1. Osteoarthritis pain: A worldwide unmet medical need.
In the United States, approximately 20% of adults suffer from chronic pain, creating a substantive personal and societal burden and costing the economy up to $635 billion per year1,2. The musculoskeletal system is one of the leading sources of chronic pain, with osteoarthritis (OA) as the leading cause. As the most prevalent form of arthritis, OA affects over 32.5 million US adults. Worldwide, 240 million individuals live with symptomatic OA3–6, and its prevalence is increasing as the world’s population is ageing7. It has been recognized that OA is associated with an increased risk of all-cause mortality, often because of reduced mobility8.
Chronic pain and disability define clinical OA, and activity-limiting pain has been shown to be the greatest burden driving patients to seek medical attention in older adults9. Chronic pain can have a profound negative impact on patients’ physical lives as well as their mental health, and knee OA is significantly correlated with poor mental health and depressive symptoms10–12.
Disease-modifying and pain-alleviating therapies for OA remain elusive. Currently available analgesics are largely inadequate in addition to having adverse side effects, resulting in a major unmet medical need for patients. Therefore, furthering our understanding of the origins and mechanisms of OA pain will be critical to developing effective treatments.
2. Pain in Osteoarthritis
OA pathology is characterized by progressive joint damage, including cartilage erosion, subchondral bone sclerosis, synovitis, bone remodeling with osteophyte formation, and meniscal damage, all of which may contribute to pain. There is no simple correlation between pain and structural pathology, and in knee OA, studies have only found weak correlations between pain and radiographic findings13–15. In contrast, MRI studies, which image soft tissues, have uncovered that synovitis and bone marrow lesions are correlated with pain. This suggests that specific aspects of joint pathology may indeed cause OA pain, offering the prospect for targeted treatment strategies, although this has not yet led to agents that have been approved by regulatory agencies16. Attempts have also been made to stratify patients based on pain phenotypes, but translating this into therapeutic strategies has so far been unsuccessful17.
Pain in OA is complex and multi-factorial, modified by genetic, psychological, and environmental determinants. Patients struggle to self-describe pain with just ‘intensity’, and describe numerous types of pain that vary in duration, depth, type of occurrence, impact, and rhythm18. Several recent reviews have thoughtfully discussed different complex aspects of the genesis of pain in OA, including peripheral mechanisms of pain and sensitization19 20, central pain pathways21, the role of innate immune responses22, and structural correlates of OA pain23. When considering the genesis of joint pain, we should bear in mind that pain is one of the cardinal signs of inflammation. While the role of low-level inflammation in the pathogenesis of OA joint damage has been extensively studied in recent years24, it is not at all clear how exactly inflammation contributes to the accompanying pain. Targeting inflammation in OA can provide pain relief for some patients, but the effect size for nonsteroidal anti-inflammatory drugs (NSAIDs), a first-line treatment for OA, is moderate at best25. Likewise, intra-articular corticosteroid injections – while providing acute pain relief – have not shown long-term analgesic effects26,27. This illustrates that joint inflammation alone is not enough for sustaining pain in OA. Inflammatory mediators can act on many cells, including neurons, and inflammation comes in many guises, operating at many levels, including in the joint, in the nervous system (“neuro-inflammation”), and systemically. In addition, inflammation can both induce pain, as well as promote the resolution of pain. In this narrative review, we discuss recent discoveries that clarify how these different levels of inflammation may initiate, maintain, and modify pain in OA. We searched PubMed for the following terms: “osteoarthritis”, “pain”, “inflammation”, “cytokines”, “NGF”, “synovium”, “DRG”, “obesity”, and principally focused on studies published in the last 5 years.
3. Local inflammation in the OA joint: contributions to pain.
The pain pathway can be broken down into signal detection and neuron stimulation, signal transmission to the dorsal horn via the peripheral nervous system, and transmission to the brain via the central nervous system (Figure 1). A range of mechanical, thermal, and chemical stimuli - both noxious and non-noxious - are sensed in peripheral tissues via sensory neurons. This generates action potentials that carry these signals to the dorsal horn via the dorsal root ganglia (DRG), where the cell bodies of sensory neurons are located. Sensory nerves are either thick, myelinated Aβ fibers which are activated by joint movement, or thinly myelinated Aδ and unmyelinated C fibers, which are activated by noxious mechanical, thermal and chemical stimuli through specialized receptors28. These high-threshold pain-sensing neurons are called nociceptors (from the Latin nocere – to harm). For example, noxious heat is detected by the capsaicin receptor, transient receptor potential vanilloid type 1 (TRPV1)29, generating action potentials that invade the DRG. Voltage-gated sodium channels are essential for the generation of action potentials. From the DRG, the painful signals are then transmitted to the dorsal horn in the spinal cord, where the first synapse occurs28. Second-order neurons, activated by the release of glutamate, project to supraspinal regions, and from there, the signal is relayed to higher regions of the brain, where pain perception occurs, and information is generated on the location and intensity of the pain, while affective aspects modify the pain experience30.
Figure 1: Pain pathway and sites of inflammation and neuroplasticity in osteoarthritis.
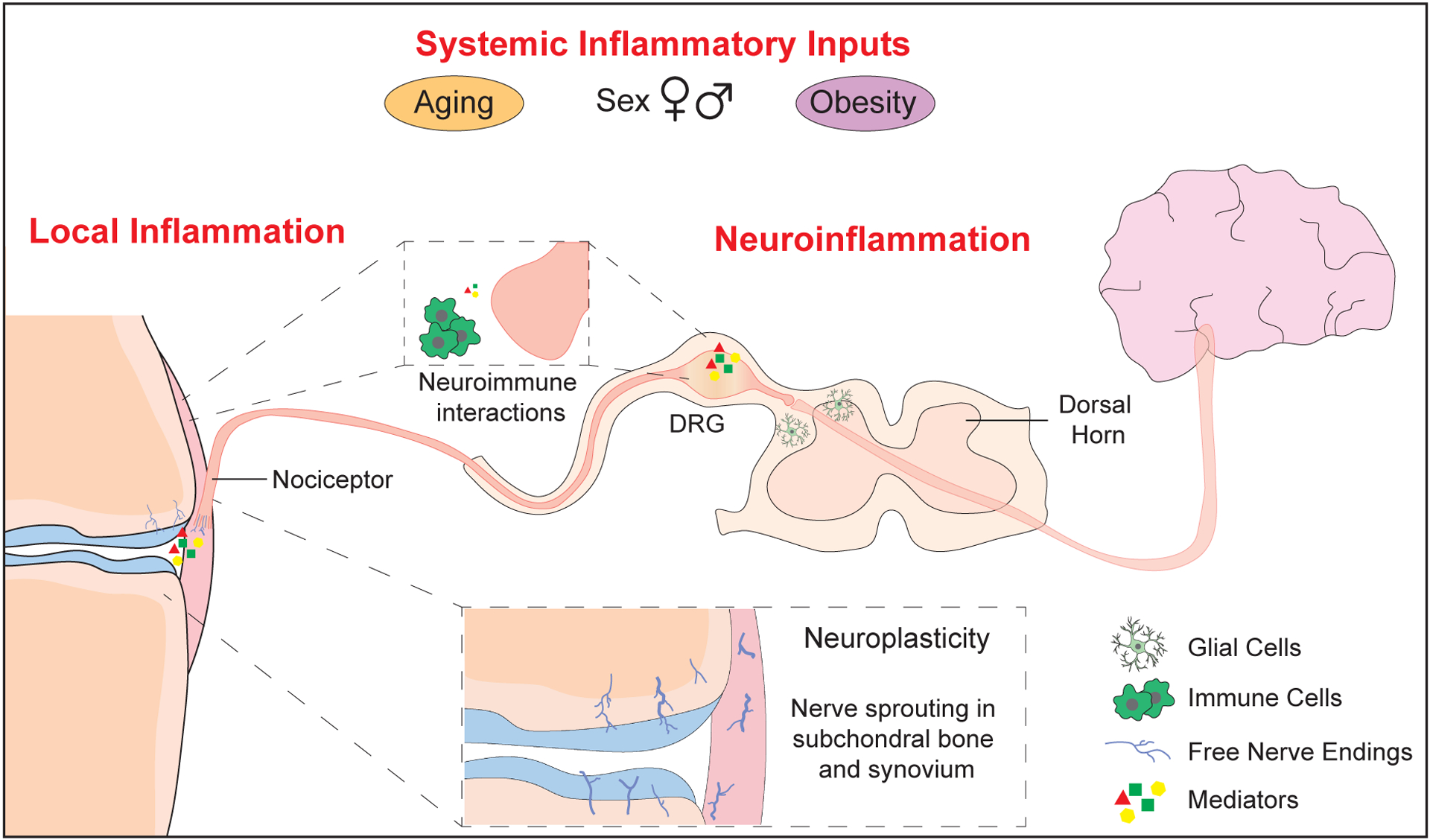
Nociceptors (Aδ or C- fibers) detect stimuli in joint tissues, generating action potentials that are transmitted to the dorsal horn via the dorsal root ganglia (DRG), and to the brain via the central nervous system. Inflammatory states and neuroimmune interactions can occur at multiple points along this pathway. Structural neuroplasticity occurs in the OA joint, with free nerve endings sprouting in the subchondral bone and synovium. Joint nociceptors become sensitized by mediators released in OA pathology, including nerve growth factor (NGF), inflammatory cytokines and chemokines, and damage-associated molecular patterns (DAMPs). Immune cell infiltration in synovium and DRG can include macrophages, T- and B- lymphocytes, and mast cells, which can further amplify pain mechanisms. Neuroimmune interactions also occur in the DRG and the dorsal horn. Finally, factors such as obesity, age, and sex may alter systemic inflammatory inputs and modify OA pain.
Below, we will discuss how inflammatory mediators generated in the OA joint may modify the nociceptive pathway through direct effects on sensory neurons that innervate joint tissues.
3.1. Local inflammatory mediators in the joint may affect neuronal activity and neuronal sprouting.
Inflammation and tissue injury are associated with sensitization of pain pathways, which results in augmented pain responses. For example, patients with OA are sensitized to mechanical stimuli, and quantitative sensory testing (QST) has revealed hyperalgesia, manifesting as lowered pain pressure thresholds when a force is applied to the joint, as well as allodynia, where non-noxious stimuli are perceived as painful. QST measures are more closely associated with pain severity than radiographic severity, and may aid in identifying people susceptible to chronic pain (reviewed in31). Sensitization to mechanical stimuli is also a feature of all animal models of OA32. Thus, it can be expected that elucidating the mechanisms underlying sensitization of joint nociceptors may be a key step in understanding the genesis of chronic OA pain33. Here, we will briefly discuss inflammatory mediators present in the joint that may promote pain through direct effects on joint nociceptors.
3.1.1. Inflammatory cytokines and chemokines -
Inflammatory mediators, including cytokines, chemokines, neurotrophins, and prostaglandins can all activate nociceptors through direct binding of their cognate receptors expressed by these cells34. OA joint tissues and the synovial fluid contain many such pro-algesic factors, including tumor necrosis factor (TNF)-α, interleukin (IL)1-β, IL-6, and chemokine (C-C motif) ligand 2 (CCL2)35, and in vitro studies have shown that OA synovial fluid induces hyperexcitability in DRG neurons36. In subjects with OA, synovial fluid levels of IL-7, IL-12, interferon (IFN)-γ, IL-10 and IL-13 have been correlated with knee pain37. Clinical trials with antibodies neutralizing traditional pro-inflammatory cytokines, including IL-1 and TNF-α, have been largely disappointing38, and more recent work in animal models deals with cytokines that were previously not a focus in OA research, including IL-17 and IL-33. IL-17A causes long-lasting sensitization of joint nociceptors to mechanical stimuli when injected into the rat knee39. In an inflammatory model (antigen-induced arthritis), Il17 null mice have less mechanical hyperalgesia, even though joint damage is as severe as in wild type mice, suggesting a direct contribution of IL-17A to the genesis of pain40. Na et al. reported that IL-1 receptor antagonist (IL-1Ra) null mice have increased pain and cartilage destruction in the monosodium iodoacetate (MIA) model of OA, but attenuation of increased IL-17 levels using IL-17/IL-1RA double-deficient mice resulted in significantly decreased pain scores41. Another cytokine that was recently studied in the context of OA is IL-33, a member of the IL-1 family that plays a role in innate and adaptive immunity42. He et al. reported increased concentrations of IL-33 in the synovial fluid of OA patients, and increased expression of IL-33 and its receptor, suppression of tumorigenicity 2 (ST2), in OA chondrocytes43. IL-33 expression is also increased in mice after DMM surgery, and intra-articular administration of recombinant IL-33 caused mechanical allodynia. Neutralizing monoclonal antibodies against IL-33 and ST2 attenuated both OA progression and pain in the DMM model.
There is also continued interest in the chemokine, CCL2, as a target for OA pain. CCL2 levels are increased in OA synovial fluid, and this has been correlated with symptoms44,45. Several laboratories have found that CCL2-CCR2 signaling is essential for the initiation and maintenance of pain-related behaviors in the DMM model (reviewed in46). This signaling pathway may contribute to OA pain through multiple mechanisms, including macrophage recruitment to the joint and to the DRG, as well as through direct neuronal activation47,48. CCR2 (the main receptor for CCL2) is expressed on intra-articular nociceptors, both in naïve mice and after destabilization of the medial meniscus (DMM)49. Furthermore, intra-articular injection of CCR2 receptor antagonist into the knee joint reduced knee hyperalgesia after DMM surgery, suggesting a local role for CCL2-CCR2 signaling in sensitization of joint nociceptors, and providing a possibility for therapeutic targeting of this pathway in early disease.
Finally, targeting the transcription factor, NF-κB, which is activated by both mechanical stress as well as cytokines and chemokines, remains an area of active investigation. In particular, recent work has focused on developing intra-articular therapeutic strategies that target this pathway in the context of post-traumatic OA, revealing analgesic effects50.
3.1.2. Nerve Growth Factor (NGF) -
The neurotrophin, NGF, has received much attention in the field in recent years, because clinical trials with monoclonal antibodies that neutralize NGF showed promising analgesic effects in OA of the knee51. However, the ill-understood risk for rapidly progressive OA in a subset of patients receiving the antibodies has prevented FDA approval of this biological therapy52.
During embryonic development, NGF is crucial for the growth and survival of neurons. Postnatally, NGF retains key functions, especially in inflammation, when levels are dramatically increased53. Pain production is a major biological effect of NGF, and local injection of NGF produces immediate and often long-lasting sensitization and pain responses in animals and humans, including when injected into the joint54–57. The mechanisms by which NGF exerts its pain-producing actions have been extensively reviewed, and involve both rapid effects and long-term neuroplastic changes58. The high-affinity receptor for NGF, tropomyosin kinase A (TrkA), is expressed by nociceptors, and NGF-TrkA signaling results in excitatory effects that lead to functional sensitization of nociceptors, both through transactivation of ion channels such as TRPV1, and through retrograde transport of the NGF/TrkA complex to the cell bodies in the DRG, where it initiates gene expression changes that lead to synthesis of pro-algesic peptides (substance P, CGRP) and ion channels such as NaV1.8.
An important property of NGF that may contribute to its pain-promoting activity, is that it can stimulate neuronal sprouting at an injured site, as has been described in models of bone fracture, bone cancer, and inflammation induced by intra-articular (i.a.) complete Freund’s adjuvant, where anti-NGF antibodies blocked neuronal sprouting and pain58. This biological property may be of key importance in the context of OA, since it is becoming increasingly clear that profound neuronal plasticity of nociceptors occurs as part of OA joint pathology. Most strikingly, in human OA knees as well as in rodent models, osteochondral channels breach the tidemark between the subchondral bone and the articular cartilage, and these channels contain neurons and blood vessels59–62. At this time, it is not clear to which extent these newly sprouted nociceptors contribute to pain in human OA knees, but the presence of CGRP-immunoreactive nociceptors in these channels has been associated with pain61. Neuronal sprouting has also been reported in the medial synovium in a surgical murine model of OA62.
Clearly, it will be important to identify the key drivers of this neuroplasticity in order to enhance our understanding of the genesis of OA joint pain. Candidate molecules have been identified, such as netrin, an osteoclast-derived molecule that has been linked to neuronal sprouting in the subchondral bone in a surgical murine model63. Importantly, NGF levels are elevated in the synovial fluid of OA joints64, and expression of NGF is increased in OA chondrocytes64,65, synovial fibroblasts66 (associated with pain) and osteochondral channels67 (also associated with pain). Therefore, it is highly likely that NGF plays a key role in the observed anatomical neuroplasticity observed in OA joints, although this has not been directly investigated.
3.1.3. Damage associated molecular patterns (DAMPs) -
Products produced as part of the joint degradation process have the potential to further amplify ongoing joint damage, and innate immune mechanisms are the focus of intense research in OA pathogenesis68, including OA pain (recently reviewed22). Indeed, joint remodeling in OA generates a host of DAMPs, such as fragments of proteoglycans or inflammation-associated alarmins (e.g., S100 proteins) that can bind receptors, including Toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are expressed by many joint tissue cells, including nociceptors. This leads to direct activation of nociceptors, resulting in pain. Tissue damage products can also signal to TLRs on innate immune cells, causing them to produce chemokines and cytokines that also act on pain-sensing neurons, further amplifying and maintaining pain responses.
3.2. The role of synovium in osteoarthritis pain
OA pathology involves all joint tissues, and inflammatory cytokines,chemokines, DAMPs, and NGF can exert their biological effects in all these tissues, and sensitize nociceptors. As we have discussed, neuronal sprouting has been observed in the subchondral bone of OA joints, as well as in the synovium (Figure 1). This begs the question as to how synovitis may contribute to OA pain. The synovial tissue lines the joint cavity and secretes synovial fluid, aiding in joint movement. Inflammation of the synovial tissue is observed in more than half of OA patients throughout disease progression, including early stages of disease69,70. Synovitis, as detected by MRI, is associated with pain in knees with and without radiographic OA, as well as with sensitization in knee OA71,72. Of interest, different anatomical patterns of synovitis have been described, and a pattern including patellar sites of synovitis have been particularly associated with pain73. Synovitis in the OA joint is characterized by an influx of a variety of leucocytes, primarily macrophages and T-lymphocytes, but mast cells, B-lymphocytes and plasma cells can also be present74. The quantity of activated macrophages has been associated with OA pain severity and radiographic OA severity75. Correlation studies have also suggested a role for lymphocytes in OA pain; for example, Zhu et al. showed a positive correlation between circulating CD4+ T cells and WOMAC pain scores76. In the synovial tissue, proinflammatory T helper cell type 1 (Th1) infiltration has been recently reported in early OA patients77. Similarly, Nees et al. recently investigated T cell subsets in the synovial tissue of OA patients, with a focus on pain and disability, and found correlations between levels of both Th1 and Th2 T cells with OA-related knee pain78. Finally, mast cell numbers have also been found to be elevated in OA synovium73, and an interesting recent study in murine MIA reported that NGF-stimulated synovial mast cells produce prostaglandin D2, which can signal to nociceptors, thus linking NGF-TrkA signaling in mast cells to OA pain79.
In order to unravel how infiltrating immune cells contribute to OA pain, it will be critical to elucidate the bi-directional crosstalk between the nervous system and the immune system. For example, targeting granulocyte macrophage colony stimulating factor (GM-CSF) prevents pain in murine collagenase-induced osteoarthritis (CiOA)80,81. Tewari et al. showed the absence of functional GM-CSF receptors on mouse nociceptors, but found that conditioned medium from GM-CSF treated macrophages could drive nociceptor transcriptional changes82. This exemplifies again how neuroimmune crosstalk may operate in the knee and offer avenues for targeted intervention.
Recent development of technologies such as bulk and single cell RNA sequencing has allowed a broader approach to mapping the immune cell environment in the synovial tissue. While most studies have not yet examined a direct link of the synovial microenvironment to OA pain, they highlight that the advancement of these technologies will allow delineation of the relationship between immune cells and OA pain in the future. In particular, Chou et al. performed single cell RNAseq on synoviocytes of three patients undergoing total knee replacement for medial compartment knee OA. This allowed them to identify 12 synovial cell types, including synovial fibroblasts, endothelial cells, and various types of immune cells. In addition, they were able to molecularly define the synovial cells producing particular cytokines, for example, inflammatory macrophages and dendritic cells expressing HLA-DQA1, HLA-DQA2, oxidized low density lipoprotein receptor 1 (OLR1), or TLR2 were linked to production of IL-1β83. Another study used bulk RNAseq to characterize macrophages purified from the synovial tissue of subjects with OA or with inflammatory arthritis84. Gene expression analyses and phenotyping revealed a heterogeneous population of macrophages within the OA samples, one of which were “inflammatory-like” macrophages that had a proliferative phenotype and aligned closely with inflammatory arthritis macrophages. While neither of these studies incorporated pain as a variable, they clearly illustrate how such approaches may aid the identification of specific cell types and their interactions with other cell types such as neurons, and how they contribute to particular aspects of the pathology, including pain. To illustrate this point, a recent single cell RNAseq study uncovered that synovial fibroblasts from painful sites of the patellar synovium were distinct from fibroblasts from non-painful areas, with an altered gene signature promoting fibrosis, inflammation and growth of neurons85, supporting the idea that synovitis is linked to neuroplasticity, and thus pain. Finally, a bulk RNAseq study analyzed synovial biopsies collected from knee OA patients with either low (0–3) or high (7–10) visual analogue scale (VAS) pain levels at the time of surgery86. Interestingly, while no major differences in the cellular composition were noted between pain groups, their analysis identified neuronal genes such as NTRK2, encoding the receptor for the neurotrophin BDNF, as differently upregulated in the high vs. low pain groups. The detailed identification and characterization of synovial tissue immune cells and molecular profiles, and their interactions with sensory neurons hold great promise for unraveling precise mechanisms of pain generation in the joint.
4. Neuroinflammation: An emerging player in OA pain
Inflammatory responses in the central and peripheral nervous system are considered “neuroinflammation”, a process increasingly recognized to contribute to chronic pain87, including in OA88. A hallmark of neuroinflammation is the activation of glial cells, such as microglia, as well as infiltrating macrophages in the DRG and the dorsal horn, which release chemokines and other mediators, and through neuroimmune interactions, modify pain signaling. The description of neuroinflammation in OA is still its infancy, as briefly summarized below.
4.1. DRG
Neuroinflammation has been described in several rodent models of OA. For example, in a rat CiOA model, DRG satellite glial cells become activated, alongside microglia activation in the dorsal horn – coinciding with pain development89. In addition to activation of resident glia, a growing body of recent research implicates immune cell infiltration of the DRG, in particular macrophages, as a contributor to pain in OA47,90. Raoof et al. showed in two rodent models of OA that M1-like macrophages accumulate in the DRG away from injury site. Furthermore, inhibition of these M1-like macrophages in the DRG by intrathecal injection of IL-4/IL-10 fusion protein or M2-like macrophages resolved persistent pain90. Microarray analysis of DRGs from a murine OA model distinguished genes differentially expressed in early vs. persistent OA pain, and identified genes that contribute to the initiation and maintenance of OA pain and neuroinflammation canonical pathway, including Cx3cl1, Ccl2, Tlr1, and Ngf91.
4.2. Dorsal horn
Ongoing C-fiber input in the dorsal horn markedly increases second-order neuron excitability, causing activation of microglia and astrocytes, which produce a host of cytokines and chemokines that further amplify the signal30. In experimental models of OA, these processes are only beginning to be studied, and activated microglia have been described in the rat MIA model92, as well as in a surgical model of OA, where microgliosis was a feature of late-stage OA and was associated with persistent pain93. At this time, it is not clear if targeting microglia activation can modify pain. A recent study in a rat MIA model reported that inhibition of glia with minocycline and fluorocitrate alleviated mechanical allodynia. This activation state was present alongside increased joint nociceptor innervation and DRG expression of Activating Transcription Factor 3 (ATF3), a neuronal stress marker. The authors hypothesized that microglial activation induced proinflammatory changes that contributed to OA pain94.
Nuclear factor-kappa B (NF-κB) is a transcription factor with well described roles in inflammatory responses. Previous studies have reported the association of NF-κB activity with chronic pain in the complete Freund’s adjuvant (CFA) model of inflammation95, vincristine-induced neuropathy model96, and an RM-1 induced bone cancer pain model97. Li et al. sought to identify the mechanism NF-κB, astrocyte proliferation and pain in the rat MIA OA model. They found that both the number of astrocytes, and expression of NF-κB/p65 in astrocytes in the dorsal horn increased significantly. Furthermore, upon spinal inhibition of NF-κB/p65, mechanical hyperalgesia was alleviated and significant decreases were measured in the expression inflammatory cytokines IL-1β, TNF-α and IL-33 in the dorsal horn98.
While we know very little about the precise mechanisms operating in OA, neuroimmune interactions clearly modify pain pathways, not just in the joint but also in the peripheral and central nervous systems. Information on microglial and astrocyte activation in the brain is scant, but we identified one recent study that reported supraspinal astrocyte activation as a mechanism underlying anxiety-augmented pain behaviors in rat MIA99. Furthermore, microglia activation was recently shown in a murine model of rheumatoid arthritis, illustrating the wide impact of joint inflammation on the nervous system100. Hence, it can be expected that the study of neuroinflammation holds great potential for identifying specific pathways that may be targeted to attenuate pain in chronic arthritis.
5. Systemic inflammatory inputs to pain
Chronic inflammation can be associated with increased systemic levels of cytokines and other mediators that may have a profound effect on the body’s homeostasis, including pain. Below, we briefly discuss factors that have been associated with systemic inflammation, including obesity, and how they may affect nociception, sensitization, and chronic pain in the context of OA.
5.1. Obesity
A correlation between obesity and OA has been historically recognized, with research showing that in as many as 24% of OA patients, disease can be attributed to being overweight or obese101. In addition, being overweight/obese is also associated with increased OA pain102. Mechanical overload of the joint may contribute to this, but obesity has also been associated with OA in non-weight bearing joints, suggesting that biomechanical loading may not be the sole cause of OA pain in obese or overweight patients and that other factors such as systemic and/or local inflammation may contribute103,104. In support of this, the association between metabolic syndrome and knee OA is greater for symptomatic OA compared to radiographic OA105. Links between obesity and non-mechanical painful disorders such as headaches and migraines have also been reported, suggesting a direct role for diet and intake of essential fatty acids in chronic pain, with suggested links to altered inflammatory states106.
Several studies in experimental models have attempted to unravel these complex relationships between obesity, adiposity, loading, inflammation, and pain. In mice with high-fat induced OA, wheel-running is protective against joint damage rather than damaging, further suggesting that increased joint loading is not sufficient to explain OA107. The authors found that exercised mice had improved glucose tolerance and disrupted proinflammatory cytokine networks in the absence of weight loss. Song et al. evaluated pain behaviors in two strains of rats subjected to high-fat diets, one strain in which obesity is induced and one strain in which obesity is not induced. Although no changes in circulating inflammatory cytokines were measured, an infiltration of macrophages into the DRG was observed, suggesting a link between high-fat diets and the nervous system in chronic pain108. Finally, a recent study showed that mice with lipodystrophy were protected from joint damage and knee hyperalgesia after DMM, even under a high fat diet. Adding back a mixture of subcutaneous and visceral adipose tissue from wildtype mice could overcome this protection109. This study suggests an important role for adipose tissue as a mediator of joint degeneration and associated pain in mice, independently of body weight. Although the link between obesity and OA progression may not be entirely due to overloading of joints, physical activity has long been a recommendation for the treatment of OA. The benefits of exercise for OA patients are clear, with studies showing clear improvements in disease burden and pain scores110–112. Conversely, during Covid-19 lockdowns, researchers found that VAS and WOMAC scores increased significantly, correlating with a reduction in physical activity113. A recent study demonstrated that diet and exercise intervention in overweight and obese people suffering from knee OA resulted in an improvement of pain and function that was independent from BMI114. This was attributed partially to a change in serum levels of inflammatory factors, including IL-6, TNF-α, soluble IL-1 receptor and C-reactive protein (CRP). The association of obesity and osteoarthritis is clear, however understanding the relative contribution of mechanical, inflammatory, and metabolic factors to OA pain requires further investigation.
5.2. Other systemic factors that can modify the inflammatory aspects of OA pain
Several modifiable and unmodifiable factors profoundly affect the inflammatory process. Inflammation is considered one of the mechanisms that contribute to age-related diseases, and chronic age-related stimulation of the innate immune system is termed “inflamm-aging”115. Aging is one of the major risk factors for radiographic as well as symptomatic OA, and research is increasingly uncovering how systemic and local age-related inflammation can contribute to OA joint damage116. With age, levels of inflammatory mediators such as IL-6 and TNFα increase systemically and in adipose tissues, while in the joint, senescent cells accumulate and the senescence-associated secretory phenotype (SASP) creates a locally damaging environment117. However, the relationship of inflamm-aging and OA pain remains understudied. It has been shown that systemic levels of CRP, TNFα and IL6 are associated with knee pain in older adults118. Interestingly, the authors found that when corrected for the presence of radiographic OA, these associations of inflammatory markers and knee pain remained.
Sex is a major determinant in the inflammatory process, and while differences between males and females in both the disease progression and pain burden of OA have been widely reported119, it is not at all yet clear how this relates to mechanisms of pain. Females have a higher symptom burden and are more likely to use health care, resulting in a higher prevalence of clinical diagnosis120,121. This gender discrepancy becomes particularly apparent in older adults, after 50 years of age120. Mun et al. hypothesized that an underlying mechanism of sex-based risk differences in developing OA might be due to exaggerated inflammatory responses to pain in women compared to men. The authors measured serum levels of IL-6 over time following laboratory-induced pain and found that IL-6 levels were significantly increased in women compared to men after painful stimulus122. Similarly, Perruccio et al. explored sex-based differences of systemic inflammatory markers in OA patients scheduled for knee arthroplasty, and found that IL-6 was positively correlated with knee pain in females only. Furthermore, they found positive correlations of IL-1β and IL-18 with knee pain in males, but these same cytokines were negatively correlated with knee pain in females123. Clearly, this will be an important area of study for the successful development of analgesics in OA.
6. Conclusions and future directions
In summary, recent research has generated crucial novel insights into the generation of OA pain. Notably, OA joint pathology is characterized by profound neuroplasticity, with the sprouting of nociceptors in the subchondral bone and the synovium. Secondly, innate immune and inflammatory mechanisms mediate OA pathology and pain at multiple levels, and interact with sensory neurons in the joint and also in the nervous system. It can be expected that the precise molecular elucidation of these neuroimmune interactions will offer a rich substrate for targeted interventions. Several modifying systemic factors will need to be taken into account when studying these mechanisms, including sex, age, and obesity.
Finally, it should be considered that OA is strongly mechanically driven, promoted by joint overload. ‘Mechanoinflammation’ has been suggested as a mechanical signaling pathway driving inflammatory signaling and pain in OA124. One potential pathway that may be of particular interest is the mechanosensitive channel, Piezo2, expressed by nociceptors. Piezo2 becomes sensitized under inflammatory conditions and plays a role in mediating mechanical allodynia125,126. Interestingly, a link between NGF and Piezo2 has also been proposed as a mechanism of activation of ‘silent nociceptors’ – nociceptors that begin to sense mechanical stimuli after sensitization127. Therefore, the interactions between inflammatory pathways and mechanical stress on the joints may well be a unique feature of OA pathogenesis and the genesis of OA pain.
Key Points:
Chronic pain is the most burdensome symptom for OA patients, yet pain is often inadequately managed in OA.
Nerve growth factor (NGF), inflammatory cytokines and chemokines, and DAMPs are pro-algesic through direct activation of nociceptors.
Neuronal sprouting occurs in the synovium and subchondral bone of OA joints, and neuroimmune interactions contribute to the genesis of pain.
Neuroinflammation in the dorsal root ganglia (DRG) and the dorsal horn further amplify pain signaling and may contribute to pain chronification.
Factors such as obesity and sex can alter systemic levels of cytokines contributing to chronic inflammation in OA, and affect sensitization and pain.
Synopsis:
Chronic pain is a substantial personal and societal burden worldwide. Osteoarthritis (OA) is one of the leading causes of chronic pain and is increasing in prevalence in accordance with a global aging population. In addition to affecting patients’ physical lives, chronic pain also adversely affects patients’ mental wellbeing. However, there remain no pharmacological interventions to slow down the progression of OA, and pain-alleviating therapies are largely unsuccessful. The presence of low-level inflammation in OA has been recognised for many years as a major pathogenic driver of joint damage. Inflammatory mechanisms can occur locally in joint tissues, such as the synovium, within the sensory nervous system, as well as systemically, caused by modifiable and unmodifiable factors. Understanding how inflammation may contribute to, and modify pain in OA will be instrumental in identifying new druggable targets for analgesic therapies. In this narrative review we discuss recent insights into inflammatory mechanisms in OA pain. We discuss how local inflammation in the joint can contribute to mechanical sensitization and to structural neuroplasticity of joint nociceptors, through pro-inflammatory factors such as nerve growth factor, cytokines and chemokines. We consider the role of synovitis, and the amplifying mechanisms of neuroimmune interactions. We then explore emerging evidence around the role of neuroinflammation in the dorsal root ganglia and the dorsal horn. Finally, we discuss how systemic inflammation associated with obesity may modify OA pain, and suggest future research directions.
Acknowledgements
The authors are grateful for the support of the Rheumatology Research Foundation (MJW and AMM funded by a Rheumatology Research Foundation 2021 Innovative Research Award). And the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS]) (R01AR060364, R01AR064251, and P30AR079206 to A.M. Malfait; R01AR077019 to R.E. Miller,).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: MJW and REM: nothing to disclose; in the last 24 months, AMM has received consulting fees from Eli Lilly, Pfizer, Vizuri, AKP, Ceva, and 23andMe.
Contributor Information
Matthew J. Wood, Department of Internal Medicine, Division of Rheumatology, Rush University Medical Center, Room 340, 1735 W Harrison Street, Chicago, IL 60612, USA..
Rachel E. Miller, Department of Internal Medicine, Division of Rheumatology, Rush University Medical Center, Room 714, 1735 W Harrison Street, Chicago, IL 60612, USA..
Anne-Marie Malfait, Department of Internal Medicine, Division of Rheumatology, Rush University Medical Center, 1611 W Harrison Street, Suite 510 Chicago, IL 60612, USA..
References
- 1.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 3.Deshpande BR, Katz JN, Solomon DH, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res. 2016;68(12):1743–1750. doi: 10.1002/acr.22897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States (BMUS). Fourth Edition. [Google Scholar]
- 5.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthr Cartil. Published online 2021. doi: 10.1016/j.joca.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safiri S, Kolahi A-A, Smith E, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819 LP–828. doi: 10.1136/annrheumdis-2019-216515 [DOI] [PubMed] [Google Scholar]
- 8.Osteoarthritis: A Serious Disease. Pre Compet Consort Osteoarthr Osteoarthr Res Soc Int. Published online 2016. [Google Scholar]
- 9.Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life and health service use among older adults with osteoarthritis. Arthritis Care Res. 2004;51(3):326–331. doi: 10.1002/art.20390 [DOI] [PubMed] [Google Scholar]
- 10.Riddle Daniel L., Xiangrong Kong GKF. Psychological Health Impact on Two-Year Changes in Pain and Function in Persons with Knee Pain: Data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2011;19(9):1095–1101. doi: 10.1016/j.joca.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H-M, Kim H-S, Lee Y-J. Knee osteoarthritis and its association with mental health and health-related quality of life: A nationwide cross-sectional study. Geriatr Gerontol Int. 2020;20(4):379–383. doi: 10.1111/ggi.13879 [DOI] [PubMed] [Google Scholar]
- 12.Rathbun AM, Stuart EA, Shardell M, Yau MS, Baumgarten M, Hochberg MC. Dynamic Effects of Depressive Symptoms on Osteoarthritis Knee Pain. Arthritis Care Res (Hoboken). 2018;70(1):80–88. doi: 10.1002/acr.23239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: Results from two cohort studies. BMJ. 2009;339(7719):498–501. doi: 10.1136/bmj.b2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon K, Lavalley MP, Jafarzadeh SR, Felson D. Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann Rheum Dis. 2020;79(8):1105–1110. doi: 10.1136/annrheumdis-2020-217363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Kim HA, Felson DT, et al. Radiographic Knee Osteoarthritis and Knee Pain: Cross-sectional study from Five Different Racial/Ethnic Populations. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-19470-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill TW, Felson DT. Mechanisms of Osteoarthritis (OA) Pain. Curr Osteoporos Rep. 2018;16(5):611–616. doi: 10.1007/s11914-018-0477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kittelson AJ, Stevens-Lapsley JE, Schmeige SJ. Determination of Pain Phenotypes in Knee Osteoarthritis: A Latent Class Analysis Using Data from the Osteoarthritis Initiative. Arthritis Care Res. 2017;68(5):612–620. doi: 10.1002/acr.22734.Determination [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cedraschi C, Delézay S, Marty M, et al. “Let’s talk about OA pain”: A qualitative analysis of the perceptions of people suffering from OA. Towards the development of a specific pain OA-related questionnaire, the Osteoarthritis Symptom Inventory Scale (OASIS). PLoS One. 2013;8(11):1–10. doi: 10.1371/journal.pone.0079988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent TL. Peripheral pain mechanisms in osteoarthritis. Pain. 2020;161. https://journals.lww.com/pain/Fulltext/2020/09001/Peripheral_pain_mechanisms_in_osteoarthritis.15.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malfait AM, Miller RE, Miller RJ. Basic Mechanisms of Pain in Osteoarthritis: Experimental Observations and New Perspectives. Rheum Dis Clin North Am. 2021;47(2):165–180. doi: 10.1016/j.rdc.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol. 2017;35(5):S79–S84. [PubMed] [Google Scholar]
- 22.Miller RJ, Malfait A-M, Miller RE. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr Cartil. 2020;28(5):562–571. doi: 10.1016/j.joca.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neogi T Structural correlates of pain in osteoarthritis. Clin Exp Rheumatol. 2017;35(5):S75–S78. [PubMed] [Google Scholar]
- 24.Griffin TM, Scanzello CR. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol. 2019;37 Suppl 1(5):57–63. https://pubmed.ncbi.nlm.nih.gov/31621560 [PMC free article] [PubMed] [Google Scholar]
- 25.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 26.Deyle GD, Allen CS, Allison SC, et al. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. N Engl J Med. 2020;382(15):1420–1429. doi: 10.1056/NEJMoa1905877 [DOI] [PubMed] [Google Scholar]
- 27.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017;317(19):1967–1975. doi: 10.1001/jama.2017.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julius D TRP Channels and Pain. Annu Rev Cell Dev Biol. 2013;29(1):355–384. doi: 10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- 30.Woller SA, Eddinger KA, Corr M, Yaksh TL. An overview of pathways encoding nociception. Clin Exp Rheumatol. 2017;35 Suppl 1(5):40–46. https://pubmed.ncbi.nlm.nih.gov/28967373 [PMC free article] [PubMed] [Google Scholar]
- 31.Arant KR, Katz JN, Neogi T. Quantitative sensory testing: identifying pain characteristics in patients with osteoarthritis. Osteoarthr Cartil. Published online 2021. doi: 10.1016/j.joca.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RE, Malfait A-M. Osteoarthritis pain: What are we learning from animal models? Best Pract Res Clin Rheumatol. 2017;31(5):676–687. doi: 10.1016/j.berh.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller RE, Malfait A-M. Can we prevent chronic osteoarthritis pain? A view from the bench. Osteoarthr Cartil. Published online 2021. doi: 10.1016/j.joca.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. doi: 10.1007/978-3-540-79090-7_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scanzello CR. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J Orthop Res. 2017;35(4):735–739. doi: 10.1002/jor.23471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti S, Jadon DR, Bulmer DC, Smith ESJ. Human osteoarthritic synovial fluid increases excitability of mouse dorsal root ganglion sensory neurons: an in-vitro translational model to study arthritic pain. Rheumatology. 2020;59(3):662–667. doi: 10.1093/rheumatology/kez331 [DOI] [PubMed] [Google Scholar]
- 37.Nees TA, Rosshirt N, Zhang JA, et al. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J Clin Med . 2019;8(9). doi: 10.3390/jcm8091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson MSM, Sarmanova A, Doherty M, Zhang W. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology. 2018;57(10):1830–1837. doi: 10.1093/rheumatology/key131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter F, Natura G, Ebbinghaus M, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum. 2012;64(12):4125–4134. doi: 10.1002/art.37695 [DOI] [PubMed] [Google Scholar]
- 40.Ebbinghaus M, Natura G, Segond von Banchet G, et al. Interleukin-17A is involved in mechanical hyperalgesia but not in the severity of murine antigen-induced arthritis. Sci Rep. 2017;7(1):10334. doi: 10.1038/s41598-017-10509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Na HS, Park J-S, Cho K-H, et al. Interleukin-1-Interleukin-17 Signaling Axis Induces Cartilage Destruction and Promotes Experimental Osteoarthritis . Front Immunol . 2020;11:730. https://www.frontiersin.org/article/10.3389/fimmu.2020.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liew FY, Girard J-P, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676–689. doi: 10.1038/nri.2016.95 [DOI] [PubMed] [Google Scholar]
- 43.He Z, Song Y, Yi Y, et al. Blockade of IL-33 signalling attenuates osteoarthritis. Clin Transl Immunol. 2020;9(10):e1185. doi: 10.1002/cti2.1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haraden CA, Huebner JL, Hsueh M-F, Li Y-J, Kraus VB. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. 2019;21(1):146. doi: 10.1186/s13075-019-1923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Jiang B-E. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2014;52(2):276–282. doi: 10.1177/0004563214545117 [DOI] [PubMed] [Google Scholar]
- 46.Miller RE, Malfait A-M. Can we target CCR2 to treat osteoarthritis? The trick is in the timing! Osteoarthr Cartil. 2017;25(6):799–801. doi: 10.1016/j.joca.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller RE, Tran PB, Das R, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109(50):20602–20607. doi: 10.1073/pnas.1209294110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miotla Zarebska J, Chanalaris A, Driscoll C, et al. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthr Cartil. 2017;25(3):406–412. doi: 10.1016/j.joca.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishihara S, Obeidat AM, Wokosin DL, et al. The role of intra-articular neuronal CCR2 receptors in knee joint pain associated with experimental osteoarthritis in mice. Arthritis Res Ther. 2021;23(1):103. doi: 10.1186/s13075-021-02486-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berke IM, Jain E, Yavuz B, et al. NF-κB-mediated effects on behavior and cartilage pathology in a non-invasive loading model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2021;29(2):248–256. doi: 10.1016/j.joca.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietz BW, Nakamura MC, Bell MT, Lane NE. Targeting Nerve Growth Factor for Pain Management in Osteoarthritis—Clinical Efficacy and Safety. Rheum Dis Clin North Am. 2021;47(2):181–195. doi: 10.1016/j.rdc.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 52.Wise BL, Seidel MF, Lane NE. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol. 2021;17(1):34–46. doi: 10.1038/s41584-020-00528-4 [DOI] [PubMed] [Google Scholar]
- 53.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27(2):85–91. doi: 10.1016/j.tips.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 54.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148(3). https://journals.lww.com/pain/Fulltext/2010/03000/NGF_induces_non_inflammatory_localized_and_lasting.11.aspx [DOI] [PubMed] [Google Scholar]
- 55.Weinkauf B, Obreja O, Schmelz M, Rukwied R. Differential time course of NGF-induced hyperalgesia to heat versus mechanical and electrical stimulation in human skin. Eur J Pain (United Kingdom). 2015;19(6):789–796. doi: 10.1002/ejp.603 [DOI] [PubMed] [Google Scholar]
- 56.Andresen T, Nilsson M, Nielsen AK, Lassen D, Arendt-Nielsen L, Drewes AM. Intradermal Injection with Nerve Growth Factor: A Reproducible Model to Induce Experimental Allodynia and Hyperalgesia. Pain Pract. 2016;16(1):12–23. doi: 10.1111/papr.12267 [DOI] [PubMed] [Google Scholar]
- 57.Ashraf S, Mapp PI, Burston J, Bennett AJ, Chapman V, Walsh DA. Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis. 2014;73(9):1710 LP–1718. doi: 10.1136/annrheumdis-2013-203416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denk F, Bennett DL, McMahon SB. Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci. 2017;40(1):307–325. doi: 10.1146/annurev-neuro-072116-031121 [DOI] [PubMed] [Google Scholar]
- 59.Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–1428. doi: 10.1136/ard.2006.063354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mapp PI, Sagar DR, Ashraf S, et al. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthr Cartil. 2013;21(9):1336–1345. doi: 10.1016/j.joca.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aso K, Shahtaheri SM, Hill R, et al. Contribution of nerves within osteochondral channels to osteoarthritis knee pain in humans and rats. Osteoarthr Cartil. 2020;28(9):1245–1254. doi: 10.1016/j.joca.2020.05.010 [DOI] [PubMed] [Google Scholar]
- 62.Obeidat AM, Miller RE, Miller RJ, Malfait A-M. The nociceptive innervation of the normal and osteoarthritic mouse knee. Osteoarthr Cartil. 2019;27(11):1669–1679. doi: 10.1016/j.joca.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu S, Zhu J, Zhen G, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129(3):1076–1093. doi: 10.1172/JCI121561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iannone F, De Bari C, Dell’Accio F, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology. 2002;41(12):1413–1418. doi: 10.1093/rheumatology/41.12.1413 [DOI] [PubMed] [Google Scholar]
- 65.Driscoll C, Chanalaris A, Knights C, et al. Nociceptive Sensitizers Are Regulated in Damaged Joint Tissues, Including Articular Cartilage, When Osteoarthritic Mice Display Pain Behavior. Arthritis Rheumatol (Hoboken, NJ). 2016;68(4):857–867. doi: 10.1002/art.39523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol (Hoboken, NJ). 2014;66(11):3018–3027. doi: 10.1002/art.38778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aso K, Shahtaheri SM, Hill R, Wilson D, McWilliams DF, Walsh DA. Associations of Symptomatic Knee Osteoarthritis With Histopathologic Features in Subchondral Bone. Arthritis Rheumatol. 2019;71(6):916–924. doi: 10.1002/art.40820 [DOI] [PubMed] [Google Scholar]
- 68.van den Bosch MHJ, van Lent PLEM, van der Kraan PM. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr Cartil. 2020;28(5):532–543. doi: 10.1016/j.joca.2020.01.016 [DOI] [PubMed] [Google Scholar]
- 69.Roemer FW, Guermazi A, Felson DT, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804 LP–1809. doi: 10.1136/ard.2011.150243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263 LP–1267. doi: 10.1136/ard.2004.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker K, Grainger A, Niu J, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69(10):1779–1783. doi: 10.1136/ard.2009.121426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neogi T, Guermazi A, Roemer F, et al. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol (Hoboken, NJ). 2016;68(3):654–661. doi: 10.1002/art.39488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Lange-Brokaar BJE, Ioan-Facsinay A, Yusuf E, et al. Association of Pain in Knee Osteoarthritis With Distinct Patterns of Synovitis. Arthritis Rheumatol. 2015;67(3):733–740. doi: 10.1002/art.38965 [DOI] [PubMed] [Google Scholar]
- 74.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi: 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraus VB, McDaniel G, Huebner JL, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr Cartil. 2016;24(9):1613–1621. doi: 10.1016/j.joca.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu W, Zhang X, Jiang Y, et al. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin Rheumatol. 2020;39(2):523–532. doi: 10.1007/s10067-019-04768-y [DOI] [PubMed] [Google Scholar]
- 77.Rosshirt N, Trauth R, Platzer H, et al. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res Ther. 2021;23(1):37. doi: 10.1186/s13075-020-02410-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nees TA, Rosshirt N, Zhang JA, et al. T Helper Cell Infiltration in Osteoarthritis-Related Knee Pain and Disability. J Clin Med. 2020;9(8):2423. doi: 10.3390/jcm9082423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sousa-Valente J, Calvo L, Vacca V, Simeoli R, Arévalo JC, Malcangio M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthr Cartil. 2018;26(1):84–94. doi: 10.1016/j.joca.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 80.Cook AD, Pobjoy J, Steidl S, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14(5):R199. doi: 10.1186/ar4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KM-C, Prasad V, Achuthan A, Fleetwood AJ, Hamilton JA, Cook AD. Targeting GM-CSF for collagenase-induced osteoarthritis pain and disease in mice. Osteoarthr Cartil. 2020;28(4):486–491. doi: 10.1016/j.joca.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 82.Tewari D, Cook AD, Lee M-C, et al. Granulocyte-Macrophage Colony Stimulating Factor As an Indirect Mediator of Nociceptor Activation and Pain. J Neurosci. 2020;40(11):2189–2199. doi: 10.1523/JNEUROSCI.2268-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou C-H, Jain V, Gibson J, et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci Rep. 2020;10(1):10868. doi: 10.1038/s41598-020-67730-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wood MJ, Leckenby A, Reynolds G, et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI insight. 2019;4(2):e125325. doi: 10.1172/jci.insight.125325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nanus DE, Badoume A, Wijesinghe SN, et al. Synovial tissue from sites of joint pain in knee osteoarthritis patients exhibits a differential phenotype with distinct fibroblast subsets. EBioMedicine. 2021;72:103618. doi: 10.1016/j.ebiom.2021.103618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bratus-Neuenschwander A, Castro-Giner F, Frank-Bertoncelj M, et al. Pain-Associated Transcriptome Changes in Synovium of Knee Osteoarthritis Patients. Genes (Basel). 2018;9(7):338. doi: 10.3390/genes9070338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsuda M, Huh Y, Ji R-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33(1):131–139. doi: 10.1007/s00540-018-2579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geraghty T, Winter DR, Miller RJ, Miller RE, Malfait A-M. Neuroimmune interactions and osteoarthritis pain: focus on macrophages. Pain reports. 2021;6(1):e892–e892. doi: 10.1097/PR9.0000000000000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adães S, Almeida L, Potes CS, et al. Glial activation in the collagenase model of nociception associated with osteoarthritis. Mol Pain. 2017;13:1744806916688219–1744806916688219. doi: 10.1177/1744806916688219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raoof R, Martin Gil C, Lafeber FPJG, et al. Dorsal root ganglia macrophages maintain osteoarthritis pain. J Neurosci. Published online August 16, 2021:JN-RM-1787–20. doi: 10.1523/JNEUROSCI.1787-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller RE, Tran PB, Ishihara S, et al. Microarray analyses of the dorsal root ganglia support a role for innate neuro-immune pathways in persistent pain in experimental osteoarthritis. Osteoarthr Cartil. 2020;28(5):581–592. doi: 10.1016/j.joca.2020.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ogbonna AC, Clark AK, Malcangio M. Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice. Age (Omaha). 2015;37(3):54. doi: 10.1007/s11357-015-9792-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tran PB, Miller RE, Ishihara S, Miller RJ, Malfait AM. Spinal microglial activation in a murine surgical model of knee osteoarthritis. Osteoarthr Cartil. 2017;25(5):718–726. doi: 10.1016/j.joca.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bourassa V, Deamond H, Yousefpour N, Fitzcharles M-A, Ribeiro-da-Silva A. Pain-related behavior is associated with increased joint innervation, ipsilateral dorsal horn gliosis, and dorsal root ganglia activating transcription factor 3 expression in a rat ankle joint model of osteoarthritis. PAIN Reports. 2020;5(5). https://journals.lww.com/painrpts/Fulltext/2020/10000/Pain_related_behavior_is_associated_with_increased.4.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hartung JE, Eskew O, Wong T, et al. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav Immun. 2015;50:196–202. doi: 10.1016/j.bbi.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou L, Hu Y, Li C, et al. Levo-corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway. Neuropharmacology. 2018;135:34–47. doi: 10.1016/j.neuropharm.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 97.Xu J, Zhu M-D, Zhang X, et al. NFκB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflammation. 2014;11:38. doi: 10.1186/1742-2094-11-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Yang Y, Guo J, Guo X, Feng Z, Zhao X. Spinal NF-kB upregulation contributes to hyperalgesia in a rat model of advanced osteoarthritis. Mol Pain. 2020;16:1744806920905691. doi: 10.1177/1744806920905691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burston JJ, Valdes AM, Woodhams SG, et al. The impact of anxiety on chronic musculoskeletal pain and the role of astrocyte activation. Pain. 2019;160(3). https://journals.lww.com/pain/Fulltext/2019/03000/The_impact_of_anxiety_on_chronic_musculoskeletal.13.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsushita T, Otani K, Oto Y, Takahashi Y, Kurosaka D, Kato F. Sustained microglial activation in the area postrema of collagen-induced arthritis mice. Arthritis Res Ther. 2021;23(1):273. doi: 10.1186/s13075-021-02657-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(4):507–515. doi: 10.1016/j.joca.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 102.Whittaker JL, Runhaar J, Bierma-Zeinstra S, Roos EM. A lifespan approach to osteoarthritis prevention. Osteoarthr Cartil. Published online 2021. doi: 10.1016/j.joca.2021.06.015 [DOI] [PubMed] [Google Scholar]
- 103.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:1–5. doi: 10.1186/1471-2474-9-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kluzek S, Newton JL, Arden NK. Is osteoarthritis a metabolic disorder? Br Med Bull. 2015;115(1):111–121. doi: 10.1093/bmb/ldv028 [DOI] [PubMed] [Google Scholar]
- 105.Berenbaum F, Griffin TM, Liu-Bryan R. Review: Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol. 2017;69(1):9–21. doi: 10.1002/art.39842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M. Association of diet and headache. J Headache Pain. 2019;20(1):1–11. doi: 10.1186/s10194-019-1057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 2012;64(2):443–453. doi: 10.1002/art.33332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song Z, Xie W, Chen S, et al. High-fat diet increases pain behaviors in rats with or without obesity. Sci Rep. 2017;7(1):10350. doi: 10.1038/s41598-017-10458-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Collins KH, Lenz KL, Pollitt EN, et al. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A. 2021;118(1):e2021096118. doi: 10.1073/pnas.2021096118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anwer S, Alghadir A, Brismeé JM. Effect of Home Exercise Program in Patients with Knee Osteoarthritis: A Systematic Review and Meta-analysis. J Geriatr Phys Ther. 2016;39(1):38–48. doi: 10.1519/JPT.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 111.Kraus VB, Sprow K, Powell KE, et al. Effects of Physical Activity in Knee and Hip Osteoarthritis: A Systematic Umbrella Review. Med Sci Sports Exerc. 2019;51(6):1324–1339. doi: 10.1249/MSS.0000000000001944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Endstrasser F, Braito M, Linser M, Spicher A, Wagner M, Brunner A. The negative impact of the COVID-19 lockdown on pain and physical function in patients with end-stage hip or knee osteoarthritis. Knee Surgery, Sport Traumatol Arthrosc. 2020;28(8):2435–2443. doi: 10.1007/s00167-020-06104-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Runhaar J, Beavers DP, Miller GD, et al. Inflammatory cytokines mediate the effects of diet and exercise on pain and function in knee osteoarthritis independent of BMI. Osteoarthr Cartil. 2019;27(8):1118–1123. doi: 10.1016/j.joca.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 115.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 116.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23(11):1966–1971. doi: 10.1016/j.joca.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coryell PR, Diekman BO, Loeser RF. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol. 2021;17(1):47–57. doi: 10.1038/s41584-020-00533-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535 LP–540. doi: 10.1136/annrheumdis-2011-201047 [DOI] [PubMed] [Google Scholar]
- 119.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 120.Laitner MH, Erickson LC, Ortman E. Understanding the Impact of Sex and Gender in Osteoarthritis: Assessing Research Gaps and Unmet Needs. J Women’s Heal. 2021;30(5):634–641. doi: 10.1089/jwh.2020.8828 [DOI] [PubMed] [Google Scholar]
- 121.Tschon M, Contartese D, Pagani S, Borsari V, Fini M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J Clin Med . 2021;10(14). doi: 10.3390/jcm10143178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mun CJ, Letzen JE, Nance S, et al. Sex Differences in Interleukin-6 Responses Over Time Following Laboratory Pain Testing Among Patients With Knee Osteoarthritis. J Pain. 2020;21(5):731–741. doi: 10.1016/j.jpain.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perruccio AV, Badley EM, Power JD, et al. Sex differences in the relationship between individual systemic markers of inflammation and pain in knee osteoarthritis. Osteoarthr Cartil Open. 2019;1(1):100004. doi: 10.1016/j.ocarto.2019.100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vincent TL. Mechanoflammation in osteoarthritis pathogenesis. Semin Arthritis Rheum. 2019;49(3, Supplement):S36–S38. doi: 10.1016/j.semarthrit.2019.09.018 [DOI] [PubMed] [Google Scholar]
- 125.Murthy SE, Loud MC, Daou I, et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med. 2018;10(462):eaat9897. doi: 10.1126/scitranslmed.aat9897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Szczot M, Liljencrantz J, Ghitani N, et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med. 2018;10(462):eaat9892. doi: 10.1126/scitranslmed.aat9892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prato V, Taberner FJ, Hockley JRF, et al. Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell Rep. 2017;21(11):3102–3115. doi: 10.1016/j.celrep.2017.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]


