ABSTRACT
Background:
In patients undergoing assisted reproduction, levels of mitochondrial DNA (mtDNA) in the trophectodermal cells of the developing blastocyst are suggested to be associated with its ability to implant. However, discrepancies exist regarding the use of mtDNA levels as a reliable biomarker to predict outcomes of assisted reproduction.
Aims:
The aim of the study is to explore the association of trophectodermal mtDNA levels to determine blastocyst quality, implantation potential of blastocyst and clinical outcomes in couples who have undergone pre-implantation genetic testing for aneuploidy (PGT-A).
Study Setting:
Private fertility centre.
Study Design:
Retrospective analysis.
Materials and Methods:
We analysed mtDNA levels in the trophectodermal cells of 287 blastocysts from 61 couples undergoing PGT-A. The levels of mtDNA were estimated by next-generation sequencing method. mtDNA levels were correlated with maternal age, blastocyst morphology, ploidy status, implantation rates, miscarriage rate and live birth rate.
Statistical Analysis Used:
Linear regression and one-way ANOVA with Tukey's all column comparison test.
Results:
The trophectodermal mtDNA levels did not correlate with maternal age. There were no significant differences in their levels in grade 1 and grade 2 blastocysts. No significant differences were seen between mtDNA levels of implanted and non-implanted blastocysts or those blastocysts that resulted in miscarriage or live birth. However, significantly lower amounts of mtDNA were seen in euploid blastocysts as compared to that in aneuploid blastocysts.
Conclusion:
mtDNA levels in the trophectodermal cells of the blastocyst do not associate with blastocyst quality (grade 1 and grade 2), implantation potential and clinical outcomes but can differentiate between aneuploid and euploid blastocysts. Our study does not support the use of trophectodermal mtDNA levels as a biomarker for blastocyst quality and predictor of clinical outcomes.
KEYWORDS: Aneuploidy, implantation, live birth, maternal age, miscarriage rate, morphology, next-generation sequencing
INTRODUCTION
Assisted reproduction technologies (ARTs) have witnessed significant progress in the last three decades and have benefitted many infertile couples. However, despite tremendous advancements in the field, take-home baby rates remain low.[1] The success of ART is determined by several crucial factors such as maternal and paternal age, gamete quality, endometrial receptivity and embryo quality.[2,3,4,5] The current methods for assessing embryo quality involve the use of qualitative grading criteria or real-time monitoring with multiple quantitative end points. Despite these add-ons, an absolute improvement in pregnancy rates has not been observed.[6,7,8] The advent of single-cell genetic analysis has made it apparent that a large number of embryos developed in vitro are chromosomally aneuploid.[9,10] This has prompted the routine evaluation of the chromosomal complement of embryos by pre-implantation genetic testing for aneuploidy (PGT-A). While the introduction of PGT-A has led to a reduction in miscarriage rates,[11] the improvements in live birth rates following PGT-A remain relatively low.[12] As many as 50% of embryos diagnosed as “euploid” by PGT-A still do not implant, and many “mosaic” embryos still result in pregnancy.[13,14] Thus, the routine use of PGT-A in IVF clinics as a tool for embryo selection has been challenged.[15,16] Thus, there is a need to identify markers that may aid in embryo selection for improving pregnancy rates in patients undergoing assisted reproduction.
The processes of fertilisation and embryonic development rely heavily on energy derived from mitochondria.[17,18] The human oocytes contain the highest number of mitochondria per cell.[19] Studies in human embryos have highlighted that conditions such as aneuploidy, advanced maternal age or chemically induced stress are associated with higher mtDNA content.[20,21] This has prompted several groups to investigate if levels of mtDNA in the developing embryos can aid as a marker for embryo selection in ART. Fragouli et al. were the first to report that embryos with high levels of mtDNA have compromised implantation potential.[22] These findings were corroborated by additional studies that reported an increased mtDNA copy number was associated with lower implantation rates of euploid embryos.[23,24,25,26,27,28,29] Collectively, these findings reinforced the notion that beyond aneuploidy, quantification of mtDNA levels could serve as a biomarker for embryo selection. However, some studies have challenged this notion and have demonstrated that after controlling for inter-patient variables, mtDNA quantitation was not able to predict the implantation potential of an embryo.[30,31,32] Studies have also failed to find a correlation of mtDNA levels with blastocyst grade, implantation rates or on-going pregnancy rates in patients undergoing euploid embryo transfer.[23,31,32,33,34,35] Thus, it is unclear if mtDNA levels can predict the implantation potential of embryos and pregnancy outcomes.
Our centre routinely offers PGT-A by next-generation sequencing (NGS) to eligible couples who are undergoing assisted reproduction. To evaluate if the mitochondrial DNA content in the blastocyst is a useful marker for determining the implantation potential of the blastocysts, we recorded these data from the NGS files and analysed its association with outcomes in ART. Herein, we report the association of trophectodermal mtDNA levels to determine blastocyst quality, implantation potential of blastocyst and clinical outcomes in couples who have undergone PGT-A.
MATERIALS AND METHODS
Ethics statement
The study is a retrospective analysis of the anonymised data collected at Craft Hospital and Research Center. The ethical review board of Craft Hospital and Research Center Hospital and Research Center approved the study (Ethics no: 002/21/3/2019). The study was conducted following the ethical standards and adhered to the Declaration of Helsinki 2013. Informed consent was obtained from every participant included in this study.
Study design
A total of 61 couples agreed to undergo PGT-A by NGS from January 2016 to July 2017. Controlled ovarian stimulation was performed with antagonist protocol using gonadotropins with doses ranging between 150 and 300 IU depending on age and body mass index (BMI). Oocytes were aspirated under local anaesthesia after 36 h of agonist trigger. Denudation was performed an hour after oocyte retrieval. Intracytoplasmic sperm injection (ICSI) was performed as described previously.[36] For patients requiring oocyte accumulation, oocytes were frozen within 30 min post-denudation and ICSI was performed later. Zygotes were cultured in VITROMED culture medium (VITROMED, Germany) for 5–6 days. Fertilisation was checked on the day following ICSI after which day 3 embryo quality was checked. Good-quality day 3 cleavage stage embryos (defined as embryos with 6–8 cells, equal size of blastomeres and cytoplasmic fragmentation less than 10%) were allowed to grow till the blastocyst stage. Blastocyst quality was graded as described previously.[37,38]
Embryo/blastocyst biopsy and vitrification
Trophectoderm biopsy of only grade 1 and grade 2 blastocysts was done on day 5 as described previously.[13,39] Grade 3 blastocyst were excluded. Briefly, on day 5, herniating blastocysts were selected, and 5–10 trophectoderm cells were removed by suction followed by laser pulsation. The trophectoderm cells were collected in phosphate-buffered saline and stored at −80°C until further processing. The vitrification method was used to freeze down blastocysts. Briefly, the blastocysts were immersed in equilibration solution for 12–15 min, followed by transfer to vitrification solution. This was finally transferred to a cryolock containing liquid nitrogen.
Whole-genome amplification and next-generation sequencing of trophectodermal cells
Whole-genome amplification (WGA) on each biopsy was performed using the Rubicon PicoPLEX WGA kit (Agilent, CA, USA) as per the manufacturer's recommendations as detailed previously.[39,40] Following WGA, NGS of the trophectodermal biopsies was carried out. For constructing WGA library, Ion Xpress Plus fragment library kit and Ion Xpress barcode adapters kit were used as per the manufacturer's instructions (Thermo Fisher Scientific, MA, USA). Briefly, 150 ng of WGA DNA was fragmented to generate 280 base pair fragments. The purified fragmented DNA was barcoded and amplified as per the manufacturer's instructions (Thermo Fisher Scientific, MA, USA). These (a pool of 24 samples) were then loaded on an Ion 520 Chip and were sequenced on the Ion Gene Studio S5 system (Ion S5 System User Guide, Thermo Fisher Scientific, MA, USA). Approximately 4.8–5 million reads were obtained for each run generating 200,000–300,000 reads/embryo.
Analysis of ploidy and mitochondrial DNA levels
The ploidy status and mtDNA levels for all the blastocysts undergoing PGT-A with NGS were assessed with Ion Reporter Cloud-based software 5.18.2.0 (Thermo Fisher Scientific, MA, USA).
Embryo transfer
The endometrium was prepared for transplantation using hormone replacement protocol. Estradiol valerate was administered from day 2 of the cycle in a dose-dependent manner. Serial ultrasound monitoring was performed to check endometrial thickness (10 mm), after which oral and vaginal progesterone was administered and frozen embryo was transferred. The vitrified blastocyst from the cryolock was first directly placed in transfer solution followed by washing with sucrose solution at 37°C. Serum β-human chorionic gonadotropin (HCG) levels were measured using HCG STAT Elecsys assay on Cobas E601 Immunology Analyser (Roche, Basel, Switzerland) after 2 weeks of embryo transfer to detect biochemical pregnancy. Clinical pregnancy was determined as visualisation of an intrauterine gestation sac by transvaginal ultrasound performed at 6 weeks.
Estimation of implantation and clinical pregnancy rate
Implantation rate was calculated as the number of gestational sacs seen at 6 weeks of gestation in ultrasound divided by the total number of embryos transferred.
Statistical analysis
As it is a retrospective analysis, sample size calculations were not done. Linear regression analysis was performed to study the correlation between maternal age at the time of oocyte retrieval and mtDNA from the trophectodermal cells of the blastocyst. Correlation between mtDNA level and blastocyst morphology, ploidy status and pregnancy outcomes was carried out using one-way ANOVA with Tukey's all column comparison test. GraphPad Prism version 5.0 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com was used to perform all statistical analysis, and P < 0.05 was accepted as statistically significant.
RESULTS
A total of 61 couples opted for PGT-A during the study period. There were 287 grade 1 and grade 2 blastocysts from 61 couples who finally underwent NGS analysis for PGT-A. Presented below are the results from these 287 blastocysts [Figure 1].
Figure 1.
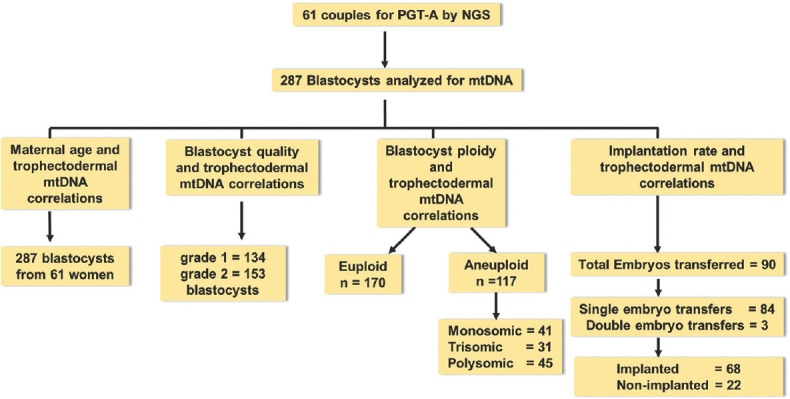
Flowchart depicting the numbers of embryos used to analyse the correlation between mtDNA levels in trophectoderm cells of the blastocyst and other parameters
Mitochondrial DNA levels in trophectodermal cells of the blastocyst and maternal age at the time of oocyte retrieval
To investigate the association of maternal age and trophectoderm mtDNA levels, data from 287 blastocysts were analysed by linear regression analysis. These embryos were generated from 61 women aged between 24 and 46 years (average age of 32.6 ± 4.13 years). The results revealed no correlation between maternal age at the time of oocyte retrieval and mtDNA levels [Figure 2]. The slope was 6.046e-005 and the R square value was 0.01327, and this was not statistically significant.
Figure 2.
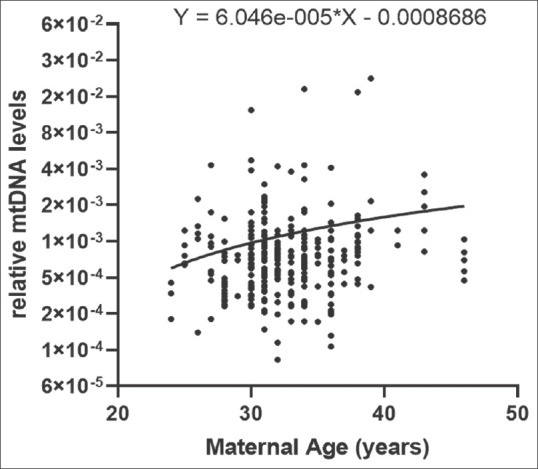
Correlation of mtDNA levels in trophectodermal cells data with maternal age at the time of oocyte retrieval. Values on X-axis are maternal age in years. Y-axis is relative mtDNA levels in the trophectodermal cells. The data are derived from 287 independent embryos. The equation of the slope is provided. mtDNA: Mitochondrial DNA
Mitochondrial DNA levels in the trophectodermal cells of the blastocyst and blastocyst morphology
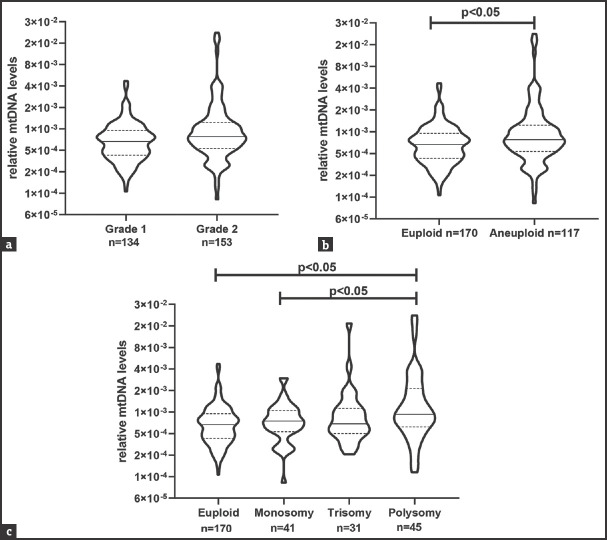
At our centre, PGT-A is only performed on grade 1 and 2 blastocysts. Thus, data for only these two grades are available. To understand if there is a correlation between the mtDNA levels in the trophectoderm cells and blastocyst morphology, mtDNA levels of grade 1 (n = 134) and grade 2 (n = 153) blastocysts were analysed. The results revealed no significant difference in the mtDNA levels between grade 1 and grade 2 blastocysts [Figure 3a].
Figure 3.
Association of levels of mtDNA in trophectodermal cells with blastocyst morphology and ploidy status. (a) Comparison of mtDNA levels in grade 1 and grade 2 blastocysts. (b) Comparison of mtDNA levels in euploid and aneuploid blastocysts. (c) Comparison of mtDNA levels in euploid, monosomic, trisomic and polysomic blastocyst. In all the graphs, the value on the Y-axis is relative mtDNA levels. In each violin, the solid horizontal bar is median and the dotted bars quartiles. The numbers (n) of blastocysts are given in each case. P < 0.05 was accepted as statistically significant. mtDNA: Mitochondrial DNA
Mitochondrial DNA levels in trophectodermal cells in aneuploid blastocysts
The blastocysts were classified as euploid and aneuploid to study the correlation between mtDNA levels and ploidy status of the blastocysts. Out of 287 blastocysts analysed, 170 were euploid and 117 were aneuploid. Of 117 aneuploid blastocysts, 41 were monosomic, 31 were trisomic and 45 were polysomic (aneuploidy of more than 1 chromosome). mtDNA levels were significantly high in aneuploid blastocysts compared to euploid blastocyst (P < 0.05) [Figure 3b]. When stratified by the type of aneuploidy, the mtDNA levels in polysomic blastocysts were significantly higher compared to euploid and monosomic blastocysts (P < 0.05) [Figure 3c].
Levels of mitochondrial DNA in trophectodermal cells of blastocyst in embryo implantation and clinical outcomes
In our study, all the couples had at least one euploid embryo for transfer. 90 embryos were transferred in all. Of these, 84 were single embryo transfers and 3 were double embryo transfers. From the 90 embryos transferred, 68 implanted successfully and 22 embryos did not implant. Thus, the overall implantation rate was 75.55%. There was no statistical difference in levels of mtDNA of implanted (n = 68) versus non-implanted blastocysts (n = 22). The couples who were positive for clinical pregnancy were followed up until term. There was no statistically significant difference in mtDNA levels and pregnancy [Figure 4] that resulted in miscarriage (n = 4) or live birth (n = 42).
Figure 4.
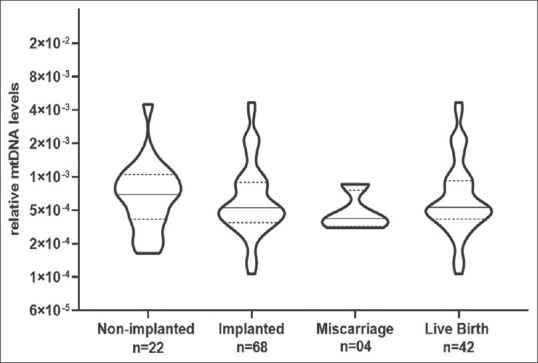
The association of mtDNA levels in trophectodermal cells with implanted euploid embryos and clinical outcomes. Values on the Y axis are relative mtDNA levels. The numbers (n) of blastocysts are given in each case. P < 0.05 was accepted as statistically significant. In each violin, the solid horizontal bar is median and the dotted bars quartiles. mtDNA: Mitochondrial DNA
DISCUSSION
In the present study, we analysed if blastocyst mtDNA levels can predict implantation potential. Our results indicate that mtDNA levels differ between euploid and aneuploid blastocysts, but mtDNA levels in the trophectoderm of grade 1 and 2 blastocysts do not correlate with their ability to implant.
The selection of a blastocyst for transfer in couples undergoing ART has been a major clinical challenge. With the discovery that a large proportion of in vitro developed human blastocysts are aneuploid,[41] the application of PGT-A to select euploid blastocysts has been integrated into mainstream clinical practice. While PGT-A has helped identify euploid embryos, one-quarter to half of these embryos still fail to implant,[42,43] suggesting that additional factors may govern embryo implantation. In recent years, measurement of mtDNA levels in the blastocyst has gained attention as a potential biomarker to predict the implantation potential of the embryo and pregnancy outcomes in ART.[44,45,46]
Mitochondria play a key role in cellular metabolism and fulfil a cell's energy requirements. In most organisms, the mitochondria are exclusively maternal in origin and any disruptions in their levels or activity can compromise cellular functions. It is well established that advanced maternal age has a detrimental effect on pregnancy outcomes due to compromised oocyte quality.[3,47] Studies have reported low mtDNA levels in cumulus cells or oocytes of women with advanced age.[48,49,50] In contrast, elevated mtDNA content is observed in blastocysts from women with advanced age.[21,22,27,28,51] In our study, however, we did not find any correlation between trophectoderm mtDNA levels and maternal age on analysing women within the age group of 24–46 years. Our findings are in agreement with other studies where a lack of correlation between maternal age and mtDNA copy number has been reported.[23,26,31,33,34,52] Differences in sample size and in the number of women with advanced maternal age could be possible reasons for such discrepancies in results across studies. Since, in our study, there were very few women with advanced maternal age (i.e. greater than 40 years) (age <35 = 45, age >35 = 16), it is possible that we have missed any correlation of mtDNA levels in women in the higher age group. However, the lack of correlation between advanced maternal age and mtDNA in the blastocyst is not unexpected because oocytes are arrested in meiosis prenatally, and hence, mitochondrial copy numbers are expected to be similar for all mothers irrespective of their age. Indeed, a large population-based study has shown that there is mtDNA turnover in oocytes during meiotic arrest, which contributes to an increase in numbers of mutations in the mtDNA, and there is a drift in heteroplasmy frequencies with age.[53] Together, the data indicate that maternal age may not contribute to alterations in mtDNA levels in the blastocysts although its effects on mtDNA genome sequence and heteroplasmy warrant further investigations.
The system of embryo grading based on morphological and or morphokinetic criteria is based on the premise that anatomically high-quality embryos must result in higher implantation. This is also evident from studies where endometrial stromal cells can differentiate between a morphologically good-quality and bad-quality embryo.[54] It is suggested that good-quality embryos have low metabolism whereas an embryo under stress tends to be more metabolically active.[55] This hypothesis has prompted several investigators to analyse levels of mtDNA in association with the developmental competence of the embryo. High levels of mtDNA in poor-quality embryos have been reported by several groups.[23,26,56] Interestingly, in our study, we did not find any correlation between mtDNA levels in the trophectodermal cells and the morphological grade of blastocysts. However, our analysis is only limited to grade 1 and 2 blastocysts as we did not perform PGT-A of grade 3 blastocysts, and hence, the data for the mtDNA in these are lacking. Indeed, it was reported that mtDNA levels best predicted the lowest and highest grades of embryos but not mid-grade embryos.[34] It must be kept in mind that these results are derived from embryos that are already visibly compromised, and hence, the clinical utility of mtDNA scores/levels for predicting blastocyst quality is rather limited.
We next tested the levels of mtDNA in trophectodermal cells and its association with the ploidy status of the blastocyst. We observed higher levels of mtDNA in trophectodermal cells of aneuploid blastocysts as compared to euploid blastocysts. Interestingly, higher levels of mtDNA in the trophectodermal cells were restricted to polysomic blastocysts; the levels in monosomic or trisomic blastocysts were comparable to those in the euploid blastocysts. Our results are consistent with earlier studies that report elevated levels of mtDNA in aneuploid embryos, specifically in polysomic embryos.[24,26,57] Elevated levels of mtDNA (above the mean ± 2SD standard deviation) were seen only in 4/45 polysomic and 1/31 trisomic blastocysts and were not a uniform feature of all polysomic or aneuploid blastocysts. These results imply that, in a clinical setup, the measurement of mtDNA in the trophectoderm cells cannot be a reliable feature to predict the ploidy status in most blastocysts.
The transfer of a morphologically and developmentally competent blastocyst to the uterus does not always result in pregnancy, and there is a constant search of endometrial and embryonic factors to predict the success of implantation.[58] Several studies have demonstrated that euploid embryos with higher mtDNA levels (above a particular cut-off) failed to implant, whereas embryos with low mtDNA implanted successfully.[21,22,24,57,59] However, we failed to detect any changes in mtDNA content in blastocysts that implanted versus those that did not implant. These results were consistent even after adjusting for single versus two embryo transfer cycles. We also failed to detect any embryos in our cohort that had mtDNA quantity above the threshold proposed by Fragouli et al.[22] In fact, in our cohort, the embryos that did not implant had mtDNA levels identical to those which implanted. Our results are in concordance with the studies that also reported that mtDNA levels or mitoscores were not associated with implantation rates and noted that there was no threshold above or below which pregnancy would or would not occur.[31,33,52] It is reported that embryos from women with higher BMI have higher mtDNA copy numbers and that high levels of maternal serum progesterone are inversely correlated with mtDNA levels.[26] However, in our study, the baseline BMI and serum progesterone levels did not significantly differ between the different groups analysed and had no effect on mtDNA levels after adjustments (data not shown). Thus, our results do not support the use of mtDNA levels (or mitoscore) to be used in ART clinics for embryo selection.
Finally, we asked if mtDNA levels could predict outcomes of implanted blastocysts. Our results revealed that levels of mtDNA in the blastocyst did not correlate with the risk of miscarriage or chance of live birth. Although the numbers of patients in the miscarriage group were few, the absence of an association between the mtDNA levels and miscarriage has also been reported in other studies.[33,52,59] However, most of these are retrospective analyses of the data with a limited sample size precluding statistical analysis.
CONCLUSION
Our results, in conjunction with the findings from other studies, reveal a lack of association of mtDNA levels with the different ART outcomes specifically in predicting blastocyst implantation potential or pregnancy outcomes. The limitations of the study are the nature of study design (retrospective analysis), small sample size (n = 61 couples) and single-centre data. Further, methodological differences in the quantification of mtDNA, number of cells during biopsy and sample storage method are other reasons for the inconsistent results between various studies.[43,45,60] There is a need to develop consistent selection criteria and a standardised procedure to study the correlation between mtDNA and ART outcomes. Multicentric data with uniform protocols and patient selection criteria are needed to arrive at a final consensus.
In summary, the present study fails to support the notion that trophectodermal mtDNA level can predict embryo quality, implantation ability of the blastocysts or pregnancy outcomes in ART. The results of our centre highlight that mtDNA copy number does not provide any advantage in embryo ranking and would lead to de-selection of blastocysts that may otherwise result in healthy pregnancies.
Availability of data and material
Raw data files can be made available upon reasonable request to the corresponding author.
Financial support and sponsorship
SL is grateful to Council of Scientific and Industrial Research, India for Senior Research Fellowship. SC is thankful to Department of Health Research for the grant YSS/2019/000118/PRCYSS.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sadeghi MR. Low success rate of ART, an illusion, a reality or simply a too high expectation? J Reprod Infertil. 2012;13:123. [PMC free article] [PubMed] [Google Scholar]
- 2.Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35:1953–68. doi: 10.1007/s10815-018-1304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–85. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- 4.Oron G, Son WY, Buckett W, Tulandi T, Holzer H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: A pilot study. Hum Reprod. 2014;29:1444–51. doi: 10.1093/humrep/deu079. [DOI] [PubMed] [Google Scholar]
- 5.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97:1028–32. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: Shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol. 2008;20:234–41. doi: 10.1097/GCO.0b013e3282fe723d. [DOI] [PubMed] [Google Scholar]
- 7.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: An observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 8.Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: A consecutive case series study. Hum Reprod. 2016;31:2245–54. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 9.Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril. 2016;105:1307–13. doi: 10.1016/j.fertnstert.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Verlinsky Y, Cieslak J, Freidine M, Ivakhnenko V, Wolf G, Kovalinskaya L, et al. Pregnancies following pre-conception diagnosis of common aneuploidies by fluorescent in-situ hybridization. Hum Reprod. 1995;10:1923–7. doi: 10.1093/oxfordjournals.humrep.a136207. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Zheng YM, Li L, Jin F. Preimplantation genetic screening: An effective testing for infertile and repeated miscarriage patients? Obstet Gynecol Int. 2010;2010:120130. doi: 10.1155/2010/120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato T, Sugiura-Ogasawara M, Ozawa F, Yamamoto T, Kato T, Kurahashi H, et al. Preimplantation genetic testing for aneuploidy: A comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. 2019;34:2340–8. doi: 10.1093/humrep/dez229. [DOI] [PubMed] [Google Scholar]
- 13.Capalbo A, Ubaldi FM, Cimadomo D, Maggiulli R, Patassini C, Dusi L, et al. Consistent and reproducible outcomes of blastocyst biopsy and aneuploidy screening across different biopsy practitioners: A multicentre study involving 2586 embryo biopsies. Hum Reprod. 2016;31:199–208. doi: 10.1093/humrep/dev294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: An analysis of the United States Assisted Reproductive Technology Surveillance Data, 2011-2012. Fertil Steril. 2016;105:394–400. doi: 10.1016/j.fertnstert.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleicher N, Barad DH, Ben-Rafael Z, Glujovsky D, Mochizuki L, Modi D, et al. Commentary on two recently published formal guidelines on management of “mosaic” embryos after preimplantation genetic testing for aneuploidy (PGT-A) Reprod Biol Endocrinol. 2021;19:23. doi: 10.1186/s12958-021-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gleicher N, Albertini DF, Barad DH, Homer H, Modi D, Murtinger M, et al. The 2019 PGDIS position statement on transfer of mosaic embryos within a context of new information on PGT-A. Reprod Biol Endocrinol. 2020;18:57. doi: 10.1186/s12958-020-00616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–53. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Jansen RP, de Boer K. The bottleneck: Mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol. 1998;145:81–8. doi: 10.1016/s0303-7207(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–95. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogur C, Findikli N, Caferler J, Gultomruk M, Capar B, Griffin DK, et al. Mitochondrial DNA (mtDNA) counts correlate to maternal age, day of biopsy and implantation potential. Reprod Biomed Online. 2019;38:e26–7. [Google Scholar]
- 22.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241. doi: 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: Less is better. Fertil Steril. 2015;104:534–41.e1. doi: 10.1016/j.fertnstert.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Fragouli E, McCaffrey C, Ravichandran K, Spath K, Grifo JA, Munné S, et al. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: A blinded prospective non-selection study. Hum Reprod. 2017;32:2340–7. doi: 10.1093/humrep/dex292. [DOI] [PubMed] [Google Scholar]
- 25.Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, et al. Mitochondrial DNA quantification as a tool for embryo viability assessment: Retrospective analysis of data from single euploid blastocyst transfers. Hum Reprod. 2017;32:1282–92. doi: 10.1093/humrep/dex070. [DOI] [PubMed] [Google Scholar]
- 26.de Los Santos MJ, Diez Juan A, Mifsud A, Mercader A, Meseguer M, Rubio C, et al. Variables associated with mitochondrial copy number in human blastocysts: What can we learn from trophectoderm biopsies? Fertil Steril. 2018;109:110–7. doi: 10.1016/j.fertnstert.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Shang W, Zhang Y, Shu M, Wang W, Ren L, Chen F, et al. Comprehensive chromosomal and mitochondrial copy number profiling in human IVF embryos. Reprod Biomed Online. 2018;36:67–74. doi: 10.1016/j.rbmo.2017.10.110. [DOI] [PubMed] [Google Scholar]
- 28.Lledo B, Ortiz JA, Morales R, García-Hernández E, Ten J, Bernabeu A, et al. Comprehensive mitochondrial DNA analysis and IVF outcome. Hum Reprod Open. 2018;2018:hoy023. doi: 10.1093/hropen/hoy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boynukalin FK, Gultomruk M, Cavkaytar S, Turgut E, Findikli N, Serdarogullari M, et al. Parameters impacting the live birth rate per transfer after frozen single euploid blastocyst transfer. PLoS One. 2020;15:e0227619. doi: 10.1371/journal.pone.0227619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;32:954–62. doi: 10.1093/humrep/dex034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2017;107:34–42.e3. doi: 10.1016/j.fertnstert.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Victor A, Griffin DK, Gardner D, Brake A, Zouves C, Barnes F, et al. Births from embryos with highly elevated levels of mitochondrial DNA. Reprod Biomed Online. 2019;39:403–12. doi: 10.1016/j.rbmo.2019.03.214. [DOI] [PubMed] [Google Scholar]
- 33.Qiu MY, Chen MS, Dayang C, Liu MP, Xia MJ, Yang BL, et al. The mitochondrial DNA content cannot predict the embryo viability. bioRxiv. 2018:445940. [doi: 10.1101/445940] [Google Scholar]
- 34.Klimczak AM, Pacheco LE, Lewis KE, Massahi N, Richards JP, Kearns WG, et al. Embryonal mitochondrial DNA: Relationship to embryo quality and transfer outcomes. J Assist Reprod Genet. 2018;35:871–7. doi: 10.1007/s10815-018-1147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YX, Chen CH, Lin SY, Lin YH, Tzeng CR. Adjusted mitochondrial DNA quantification in human embryos may not be applicable as a biomarker of implantation potential. J Assist Reprod Genet. 2019;36:1855–65. doi: 10.1007/s10815-019-01542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Velde H, De Vos A, Joris H, Nagy ZP, Van Steirteghem AC. Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3160–4. doi: 10.1093/humrep/13.11.3160. [DOI] [PubMed] [Google Scholar]
- 37.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Toward Reproductive Certainty: Fertility and Genetics Beyond. Carnforth: Parthenon Publishing; 1999. pp. 378–88. [Google Scholar]
- 38.Sen S, Dixit A, Thakur C, Gokral J, Hinduja I, Zaveri K, et al. Association of progesterone receptor gene polymorphism with male infertility and clinical outcome of ICSI. J Assist Reprod Genet. 2013;30:1133–9. doi: 10.1007/s10815-013-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritu G, Ashraf M. Birth of healthy twins after PGT-M for achondroplasia – First reported case in India and second in the world. Polymorphism. 2020;5:27–32. [Google Scholar]
- 40.Pai H, Palshetkar N, Garikipati R, Pai R, Charumati P, Palshetkar R. Successful outcome in a patient of recurrent implantation failures using non-invasive PGT-A: A case report. Polymorphism. 2021;7:31–5. [Google Scholar]
- 41.Shahbazi MN, Wang T, Tao X, Weatherbee BA, Sun L, Zhan Y, et al. Developmental potential of aneuploid human embryos cultured beyond implantation. Nat Commun. 2020;11:3987. doi: 10.1038/s41467-020-17764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seli E. Mitochondrial DNA as a biomarker for in-vitro fertilization outcome. Curr Opin Obstet Gynecol. 2016;28:158–63. doi: 10.1097/GCO.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 43.Wells D. Mitochondrial DNA quantity as a biomarker for blastocyst implantation potential. Fertil Steril. 2017;108:742–7. doi: 10.1016/j.fertnstert.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Cozzolino M, Marin D, Sisti G. New frontiers in IVF: mtDNA and autologous germline mitochondrial energy transfer. Reprod Biol Endocrinol. 2019;17:55. doi: 10.1186/s12958-019-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humaidan P, Kristensen SG, Coetzee K. Mitochondrial DNA, a new biomarker of embryonic implantation potential: Fact or fiction? Fertil Steril. 2018;109:61–2. doi: 10.1016/j.fertnstert.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Seli E. Mitochondria as a biomarker for IVF outcome. Reproduction. 2019;157:R235–42. doi: 10.1530/REP-18-0580. [DOI] [PubMed] [Google Scholar]
- 47.Londero AP, Rossetti E, Pittini C, Cagnacci A, Driul L. Maternal age and the risk of adverse pregnancy outcomes: A retrospective cohort study. BMC Pregnancy Childbirth. 2019;19:261. doi: 10.1186/s12884-019-2400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boucret L, Chao de la Barca JM, Morinière C, Desquiret V, Ferré-L’Hôtellier V, Descamps P, et al. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30:1653–64. doi: 10.1093/humrep/dev114. [DOI] [PubMed] [Google Scholar]
- 49.Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11:843–6. doi: 10.1093/molehr/gah243. [DOI] [PubMed] [Google Scholar]
- 50.Ogino M, Tsubamoto H, Sakata K, Oohama N, Hayakawa H, Kojima T, et al. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016;33:367–71. doi: 10.1007/s10815-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Munck N, Linana A, Elkhatib I, Bayram A, Arnanz A, Rubio C, et al. mtDNA dynamics between cleavage-stage embryos and blastocysts. J Assist Reprod Genet. 2019;36:1867–75. doi: 10.1007/s10815-019-01544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott RT, 3rd, Sun L, Zhan Y, Marin D, Tao X, Seli E. Mitochondrial DNA content is not predictive of reproductive competence in euploid blastocysts. Reprod Biomed Online. 2020;41:183–90. doi: 10.1016/j.rbmo.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Zaidi AA, Wilton PR, Su MS, Paul IM, Arbeithuber B, Anthony K, et al. Bottleneck and selection in the germline and maternal age influence transmission of mitochondrial DNA in human pedigrees. Proc Natl Acad Sci U S A. 2019;116:25172–8. doi: 10.1073/pnas.1906331116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashary N, Tiwari A, Modi D. Embryo implantation: War in times of love. Endocrinology. 2018;159:1188–98. doi: 10.1210/en.2017-03082. [DOI] [PubMed] [Google Scholar]
- 55.Leese HJ. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–9. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- 56.Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–75. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tüfekçi M, Cetinkaya C, Colakoglu Y, Cetinkaya M, Kahraman S. Mitochondrial DNA content is not related with pregnancy but with euploidy. Reprod Biomed Online. 2019;38:e26. [Google Scholar]
- 58.Mishra A, Ashary N, Sharma R, Modi D. Extracellular vesicles in embryo implantation and disorders of the endometrium. Am J Reprod Immunol. 2021;85:e13360. doi: 10.1111/aji.13360. [DOI] [PubMed] [Google Scholar]
- 59.Rechistsky S, Cram D, Leigh D, Cao Y, Wang L, Yao Y, et al. Mitochondrial DNA levels are not a simple indicator for preselection of euploid embrios with higher developmental potential. Reprod Biomed Online. 2019;38:e25. [Google Scholar]
- 60.Fragouli E, Alfarawati S, Spath K, Jaroudi S, Sarasa J, Enciso M, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132:1001–13. doi: 10.1007/s00439-013-1309-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data files can be made available upon reasonable request to the corresponding author.