that there are a number of conflicting reports of the role of ANGPTL4 in permeability. For example, studies reveal ANGPTL4 can reduce permeability in the brain in stroke and can specifically attenuate VEGF induced permeability by inhibiting Src phosphorylation and activation91 and recent findings reveal ANGPTL4 can inhibit pro-inflammatory genes and promote anti-inflammatory genes in macrophages in cell culture and in a myocardial infarct model92. Clearly additional studies on the complex role of ANGPTL4 are needed. However, a previous study identified another neuropilin binding protein semaphorin 3A (Sema3A) also induces retinal permeability and conditional knockout of neuropilin prevented Sema3A induced permeability but not VEGF induced permeability93. Targeting neuropilin may provide a novel option to treat DR and may potentially prevent induction of permeability from multiple sources if no toxicity is associated with this therapy.
Gene expression studies of pathological angiogenesis identified elevated expression of leucine-rich alpha-2-glycoprotein 1 (Lrg1) that promotes neovascularization. Lrg1 modifies transforming growth factor β (TGF-β) signaling by binding to the accessory receptor endoglin and promoting a pro-angiogenic signaling pathway94. Lrg1 knockout mice have a modest delay in retinal vascular development but both the knock out mice or an anti-Lrg1 antibody could dramatically reduce pathological angiogenesis in animal models. Currently, a humanized monoclonal antibody to LRG1 called Magacizumab is undergoing phase I/IIa clinical trial and could potentially provide an additional therapeutic option for PDR.
Finally, the direct role of hyperglycemia on endothelial cells has been extensively studies. However, a recent study provides an intriguing model of hyperglycemia regulated epigenetic control of oxidative stress in endothelial cells. Using siRNA and pharmacological inhibition of DNA methylation and hydroxymethylation, the investigators provide evidence for hyperglycemia induced increase in 5-hydroxy methyl cytosine and NFkB induced gene activation of Ras-related C3 botulinum toxin substrate 1 (Rac1)95. Rac1 is an essential component of NADPH oxidase 2 (Nox2) promoting reactive oxygen species (ROS) production, which is activated early in hyperglycemia induced endothelial cell dysfunction and contributes to mitochondrial production of ROS. These studies provide a novel model to link hyperglycemia induced epigentic gene regulation to ROS production and mitochondrial dysfunction (reviewed in96).
Altered Neuronal Function
While most clinical focus has been on vascular pathology during DR, there is now wide recognition that the impact of diabetes more broadly affects the cells of the retina. In addition to the clinically visible vascular defects, evidence points to changes occurring in the neural retina as well. For example, apoptosis of non-vascular cells has been consistently identified in animal models of DR97. Longitudinal studies of patients with diabetes suggests retinal degeneration as observed by thinning of the nerve fiber and ganglion cell layer, termed retinal neurodegeneration, without evidence of vascular pathology98. A number of changes in retinal function have been characterized that may occur before clinically observable vascular pathology including reduced electrical response as measured by ERG and diminished contrast sensitivity (reviewed in99,100). A recent animal study found that apoptosis in the diabetic retina depended on protein regulated in development and DNA damage response 1 (REDD1)101. REDD1 promotes dephosphorylation and inhibition of Akt kinase activity allowing the transcription factor FOXO1 to promote cell death. Depletion of REDD1 in retinal neural cell culture prevented hyperglycemia induced apoptosis and deletion of REDD1 in mouse reduced diabetes induced retinal apoptosis and attenuated aspects of visual loss, most prominently loss of b-wave intensity in scotopic ERG and loss of contrast sensitivity. Some factors may directly impact both vascular and neural tissue such as endothelin which impacts vascular and neural tissue through different receptors subtypes. Recent data revealed topical administration of an endothelin antagonist in a diabetic mouse model prevented neurodegeneration102.
While vascular and neuronal changes clearly both occur during DR, the question remains whether neural or vascular dysfunction initiates the disease process or whether the alterations are coincident but unrelated. The European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) recently studied 449 diabetic patients with no versus mild vascular defects as assessed by ETDRS scoring and measured alterations in retinal function using multifocal ERG (mfERG) or retinal structure measured by OCT. The study found 61% of patients without microvascular disease presented abnormalities related to neurodegeneration assessed by mfERG or OCT103. Conversely, 32% of patients with visible microvascular disease did not present any sign of neurodegeneration. It is important to note that the lack of observable vascular defects do not confirm unaltered vessel function. However, the authors raise the possibility of distinct disease etiology in DR. The use of conditional gene regulation targeting specific cell types is necessary to begin to elucidate the causal relationship between retinal vascular and neural changes observed in animal models of diabetes. Further, longitudinal studies of patient populations assessing vascular and neuronal alterations and retinal function are needed to clarify potential differences in disease progression that will inform therapeutic approaches.
There is growing clinical evidence that neurovascular changes occur in the brain of patients with diabetes, especially in the context of T2DM leading to increased risk of dementia104 or Alzheimer disease105. There is also growing evidence of an association between retinal vessel abnormalities and cognitive impairment and dementia106 with the possibility of retinal imaging as an effective biomarker for neurodegenerative diseases. Recent proteomic analysis of the vitreous has identified changes in proteins associated with Alzheimer’s and Parkinson’s disease107. While these studies are intriguing, significant research with mechanistic detail is needed to explore a potential role for diabetes in brain neurodegeneration and similarities or differences with the retina.
Extensive studies have illuminated a role of oxidative stress in contributing to the pathology of DR (reviewed in108). NF-E2-related factor 2 (Nrf2) is a transcription factor that is a master regulator of a host of genes that act in a cytoprotective manner and provide cellular antioxidant gene products. Nrf2 is normally bound and inhibited by Keap1, targeting Nrf2 for degradation. Stress induced alteration in Keap1 binding stabilize Nrf2 and provide cellular protection. Gene deletion of Nrf2 exacerbates the degree of retinal ischemia and increases pre-retinal neovascularization in the oxygen induced retinopathy (OIR) model109. The OIR model takes advantage of the plasticity of the neonatal retina and creates a central retinal ischemia which drives pathological angiogenesis. By using broad neuronal conditional knockout, glial specific knockout and endothelial knockout, the investigators demonstrated this effect on vascular development, particularly the increased avascular area, was driven by neuronal cells. Loss of Nrf2 increases expression of semaphorin (Sema)6a that acts extracellularly on endothelial cells through notch signaling. The vascular pathologies in the Nrf2 knockout animals were reversed by lentiviral delivery of shRNA targeting Sema6a. A role of Nrf2 signaling in diabetes was demonstrated with increased vessel permeability and loss of visual acuity in Nrf2 gene deleted diabetic animals110 and an ischemia reperfusion (IR) model was used to demonstrate a role of Nrf2 in retinal ganglion cell protection111. IR was previously shown to model aspects of VEGF-dependent vascular permeability, inflammation and retinal cell loss observed in diabetes but more dramatically and over a shorter time-frame112,113. Recent studies reveal an impressive protection of visual acuity with a Nrf2 activating drug in the IR model114 suggesting this approach may provide therapeutic benefit for neurons in ischemic retinal diseases including DR.
Potential for Regenerative Medicine
Recent studies have begun to explore the potential for retinal vascular regeneration. In a clinical case study, spontaneous re-perfusion of ischemic retina followed by recovery of visual acuity has been reported following radiation retinopathy115 thus suggesting that return of adequate blood flow can restore retinal function. Spontaneous re-perfusion of the ischemic diabetic retina has been reported116 117 although this is generally a rare occurrence in DR and there is a general assertion that normal vascular reparative processes are defective in early diabetes and can, at least in part, account for the observed progressive vascular degeneration. The diabetes-related deficiencies in vascular repair processes are not well-understood although it is widely appreciated that diabetic patients suffer exacerbated cardiac and peripheral limb ischemia through reduced collateral vessel development and abnormal repair following infarct118. Interestingly, the Joslin Medalist T1DM cohort show normal levels of endothelial progenitor cells (EPC) and circulating progenitor cells when compared to other patient cohorts with diabetes suggesting endogenous, protective factors may serve to provide a protective effect in the Medalist119. Indeed, there is increasing evidence that diabetes suppresses resident progenitor cells that would normally be activated by injury120,121. This is especially true for the recently identified, side population cells that possess a progenitor phenotype in the endothelium122,123. Lineage tracing experiments in mice show that these self-renewing progenitors can become activated by vessel damage after which they re-establish a viable endothelium and restore perfusion123–127. Although currently unknown, altered retinal progenitor cells could account for the observed deficits in repair in DR.
In view of diabetes-related damage to the retinal NVU, a strategy that could replace damaged endothelium is attractive and has clear translational potential. Cell therapy using vasoactive progenitors has received attention since such cells are recruited to sites of capillary loss where they promote re-perfusion128. Various cell-types including CD34+ cells129, Lin− hematopoietic stem cells (HSCs)130, CD44hi cells131 and circulating angiogenic cells132 have all been shown to enhance tissue repair of ischemic tissues in pre-clinical models, including the retina. Although described as endothelial progenitor cells (EPCs), many such populations are, in fact, heterogenous mixtures of myeloid cell types with no evidence of incorporation into the vasculature133. Unfortunately, the majority of clinical trials using the heterogenous and poorly defined EPCs have been disappointing134. However, an ongoing, retina-focused trial using CD34+ cells has demonstrated in Phase I that intravitreous delivery is safe135 and clinical trial for various retinopathies, including DR, is currently ongoing (ClinicalTrials.gov Identifier: NCT01736059). This is encouraging, although in the context of DR there may need to be some caution since recent evidence suggests that the use of progenitors that carry myeloid markers may actively participate in pro-inflammatory responses136.
Perhaps the most promising cell from a therapeutic perspective is the EPC-type called endothelial colony forming cells (ECFCs) isolated from peripheral adult blood or umbilical cord blood. These have proven to be homogenous and distinct from HSCs and cells sorted on CD34+ 133. ECFCs possess many endothelial and progenitor cell characteristics and lack the hematopoietic markers CD45 and CD14. They also possess de novo endothelial tube forming potential in vitro and in vivo and can form de novo vessels or directly incorporate into pre-existing capillaries137–140. In vivo, ECFCs appear to share properties with side population cells that are present in the vasculature122 and can become activated by vessel damage upon which they incorporate into the endothelium124–126. Emerging pre-clinical studies validate the potential for ECFCs in diseases where vascular insufficiency is a cardinal feature such as stroke, peripheral artery disease, heart disease, and DR141. Intravitreous delivered ECFCs have been shown to migrate to ischemic retina and activate vascular repair142–144. In diabetic mice, ECFCs combined with recombinant angiopoietin 1 gene therapy, prevent barrier dysfunction and restores vision as measured by opto-kinetic functional readouts145.
While most attention has been focused on replacement of damaged endothelial cells, in the diabetic retina there is also a need to restore other damaged cells. For example, replacing lost pericytes may be possible using mesenchymal stromal cells (MSCs) since their potential has been shown to reside adjacent to retinal vessels and adopt pericyte-like phenotype which maintains vascular integrity146,147. Although not yet studied in diabetic retina, there is likewise potential for replacement of defective Muller glia, RPE and perhaps even neurons148.
Patient-Specific Cells and Organelles
The potential of using induced pluripotent stem cell (iPSC) technology to produce retinal organoids has already led to significant impacts in ophthalmology and vision science. So-called “retina in a dish” approaches have been developing apace in recent years and they have provided great insight into both developmental biology and retinal neurodegenerative diseases149,150. Indeed, iPS-derived, patient-linked cells are already advancing other ophthalmic disease fields such as informing the pathogenesis of drusen formation in iPSC-RPE from patients with macular degenerative disease151. DR research has been heavily reliant on animal models and while this has led to many important advances, there have always been limits in the clinical fidelity. Studies using retinal organoids for DR research may help circumvent this limitation. There are already findings on iPSC-RPE derived from T2DM patient donors revealing decreased barrier function and attenuated autophagic capacity when compared to iPSC-RPE from non-diabetic controls have been reported152. Depending on the pluripotency approach used, these iPSC-derived cells may carry an epigenetic imprint and harbor DNA methylation signatures characteristic of their somatic tissue of origin153. Use of iPSC has the potential to combine laboratory studies with clinically relevant cells to more fully understand DR phenotypes from a molecular perspective with the clear potential to develop more patient-specific therapeutic approaches.
Conclusions
Diabetes remains a leading cause of vision impairment worldwide. While the precise etiology of metabolic dysregulation contributing to loss of retinal functions remains to be fully elucidated, targeting VEGF-A cytokine signaling driving microvascular pathologies has proven effective in preventing disease progression and improving vision for many patients. Studies exploring the cellular communication of the NVU in the retina and the alterations that occur in diabetes may provide additional targets to treat those patients that fail to respond to current therapy. Disease management of DR may by further improved with the development of novel biomarkers that take advantage of the unique availability of retinal imaging. However, a better understanding of the disease etiology, what factors may drive DME or PDR, what specific cytokines or factors mediate specific disease processes and additional information on the genetic basis of susceptibility or protection to DR is needed. Accurate phenotypic description of patient populations coupled with analysis of altered cytokine profiles in vitreous or aqueous fluid may lead to precision medicine with improved patient outcomes. In basic research, studies that utilize conditional gene targeting to explore the cell communications in vivo are needed to elucidate the functional relationship of the cells in the neurovascular unit and the contribution to vascular and neuronal dysfunction in DR. Finally, regenerative and restorative approaches provide hope to restore retinal function lost by diabetes.
Contributor Information
David A. Antonetti, Department of Ophthalmology and Visual Sciences, Department of Molecular and Integrative Physiology, Kellogg Eye Center, University of Michigan, MI, USA.
Paolo S. Silva, Department of Ophthalmology, Harvard Medical School, Staff Ophthalmologist and Assistant Chief of Telemedicine, Beetham Eye Institute, Joslin Diabetes Center, Boston, MA, USA.
Alan W. Stitt, Centre for Experimental Medicine, Queen’s University, Belfast, Northern Ireland, UK.
References
- 1.Ogurtsova K et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes research and clinical practice 128, 40–50, doi: 10.1016/j.diabres.2017.03.024 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98, 786–806 (1991). [PubMed] [Google Scholar]
- 3.Solomon SD et al. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 40, 412–418, doi: 10.2337/dc16-2641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohner EM, Patel V & Rassam SM Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes 44, 603–607, doi: 10.2337/diab.44.6.603 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Roy S, Ha J, Trudeau K & Beglova E Vascular basement membrane thickening in diabetic retinopathy. Curr Eye Res 35, 1045–1056, doi: 10.3109/02713683.2010.514659 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Enge M et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. Embo J 21, 4307–4316 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98, 823–833 (1991). [PubMed] [Google Scholar]
- 8.Hovind P & Klein R, K. M, Lee KE,Gangnon R,Klein BEK. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning DJ et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 114, 525–536, doi:S0161–6420(06)01120–1 [pii] 10.1016/j.ophtha.2006.06.052 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner TW, Larsen M, Girach A & Zhi X Diabetic macular oedema and visual loss: relationship to location, severity and duration. Acta Ophthalmol, doi:AOS1545 [pii] 10.1111/j.1755-3768.2009.01545.x (2009). [DOI] [PubMed] [Google Scholar]
- 11.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 103, 1796–1806 (1985). [PubMed] [Google Scholar]
- 12.Cunha-Vaz J Mechanisms of Retinal Fluid Accumulation and Blood-Retinal Barrier Breakdown. Dev Ophthalmol 58, 11–20, doi: 10.1159/000455265 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Samuels IS, Bell BA, Pereira A, Saxon J & Peachey NS Early retinal pigment epithelium dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes. Journal of neurophysiology 113, 1085–1099, doi: 10.1152/jn.00761.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentley K et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 16, 309–321, doi: 10.1038/ncb2926 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Desjardins DM et al. Progressive Early Breakdown of Retinal Pigment Epithelium Function in Hyperglycemic Rats. Invest Ophthalmol Vis Sci 57, 2706–2713, doi: 10.1167/iovs.15-18397 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutty GA Effects of diabetes on the eye. Invest Ophthalmol Vis Sci 54, ORSF81–87, doi: 10.1167/iovs.13-12979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson CP et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110, 1677–1682 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Abramoff MD et al. Approach for a Clinically Useful Comprehensive Classification of Vascular and Neural Aspects of Diabetic Retinal Disease. Invest Ophthalmol Vis Sci 59, 519–527, doi: 10.1167/iovs.17-21873 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein BE Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 14, 179–183 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Moss SE & Cruickshanks KJ The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 102, 7–16 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Duh EJ, Sun JK & Stitt AW Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2, doi: 10.1172/jci.insight.93751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronald Klein M, MPH. in Contemporary Diabetes: Diabetic Retinopathy Vol. 1 Contemporary Diabetes (ed MD Duh Elia J.) Ch. 3, 67–107 (Humana Press, 2008). [Google Scholar]
- 23.Kady NM et al. ELOVL4-Mediated Production of Very Long-Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability. Diabetes 67, 769–781, doi: 10.2337/db17-1034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tikhonenko M et al. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59, 219–227, doi: 10.2337/db09-0728 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva PS et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology 120, 2587–2595, doi:S0161–6420(13)00413–2 [pii]; 10.1016/j.ophtha.2013.05.004 [doi] (2013). [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen ML et al. Comparison between Early Treatment Diabetic Retinopathy Study 7-field retinal photos and non-mydriatic, mydriatic and mydriatic steered widefield scanning laser ophthalmoscopy for assessment of diabetic retinopathy. J Diabetes Complications, doi:S1056–8727(14)00255–4 [pii]; 10.1016/j.jdiacomp.2014.08.009 [doi] (2014). [DOI] [PubMed] [Google Scholar]
- 27.Silva PS et al. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 122, 949–956, doi:S0161–6420(15)00046–9 [pii]; 10.1016/j.ophtha.2015.01.008 [doi] (2015). [DOI] [PubMed] [Google Scholar]
- 28.Talks SJ, Manjunath V, Steel DH, Peto T & Taylor R New vessels detected on wide-field imaging compared to two-field and seven-field imaging: implications for diabetic retinopathy screening image analysis. Br J Ophthalmol. 99, 1606–1609, doi:bjophthalmol-2015–306719 [pii]; 10.1136/bjophthalmol-2015-306719 [doi] (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiello LP et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging With Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy. JAMA Ophthalmol, doi: 10.1001/jamaophthalmol.2018.4982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessel MM et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina 32, 785–791, doi: 10.1097/IAE.0b013e3182278b64 [doi] (2012). [DOI] [PubMed] [Google Scholar]
- 31.Silva PS et al. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 122, 2465–2472, doi: 10.1016/j.ophtha.2015.07.034 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Korot E, Comer G, Steffens T & Antonetti DA Algorithm for the Measure of Vitreous Hyperreflective Foci in Optical Coherence Tomographic Scans of Patients With Diabetic Macular Edema. JAMA Ophthalmol 134, 15–20, doi: 10.1001/jamaophthalmol.2015.3949 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia Y et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 20, 4710–4725, doi: 10.1364/OE.20.004710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vujosevic S et al. Early Microvascular and Neural Changes in Patients with Type 1 and Type 2 Diabetes Mellitus without Clinical Signs of Diabetic Retinopathy. Retina 39, 435–445, doi: 10.1097/IAE.0000000000001990 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR & Staurenghi G Optical coherence tomography angiography. Prog Retin Eye Res 64, 1–55, doi: 10.1016/j.preteyeres.2017.11.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haines NR, Manoharan N, Olson JL, D’Alessandro A & Reisz JA Metabolomics Analysis of Human Vitreous in Diabetic Retinopathy and Rhegmatogenous Retinal Detachment. J Proteome Res 17, 2421–2427, doi: 10.1021/acs.jproteome.8b00169 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Liew G et al. Metabolomics of Diabetic Retinopathy. Curr Diab Rep 17, 102, doi: 10.1007/s11892-017-0939-3 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Mishra A & Newman EA Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia 58, 1996–2004, doi: 10.1002/glia.21068 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen TT et al. Correlation of light-flicker-induced retinal vasodilation and retinal vascular caliber measurements in diabetes. Invest Ophthalmol Vis Sci 50, 5609–5613, doi: 10.1167/iovs.09-3442 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Pannicke T et al. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes 55, 633–639, doi: 10.2337/diabetes.55.03.06.db05-1349 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Thompson K et al. Advanced glycation end (AGE) product modification of laminin downregulates Kir4.1 in retinal Muller cells. PloS one 13, e0193280, doi: 10.1371/journal.pone.0193280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDowell RE et al. Muller glial dysfunction during diabetic retinopathy in rats is reduced by the acrolein-scavenging drug, 2-hydrazino-4,6-dimethylpyrimidine. Diabetologia 61, 2654–2667, doi: 10.1007/s00125-018-4707-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portillo JC et al. CD40 in Retinal Muller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes 66, 483–493, doi: 10.2337/db16-0051 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlstetter M et al. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45, 30–57, doi: 10.1016/j.preteyeres.2014.11.004 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Mohamed Q, Gillies MC & Wong TY Management of diabetic retinopathy: a systematic review. JAMA 298, 902–916, doi: 10.1001/jama.298.8.902 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Simo R, Sundstrom JM & Antonetti DA Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 37, 893–899, doi: 10.2337/dc13-2002 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Titchenell PM & Antonetti DA Using the past to inform the future: anti-VEGF therapy as a road map to develop novel therapies for diabetic retinopathy. Diabetes 62, 1808–1815, doi: 10.2337/db12-1744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen QD et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119, 789–801, doi: 10.1016/j.ophtha.2011.12.039 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Do DV et al. One-Year Outcomes of the DA VINCI Study of VEGF Trap-Eye in Eyes with Diabetic Macular Edema. Ophthalmology 119, 1658–1665, doi: 10.1016/j.ophtha.2012.02.010 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Heier JS et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology 123, 2376–2385, doi: 10.1016/j.ophtha.2016.07.032 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Sun JK et al. Rationale and Application of the Protocol S Anti-Vascular Endothelial Growth Factor Algorithm for Proliferative Diabetic Retinopathy. Ophthalmology 126, 87–95, doi: 10.1016/j.ophtha.2018.08.001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wells JA et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl.J Med 372, 1193–1203, doi: 10.1056/NEJMoa1414264 [doi] (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arima M et al. Claudin-5 Redistribution Induced by Inflammation Leads to Anti-VEGF-Resistant Diabetic Macular Edema. Diabetes 69, 981–999, doi: 10.2337/db19-1121 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Potente M, Gerhardt H & Carmeliet P Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887, doi: 10.1016/j.cell.2011.08.039 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Miloudi K et al. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proc Natl Acad Sci U S A 116, 4538–4547, doi: 10.1073/pnas.1814711116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daneman R, Zhou L, Kebede AA & Barres BA Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566, doi: 10.1038/nature09513 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindblom P et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes & development 17, 1835–1840, doi: 10.1101/gad.266803 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park DY et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun 8, 15296, doi: 10.1038/ncomms15296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geraldes P et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 15, 1298–1306, doi:nm.2052 [pii] 10.1038/nm.2052 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geraldes P & King GL Activation of protein kinase C isoforms and its impact on diabetic complications. Circ.Res 106, 1319–1331 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Q et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Ye X et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139, 285–298, doi: 10.1016/j.cell.2009.07.047 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344, doi: 10.1016/j.cell.2012.10.042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y et al. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest 124, 3825–3846, doi: 10.1172/JCI76431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tokunaga CC, Chen YH, Dailey W, Cheng M & Drenser KA Retinal vascular rescue of oxygen-induced retinopathy in mice by norrin. Invest Ophthalmol Vis Sci 54, 222–229, doi: 10.1167/iovs.12-10127 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Ohlmann A et al. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci 30, 183–193, doi: 10.1523/JNEUROSCI.3210-09.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeilbeck LF et al. Norrin mediates angiogenic properties via the induction of insulin-like growth factor-1. Exp Eye Res 145, 317–326, doi: 10.1016/j.exer.2015.12.001 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Diaz-Coranguez M, Lin CM, Liebner S & Antonetti DA Norrin restores blood-retinal barrier properties after vascular endothelial growth factor-induced permeability. J Biol Chem, doi: 10.1074/jbc.RA119.011273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simo-Servat O, Hernandez C & Simo R Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm 2012, 872978, doi: 10.1155/2012/872978 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vujosevic S & Simo R Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Invest Ophthalmol Vis Sci 58, BIO68–BIO75, doi: 10.1167/iovs.17-21769 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Joussen AM et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. Faseb J 16, 438–440 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Huang H et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci 52, 1336–1344, doi:iovs.10–5768 [pii] 10.1167/iovs.10-5768 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joussen AM et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J 18, 1450–1452 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Demircan N, Safran BG, Soylu M, Ozcan AA & Sizmaz S Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (2005). [DOI] [PubMed] [Google Scholar]
- 75.Koleva-Georgieva DN, Sivkova NP & Terzieva D Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 53, 44–50 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Schoenberger SD et al. Increased Prostaglandin E2 (PGE2) Levels in Proliferative Diabetic Retinopathy and Correlation with VEGF and Inflammatory Cytokines. Investigative Ophthalmology & Visual Science, doi: 10.1167/iovs.12-10410 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Yoshimura T et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PloS one 4, e8158, doi: 10.1371/journal.pone.0008158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogata N et al. Pigment epithelium-derived factor in the vitreous is low in diabetic retinopathy and high in rhegmatogenous retinal detachment. Am J Ophthalmol 132, 378–382 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF & Antonetti DA TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59, 2872–2882, doi:db09–1606 [pii] 10.2337/db09-1606 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Titchenell PM et al. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem J 446, 455–467, doi: 10.1042/BJ20111961 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin CM et al. Inhibition of Atypical Protein Kinase C Reduces Inflammation-Induced Retinal Vascular Permeability. Am J Pathol 188, 2392–2405, doi: 10.1016/j.ajpath.2018.06.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gardner TW & Sundstrom JM A proposal for early and personalized treatment of diabetic retinopathy based on clinical pathophysiology and molecular phenotyping. Vision Res 139, 153–160, doi: 10.1016/j.visres.2017.03.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun JK et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 34, 968–974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Praidou A et al. Angiogenic growth factors and their inhibitors in diabetic retinopathy. Curr Diabetes Rev 6, 304–312 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Yokomizo H et al. Retinol binding protein 3 is increased in the retina of patients with diabetes resistant to diabetic retinopathy. Sci Transl Med 11, doi: 10.1126/scitranslmed.aau6627 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Ramirez M et al. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 52, 2633–2641, doi: 10.1007/s00125-009-1548-8 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Gao BB et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med 13, 181–188 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Clermont A et al. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes 60, 1590–1598, doi:db10–1260 [pii] 10.2337/db10-1260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sodhi A et al. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J Clin Invest 129, 4593–4608, doi: 10.1172/JCI120879 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xin X et al. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A 110, E3425–3434, doi: 10.1073/pnas.1217091110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouleti C et al. Protective effects of angiopoietin-like 4 on cerebrovascular and functional damages in ischaemic stroke. Eur Heart J 34, 3657–3668, doi: 10.1093/eurheartj/eht153 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Cho DI et al. Antiinflammatory activity of ANGPTL4 facilitates macrophage polarization to induce cardiac repair. JCI Insight 4, doi: 10.1172/jci.insight.125437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cerani A et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab 18, 505–518, doi: 10.1016/j.cmet.2013.09.003 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Wang X et al. LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature 499, 306–311, doi: 10.1038/nature12345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duraisamy AJ, Mishra M, Kowluru A & Kowluru RA Epigenetics and Regulation of Oxidative Stress in Diabetic Retinopathy. Invest Ophthalmol Vis Sci 59, 4831–4840, doi: 10.1167/iovs.18-24548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kowluru RA Diabetic retinopathy, metabolic memory and epigenetic modifications. Vision Res 139, 30–38, doi: 10.1016/j.visres.2017.02.011 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Barber AJ et al. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. Journal of Clinical Investigation 102, 783–791 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sohn EH et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A 113, E2655–2664, doi: 10.1073/pnas.1522014113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gardner TW, Abcouwer SF, Barber AJ & Jackson GR An integrated approach to diabetic retinopathy research. Arch Ophthalmol 129, 230–235, doi: 10.1001/archophthalmol.2010.362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lynch SK & Abramoff MD Diabetic retinopathy is a neurodegenerative disorder. Vision Res 139, 101–107, doi: 10.1016/j.visres.2017.03.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller WP et al. Deletion of the Akt/mTORC1 Repressor REDD1 Prevents Visual Dysfunction in a Rodent Model of Type 1 Diabetes. Diabetes 67, 110–119, doi: 10.2337/db17-0728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bogdanov P et al. Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes. Int J Mol Sci 19, doi: 10.3390/ijms19113578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santos AR et al. Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes 66, 2503–2510, doi: 10.2337/db16-1453 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Biessels GJ, Staekenborg S, Brunner E, Brayne C & Scheltens P Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5, 64–74, doi: 10.1016/S1474-4422(05)70284-2 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Kopf D & Frolich L Risk of incident Alzheimer’s disease in diabetic patients: a systematic review of prospective trials. J Alzheimers Dis 16, 677–685, doi: 10.3233/JAD-2009-1011 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Cheung CY, Ikram MK, Chen C & Wong TY Imaging retina to study dementia and stroke. Prog Retin Eye Res 57, 89–107, doi: 10.1016/j.preteyeres.2017.01.001 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Sundstrom JM et al. Proteomic Analysis of Early Diabetic Retinopathy Reveals Mediators of Neurodegenerative Brain Diseases. Invest Ophthalmol Vis Sci 59, 2264–2274, doi: 10.1167/iovs.17-23678 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stitt AW et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51, 156–186, doi: 10.1016/j.preteyeres.2015.08.001 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Wei Y et al. Nrf2 in ischemic neurons promotes retinal vascular regeneration through regulation of semaphorin 6A. Proc Natl Acad Sci U S A 112, E6927–6936, doi: 10.1073/pnas.1512683112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu Z et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 57, 204–213, doi: 10.1007/s00125-013-3093-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Z et al. Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. J Neurochem 133, 233–241, doi: 10.1111/jnc.13064 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abcouwer SF et al. Effects of ischemic preconditioning and bevacizumab on apoptosis and vascular permeability following retinal ischemia-reperfusion injury. Investigative Ophthalmology & Visual Science 51, 5920–5933, doi: 10.1167/iovs.10-5264 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Muthusamy A et al. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood Flow Metab 34, 522–531, doi: 10.1038/jcbfm.2013.230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hui Q et al. Inhibition of the Keap1-Nrf2 protein-protein interaction protects retinal cells and ameliorates retinal ischemia-reperfusion injury. Free Radic Biol Med 146, 181–188, doi: 10.1016/j.freeradbiomed.2019.10.414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stitt AW et al. Vascular stem cells and ischaemic retinopathies. Prog Retin Eye Res 30, 149–166, doi: 10.1016/j.preteyeres.2011.02.001 (2011). [DOI] [PubMed] [Google Scholar]
- 116.Mohan R & Kohner EM Retinal revascularisation in diabetic retinopathy. Br J Ophthalmol 70, 114–117, doi: 10.1136/bjo.70.2.114 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi K, Kishi S, Muraoka K & Shimizu K Reperfusion of occluded capillary beds in diabetic retinopathy. Am J Ophthalmol 126, 791–797, doi: 10.1016/s0002-9394(98)00242-6 (1998). [DOI] [PubMed] [Google Scholar]
- 118.Abaci A et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99, 2239–2242 (1999). [DOI] [PubMed] [Google Scholar]
- 119.Hernandez SL et al. Characterization of circulating and endothelial progenitor cells in patients with extreme-duration type 1 diabetes. Diabetes Care 37, 2193–2201, doi: 10.2337/dc13-2547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Altabas V Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye on? Int J Endocrinol 2015, 848272, doi: 10.1155/2015/848272 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel J et al. Functional Definition of Progenitors Versus Mature Endothelial Cells Reveals Key SoxF-Dependent Differentiation Process. Circulation 135, 786–805, doi: 10.1161/CIRCULATIONAHA.116.024754 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Naito H et al. Endothelial Side Population Cells Contribute to Tumor Angiogenesis and Antiangiogenic Drug Resistance. Cancer Res 76, 3200–3210, doi: 10.1158/0008-5472.CAN-15-2998 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Iba T et al. Isolation of tissue-resident endothelial stem cells and their use in regenerative medicine. Inflamm Regen 39, 9, doi: 10.1186/s41232-019-0098-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ingram DA et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105, 2783–2786 (2005). [DOI] [PubMed] [Google Scholar]
- 125.Kovacic JC & Boehm M Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res 2, 2–15, doi: 10.1016/j.scr.2008.05.005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naito H, Kidoya H, Sakimoto S, Wakabayashi T & Takakura N Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J 31, 842–855, doi: 10.1038/emboj.2011.465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wakabayashi T et al. CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell 22, 384–397 e386, doi: 10.1016/j.stem.2018.01.010 (2018). [DOI] [PubMed] [Google Scholar]
- 128.Sekiguchi H, Ii M & Losordo DW The relative potency and safety of endothelial progenitor cells and unselected mononuclear cells for recovery from myocardial infarction and ischemia. J Cell Physiol 219, 235–242 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Grant MB et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8, 607–612 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Otani A et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest 114, 765–774 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Otani A et al. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 8, 1004–1010 (2002). [DOI] [PubMed] [Google Scholar]
- 132.O’Neill CL et al. Endothelial cell-derived pentraxin 3 limits the vasoreparative therapeutic potential of circulating angiogenic cells. Cardiovasc Res 112, 677–688, doi: 10.1093/cvr/cvw209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Medina RJ et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl Med 6, 1316–1320, doi: 10.1002/sctm.16-0360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Neill CL et al. Therapeutic revascularisation of ischaemic tissue: the opportunities and challenges for therapy using vascular stem/progenitor cells. Stem cell research & therapy 3, 31, doi: 10.1186/scrt122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park SS et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci 56, 81–89, doi: 10.1167/iovs.14-15415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chambers SEJ et al. The Vasoreparative Function of Myeloid Angiogenic Cells Is Impaired in Diabetes Through the Induction of IL1beta. Stem Cells 36, 834–843, doi: 10.1002/stem.2810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Medina MJ et al. Outgrowth endothelial cells characterisation and their potential for reversing ischemic retinopathy. Investigative Ophthalmology & Visual Science 51, 5906–5913 (2010). [DOI] [PubMed] [Google Scholar]
- 138.Medina RJ et al. Ex-vivo Expansion of Human Endothelial Progenitors Leads to IL-8-Mediated Replicative Senescence and Impaired Vasoreparative Function Stem Cells (under 3rd revision) (2013). [DOI] [PubMed] [Google Scholar]
- 139.Yoder MC Defining human endothelial progenitor cells. J Thromb Haemost 7 Suppl 1, 49–52 (2009). [DOI] [PubMed] [Google Scholar]
- 140.Heo SC et al. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells 32, 779–790, doi: 10.1002/stem.1578 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Bertelli PM et al. Vascular Regeneration for Ischemic Retinopathies: Hope from Cell Therapies. Curr Eye Res, 1–13, doi: 10.1080/02713683.2019.1681004 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Prasain N et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol 32, 1151–1157, doi: 10.1038/nbt.3048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reid E et al. Preclinical Evaluation and Optimization of a Cell Therapy Using Human Cord Blood-Derived Endothelial Colony-Forming Cells for Ischemic Retinopathies. Stem Cells Transl Med 7, 59–67, doi: 10.1002/sctm.17-0187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakimoto S et al. CD44 expression in endothelial colony-forming cells regulates neurovascular trophic effect. JCI Insight 2, e89906, doi: 10.1172/jci.insight.89906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cahoon JM et al. Intravitreal AAV2.COMP-Ang1 Prevents Neurovascular Degeneration in a Murine Model of Diabetic Retinopathy. Diabetes 64, 4247–4259, doi: 10.2337/db14-1030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mendel TA et al. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PloS one 8, e65691, doi: 10.1371/journal.pone.0065691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hajmousa G et al. Human adipose tissue-derived stromal cells act as functional pericytes in mice and suppress high-glucose-induced proinflammatory activation of bovine retinal endothelial cells. Diabetologia 61, 2371–2385, doi: 10.1007/s00125-018-4713-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langhe R & Pearson RA Rebuilding the Retina: Prospects for Muller Glial-mediated Self-repair. Curr Eye Res, 1–12, doi: 10.1080/02713683.2019.1669665 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Ahmad I, Teotia P, Erickson H & Xia X Recapitulating developmental mechanisms for retinal regeneration. Prog Retin Eye Res, 100824, doi: 10.1016/j.preteyeres.2019.100824 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Zerti D, Collin J, Queen R, Cockell SJ & Lako M Understanding the complexity of retina and pluripotent stem cell derived retinal organoids with single cell RNA sequencing: current progress, remaining challenges and future prospective. Curr Eye Res, doi: 10.1080/02713683.2019.1697453 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Galloway CA et al. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. Proc Natl Acad Sci U S A 114, E8214–E8223, doi: 10.1073/pnas.1710430114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kiamehr M et al. Compromised Barrier Function in Human Induced Pluripotent Stem-Cell-Derived Retinal Pigment Epithelial Cells from Type 2 Diabetic Patients. Int J Mol Sci 20, doi: 10.3390/ijms20153773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim K et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290, doi: 10.1038/nature09342 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


