Abstract
Patients with chronic liver disease (CLD) and liver transplant recipients are at increased risk for infections from vaccine-preventable diseases. Gastroenterologists and hepatologists should assess patient immunization history, and necessary vaccinations should be given as soon as possible. Vaccines demonstrate superior immunogenicity when given earlier in the course of liver disease and prior to transplant. This article summarizes recommendations from the Advisory Committee on Immunization Practices for vaccinations in patients with CLD and liver transplant recipients, and includes a discussion of the influenza, herpes zoster, hepatitis A, hepatitis B, pneumococcal, human papillomavirus, and COVID-19 vaccines.
Keywords: Vaccines, vaccine-preventable diseases, liver transplantation, liver diseases
Patients with liver disease are at increased risk for infections through multiple pathophysiologic mechanisms, including intestinal dysbiosis, increased bacterial translocation, portosystemic shunting, and cirrhosis-associated immune dysfunction. Cirrhosis-associated immune dysfunction is a multifactorial state involving dysregulation of both the adaptive and the innate immune systems as well as persistent immune system activation. Other factors contributing to the increased risk for infections in patients with liver disease include malnutrition and, in the case of liver transplant recipients, immunosuppressive medications.1
Both patients with chronic liver disease (CLD) and liver transplant recipients are at high risk for acquiring and experiencing complications from vaccine-preventable diseases (VPDs).2,3 Despite this risk, multiple studies have reported suboptimal vaccination rates in both patients with CLD and liver transplant recipients.4,5 Reasons for this may include lack of education on the recommended vaccine schedule among providers and patients, concern about vaccine safety and efficacy, and confusion about which medical provider is primarily responsible for recommending vaccines. Clinical practice guidelines for vaccination of immunosuppressed patients recommend that specialists share responsibility for ensuring that their patients receive the appropriate vaccines.6 Therefore, gastroenterologists and hepatologists should assume an active role in the appropriate immunization of their patients with CLD. It is important to address immunization needs as soon as possible, as immunologic responses earlier in the course of liver disease are stronger.7,8 For patients undergoing liver transplant evaluation, vaccinations should be given prior to transplant when possible, as vaccine responses are typically higher pretransplant.9 Live vaccines should be administered at least 4 months prior to transplant and are contraindicated after transplant.10
The Advisory Committee on Immunization Practices (ACIP) is a committee of medical and public health experts that develops recommendations on the use of vaccines. The Centers for Disease Control and Prevention sets the US adult and childhood immunization schedules based on ACIP recommendations. This article focuses on appropriate immunization strategies both for patients with CLD and for liver transplant recipients, as outlined by the ACIP and the American Society of Transplantation Infectious Disease Community of Practice.11,12
Influenza Vaccine
Influenza is a common respiratory illness that results in significant morbidity and mortality and resulted in an estimated 490,600 hospitalizations and 34,200 deaths from 2018 to 2019 in the United States.13 Disease complications and hospitalizations are highest among adults ages 65 years and older and immunosuppressed patients, such as patients with CLD and solid organ transplant recipients.14 Influenza infection in patients with liver disease portends a poor outcome. One global analysis found that, among patients infected with the 2009 influenza A virus (H1N1), patients with liver disease had a 5-fold increased risk of influenza-related hospitalization and a 17-fold increased risk of death compared with healthy individuals.15 Another study found a 2-fold increased risk of hospital admission for influenza virus from 2013 to 2014 in patients with liver disease.16 The influenza vaccine can decrease the risk of disease and severity of illness. An observational, prospective, multicenter study demonstrated that solid organ transplant recipients (including liver transplant recipients) and hematopoietic stem cell transplant recipients who had laboratory-confirmed influenza A were less likely to develop pneumonia or require intensive care unit admission if they had received the influenza vaccine during the same influenza season.17 Influenza infection is also associated with hepatic decompensation in patients with cirrhosis, and administration of the influenza vaccine can reduce the risk of this complication.18,19
To decrease morbidity and mortality from influenza, the ACIP recommends annual influenza immunization for all adults who do not have contraindications (Table). The vaccine should be offered ideally by the end of October in the United States and before the onset of influenza activity in the community.14 All influenza vaccines in the United States are now quadrivalent, containing 2 influenza A virus strains and 2 influenza B virus strains. The influenza vaccines licensed in the United States are the inactivated vaccine (available in both the quadrivalent high dose [HD], which was licensed by the US Food and Drug Administration [FDA] in 2019, or the quadrivalent standard dose [SD]), adjuvanted quadrivalent vaccine, recombinant quadrivalent vaccine, and live attenuated quadrivalent vaccine. Because the live attenuated influenza vaccine is indicated for healthy individuals 2 to 49 years of age, patients with CLD are not candidates for this vaccine.14 The live attenuated influenza vaccine should not be given to transplant recipients, along with other live virus vaccines, including the measles, mumps, and rubella vaccine and the varicella vaccine.12 The HD trivalent vaccine demonstrated superior efficacy against influenza compared with that of the SD trivalent vaccine in a randomized controlled trial among persons ages 65 years and older.20 The quadrivalent influenza vaccine with an adjuvant demonstrated acceptable vaccine efficacy in individuals ages 65 years and older in a clinical trial during an influenza season with poor vaccine strain match.21
Table.
Adult Immunization Schedule for Patients With Chronic Liver Disease and Liver Transplant Recipients Based on ACIP Recommendations
| Dosing Schedule | ||
|---|---|---|
| Vaccine | Patients With Chronic Liver Disease | Liver Transplant Recipients |
| SD quadrivalent influenza, inactivated | 1 dose seasonally for all patients 18-64 years of age | |
| HD influenza, inactivated | 1 dose seasonally for patients ≥65 years of agea | 1 dose seasonally for liver transplant recipients ≥18 years of ageb |
| Influenza, live attenuated | Contraindicated for patients with chronic liver disease and liver transplant recipients | |
| Herpes zoster (Shingrix) | 2 doses (2-6 months apart) for all patients ≥50 years of age | 2 doses (2-6 months apart) for patients ≥19 years of age; can be given 1-2 months apart |
| Hepatitis A (Vaqta; Havrix; Hep A-Hep B [Twinrix]) | 2 doses of Vaqta (6-18 months apart) or Havrix (6-12 months apart) at higher dose for adults ≥19 years of age OR 3 doses of Hep A-Hep B at 0, 1, and 6 months or accelerated 4 doses at 0, 7, 21-30 days, and 12 months |
|
| Hepatitis B (Engerix-B; Recombivax HB; Hep A-Hep B [Twinrix]; HepB-CpG [Heplisav-B]) | 3 doses of Engerix-B or Recombivax HB at 0, 1, and 6 months OR 3 doses of Hep A-Hep B at 0, 1, and 6 months or accelerated 4 doses at 0, 7, 21-30 days, and 12 months OR 2 doses of HepB-CpG at 0 and 1 monthc |
|
| Pneumococcal |
Vaccine-naive: PCV15 followed by PPSV23 1 year laterd OR PCV20 alone Previously immunized: Received PCV13: If 19-64 years of age, 1 dose of PPSV23 at least 8 weeks after PCV13 and second dose 5 years later If ≥65 years of age, 1 dose of PPSV23 Received PPSV23: Single dose of PCV15 or PCV20 at least 1 year after PPSV23 |
|
| Human papillomavirus (Gardasil 9) | 3 doses (0, 1-2, and 6 months) for patients 15-26 years of age; consider 3-dose series for at-risk patients 27-45 years of age | |
| COVID-19 (Pfizer-BioNTech COVID-19 vaccine [Comirnaty]; Moderna COVID-19 vaccine [Spikevax]; Johnson & Johnson/Janssen COVID-19 vaccine) | Moderna or Pfizer-BioNTech vaccine preferred Pfizer-BioNTech: 2 doses (21 days apart) Moderna: 2 doses (28 days apart) Booster dose of mRNA vaccine at least 5 months after completing initial series |
Moderna or Pfizer-BioNTech vaccine preferred Pfizer-BioNTech: 2 doses (21 days apart) Moderna: 2 doses (28 days apart) Additional primary dose of mRNA vaccine at least 28 days after second dose Booster dose of mRNA vaccine at least 3 months after completing initial series Posttransplant, postpone immunization if undergoing treatment for acute rejection |
ACIP, Advisory Committee on Immunization Practices; HD, high dose; mRNA, messenger RNA; PCV13, pneumococcal conjugate 13-valent vaccine; PCV15, pneumococcal conjugate 15-valent vaccine; PCV20, pneumococcal conjugate 20-valent vaccine; PPSV23, pneumococcal polysaccharide 23-valent vaccine; SD, standard dose.
aThe HD influenza vaccine contains 4 times more hemagglutinin than the SD vaccine, and induces higher antibody concentrations. It provides better protection against influenza in adults 65 years of age and older.20
bThe HD influenza vaccine demonstrated better immunogenicity than the SD vaccine in adult transplant recipients in a randomized controlled trial.26
cA higher dose of vaccine may be offered in the posttransplant setting, although response rates may be poor. Protective antibodies should be closely monitored. Antibodies to hepatitis B surface antigen should be checked at least 4 weeks after completing the series.
dFor patients who are immunocompromised or anticipating immunosuppressive therapy, an 8-week interval should be used.
Contraindications to the influenza vaccine include history of severe allergic reaction to any component of the vaccine (other than egg) or to a previous dose of any influenza vaccine. Contraindications to the live attenuated vaccine also include concomitant aspirin or salicylate-containing therapy, immunocompromised state, pregnancy, cerebrospinal fluid leak, recent receipt of influenza antiviral medication, and cochlear implants.14 The inactivated influenza vaccines are safe after solid organ transplant. Several studies have demonstrated that there is no increased risk of allograft rejection or dysfunction following influenza vaccination in solid organ transplant recipients.22 The influenza vaccine should be given annually to all patients with CLD and to all liver transplant recipients. The ACIP does not express preference among HD, adjuvanted, or SD vaccines for immunosuppressed patients or for the general population.14
Although most inactivated vaccines are administered at least 3 months posttransplant to allow for achievement of steady maintenance immunosuppression medication levels, the influenza vaccine can be given as early as 1 month following transplant.12 In general, the immune response to the influenza vaccine is less robust in solid organ transplant recipients, but the vaccine is still effective and should be given when due.23,24 Although studies specifically in liver transplant recipients have not been published, a study of kidney transplant patients failed to show superiority of adjuvanted vaccines over nonadjuvanted influenza vaccines.25 In a randomized, double-blind trial of 172 solid organ transplant recipients, seroconversion rates and postimmunization geometric mean titers were significantly higher in patients who received the HD trivalent influenza vaccine compared with patients who received the SD trivalent vaccine.26 Both the SD and HD vaccines are acceptable options for liver transplant recipients; however, the HD vaccine has demonstrated superior immunogenicity in liver transplant recipients. The ACIP and the American Society of Transplantation recommend the HD, adjuvanted, or SD influenza vaccine for liver transplant recipients.
Herpes Zoster Vaccine
Shingles, or herpes zoster (HZ), is caused by reactivation of latent varicella zoster virus, which is the virus that causes chicken pox. HZ often presents with a unilateral, localized vesicular eruption and pain in the affected dermatome. Chronic neuropathic pain following resolution of the acute vesicular eruption, called postherpetic neuralgia (PHN), is a common complication that occurs in approximately 10% of the general population.27 Other complications include cranial and peripheral nerve palsies, vasculopathies, and ophthalmic involvement. Immunosuppressed patients, including solid organ transplant recipients, have a greater incidence of HZ and a higher risk for severe and disseminated disease.28 In 1 study, PHN occurred in up to one-third of liver transplant recipients.29 There is a high incidence of HZ specifically in liver transplant recipients, with an even greater risk among liver transplant recipients older than 50 years of age or those taking mycophenolate mofetil.3,29
A population-based study in Taiwan showed that there was no increased risk of HZ in patients ages 18 years and older with liver cirrhosis compared with a matched control population without cirrhosis.30 However, this study did not differentiate between patients with decompensated liver cirrhosis and those with compensated liver cirrhosis, and it did not consider zoster immunization history. The results of this study suggest that certain patients with cirrhosis may benefit from HZ immunization.
The recombinant HZ vaccine (RZV; Shingrix, GlaxoSmithKline) combines glycoprotein E with a novel adjuvant to amplify immune response, and is administered as a series of 2 doses separated by 2 to 6 months. RZV is highly immunogenic, with 97% efficacy demonstrated in individuals ages 50 years and older and 89% efficacy in individuals ages 70 years and older in 2 clinical trials.31,32 The live attenuated zoster vaccine (ZVL) that was licensed in 2006 is no longer available in the United States as of November 2020.
The ACIP recommends administration of RZV to immunocompetent adults ages 50 years and older. Adults should receive RZV if they have had shingles or have previously received ZVL. RZV is also now recommended for the prevention of HZ and related complications in immunodeficient or immunosuppressed adults ages 19 years and older. The second dose should be given at least 1 to 2 months after the first in this population (Table).33
There are no published studies evaluating the immunogenicity of RZV in liver transplant recipients specifically. The vaccine has been shown to be safe and immunogenic in adults with hematologic malignancies receiving immunosuppressive cancer therapies and in autologous stem cell transplant recipients.34,35 In a multicenter, randomized, placebo-controlled trial studying RZV in renal transplant recipients ages 18 years and older receiving daily immunosuppressive therapy, RZV was immunogenic and immune response persisted through 12 months postvaccination.36 Overall occurrence of renal function changes, rejections, and serious adverse events were similar between the vaccine and placebo groups. Local adverse events were more frequent in the RZV group, as expected. History of severe allergic reaction to any component of the vaccine is the only contraindication.33 RZV is recommended in all adults ages 50 years and older with CLD, and in liver transplant recipients ages 19 years and older who have not yet received the vaccine.
Hepatitis A Vaccine
Hepatitis A virus (HAV) is a common virus worldwide that is spread through fecal-oral transmission. It typically causes an acute, self-limited infection in healthy adults, although some prolonged or relapsing cases have been reported.37,38 Patients may be asymptomatic or can develop fever, fatigue, nausea, vomiting, anorexia, jaundice, dark urine, and pale stools. HAV is clinically indistinguishable from other acute viral hepatitides. In severe cases, HAV infection can lead to fulminant hepatitis, which is more likely in adults 50 years of age and older and in patients with underlying CLD.39 Patients with CLD are not at increased risk for acquiring HAV infection, but they are at an increased risk of mortality and for developing fulminant hepatitis once infected.40-42
There are 3 vaccines available in the United States for hepatitis A. Two are single-antigen inactivated hepatitis A vaccines given in 2 doses 6 to 12 months apart (Havrix, GlaxoSmithKline) or 6 to 18 months apart (Vaqta, Merck) (Table). These vaccines are approved for children 12 months of age and older. For adults 19 years of age and older, both vaccines are given at an increased dose. The third available vaccine is a combination-inactivated vaccine (Twinrix, GlaxoSmithKline), which contains both a hepatitis A and a hepatitis B vaccine (Havrix and Engerix-B, GlaxoSmithKline). This combination vaccine is given on either a standard dosing schedule of 3 doses at 0, 1, and 6 months, or on an accelerated dosing schedule of 4 doses at 0 days, 7 days, and 21 to 30 days, followed by a booster at 12 months. The safety profiles of the hepatitis A vaccines are similar.43 Contraindications include allergic reaction after a previous dose or to any component of the hepatitis A vaccine, including neomycin and yeast.44 The ACIP recommends hepatitis A vaccination for adults at risk for HAV infection or severe disease from HAV infection, and for adults requesting protection against HAV without acknowledgment of a risk factor.44
The hepatitis A vaccine is indicated in all adults with CLD and in all liver transplant recipients. Limited data are available to guide whether immunocompromised patients should receive booster doses.
As with other vaccines, the hepatitis A vaccine should be given as early as possible in the course of liver disease because vaccine immunogenicity declines with severity of liver disease and in decompensated cirrhosis.2,8 Sero-protection in liver transplant recipients is variable.45,46 Because of their risk for severe disease if infected, hepatitis A vaccination for patients with liver disease is a priority.
Hepatitis B Vaccine
Compared with patients without liver disease, patients with CLD are at greater risk for severe complications and mortality from superimposed acute or chronic hepatitis B virus (HBV) infection.47 There is also a small risk of HBV infection after orthotopic liver transplant, including from donor-derived hepatitis B acquisition. Despite the risks of HBV, patients with CLD have suboptimal vaccination rates against hepatitis B.48,49
Patients with cirrhosis and patients who receive the hepatitis B vaccine after liver transplant have low serocon-version rates from the SD recombinant hepatitis B vaccine series. However, the vaccine is still immunogenic in early mild or moderate CLD.50,51 Patients with a lower Model for End-Stage Liver Disease score have a greater response to the recombinant hepatitis B vaccine.52 Therefore, the hepatitis B vaccine should be given as early as possible in CLD. In patients undergoing evaluation for a liver transplant, the vaccine series ideally is completed prior to transplant. Response to the hepatitis B vaccine after transplant varies greatly, and antibody titers may decline more rapidly among transplant recipients.53
There are several recombinant hepatitis B vaccines licensed in the United States. Engerix-B, Twinrix, and Recombivax HB (Merck) are administered as a 3-dose series at 0, 1, and 6 months. The Twinrix vaccine can also be given at an accelerated dosing schedule with 4 doses. Each of these products contains an aluminum-based adjuvant. There is a dialysis formulation available for the Recombivax HB vaccine, which contains a higher dose of antigen. HepB-CpG (Heplisav-B, Dynavax) was licensed in 2018 as a 2-dose series with at least 4 weeks between doses. HepB-CpG also contains recombinant hepatitis B surface antigen, but the adjuvant is an oligodeoxy-nucleotide that acts as a buffer. HepB-CpG has higher seroconversion rates when compared with Engerix-B in patients with type 2 diabetes mellitus, in healthy individuals ages 40 to 70 years, and in healthy adults ages 18 to 55 years.54,55 At this time, the ACIP recommends the 2-and 3-dose series vaccines, without a preference (Table).56 The ACIP recommends that the hepatitis B vaccine be given to all adults with risk factors for infection by sexual exposure or intravenous drug use, patients with HIV, persons seeking protection from HBV infection, health care personnel at risk for blood and body fluid exposure, patients on dialysis, household contacts of persons with HBV, and patients with CLD. Hepatitis B vaccines are contraindicated in persons who have had an allergic reaction after a previous dose or to any component of a hepatitis B vaccine, including neomycin and yeast.56
Because patients with cirrhosis may have a suboptimal response to the hepatitis B vaccine, patients who have previously been immunized against HBV should undergo testing to confirm their immunity. Antibodies to the hepatitis B surface antigen (anti-HBs) should be checked at least 4 weeks after the completion of the hepatitis B vaccine series. An anti-HBs level of 10 mIU/mL or greater is protective and indicates that no additional doses of hepatitis B vaccine are needed. If the anti-HBs level is less than 10 mIU/mL at this time, a challenge dose of hepatitis B vaccine should be administered, and another anti-HBs level should be obtained 4 weeks later to evaluate for an anamnestic response that would indicate the patient was a vaccine responder for whom antibodies waned. If the anti-HBs level is less than 10 mIU/mL after the challenge dose, the patient should complete another hepatitis B vaccine series.56 Different strategies to improve the immunogenicity of hepatitis B vaccines in patients with CLD include the use of HD and accelerated vaccine schedules and booster doses.52,57,58
A recently published retrospective study showed that the 2-dose series of HepB-CpG resulted in higher rates of seroconversion than the 3-dose Engerix-B vaccine series in patients with CLD.59 In multivariate analyses, patients who received HepB-CpG had a 2.7-fold greater likelihood of achieving immunity than patients who received the Engerix-B vaccine. The presence of cirrhosis, chronic obstructive pulmonary disease, or renal failure portended a lower likelihood of achieving immunity. This suggests that HepB-CpG may be preferential to the Engerix-B vaccine in patients with CLD; however, prospective studies in this population are needed to determine whether HepB-CpG achieves higher rates of seroconversion than other vaccine series.
Pretransplant serologies should be obtained for HBV. The hepatitis B vaccine is indicated in solid organ transplant candidates who are not immune. The ACIP also recommends testing for anti-HBs level after series completion in these patients. Hepatologists should play an active role in ensuring that their patients are immunized with a hepatitis B vaccine series prior to transplant. Vaccine adherence can be improved by providing the hepatitis B vaccine in clinic.60 Additionally, initiating the hepatitis B vaccination during hospitalization among patients with CLD and prior to liver transplant is an effective intervention for improving vaccination rates.61 Because HepB-CpG can be administered in 2 doses with only 4 weeks between doses, using this vaccination series can facilitate vaccine completion prior to transplant.
Pneumococcal Vaccine
Invasive pneumococcal disease (IPD) from Streptococcus pneumoniae causes significant morbidity and mortality from infections, including bacteremia, pneumonia, osteomyelitis, and meningitis. There is a greater risk of IPD in solid organ transplant recipients, and there is greater mortality following IPD in patients with cirrhosis than in the general population.62-64 IPD is most common within the first 3 years posttransplant but can occur at any time.63 In hospitalized patients with cirrhosis, pneumonia carries one of the highest risks for mortality, and the most common pathogen for community-acquired pneumonia in these patients is S pneumoniae.65 Spontaneous bacterial peritonitis, a serious complication in patients with cirrhosis and ascites, is caused by S pneumoniae in some cases (approximately 3%-5%).66 Despite the severity of disease in immunosuppressed populations, there remains suboptimal pneumococcal vaccine adherence. In 1 study, only 60% of liver transplant recipients with evaluable vaccination documents had received at least 1 pneumococcal vaccination.67
Pneumococcal vaccine recommendations have been updated recently. The pneumococcal conjugate 13-valent vaccine (PCV13) contains 13 serotypes and is no longer recommended for use in adults. The pneumococcal polysaccharide 23-valent vaccine (PPSV23) includes 12 of the serotypes included in PCV13 plus 11 additional serotypes. The pneumococcal conjugate 15-valent vaccine (PCV15) contains the PCV13 serotypes and 2 additional serotypes. The pneumococcal conjugate 20-valent vaccine (PCV20) also contains the PCV13 serotypes and 7 additional serotypes.68
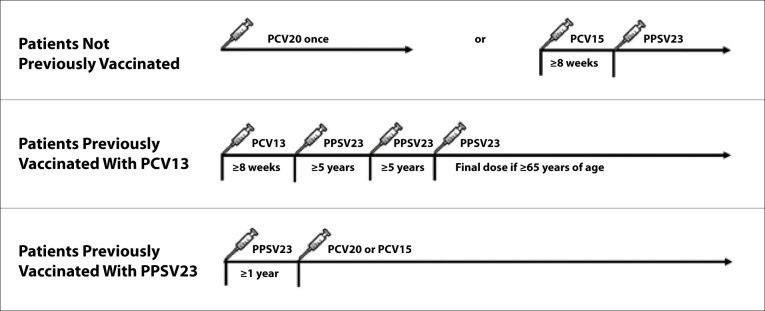
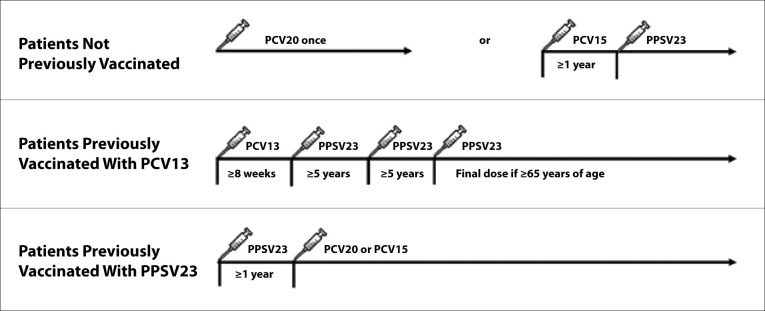
Pneumococcal vaccines are recommended for patients with CLD and liver transplant recipients (Figures 1 and 2).68 Vaccine-naive patients ages 19 and older should receive PCV20, or PCV15 followed by PPSV23. The interval between PCV15 and PPSV23 should be at least 8 weeks in those who are immunocompromised; the interval should be extended to at least 1 year in the general population. Immunocompromised patients who have received PCV13 previously should receive PPSV23 at least 8 weeks after PCV13. Patients who have received PPSV23 previously should receive a dose of PCV15 or PCV20 at least 1 year after their PPSV23 dose. Contra-indications to any pneumococcal vaccine include severe allergy after a previous dose, allergy to any component of the vaccine, or allergy after any vaccine containing diphtheria toxoid.68
Figure 1.
ACIP-recommended pneumococcal vaccine schedule for liver transplant recipients and patients with chronic liver disease who are immunosuppressed.
ACIP, Advisory Committee on Immunization Practices; PCV13, pneumococcal conjugate 13-valent vaccine; PCV15, pneumococcal conjugate 15-valent vaccine; PCV20, pneumococcal conjugate 20-valent vaccine; PPSV23, pneumococcal polysaccharide 23-valent vaccine.
Figure 2.
ACIP-recommended pneumococcal vaccine schedule for patients with chronic liver disease who are not immunosuppressed.
ACIP, Advisory Committee on Immunization Practices; PCV13, pneumococcal conjugate 13-valent vaccine; PCV15, pneumococcal conjugate 15-valent vaccine; PCV20, pneumococcal conjugate 20-valent vaccine; PPSV23, pneumococcal polysaccharide 23-valent vaccine.
Data on the immunogenicity of pneumococcal vaccines in patients with CLD are limited. Results of 1 small study suggest that liver transplant recipients may have a suboptimal response to PPSV23.69 There was no association between vaccination and allograft rejection or de novo donor-specific antibody formation in solid organ transplant recipients receiving PPSV23.22
Human Papillomavirus Vaccine
Human papillomavirus (HPV) is a common and typically asymptomatic sexually transmitted infection that can cause cervical, vaginal, vulvar, penile, anal, and some oropharyngeal cancers.70 HPV vaccines have been found to be highly effective in reducing HPV infections and HPV-associated cancers.71 HPV vaccines do not treat existing lesions caused by HPV and do not prevent the progression of HPV infection into disease. There is an increased risk for HPV-associated cancers in solid organ transplant recipients, particularly for vulvar, anal, and penile cancers.72 This highlights the importance of HPV vaccination, especially among immunosuppressed patients. The 9-valent HPV vaccine (Gardasil 9, Merck) is available in the United States and protects against oncogenic HPV types 16 and 18 (which cause the majority of HPV-associated cancers), and types 31, 33, 45, 52, and 58, as well as nononcogenic HPV types 6 and 11, which cause genital warts.
The ACIP recommends routine HPV vaccination at age 11 or 12 years, but the series can be initiated at 9 or 10 years of age if the clinician deems it appropriate.71 The vaccine is recommended for all persons through age 26 years. A 2-dose series is recommended if initiating the HPV series before the individual reaches 15 years old, with the second dose administered 6 to 12 months after the first dose. If the HPV series is initiated when the individual is 15 years or older or is immunosuppressed, a 3-dose series is indicated. The 3-dose series has a scheduled administration at 0, 1 to 2, and 6 months (Table). In June 2019, the ACIP recommended catch-up HPV vaccination for all persons through age 26 years.71 The ACIP recommends the vaccine series for persons 27 to 45 years old based on shared clinical decision making. Although the ACIP warns that vaccine efficacy may be affected by immunosuppression, the HPV vaccine series should still be administered to adults with risk factors, such as new sexual partners.71 The HPV vaccine is contraindicated in persons with severe allergic reaction after a previous dose or to a vaccine component, including yeast.71
In 1 study, solid organ transplant recipients receiving the quadrivalent HPV vaccine demonstrated lower antibody responses compared with healthy individuals.73 However, there are limited data on the immunogenicity of the 9-valent HPV vaccine among patients with CLD and liver transplant recipients, with further studies needed. Solid organ transplant recipients remain at high risk for HPV-associated malignancy.
COVID-19 Vaccine
COVID-19, the illness caused by SARS-CoV-2, emerged in Wuhan, China, and has spread worldwide since 2019. Many patients with COVID-19 are asymptomatic or have mild symptoms such as fatigue, fever, chills, cough, headache, myalgia, and anosmia. More severe infections result in pulmonary disease, coagulopathies, multiorgan failure, and death.
Older adults and patients with underlying medical comorbidities, including chronic lung disease, heart disease, diabetes, and obesity, are at greater risk for serious complications from COVID-19. Patients with CLD may also be at a higher risk. Patients with cirrhosis experience high rates of hepatic decompensation, acute-on-chronic liver failure, and death from respiratory failure following acute COVID-19 infection.74 The SECURE-Liver and COVID-Hep registries show that hepatic decompensation events are more frequent with increasing severity of liver disease. Additionally, mortality from COVID-19 is particularly high among patients with alcohol-related liver disease and advanced cirrhosis.75
Early data suggest that liver transplant recipients infected with COVID-19 may be at a similar risk for death as the general population.76 One study demonstrated that mortality and the highest level of supplemental oxygen required during hospitalization did not significantly differ between solid organ transplant recipients and non–solid organ transplant patients hospitalized with COVID-19.77 These results suggest that chronic immunosuppression may not be an independent driver of poor outcomes in COVID-19 infections. Regardless, infection with COVID-19 in solid organ transplantrecipients portends a poor outcome.78,79 The 28-day mortality among hospitalized solid-organ transplant recipients is high, and up to 20.5% in 1 study.78
Currently, there are 3 vaccines available for COVID-19 in the United States: 2 messenger RNA (mRNA)-based vaccines given in 2 doses (Pfizer-BioNTech [Comirnaty] and Moderna [Spikevax]) and an adenoviral vector vaccine given as a single dose (Johnson & John-son/Janssen). The Pfizer-BioNTech mRNA-based 2-dose vaccine now has full FDA approval in individuals ages 16 years and older. It has also been granted Emergency Use Authorization (EUA) for children ages 5 to 11 years. The Moderna mRNA-based 2-dose vaccine recently received full FDA approval, and the Johnson & Johnson/Janssen vaccine is available under EUA from the FDA. The COVID-19 vaccines target the viral spike glycoprotein that is necessary for the attachment of SARS-CoV-2 to the angiotensin-converting enzyme 2 receptor on human epithelial cells. The Moderna and Pfizer-BioNTech vaccines contain synthetic nucleoside-modified mRNA that encodes this spike glycoprotein.80,81 The Johnson & Johnson/Janssen vaccine uses a replication-incompetent adenoviral vector that contains the DNA encoding for this spike glycoprotein.82
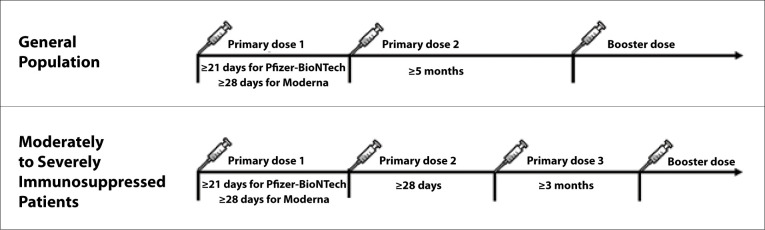
The ACIP recommends the Pfizer-BioNTech or Moderna mRNA COVID-19 vaccines over the Johnson & Johnson/Janssen COVID-19 vaccine for primary and booster vaccinations.83 The Pfizer-BioNTech vaccine can be given to individuals 5 years of age and older. An additional primary dose of mRNA vaccine is recommended in certain immunocompromised recipients of an initial mRNA COVID-19 vaccination series. The purpose of an additional primary dose is to confer additional protection to persons who likely did not mount a protective immune response after initial vaccination. Moderately to severely immunosuppressed individuals 12 years of age and older (Pfizer-BioNTech) or 18 years and older (Moderna) should receive an additional primary mRNA vaccine dose at least 28 days after their second dose (Table).84,85
Booster doses are now recommended for all individuals 12 years and older. A booster dose is intended to enhance or restore immune protection that may have waned over time. Booster doses are recommended 5 months after the last dose in the general population, and 3 months after the last dose in patients who are moderately to severely immunosuppressed (including liver transplant recipients) (Figure 3).85,86
Figure 3.
ACIP-recommended COVID-19 mRNA vaccine schedule.
ACIP, Advisory Committee on Immunization Practices; mRNA, messenger RNA.
Adults 18 years and older who received primary vaccination with the Johnson & Johnson/Janssen COVID-19 vaccine should receive a booster dose 2 months later. Individuals who are moderately or severely immunocom-promised who had an initial Johnson & Johnson/Janssen COVID-19 vaccine dose should receive an additional primary dose of an mRNA vaccine at least 28 days later. These patients should then receive a booster dose with an mRNA vaccine at least 2 months after the second primary dose, for a total of 3 doses.85
The American Association for the Study of Liver Diseases (AASLD) recommends that all adults with CLD and liver transplant recipients receive the COVID-19 vaccine, regardless of prior diagnosis of COVID-19 or the presence of antibodies to SARS-CoV-2.87 Several studies of the immunogenicity of the mRNA COVID-19 vaccine series among solid organ transplant recipients have demonstrated an antibody response among solid organ transplant recipients that was lower than expected.88-90 Other studies have demonstrated low serologic response to the mRNA COVID-19 vaccines among solid organ transplant recipients when compared with immunocompetent controls.91,92 The significant proportion of liver transplant recipients taking antimetabolite immunosuppressants (mycophenolate mofetil) demonstrated even less immunogenicity to the vaccine.91,92 Overall, immunogenicity in this population is low after vaccination. Currently, there is no evidence to support checking antibody titers to determine which patients need another vaccine dose, as the level of protective antibody is unknown.
The results of a double-blind, randomized controlled trial of a third dose of the Moderna vaccine vs placebo among 120 solid organ transplant recipients demonstrated improved immunogenicity following the third dose.93 An increase in immunogenicity following a third dose of the Pfizer-BioNTech vaccine among solid organ transplant recipients has also been demonstrated.94 Therefore, the AASLD recommends a third dose of the mRNA vaccine (Pfizer-BioNTech or Moderna) at least 28 days after the second dose among immunocompromised individuals, including solid organ transplant recipients. The AASLD also recommends this for patients with hepatocellular carcinoma and patients with CLD receiving prednisone, antimetabolites, and/or biologic therapies.87
Liver transplant candidates should proceed with COVID-19 vaccination even if their liver transplant is likely to occur before the second mRNA vaccine dose can be administered. The second dose of mRNA vaccine should be given at the earliest appropriate interval after transplant. If patients are unable to receive the vaccine prior to liver transplant, it should be given at least 3 months posttransplant and as early as 4 weeks posttrans-plant if there is ongoing community spread.87 Reduction of immunosuppressive medications to elicit a greater immune response to the vaccine is not recommended owing to risk of acute cellular rejection (ACR). The COVID-19 vaccine should not be given in liver transplant recipients who have active ACR, are being treated for ACR, or are on high daily doses of steroids. Immunization should be initiated after their ACR is resolved and their baseline immunosuppression is reestablished. Patients with CLD who are receiving therapy for HBV, hepatitis C virus, primary biliary cholangitis, or autoimmune hepatitis should continue their medications while receiving the COVID-19 vaccine.87
Contraindications to the COVID-19 vaccine include severe allergic reaction after a previous dose, allergy to a component of the COVID-19 vaccine, or development of thrombosis with thrombocytopenia after the Johnson & Johnson/Janssen vaccine.
Conclusion
Optimizing immunization rates in patients with CLD and in liver transplant recipients can prevent the morbidity and mortality associated with VPDs. Obtaining a careful immunization history, counseling on vaccine recommendations, and providing necessary vaccines are important for the health maintenance of these patients. Gastroenterologists and hepatologists should be aware of ACIP and the American Society of Transplantation vaccine recommendations, and should share responsibility with primary care providers to ensure that their patients’ vaccines are up to date.
References
- Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(9):727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Keeffe EB. Acute hepatitis A and B in patients with chronic liver disease: prevention through vaccination. Am J Med. 2005;118(suppl 10A):21S–27S. doi: 10.1016/j.amjmed.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim S, Oh J et al. Incidence and risk factors for herpes zoster after adult liver transplantation. Ann Surg Treat Res. 2019;96(2):95–99. doi: 10.4174/astr.2019.96.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajammal R, Ali IA, Syed T, Nusrat S. Immunization against hepatitis A virus and hepatitis B virus in patients with chronic liver disease: are we doing a good job? Cureus. 2018;10(4):e2528. doi: 10.7759/cureus.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghray A, Waghray N, Khallafi H, Menon KV. Vaccinating adult patients with cirrhosis: trends over a decade in the United States. Gastroenterol Res Pract. 2016;2016:5795712. doi: 10.1155/2016/5795712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LG, Levin MJ, Ljungman P et al. Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44–e100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- Andersson D, Castedal M, Friman V. Are liver transplant recipients protected against hepatitis A and B? Transplant Proc. 2013;45(3):1193–1197. doi: 10.1016/j.transproceed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34(1):28–31. doi: 10.1053/jhep.2001.25883. [DOI] [PubMed] [Google Scholar]
- Chong PP, Avery RK. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther. 2017;39(8):1581–1598. doi: 10.1016/j.clinthera.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Rhee Y, Sha BE, Santos CAQ. Optimizing vaccination in adult patients with liver disease and liver transplantation. Clin Liver Dis (Hoboken). 2020;15(2):63–68. doi: 10.1002/cld.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MS, Bernstein H, Ault KA. Advisory Committee on Immunization Practices. Recommended adult immunization schedule, United States, 2021. Ann Intern Med. 2021;174(3):374–384. doi: 10.7326/M20-8080. [DOI] [PubMed] [Google Scholar]
- Danziger-Isakov L, Kumar D. AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13563. doi: 10.1111/ctr.13563. [DOI] [PubMed] [Google Scholar]
- https://www.cdc.gov/flu/about/burden/2018-2019.html Centers for Disease Control and Prevention. Estimated flu-related illnesses, medical visits, hospitalizations, and deaths in the United States—2018-2019 flu season. Published January 8, 2020. Accessed April 7, 2021.
- Grohskopf LA, Alyanak E, Broder KR et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 influenza season. MMWR Recomm Rep. 2020;69(8):1–24. doi: 10.15585/mmwr.rr6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove MD, Vandemaele KA, Shinde V et al. WHO Working Group for Risk Factors for Severe H1N1pdm Infection. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Barberà J, Natividad-Sancho A, Trushakova S et al. Global Influenza Hospital Surveillance Study Group (GIHSN). Epidemiology of hospital admissions with influenza during the 2013/2014 Northern Hemisphere influenza season: results from the Global Influenza Hospital Surveillance Network. PLoS One. 2016;11(5):e0154970. doi: 10.1371/journal.pone.0154970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Ferreira VH, Blumberg E et al. A 5-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- Duchini A, Viernes ME, Nyberg LM, Hendry RM, Pockros PJ. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000;160(1):113–115. doi: 10.1001/archinte.160.1.113. [DOI] [PubMed] [Google Scholar]
- Song JY, Cheong HJ, Ha SH et al. Clinical impact of influenza immunization in patients with liver cirrhosis. J Clin Virol. 2007;39(3):159–163. doi: 10.1016/j.jcv.2007.04.018. [DOI] [PubMed] [Google Scholar]
- DiazGranados CA, Dunning AJ, Kimmel M et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- Beran J, Reynales H, Poder A et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: a randomised, controlled, multicentre, phase 3 efficacy study. Lancet Infect Dis. 2021;21(7):1027–1037. doi: 10.1016/S1473-3099(20)30694-0. [DOI] [PubMed] [Google Scholar]
- Mulley WR, Dendle C, Ling JEH, Knight SR. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J Heart Lung Transplant. 2018;37(7):844–852. doi: 10.1016/j.healun.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Burbach G, Bienzle U, Stark K et al. Influenza vaccination in liver transplant recipients. Transplantation. 1999;67(5):753–755. doi: 10.1097/00007890-199903150-00019. [DOI] [PubMed] [Google Scholar]
- Scharpé J, Evenepoel P, Maes B et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8(2):332–337. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- Kumar D, Campbell P, Hoschler K et al. Randomized controlled trial of adjuvanted versus nonadjuvanted influenza vaccine in kidney transplant recipients. Transplantation. 2016;100(3):662–669. doi: 10.1097/TP.0000000000000861. [DOI] [PubMed] [Google Scholar]
- Natori Y, Shiotsuka M, Slomovic J et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66(11):1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- Friesen KJ, Chateau D, Falk J, Alessi-Severini S, Bugden S. Cost of shingles: population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis. 2017;17(1):69. doi: 10.1186/s12879-017-2185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JI, Quiroga J, Sangro B et al. Herpes zoster after liver transplantation: incidence, risk factors, and complications. Liver Transpl. 2004;10(9):1140–1143. doi: 10.1002/lt.20219. [DOI] [PubMed] [Google Scholar]
- Wu PH, Lin YT, Kuo CN, Chang WC, Chang WP. No increased risk of herpes zoster found in cirrhotic patients: a nationwide population-based study in Taiwan. PLoS One. 2014;9(4):e93443. doi: 10.1371/journal.pone.0093443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Cunningham AL, Godeaux O et al. ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- Cunningham AL, Lal H, Kovac M et al. ZOE-70 Study Group. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- Anderson TC, Masters NB, Guo A et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnew AF, Ilhan O, Lee WS et al. Zoster-039 study group. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haemato-logical malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- Bastidas A, de la Serna J, El Idrissi M et al. ZOE-HSCT Study Group Collaborators. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–133. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink P, Ramon Torrell JM, Sanchez Fructuoso A et al. Z-041 Study Group. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis. 2020;70(2):181–190. doi: 10.1093/cid/ciz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D. Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine (Baltimore). 1992;71(1):14–23. doi: 10.1097/00005792-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Kassas AL, Telegdy L, Méhesfalvi E, Szilágyi T, Mihály I. Polyphasic and protracted patterns of hepatitis A infection: a retrospective study. Acta Med Hung. 1994;50(1-2):93–98. [PubMed] [Google Scholar]
- Keeffe EB. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006;117:227–237. [PMC free article] [PubMed] [Google Scholar]
- Cooksley WG. What did we learn from the Shanghai hepatitis A epidemic? J Viral Hepat. 2000;7(suppl 1):1–3. doi: 10.1046/j.1365-2893.2000.00021.x. [DOI] [PubMed] [Google Scholar]
- Lefilliatre P, Villeneuve JP. Fulminant hepatitis A in patients with chronic liver disease. Can J Public Health. 2000;91(3):168–170. doi: 10.1007/BF03404264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento S. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. J Viral Hepat. 2000;7(suppl 1):7–8. doi: 10.1046/j.1365-2893.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- Bakker M, Bunge EM, Marano C, de Ridder M, De Moerlooze L. Immunogenicity, effectiveness and safety of combined hepatitis A and B vaccine: a systematic literature review. Expert Rev Vaccines. 2016;15(7):829–851. doi: 10.1586/14760584.2016.1150182. [DOI] [PubMed] [Google Scholar]
- Nelson NP, Weng MK, Hofmeister MG et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep. 2020;69(5):1–38. doi: 10.15585/mmwr.rr6905a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan M, Wiesner RH, Poterucha JJ, Zein NN. Safety and efficacy of hepatitis A vaccination in liver transplantation recipients. Transplantation. 2001;72(2):272–276. doi: 10.1097/00007890-200107270-00019. [DOI] [PubMed] [Google Scholar]
- Günther M, Stark K, Neuhaus R, Reinke P, Schröder K, Bienzle U. Rapid decline of antibodies after hepatitis A immunization in liver and renal transplant recipients. Transplantation. 2001;71(3):477–479. doi: 10.1097/00007890-200102150-00023. [DOI] [PubMed] [Google Scholar]
- Reiss G, Keeffe EB. Review article: hepatitis vaccination in patients with chronic liver disease. Aliment Pharmacol Ther. 2004;19(7):715–727. doi: 10.1111/j.1365-2036.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- Ahmmad EM, Roberts LR. Quality of care in patients with cirrhosis: trends in recommended adult vaccination coverage. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):667–682. doi: 10.1016/j.mayocpiqo.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M. Changes in hepatitis A and B vaccination rates in adult patients with chronic liver diseases and diabetes in the U.S. population. Hepatology. 2011;54(4):1167–1178. doi: 10.1002/hep.24510. [DOI] [PubMed] [Google Scholar]
- Roni DA, Pathapati RM, Kumar AS, Nihal L, Sridhar K, Tumkur Rajashekar S. Safety and efficacy of hepatitis B vaccination in cirrhosis of liver. Adv Virol. 2013;2013:196704. doi: 10.1155/2013/196704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Chan CY, Yu MI, Lu RH, Chang FY, Lo KJ. Hepatitis B vaccination in patients with chronic hepatitis C. J Med Virol. 1999;59(4):463–468. [PubMed] [Google Scholar]
- Gutierrez Domingo I, Pascasio Acevedo JM, Alcalde Vargas A et al. Response to vaccination against hepatitis B virus with a schedule of four 40-μg doses in cirrhotic patients evaluated for liver transplantation: factors associated with a response. Transplant Proc. 2012;44(6):1499–1501. doi: 10.1016/j.transproceed.2012.05.071. [DOI] [PubMed] [Google Scholar]
- Loinaz C, de Juanes JR, Gonzalez EM et al. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterology. 1997;44(13):235–238. [PubMed] [Google Scholar]
- Heyward WL, Kyle M, Blumenau J et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31(46):5300–5305. doi: 10.1016/j.vaccine.2013.05.068. [DOI] [PubMed] [Google Scholar]
- Halperin SA, Ward B, Cooper C et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine. 2012;30(15):2556–2563. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67(15):455–458. doi: 10.15585/mmwr.mm6715a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi PR, Bacchella T, Freitas AC et al. Double-dose hepatitis B vacci-nation in cirrhotic patients on a liver transplant waiting list. Braz J Infect Dis. 2008;12(4):306–309. doi: 10.1590/s1413-86702008000400009. [DOI] [PubMed] [Google Scholar]
- Arslan M, Wiesner RH, Sievers C, Egan K, Zein NN. Double-dose accelerated hepatitis B vaccine in patients with end-stage liver disease. Liver Transpl. 2001;7(4):314–320. doi: 10.1053/jlts.2001.23069. [DOI] [PubMed] [Google Scholar]
- Amjad W, Alukal J, Zhang T, Maheshwari A, Thuluvath PJ. Two-dose hepatitis B vaccine (Heplisav-B) results in better seroconversion than three-dose vaccine (Engerix-B) in chronic liver disease. Dig Dis Sci. 2021;66(6):2101–2106. doi: 10.1007/s10620-020-06437-6. [DOI] [PubMed] [Google Scholar]
- https://www.thecommunityguide.org/sites/default/files/Vaccination-Health-Care-System-Based-Interventions-Implemented-in-Combination-Archive.pdf Guide to Community Preventive Services. Increasing appropriate vaccination: health care system-based interventions implemented in combination. 2010 archived review. Updated June 5, 2015. Accessed March 17, 2022.
- Herta T, Petroff D, Engelmann C et al. Hepatitis B vaccination in patients with liver cirrhosis evaluated for liver transplantation—a simple intervention ensures high adherence. Ann Transplant. 2019;24:527–531. doi: 10.12659/AOT.917198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viasus D, Garcia-Vidal C, Castellote J et al. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore). 2011;90(2):110–118. doi: 10.1097/MD.0b013e318210504c. [DOI] [PubMed] [Google Scholar]
- Kumar D, Humar A, Plevneshi A et al. Toronto Invasive Bacterial Diseases Network. Invasive pneumococcal disease in solid organ transplant recipients—10-year prospective population surveillance. Am J Transplant. 2007;7(5):1209–1214. doi: 10.1111/j.1600-6143.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Park HG, Jun JB et al. Clinical characteristics and outcomes of pneumococcal bacteremia in adult patients with liver cirrhosis. Diagn Microbiol Infect Dis. 2009;63(2):160–164. doi: 10.1016/j.diagmicrobio.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Ekpanyapong S, Reddy KR. Infections in cirrhosis. Curr Treat Options Gastroenterol. 2019;17(2):254–270. doi: 10.1007/s11938-019-00229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Hong SI, Park SY et al. Clinical features and outcomes of spontaneous bacterial peritonitis caused by Streptococcus pneumoniae: a matched case-control study. Medicine (Baltimore). 2016;95(22):e3796. doi: 10.1097/MD.0000000000003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltermann B, Herwig A, Dehnen D, Herzer K. Vaccination status of pneumococcal and other vaccines in 444 liver transplant patients compared to a representative population sample. Ann Transplant. 2016;21:200–207. doi: 10.12659/aot.896436. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Farrar JL, Gierke R et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–117. doi: 10.15585/mmwr.mm7104a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCashland TM, Preheim LC, Gentry MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis. 2000;181(2):757–760. doi: 10.1086/315245. [DOI] [PubMed] [Google Scholar]
- Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus-attributable cancers—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724–728. doi: 10.15585/mmwr.mm6833a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13(12):3202–3209. doi: 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. 2013;13(9):2411–2417. doi: 10.1111/ajt.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjot T, Webb GJ, Barritt AS IV et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjot T, Moon AM, Cook JA et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero J, Rodríguez-Perálvarez M, Salcedo M et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MR, Arcasoy S, Farr MA et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23(4):e13637. doi: 10.1111/tid.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates OS, Haydel BM, Florman SS et al. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 2021;73(11):e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalin E, Azzi Y, Bartash R et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Anderson EJ, Rouphael NG et al. mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.fda.gov/media/146217/download Janssen Biotech, Inc. Vaccines and Related Biological Products Advisory Committee Meeting. FDA briefing document. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Published February 26, 2021. Accessed April 2021.
- Oliver SE, Wallace M, See I et al. Use of the Janssen (Johnson & Johnson) COVID-19 vaccine: updated interim recommendations from the Advisory Committee on Immunization Practices—United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):90–95. doi: 10.15585/mmwr.mm7103a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaeyi S, Oliver SE, Collins JP et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cdc.gov/vaccines/ covid-19/clinical-considerations/covid-19-vaccines-us.html Centers for Disease Control and Prevention. Use of COVID-19 vaccines in the United States. Interim clinical considerations. Published February 2022. Accessed March 2022.
- https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html Centers for Disease Control and Prevention. COVID-19 ACIP vaccine recommendations. Updated March 18, 2022. Accessed March 2022.
- Fix OK, Blumberg EA, Chang KM et al. AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: vaccines to prevent Coronavirus disease 2019 infection in patients with liver disease. Hepatology. 2021;74(2):1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky BJ, Werbel WA, Avery RK et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaki S, Adamopoulos S, Degiannis D et al. Immunogenicity of SARSCoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden IK, Bistrup C, Nilsson AC et al. Immunogenicity of SARSCoV-2 mRNA vaccine in solid organ transplant recipients. J Intern Med. 2021;290(6):1264–1267. doi: 10.1111/joim.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidi-Alavijeh J, Frey A, Passenberg M et al. Humoral response to SARSCov-2 vaccination in liver transplant recipients—a single-center experience. Vaccines (Basel). 2021;9(7):738. doi: 10.3390/vaccines9070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowich L, Grupper A, Baruch R et al. Low immunogenicity to SARSCoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VG, Ferreira VH, Ku T et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]