Abstract
The 22q11.2 deletion is associated with >20-fold increased risk for schizophrenia. The presence of gene DGCR8 in the 22q11.2 deletion region has suggested microRNA (miRNA) dysregulation as possibly contributing to this risk. We therefore investigated the role of miRNA target genes in the context of previously identified genome-wide risk for schizophrenia conveyed by additional copy number variation (CNV) in 22q11.2 deletion syndrome (22q11.2DS). Using a cohort of individuals with 22q11.2DS and documented additional rare CNVs overlapping protein coding genes, we compared those with schizophrenia (n = 100) to those with no psychotic illness (n = 118), assessing for rare CNVs that overlapped experimentally supported miRNA target genes. We further characterized the contributing miRNA target genes using gene set enrichment analyses and identified the miRNAs most implicated. Consistent with our hypothesis, we found a significantly higher proportion of individuals in the schizophrenia than in the non-psychotic group to have an additional rare CNV that overlapped one or more miRNA target genes (odds ratio = 2.12, p = 0.0138). Gene set analyses identified an enrichment of FMRP targets and genes involved in nervous system development and postsynaptic density amongst these miRNA target genes in the schizophrenia group. The miRNAs most implicated included miR-17-5p, miR-34a-5p and miR-124-3p. These results provide initial correlational evidence in support of a possible role for miRNA perturbation involving genes affected by rare genome-wide CNVs in the elevated risk for schizophrenia in 22q11.2DS, consistent with the multi-hit and multi-layered genetic mechanisms implicated in this and other forms of schizophrenia.
Keywords: schizophrenia, pathway enrichment analysis, neurodevelopmental disorders, DiGeorge syndrome, model system, DGCR8, FMRP, miR-17-5p
Introduction
The recurrent chromosome 22q11.2 microdeletion that defines 22q11.2 deletion syndrome (22q11.2DS), occurs in an estimated 1 in 2,148 live births and is associated with an over 20-fold increased risk for schizophrenia (McDonald-McGinn et al., 2015; Blagojevic et al., 2021). Amongst mechanisms proposed to confer this schizophrenia risk is microRNA (miRNA) perturbation (Brzustowicz and Bassett, 2012; Forstner et al., 2013; Merico et al., 2014). miRNAs are small regulatory RNA molecules (∼22 nucleotides long) that typically repress translation. The 22q11.2 deletion region contains DGCR8, which encodes a critical microprocessor complex in miRNA biogenesis, as well as genes encoding seven miRNAs (Guna et al., 2015). Widespread miRNA perturbance expected due to hemizygosity of these components is supported by miRNA profiling studies in experimental models of the 22q11.2 deletion (Stark et al., 2008; Earls et al., 2012; Zhao et al., 2015; Toyoshima et al., 2016; Chun et al., 2017; Saito et al., 2020). For idiopathic or genetically uncharacterized schizophrenia, miRNA dysregulation has been implicated in studies of post-mortem brain tissue (Beveridge et al., 2010; Moreau et al., 2011; Santarelli et al., 2011) and blood serum (van den Berg et al., 2020), in genome-wide association studies (Ripke et al., 2014; Hauberg et al., 2016), and in miRNA-target gene regulatory network analyses (Guo et al., 2010; Santarelli et al., 2019).
Genome-wide rare copy number variants (CNVs), including but not limited to 22q11.2 deletions, contribute significantly to genetic risk for schizophrenia in the general population (Walsh et al., 2008; Costain et al., 2013; Marshall et al., 2017) and can impart additional risk for schizophrenia in 22q11.2DS (Bassett et al., 2017). Both miRNAs and effects of altered gene dosage related to CNVs can affect gene expression, and their interaction has been explored in both evolutionary (Duan et al., 2009; Felekkis et al., 2011; Marcinkowska et al., 2011) and disease contexts (Schiffman et al., 2011; Willemsen et al., 2011; Qiao et al., 2013; Vaishnavi et al., 2013; Merico et al., 2014; Warnica et al., 2015). Based on the theory that miRNAs can buffer against genetic variation that would otherwise disrupt gene expression (Hornstein and Shomron, 2006), a previous report proposed that miRNA dysregulation imparted by the 22q11.2 deletion may be lowering the tolerance for potentially deleterious variants involving genes for which precise regulation by miRNAs is required for gene function or for maintaining cellular homeostasis (Brzustowicz and Bassett, 2012).
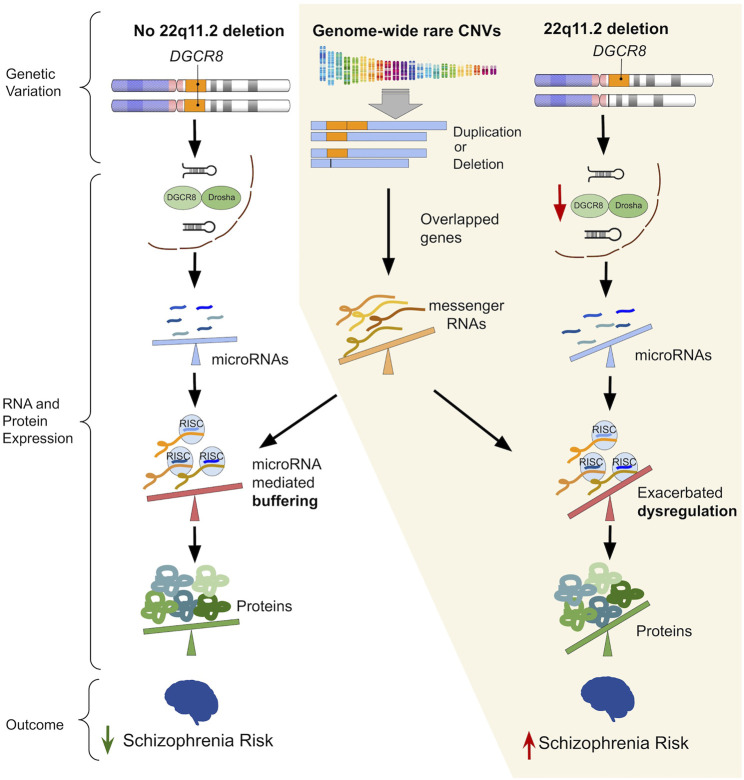
Given the known risk contribution of rare CNVs to schizophrenia expression and the possibility of miRNA effects, we proposed a multi-hit model whereby 22q11.2 deletion-related miRNA perturbance, that could affect genome-wide protein abundance, may exacerbate altered gene-dosage effects of genome-wide rare CNVs (at the level of transcription) (Figure 1). Using the subset of a previously characterized 22q11.2DS cohort with additional (non-22q11.2 deletion) rare CNVs (Bassett et al., 2017), we tested the hypothesis that the individuals with schizophrenia would be more likely to have a miRNA target gene affected by an additional rare CNV, compared to the individuals with no psychotic illness. We also used gene set analyses to examine the putative relevance to schizophrenia of the miRNA target genes involved, and we identified the miRNAs most implicated. To enable this study, we compiled a list of experimentally supported miRNA target genes. We also conducted a review of miRNAs showing evidence of differential expression in available experimental models of the 22q11.2 deletion to provide further context for the findings.
FIGURE 1.
The left-hand column (“No 22q11.2 deletion”) illustrates the ability of an intact microRNA (miRNA) regulatory system to buffer the effects of genetic variation that would otherwise perturb gene expression. The parts grouped by the yellow background illustrate the proposed mechanism of how widespread miRNA dysregulation imparted by the 22q11.2 deletion may exacerbate the downstream impact of rare genome-wide copy number variants (CNVs), thereby increasing risk for schizophrenia. Hemizygosity of DGCR8 due to the 22q11.2 deletion leads to reduced processing of primary-to pre-miRNAs in miRNA biogenesis, thus resulting in widespread miRNA dysregulation. Subsequently, an altered degree of miRNA targeting of differentially expressed messenger RNA (mRNA) transcripts (due to overlapping rare CNVs) may amplify dysregulation of protein expression. A steeper tilt of the seesaws represents a greater degree of dysregulation (i.e., upregulation or downregulation) of miRNAs, mRNAs, proteins, or miRNA mediated repression/degradation of its target mRNA.
Materials and Methods
22q11.2DS Cohort, Psychiatric Status, and CNV Detection
We used previously published data on additional rare genome-wide CNVs identified in a cohort of 22q11.2DS (Bassett et al., 2017). Details on the original genotyping and phenotyping, and informed consent from all individuals and/or their legal guardian can be found in Bassett et al., 2017. Briefly, among 329 individuals with 22q11.2DS, those diagnosed with schizophrenia or another psychotic illness (e.g., schizoaffective disorder) were assigned to the “schizophrenia group” (n = 158) and those with no history of psychotic illness at age ≥25 years were assigned to the “non-psychotic” group (n = 171). For this study we identified the subset of individuals (n = 218) with one or more autosomal, rare CNVs (additional to the 22q11.2 deletion) overlapping a protein coding gene (i.e., “genic CNVs”) and found no significant differences between groups in the selection of this subset (100 of 158 in schizophrenia vs. 118 of 171 in non-psychotic, p = 0.2948, odds ratio (OR) = 0.775). We specifically selected for genic CNVs, defined as overlapping one or more base pairs in an exonic, intronic or untranslated region of a protein coding gene, to encompass all manners in which CNVs may perturb regulation of gene expression (Supplementary Table S1). We defined rare as present in <0.1% of population control databases and all CNVs reported were ≥10 kb in length (Bassett et al., 2017).
As was the case for the whole cohort (n = 329) (Bassett et al., 2017), in this subset (n = 218) there was a significantly higher proportion of female individuals in the non-psychotic group (n = 76; 64%) compared to the schizophrenia group (n = 48; 48%) (p = 0.020, OR = 1.954 [95% confidence interval (95% CI) = 1.10–3.50].
miRNA Target Genes
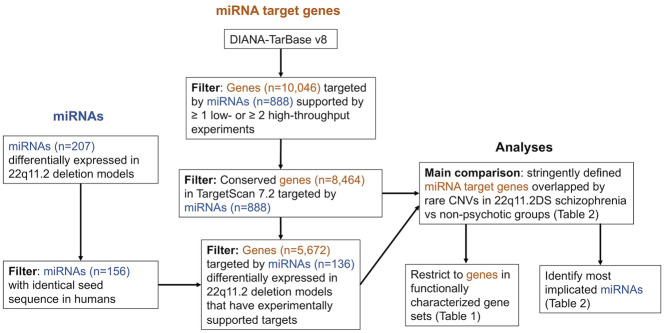
To assess the overlap between rare genome-wide CNVs in the 22q11.2DS cohort and miRNA target genes, we created two sets of miRNA targets: 1) all high-confidence miRNA targets and 2) the subset of genes targeted by a compiled list of miRNAs with evidence of differential expression in 22q11.2 deletion experimental models.
To obtain a set of all high-confidence miRNA target genes, we opted to use experimentally supported miRNA target genes from DIANA-TarBase v8 (obtained 3 September 2020) (Karagkouni et al., 2018). We used a set of filtering criteria designed to improve stringency, as follows: 1) the positive miRNA-gene interaction is supported by at least one low throughput experiment (e.g., luciferase reporter assay) or two high throughput experiments (e.g., CLIP-seq), 2) the effect on gene expression is listed as downregulated, and 3) the miRNA species is listed as human. We then further restricted these filtered miRNA target genes to genes listed as conserved, human miRNA targets in TargetScan 7.2 (Agarwal et al., 2015). This resulted in 8,464 genes representing high-confidence human miRNA targets (Figure 2, Supplementary Tables S2, S3).
FIGURE 2.
Integrated workflow for the retrieval of microRNAs (miRNAs) with evidence of differential expression in 22q11.2 deletion experimental models, filtering of experimentally supported miRNA target genes from DIANA-TarBase v8 (Karagkouni et al., 2018), and analyses comparing the proportion of individuals between groups with a miRNA target genes overlapped by an additional rare CNV. Each miRNA may target multiple genes and each gene may be targeted by multiple miRNAs.
We also created a subset these 8,464 high-confidence target genes by restricting to those targeted by a list of miRNAs we compiled that showed evidence of differential expression in 22q11.2 deletion experimental models (Supplementary Tables S4, S5). This provided a set of 5,672 miRNA targets (Figure 2).
miRNAs With Evidence of Differential Expression in 22q11.2 Deletion Experimental Models
We compiled a list of miRNAs with evidence of differential expression in all studies of 22q11.2 deletion experimental models with available published data of miRNA profiling in brain or model-brain tissue (Stark et al., 2008; Earls et al., 2012; Zhao et al., 2015; Toyoshima et al., 2016; Chun et al., 2017). These comprised of three studies using mouse models with a 1.2 or 1.3 Mb hemizygous deletion on mouse chromosome 16 (orthologous to the 1.5 Mb deletion on chromosome 22q11.2 in humans) (Stark et al., 2008; Earls et al., 2012; Chun et al., 2017) and two human induced pluripotent stem cell (hiPSC) models derived from individuals with the 3 Mb 22q11.2 deletion (Zhao et al., 2015; Toyoshima et al., 2016) (Supplementary Tables S4, S5).
To resolve discrepancies in miRNA nomenclature and differences between miRNA species, first all miRNA names were converted to miRBase v21 nomenclature using the R package miRBaseConverter (Xu et al., 2018). If a miRNA named according to a previous nomenclature system mapped onto multiple miRNAs using miRBase v21 nomenclature, all mappings were retained for subsequent analyses. To ensure that miRNAs from mouse models (with the “mmu-” prefix) were applicable for human analyses, we included only mouse miRNAs where the human miRNA (“hsa-” prefix) of the same number listed in miRBase had an identical 6-mer seed sequence (Supplementary Table S5). Mature miRNAs that are different arms (i.e., -5p vs. -3p) of the same precursor (i.e., same number) were considered distinct miRNAs. We used the Van de Peer Lab’s Venn diagram tool 1 to determine the overlap and generate a multi-group Venn diagram for differentially expressed miRNAs from different studies (Figure 3).
FIGURE 3.
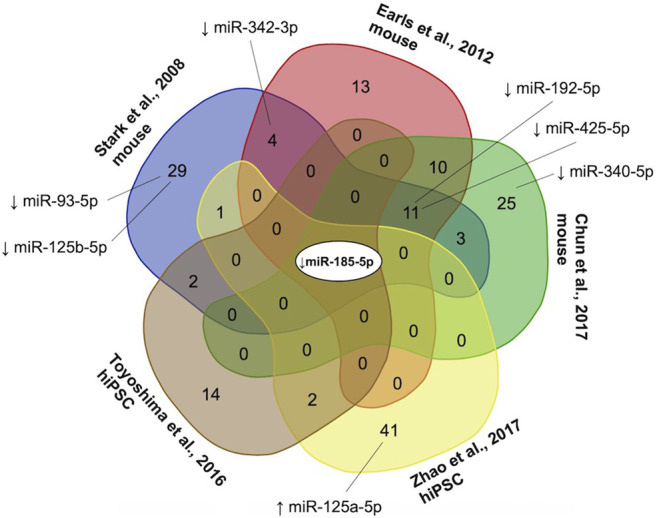
Convergence of microRNAs (miRNAs) reported as differentially expressed in three mouse model studies (Stark et al., 2008; Earls et al., 2012; Chun et al., 2017) and two human induced pluripotent stem cell models (Zhao et al., 2015; Toyoshima et al., 2016) of the 22q11.2 deletion. Collectively, 156 miRNAs were reported as differentially expressed (using various criteria), of which 122 (78.2%) were downregulated, 40 (25.6%) upregulated, and 6 (3.8%) up-or down-regulated in different studies. The only miRNA downregulated in all five studies was miR-185-5p, a conserved miRNA located within the proximal 22q11.2 deletion region (Guna et al., 2015). miRNAs with targets that were found to be overlapped by one or more rare genome-wide CNVs in significantly more individuals with 22q11.2 deletion syndrome in the schizophrenia than non-psychotic group in the current study are shown with a black line connecting to the studies reporting differential miRNA expression. ↑, increased expression; ↓, decreased expression.
miRNA Target-CNV Burden, Gene Set Enrichment, and miRNA Analyses
We compared the proportion of individuals between the schizophrenia and non-psychotic groups with one or more miRNA target gene overlapped by an additional rare CNV. This comparison was done first using all high-confidence miRNA targets (n = 8,464) and then restricted to the targets of miRNAs with evidence of differential brain tissue expression in 22q11.2 deletion models (n = 5,672) (Table 1; Supplementary Table S6). The proportions of individuals between groups were first compared using a two-sided Fisher’s exact test. Additionally, we created a logistic regression model for schizophrenia expression that included the following variables: the presence of a miRNA target gene overlapped by an additional rare genome-wide CNV, the summed size in base pairs of all rare CNVs (excluding the 22q11.2 deletion) per individual, the number of protein coding genes overlapped by all rare CNVs (excluding the 22q11.2 deletion) per individual, and sex.
TABLE 1.
Schizophrenia expression in 22q11.2DS and its relationship to having a miRNA target gene overlapped by an additional rare CNV.
| Individuals with an additional genome-wide rare genic CNV overlapping a miRNA target gene | Unadjusted analysis | Adjusted analysis a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia (n = 100) | Non-psychotic (n = 118) | |||||||||||
| n | % | n | % | OR | 95% CI | p | OR | 95% CI | p | |||
| All miRNA target genes (n = 8,464) b | ||||||||||||
| All Rare CNVs c | 69 | 69.0 | 58 | 49.2 | 2.29 | 1.27 | 4.18 | 0.0038 | 2.12 | 1.17 | 3.85 | 0.0138 |
| Gene Set Restriction d | ||||||||||||
| FMRP targets Darnell e | 11 | 11.0 | 4 | 3.4 | 3.50 | 1.00 | 15.59 | 0.0327 | 3.45 | 1.03 | 11.62 | 0.0455 |
| FMRP targets Ascano e | 16 | 16.0 | 8 | 6.8 | 2.61 | 1.00 | 7.39 | 0.0488 | 2.73 | 1.03 | 7.26 | 0.0437 |
| Nervous system development e | 23 | 23.0 | 13 | 11.0 | 2.40 | 1.09 | 5.51 | 0.0271 | 1.85 | 0.84 | 4.10 | 0.1273 |
| Neuro-function union inclusive e | 28 | 28.0 | 18 | 15.3 | 2.15 | 1.06 | 4.47 | 0.0298 | 1.65 | 0.81 | 3.37 | 0.1661 |
| Brain expression high/medium f | 42 | 42.0 | 31 | 26.3 | 2.03 | 1.10 | 3.75 | 0.0151 | 1.84 | 0.99 | 1.22 | 0.0555 |
| Endocrine/exocrine/reproduction g | 20 | 20.0 | 10 | 8.5 | 2.69 | 1.13 | 6.80 | 0.0175 | 2.55 | 1.09 | 1.45 | 0.0311 |
| 22q11.2 deletion models miRNA targets subset (n = 5,672) h | ||||||||||||
| All Rare CNVs c | 58 | 58.0 | 49 | 41.5 | 1.82 | 1.02 | 3.25 | 0.0398 | 1.64 | 0.91 | 2.97 | 0.097 |
CNV, copy number variant; Unadjusted analysis, two-sided Fisher’s exact test; OR, odds ratio; 95% CI, 95% confidence interval of the odds ratio; miRNA, microRNA; FRMP, Fragile X Mental Retardation Protein.
The p-values, ORs, and 95% CIs listed are for the presence of a miRNA target overlapped by a rare CNV in a logistic regression model with the following additional variables: the total rare CNV size per individual, total number of protein coding genes overlapped by a rare CNV per individual, and sex.
miRNA, target genes retrieved from DIANA-TarBase v8, supported by experimental evidence (see Methods for filtering criteria).
Rare CNVs, with <0.1% frequency in control databases and ≥10 kb in length.
Restricting to individuals with a rare CNV overlapping a miRNA target gene that belongs to a gene set.
Among 19 gene sets belonging to Neuro-functional category of gene sets (Supplementary Table S7). The larger Neuro-function union inclusive gene set includes all genes from the Nervous system development gene set (Supplementary Table S9).
Among seven gene sets belonging to BrainSpan category of gene sets (Supplementary Table S7).
Among seven gene sets belonging to mouse organ systems category of gene sets (Supplementary Table S7).
Subset of the 8,464 genes that are targets of miRNAs, that were found to be differentially expressed in experimental models of the 22q11.2 deletion (Figure 3).
To assess the representation of miRNA target genes overlapped by CNVs with respect to gene function related to neurodevelopmental pathways, brain expression, and other organ systems, we used 36 gene sets from a large study of rare CNVs in idiopathic schizophrenia (Marshall et al., 2017) (Supplementary Table S7). We used two approaches for gene set enrichment analyses to accommodate for the potential biases of each method (see Supplementary Methods for detailed explanation). Briefly, we first applied a “self-contained” method by comparing the proportion of individuals between groups with a rare CNV overlapping a miRNA target gene that also belongs to a gene set, using a two-sided Fisher’s exact test and the previously described logistic regression model. Second, using a “competitive” approach, we divided the genes overlapped by CNVs in the schizophrenia and non-psychotic groups by those that were or were not deemed stringent miRNA targets genes, resulting in four subgroups: 1) miRNA targets and 2) non-miRNA targets from the schizophrenia group, and 3) miRNA targets and 4) non-miRNAs targets from the non-psychotic group. For each subgroup we used a one-sided Fisher’s exact test (i.e., conventional hypergeometric test for enrichment) (Reimand et al., 2019) to compare the proportion of genes in the subgroup versus all other genes in the genome that belong to a particular gene set, using 19,203 distinct Ensembl IDs of protein coding genes with from HGNC 2 as the background. The self-contained approach measures the relative degree of enrichment between the schizophrenia and non-psychotic groups and is resistant to findings that may be driven by an anonymously large number of genes being overlapped by CNVs in small number of individuals, which may occur when using the competitive method. In contrast, the competitive method measures enrichment relative to the background genome and is resistant to findings driven by the same (or a small number of genes) repeatedly being overlapped by CNVs in an anonymously large number of individuals, which may occur when using the self-contained method. Benjamini–Hochberg false discovery rate (FDR) was used to adjust for multiple testing separately for each of these gene set categories: neuro-functional (n = 19), mouse-neuro (n = 3), brain expression (n = 7), and mouse body systems (n = 7).
To determine which miRNAs were the ones whose target genes contributed most to the differential burden of miRNA targets overlapped by CNVs between the schizophrenia and non-psychotic groups, we defined two categories of miRNAs. The first are “broadly targeting” miRNAs which we defined as miRNAs that contributed to the results of one or more individuals in both groups. The second are “exclusively targeting” miRNAs which contributed to the results of only one group. For each category, we determined the miRNAs that contributed to the results of significantly more individuals in one group compared to the other. Using a two-sided Fisher’s exact test, with the threshold of p < 0.05, we determined a priori that a miRNA deemed exclusively targeting would need to contribute to the results of either 4 of 100 individuals in the schizophrenia group (versus 0 of 118 in non-psychotic) or 6 of 118 individuals in the non-psychotic group (versus 0 of 100 in schizophrenia) to reach significance (Table 2).
TABLE 2.
miRNAs with target genes overlapped by rare CNVs in significantly more individuals in the 22q11.2DS schizophrenia group than in the 22q11.2DS non-psychotic group.
| Individuals with rare genic CNV overlapping a miRNA target a | Analysis | Evidence of miRNA dysregulation in | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia (n = 100) | Non-psychotic (n = 118) | |||||||||
| Target genes of miRNA overlapped by CNVs in | miRNA (hsa-miR-) | n | % | n | % | OR | p | FDR b | 22q11.2 deletion experimental models c | Idiopathic schizophrenia and other neuropsychiatric disorders |
| 1) ≥1 individuals in both the schizophrenia and non-psychotic groups (i.e. “broadly targeting”) d | 17-5p | 15 | 15.0 | 5 | 4.2 | 3.96 | 0.0085 | 0.6747 | 17-3p ↓ hiPSC Toyoshima et al. (2016) | - ↑ expression in schizophrenia post-mortem brain tissue (cortex) Beveridge et al. (2010); Santarelli et al. (2011); Wong et al. (2013) |
| - ↓ circulating levels in schizophrenia Shi et al. (2012) | ||||||||||
| 124-3p | 18 | 18.0 | 8 | 6.8 | 3.00 | 0.0123 | 0.6747 | None | - ↑ circulating levels in major depression Roy et al. (2017) | |
| - Essential role in neural precursor differentiation Fineberg et al. (2009) | ||||||||||
| 34a-5p | 18 | 18.0 | 9 | 7.6 | 2.65 | 0.0238 | 0.6747 | 34b-3p & 34c-5p ↑ hiPSC Zhao et al. (2015) | - ↑circulating levels in schizophrenia and dysregulated in major depression, bipolar, Alzheimer’s, and Parkinson’s Lai et al. (2011); van den Berg et al. (2020) | |
| - ↑circulating levels in early onset schizophrenia Chen et al. (2021) | ||||||||||
| 128-3p | 7 | 7.0 | 1 | 0.8 | 8.73 | 0.0253 | 0.6747 | None | -Involved in neural migration, excitability, apoptosis, development, and has been implicated in schizophrenia, intellectual disability, epilepsy, Alzheimer’s, and motor dysfunction Tan et al. (2013); Ching and Ahmad-Annuar (2015); Franzoni et al. (2015); Zhou et al. (2018); Shu et al. (2019) | |
| -Regulates astroglia receptor signalling in an FMRP dependent manner Men et al. (2020) | ||||||||||
| 20b-5p | 7 | 7.0 | 1 | 0.8 | 8.73 | 0.0253 | 0.6747 | None | - Aggravates apoptosis in Alzheimer’s mouse model Tian et al. (2021) | |
| 93-5p | 13 | 13.0 | 5 | 4.2 | 3.36 | 0.0253 | 0.6747 | ↓ mouse Stark et al. (2008) | - Target gene overlapped by de novo CNV in intellectual disability Qiao et al. (2013) | |
| - Ortholog of the miR-17/92 cluster Mogilyansky and Rigoutsos (2013) | ||||||||||
| 192-5p | 6 | 6.0 | 1 | 0.8 | 7.41 | 0.0490 | 1.0000 | ↓ mouse Stark et al. (2008); Earls et al. (2012); Chun et al. (2017) | - Upregulation rescues cognitive impairment in a depression mouse model Tang et al. (2019) | |
| 2) Only the schizophrenia group (i.e., “exclusively targeting”) e | 342-3p | 5 | 5.0 | 0 | 0 | Inf | 0.0192 | 0.0428 | ↓ mouse Stark et al. (2008); Earls et al. (2012) | - Circulating miR-342-5p↓ in schizophrenia Gardiner et al. (2012) |
| 125a-5p | 4 | 4.0 | 0 | 0 | Inf | 0.0428 | 0.0428 | ↑ hiPSC Zhao et al. (2015) | - Circulating miR-125a-3p & miR-125b-3p ↑ in schizophrenia Camkurt et al. (2016) | |
| 125b-5p | 4 | 4.0 | 0 | 0 | Inf | 0.0428 | 0.0428 | ↓ mouse Stark et al. (2008) | -↑ expression in schizophrenia post-mortem brain tissue (cortex) Beveridge et al. (2010) | |
| - Interacts with FMRP in mouse brain to affect synaptic plasticity Edbauer et al. (2010) | ||||||||||
| 340-5p | 4 | 4.0 | 0 | 0 | Inf | 0.0428 | 0.0428 | ↓ mouse Chun et al. (2017) | - ↑ expression in schizophrenia post-mortem brain tissue (cortex) Beveridge et al. (2010) | |
| -↑ expression in FMRP knock out mice Liu et al. (2015) | ||||||||||
| 425-5p | 4 | 4.0 | 0 | 0 | Inf | 0.0428 | 0.0428 | ↓ mouse Stark et al. (2008); Earls et al. (2012); Chun et al. (2017) | - Dysregulated expression in schizophrenia post-mortem brain tissue (cortex) Moreau et al. (2011); Santarelli et al. (2011) | |
CNV, copy number variant; Analysis, two-sided Fisher’s exact test; miRNA, microRNA; FDR, Benjamini-Hochberg false discovery rate; ↑, increased expression; ↓, decreased expression.
Number of individuals in each group with one or more genes targeted by the miRNA of interest and overlapped by an additional rare genome-wide CNV.
Calculated separately for each set of miRNAs satisfying the criteria for the “broadly targeting” (n = 160) and “exclusively targeting” (n = 5) criterion.
Indicates if the miRNA of interest or a closely related form is in the list compiled in this study of miRNAs with evidence of differential expression in 22q11.2 deletion models. Differences in closely related forms are bolded.
Between group comparisons were made for miRNAs that targeted genes overlapped by rare CNVs in ≥1 individual(s) in both groups. One hundred sixty miRNAs satisfied this criterion and the seven that reached significance (p < 0.05) are shown.
Between group comparisons were made for miRNAs that targeted genes overlapped by one or more rare CNVs in ≥4 individuals in the schizophrenia group and none in the non-psychotic group (p < 0.05).
All statistical analyses were performed using R 4.0.3. Statistical significance was defined as p < 0.05. This study used entirely previously published or open-source data (Stark et al., 2008; Earls et al., 2012; Agarwal et al., 2015; Zhao et al., 2015; Toyoshima et al., 2016; Bassett et al., 2017; Chun et al., 2017; Marshall et al., 2017; Karagkouni et al., 2018).
Results
Increased Burden of miRNA Target Genes Overlapped by Rare CNVs in 22q11.2DS Schizophrenia
Using the list of all high-confidence miRNA target genes (n = 8,464), consistent with our hypothesis, we found a significantly higher proportion of individuals in the schizophrenia group compared to the non-psychotic group to have one or more miRNA target genes overlapped by an additional rare genome-wide CNV (69 of 100 vs. 58 of 118; p = 0.0038; OR = 2.29 [95% CI = 1.47–4.18]) (Table 1). Presence of a miRNA target gene overlapped by a rare CNV remained a significant predictor of schizophrenia expression in a multivariable logistic regression model that adjusted for total rare CNV size, total number of genes overlapped by a rare CNV, and sex (p = 0.0138, OR = 2.12 [95% CI = 1.17–3.85]). Male sex was also a significant covariate (p = 0.0125). When considering rare duplications or deletions separately, the differential burden of miRNA target genes overlapped by CNVs between groups trended in the same direction, but did not reach statistical significance for either dosage subgroup (Supplementary Table S6).
We tested the effect of this miRNA target gene-CNV burden if we restricted to gene targets of the miRNAs that showed differential expression compared to controls identified in our review of available experimental models (n = 5) of the 22q11.2 deletion (Stark et al., 2008; Earls et al., 2012; Zhao et al., 2015; Toyoshima et al., 2016; Chun et al., 2017). Of the 156 miRNAs with available evidence of differential expression, convergence was low and most (n = 122, 78.2%) were reported in only a single study (Figure 3). Proceeding with the 136 miRNAs with experimentally supported target genes (n = 5,672, Figure 3, Supplementary Tables S4, S5), and rerunning analyses revealed more modest effect size for miRNA targets overlapped by a rare CNV as a predictor of schizophrenia status, and results no longer reached significance when adjusted for other variables in the logistic regression model (Table 1). We therefore used all high-confidence miRNA target genes (n = 8,464) for subsequent analyses.
Gene Set Enrichment Analyses
We assessed for gene set enrichment using two approaches. First, we used a “self-contained” approach by comparing the proportion of individuals between the schizophrenia and non-psychotic groups with an additional rare CNV overlapping a miRNA target gene that also belongs to a gene set. Second, we used a “competitive” approach that pools the genes overlapped by CNVs into four subgroups (divided by schizophrenia versus non-psychotic status, and whether the gene is or is not a miRNA target), and tests for enrichment compared to all other genes in the genome (i.e., “background”).
Using the self-contained approach, six gene sets were nominally enriched in the schizophrenia group (p < 0.05) (Table 1, Supplementary Tables S8, S9). Among the four in the neuro-functional category, only the two gene sets representing targets of the Fragile X Mental Retardation Protein (FMRP) (Darnell et al., 2011; Ascano et al., 2012) remained significant after adjusting for other variables using the previously described logistic regression model. We noted that 23 of the 29 genes in the neuro-functional union inclusive gene set contributing to these results are from the nervous system development gene set (Supplementary Table S9), demonstrating the nervous system development set as the main driver of the enrichment observed in the union set. Among the other two gene set categories, we observed enrichment in the schizophrenia group for genes with high/medium expression in the brain and with endocrine/exocrine/reproductive involvement. After correcting for multiple testing (FDR <0.05), no gene sets using the self-contained approach reached significance.
Using the competitive approach, we observed convergence with the self-contained approach in enrichment, after correcting for multiple testing (FDR <0.05), among miRNA target genes overlapped by rare CNVs in the schizophrenia group for the FMRP targets Ascano (OR = 3.51, FDR = 1.27 × 10–4) and the nervous system development (OR = 1.93, FDR = 0.0331) gene sets (Supplementary Table S10). We also found that in both the schizophrenia and non-psychotic groups, miRNA targets overlapped by rare CNVs were enriched for genes with high/medium, and prenatal expression in the brain. However, the self-contained approach demonstrated that the enrichment for high/medium brain expression is relatively greater in the schizophrenia group (Table 1; Supplementary Table S10). In contrast, non-miRNA target genes were enriched in both schizophrenia and non-psychotic groups for genes with minimum brain expression (FDR <0.05). Lastly, we found two gene sets, post synaptic density genes (OR = 2.24, FDR = 0.0171) and genes with both pre- and post-natal brain expression (OR = 1.61, FDR = 0.0391) to be enriched only among miRNA targets in the schizophrenia group (Supplementary Table S10). However, these two gene sets did not reach nominal significance using the self-contained approach (Supplementary Table S8).
miRNAs That Target Genes Overlapped by Rare CNVs in 22q11.2DS
We subsequently performed an exploratory analysis to determine which individual miRNAs contributed most to the differential CNV-target gene burden observed (Table 2; Supplementary Table S11). Among “broadly targeting” miRNAs (defined as contributing to the results of at least one individual in both groups), seven miRNAs (miR-17-5p, miR-124-3p, miR-34a-5p, miR-128-3p, miR-20b-5p, miR-93-5p, and miR-192-5p) had target genes overlapped by CNVs in significantly more individuals in the 22q11.2DS schizophrenia than the non-psychotic group (Table 2). No miRNAs deemed broadly targeting contributed to the results of significantly more individuals in the non-psychotic group compared to the schizophrenia group (Supplementary Table S11). Additionally, among “exclusively targeting” miRNAs (defined as contributing to the results of only one group), there were five miRNAs (miR-342-3p, miR-125a-5p, miR-125b-5p, miR-340-5p, and miR-425-5p) that targeted genes overlapped by rare CNVs exclusively in the schizophrenia group (p < 0.05) (Table 2) while no miRNAs contributing exclusively to the non-psychotic group reached significance (Supplementary Table S11).
When we examined the targets of the only miRNA reported as downregulated in all experimental models of the 22q11.2 deletion, miR-185-5p (located in the 22q11.2 deletion region) (Figure 3), that were overlapped by rare CNVs, we found no differential burden between the schizophrenia and non-psychotic groups (3 of 100 vs. 3 of 118; p = 1.0000, OR = 1.18 [95% CI = 0.15–9.05]).
Discussion
In this study, we explore a theoretical proposition that additional rare genome-wide CNVs overlapping genes targeted by miRNAs may contribute to risk for schizophrenia in individuals with a 22q11.2 deletion—the largest known molecular genetic risk contributor for schizophrenia (Bassett et al., 2017; Cleynen et al., 2020). Using a well-characterized 22q11.2DS cohort and stringently defined miRNA targets, we provide correlational evidence in support of our hypothesis by demonstrating that in individuals with a 22q11.2 deletion and an additional rare genic CNV, a significantly higher proportion of those in the schizophrenia group than those with no psychotic illness had one or more miRNA target genes overlapped by an additional rare CNV. This difference remained significant after correcting for the size and genic content of the CNVs. Results of gene set enrichment and selected miRNA analyses support the possibility that dysregulation involving both altered gene dosage and miRNA-related effects may differentially impact schizophrenia-relevant pathways, including genes with high expression in the brain. Although limited by sample size and the reliability/reproducibility of available miRNA data, the results support our proposed model. This model and the evidence available contribute novel insights into possible mechanisms behind how miRNA perturbance, that is likely to be amplified by the 22q11.2 deletion, together with genome-wide variation, may increase risk for schizophrenia in 22q11.2DS, as part of the polygenic contributors to this complex disease.
Genetic Contributors to Expression of Schizophrenia in 22q11.2DS
Previous evidence supports genome-wide variation in modifying the risk imparted by the 22q11.2 deletion (Bassett et al., 2017; Cleynen et al., 2020). These findings for 22q11.2DS are consistent with those in idiopathic schizophrenia of an enrichment of genome-wide rare CNVs overlapping both protein coding genes (Costain et al., 2013; Marshall et al., 2017) and miRNA coding sequences (Warnica et al., 2015), as well as association with common variants mostly residing in non-coding regions (Ripke et al., 2014). Together, the evidence suggests that multiple mechanisms likely contribute to the dysregulation of gene expression in the etiology of schizophrenia.
Results of the present study suggest an additional layer to how rare genome-wide CNVs overlapping a broad category of genes—those with evidence of being targeted by miRNAs—may contribute to schizophrenia risk in 22q11.2DS. This finding is consistent with both the elevated miRNA dysregulation (Stark et al., 2008; Earls et al., 2012; Zhao et al., 2015; Toyoshima et al., 2016; Chun et al., 2017) and increased penetrance of schizophrenia (Bassett and Chow, 2008; Marshall et al., 2017) in 22q11.2DS. Widespread miRNA dysregulation attributable to DGCR8 hemizygosity may serve as part of the “threshold lowering” mechanism of the 22q11.2 deletion for schizophrenia risk. Results of the current study are consistent with our proposed theory that part of this lowered threshold may be mediated by miRNA dysregulation exacerbating the pathogenicity of additional rare CNVs in individuals where such CNVs overlap a miRNA target gene (Figure 1).
The results also support miRNA and CNV mediated dysregulation of gene expression differentially impacting genes in schizophrenia-relevant pathways. Consistent with studies of idiopathic schizophrenia (Clifton et al., 2020), the results of gene set analyses in the current study indicate that miRNA target genes overlapped by rare CNVs are enriched for genes that are also targets of FMRP in individuals with 22q11.2DS-schizophrenia (Table 1; Supplementary Tables S8, S10). FMRP, like miRNAs, primarily functions to suppress translation (Laggerbauer et al., 2001; Li et al., 2001; Napoli et al., 2008; Darnell et al., 2011; Ascano et al., 2012) and may act, in some cases, in a miRNA-dependent manner (Jin et al., 2004; Edbauer et al., 2010; Muddashetty et al., 2011; Liu et al., 2015). It is conceivable that miRNA dysregulation may alter the ability of FMRP to regulate a shared target gene, adding yet another layer of perturbation. Notably, the previous analysis of rare CNVs in this cohort did not identify an enrichment of the FMRP gene sets in schizophrenia (Bassett et al., 2017), demonstrating the added utility of the focus on miRNA target genes. Additionally, we found an enrichment in miRNA target genes overlapped by rare CNVs in the schizophrenia group for genes involved in nervous system development (Table 1; Supplementary Table S8, S10). This result is consistent with perturbations in gene expression in early development posited by the neurodevelopmental model of schizophrenia (Birnbaum and Weinberger, 2017) and the established roles of miRNAs in neurodevelopment (Fineberg et al., 2009).
Further supporting the involvement of miRNA dysregulation in this proposed mechanism, there is independent evidence for dysregulation in schizophrenia and/or other neuropsychiatric disorders of the miRNAs most implicated in the findings in this study (Table 2). Amongst these miRNAs are miR-125b, miR-340, and miR-128-3p, which have been demonstrated in mouse functional studies to interact with FMRP (Muddashetty et al., 2011; Liu et al., 2015; Men et al., 2020). The absence of miR-185-5p amongst these miRNAs most implicated suggests that miRNA perturbance imparted by DGCR8 hemizygosity may affect pathways that are beyond the downstream targets of miR-185-5p (Xu et al., 2013).
Advantages and Limitations
We used the largest cohort of 22q11.2DS, with and without schizophrenia, with publicly available data on additional rare CNVs (Bassett et al., 2017). The advantages of the sampling strategy and rigorous CNV analytic methods used have been previously described (Bassett et al., 2017). Nonetheless, the cohort is small compared to several other genetic studies of schizophrenia that do not focus on a rare genetic model of schizophrenia (Marshall et al., 2017). The cohort size limited power, particularly for secondary analyses that required restricting to smaller subsets (e.g., evaluating duplications and deletions separately, gene set analyses involving multiple testing).
A challenge faced by all studies that involve miRNA targets is developing a set of accurate miRNA-target gene interactions based on currently available prediction algorithms and experimental databases (Riolo et al., 2021). Due to the high false positive rate of bioinformatically driven miRNA target prediction programs (Pinzón et al., 2017), we opted to rely primarily on experimentally supported miRNA targets (Karagkouni et al., 2018) with additional filtering criteria for added stringency. However, experimentally supported targets are likely biased towards more intensively studied miRNAs (most often those with lower miR-numbers) and genes (e.g., cancer-related PTEN and NF1).
A combination of factors may be involved in the stronger signal observed when using all miRNA target genes than when restricting to the targets of differentially expressed miRNAs derived from available studies of 22q11.2 deletion experimental models (Table 1). We opted to use brain derived profiling for relevance to schizophrenia. Using other tissues (e.g., circulating miRNAs from blood serum (de la Morena et al., 2013; Sellier et al., 2014)) may produce different results. Our review of these model-derived differentially expressed miRNAs showed little convergence across studies (Figure 3; Supplementary Table S4, S5). Low reproducibility is common in miRNA profiling studies (Baker, 2010; Wang et al., 2011; Chugh and Dittmer, 2012; Witwer and Halushka, 2016) and differences in methodology and other biological factors may have contributed to discordant findings (e.g., statistical criteria, miRNA profiling technologies, mouse models versus hiPSCs, differing 22q11.2 deletion extents, and developmental timing) (Supplementary Table S4). There is also the possibility that because miRNA expression is temporally regulated across developmental stages (Sempere et al., 2003; Caygill and Johnston, 2008), finding miRNAs that were uniquely differentially expressed in each study may imply that the scope of 22q11.2 deletion-related miRNA dysregulation across all developmental stages is broader than what is reported in any individual study, and that currently available results from model systems may underestimate the entirety of miRNA dysregulation effects in 22q11.2DS.
In summary, within the limitations of the sample size and miRNA source data as discussed above, the results of this study offer correlational evidence to suggest that genes targeted by miRNAs and overlapped by additional rare genome-wide CNVs may be conferring part of the risk for schizophrenia in individuals with a 22q11.2 deletion. We postulate that this effect is mediated by widespread miRNA dysregulation imparted by the 22q11.2 deletion through hemizygosity of DGCR8. Our proposed theory supports a model of schizophrenia risk that involves multiple layers of perturbance across many genes, pathways, and mechanisms, consistent with the complex genetic architecture of schizophrenia. This study would benefit from being replicated to test for reproducibility as larger cohorts of individuals with 22q11.2DS and CNV data become available, and as miRNA profiling studies and target gene databases/prediction programs improve in their degree of reliability. Additionally, future studies of 22q11.2DS may consider investigating other rare variant types impacting miRNA target genes. It would also be valuable to assess if this category of genes, or other genetic mechanisms involving miRNA dysregulation, also increases risk for schizophrenia in the general population.
Acknowledgments
The authors are grateful both to the local participants with 22q11.2 deletion syndrome, and to those worldwide who were part of the International 22q11.2DS Consortium on Brain and Behavior cohort, who through participating in research, together with the efforts of researchers over many years, enabled the previously published dataset that was used in the current study.
Footnotes
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SY and ASB conceptualized the study and analysis framework. SY performed the statistical analyses and manuscript writing. RKCY and ZZ contributed to analysis interpretation and provided feedback. TH performed data pre-processing. SY, ASB, RKCY, and ZZ contributed to manuscript reviewing and editing.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) (PJT-169161, PJT-148924). ASB holds the Dalglish Chair in 22q11.2 Deletion Syndrome at the University Health Network and University of Toronto. SY is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Master’s Award and the University of Toronto Joseph Wong Memorial Award. National Institute of Mental Health (5U01MH101723-02), the Canadian Institutes of Health Research (ASB, MOP #97800, MOP #111238), the Canada Research Chairs in Schizophrenia Genetics and Genomic Disorders (ASB), Canada-Latin America and Caribbean Research Exchange Grants (LACREG) (ASB), and the University of Toronto McLaughlin Centre (MC-2015-01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.812183/full#supplementary-material
References
- Agarwal V., Bell G. W., Nam J.-W., Bartel D. P. (2015). Predicting Effective microRNA Target Sites in Mammalian mRNAs. eLife 4, e05005. 10.7554/eLife.05005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascano M., Mukherjee N., Bandaru P., Miller J. B., Nusbaum J. D., Corcoran D. L., et al. (2012). FMRP Targets Distinct mRNA Sequence Elements to Regulate Protein Expression. Nature 492, 382–386. 10.1038/nature11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. (2010). MicroRNA Profiling: Separating Signal from Noise. Nat. Methods 7, 687–692. 10.1038/nmeth0910-687 [DOI] [PubMed] [Google Scholar]
- Bassett A. S., Chow E. W. C. (2008). Schizophrenia and 22q11.2 Deletion Syndrome. Curr. Psychiatry Rep. 10, 148–157. 10.1007/s11920-008-0026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett A. S., Lowther C., Merico D., Costain G., Chow E. W. C., van Amelsvoort T., et al. (2017). Rare Genome-wide Copy Number Variation and Expression of Schizophrenia in 22q11.2 Deletion Syndrome. Ajp 174, 1054–1063. 10.1176/appi.ajp.2017.16121417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge N. J., Gardiner E., Carroll A. P., Tooney P. A., Cairns M. J. (2010). Schizophrenia Is Associated with an Increase in Cortical microRNA Biogenesis. Mol. Psychiatry 15, 1176–1189. 10.1038/mp.2009.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum R., Weinberger D. R. (2017). Genetic Insights into the Neurodevelopmental Origins of Schizophrenia. Nat. Rev. Neurosci. 18, 727–740. 10.1038/nrn.2017.125 [DOI] [PubMed] [Google Scholar]
- Blagojevic C., Heung T., Theriault M., Tomita-Mitchell A., Chakraborty P., Kernohan K., et al. (2021). Estimate of the Contemporary Live-Birth Prevalence of Recurrent 22q11.2 Deletions: a Cross-Sectional Analysis from Population-Based Newborn Screening. cmajo 9, E802–E809. 10.9778/cmajo.20200294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzustowicz L. M., Bassett A. S. (2012). miRNA-Mediated Risk for Schizophrenia in 22q11.2 Deletion Syndrome. Front. Gene 3, 291. 10.3389/fgene.2012.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camkurt M. A., Karababa F., Erdal M. E., Bayazıt H., Kandemir S. B., Ay M. E., et al. (2016). Investigation of Dysregulation of Several MicroRNAs in Peripheral Blood of Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 14, 256–260. 10.9758/cpn.2016.14.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caygill E. E., Johnston L. A. (2008). Temporal Regulation of Metamorphic Processes in Drosophila by the Let-7 and miR-125 Heterochronic MicroRNAs. Curr. Biol. 18, 943–950. 10.1016/j.cub.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.-Y., Lin J.-J., Lu M.-K., Tan H.-P., Jang F.-L., Lin S.-H. (2021). Neurodevelopment Regulators miR-137 and miR-34 Family as Biomarkers for Early and Adult Onset Schizophrenia. npj Schizophr 7, 1–9. 10.1038/s41537-021-00164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching A.-S., Ahmad-Annuar A. (2015). A Perspective on the Role of microRNA-128 Regulation in Mental and Behavioral Disorders. Front. Cel. Neurosci. 9, 465. 10.3389/fncel.2015.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh P., Dittmer D. P. (2012). Potential Pitfalls in microRNA Profiling. WIREs RNA 3, 601–616. 10.1002/wrna.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S., Du F., Westmoreland J. J., Han S. B., Wang Y.-D., Eddins D., et al. (2017). Thalamic miR-338-3p Mediates Auditory Thalamocortical Disruption and its Late Onset in Models of 22q11.2 Microdeletion. Nat. Med. 23, 39–48. 10.1038/nm.4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleynen I., Engchuan W., Hestand M. S., Heung T., Holleman A. M., Johnston H. R., et al. (2020). Genetic Contributors to Risk of Schizophrenia in the Presence of a 22q11.2 Deletion. Mol. Psychiatry 26, 4496. 10.1038/s41380-020-0654-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton N. E., Rees E., Holmans P. A., Pardiñas A. F., Harwood J. C., Di Florio A., et al. (2020). Genetic Association of FMRP Targets with Psychiatric Disorders. Mol. Psychiatry 26, 2977–2990. 10.1038/s41380-020-00912-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G., Lionel A. C., Merico D., Forsythe P., Russell K., Lowther C., et al. (2013). Pathogenic Rare Copy Number Variants in Community-Based Schizophrenia Suggest a Potential Role for Clinical Microarrays. Hum. Mol. Genet. 22, 4485–4501. 10.1093/hmg/ddt297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. C., Van Driesche S. J., Zhang C., Hung K. Y. S., Mele A., Fraser C. E., et al. (2011). FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell 146, 247–261. 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Morena M. T., Eitson J. L., Dozmorov I. M., Belkaya S., Hoover A. R., Anguiano E., et al. (2013). Signature MicroRNA Expression Patterns Identified in Humans with 22q11.2 deletion/DiGeorge Syndrome. Clin. Immunol. 147, 11–22. 10.1016/j.clim.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S., Mi S., Zhang W., Dolan M. E. (2009). Comprehensive Analysis of the Impact of SNPs and CNVs on Human microRNAs and Their Regulatory Genes. RNA Biol. 6, 412–425. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2783774/ (Accessed June 24, 2021). 10.4161/rna.6.4.8830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls L. R., Fricke R. G., Yu J., Berry R. B., Baldwin L. T., Zakharenko S. S. (2012). Age-Dependent MicroRNA Control of Synaptic Plasticity in 22q11 Deletion Syndrome and Schizophrenia. J. Neurosci. 32, 14132–14144. 10.1523/JNEUROSCI.1312-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D., Neilson J. R., Foster K. A., Wang C.-F., Seeburg D. P., Batterton M. N., et al. (2010). Regulation of Synaptic Structure and Function by FMRP-Associated microRNAs miR-125b and miR-132. Neuron 65, 373–384. 10.1016/j.neuron.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felekkis K., Voskarides K., Dweep H., Sticht C., Gretz N., Deltas C. (2011). Increased Number of MicroRNA Target Sites in Genes Encoded in CNV Regions. Evidence for an Evolutionary Genomic Interaction. Mol. Biol. Evol. 28, 2421–2424. 10.1093/molbev/msr078 [DOI] [PubMed] [Google Scholar]
- Fineberg S. K., Kosik K. S., Davidson B. L. (2009). MicroRNAs Potentiate Neural Development. Neuron 64, 303–309. 10.1016/j.neuron.2009.10.020 [DOI] [PubMed] [Google Scholar]
- Forstner A. J., Degenhardt F., Schratt G., Nöthen M. M. (2013). MicroRNAs as the Cause of Schizophrenia in 22q11.2 Deletion Carriers, and Possible Implications for Idiopathic Disease: a Mini-Review. Front. Mol. Neurosci. 6, 47. 10.3389/fnmol.2013.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni E., Booker S. A., Parthasarathy S., Rehfeld F., Grosser S., Srivatsa S., et al. (2015). miR-128 Regulates Neuronal Migration, Outgrowth and Intrinsic Excitability via the Intellectual Disability Gene Phf6. eLife 4, e04263. 10.7554/eLife.04263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner E., Beveridge N. J., Wu J. Q., Carr V., Scott R. J., Tooney P. A., et al. (2012). Imprinted DLK1-DIO3 Region of 14q32 Defines a Schizophrenia-Associated miRNA Signature in Peripheral Blood Mononuclear Cells. Mol. Psychiatry 17, 827–840. 10.1038/mp.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guna A., Butcher N. J., Bassett A. S. (2015). Comparative Mapping of the 22q11.2 Deletion Region and the Potential of Simple Model Organisms. J. Neurodevelop Disord. 7, 18. 10.1186/s11689-015-9113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A.-Y., Sun J., Jia P., Zhao Z. (2010). A Novel microRNA and Transcription Factor Mediated Regulatory Network in Schizophrenia. BMC Syst. Biol. 4, 10. 10.1186/1752-0509-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauberg M. E., Roussos P., Grove J., Børglum A. D., Mattheisen M. and for the Schizophrenia Working Group of the Psychiatric Genomics Consortium (2016). Analyzing the Role of MicroRNAs in Schizophrenia in the Context of Common Genetic Risk Variants. JAMA Psychiatry 73, 369. 10.1001/jamapsychiatry.2015.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein E., Shomron N. (2006). Canalization of Development by microRNAs. Nat. Genet. 38, S20–S24. 10.1038/ng1803 [DOI] [PubMed] [Google Scholar]
- Jin P., Zarnescu D. C., Ceman S., Nakamoto M., Mowrey J., Jongens T. A., et al. (2004). Biochemical and Genetic Interaction between the Fragile X Mental Retardation Protein and the microRNA Pathway. Nat. Neurosci. 7, 113–117. 10.1038/nn1174 [DOI] [PubMed] [Google Scholar]
- Karagkouni D., Paraskevopoulou M. D., Chatzopoulos S., Vlachos I. S., Tastsoglou S., Kanellos I., et al. (2018). DIANA-TarBase V8: a Decade-Long Collection of Experimentally Supported miRNA-Gene Interactions. Nucleic Acids Res. 46, D239–D245. 10.1093/nar/gkx1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B., Ostareck D., Keidel E.-M., Ostareck-Lederer A., Fischer U. (2001). Evidence that Fragile X Mental Retardation Protein Is a Negative Regulator of Translation. Hum. Mol. Genet. 10, 329–338. 10.1093/hmg/10.4.329 [DOI] [PubMed] [Google Scholar]
- Lai C.-Y., Yu S.-L., Hsieh M. H., Chen C.-H., Chen H.-Y., Wen C.-C., et al. (2011). MicroRNA Expression Aberration as Potential Peripheral Blood Biomarkers for Schizophrenia. PLOS ONE 6, e21635. 10.1371/journal.pone.0021635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang Y., Ku L., Wilkinson K. D., Warren S. T., Feng Y. (2001). The Fragile X Mental Retardation Protein Inhibits Translation via Interacting with mRNA. Nucleic Acids Res. 29, 2276–2283. 10.1093/nar/29.11.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Wan R.-P., Tang L.-J., Liu S.-J., Li H.-J., Zhao Q.-H., et al. (2015). A MicroRNA Profile in Fmr1 Knockout Mice Reveals MicroRNA Expression Alterations with Possible Roles in Fragile X Syndrome. Mol. Neurobiol. 51, 1053–1063. 10.1007/s12035-014-8770-1 [DOI] [PubMed] [Google Scholar]
- Marcinkowska M., Szymanski M., Krzyzosiak W. J., Kozlowski P. (2011). Copy Number Variation of microRNA Genes in the Human Genome. BMC Genomics 12, 183. 10.1186/1471-2164-12-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. R., Howrigan D. P., Merico D., Thiruvahindrapuram B., Wu W., Greer D. S., et al. (2017). Contribution of Copy Number Variants to Schizophrenia from a Genome-wide Study of 41,321 Subjects. Nat. Genet. 49, 27–35. 10.1038/ng.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn D. M., Sullivan K. E., Marino B., Philip N., Swillen A., Vorstman J. A. S., et al. (2015). 22q11.2 Deletion Syndrome. Nat. Rev. Dis. Primers 1, 1–19. 10.1038/nrdp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y., Ye L., Risgaard R. D., Promes V., Zhao X., Paukert M., et al. (2020). Astroglial FMRP Deficiency Cell-Autonomously Up-Regulates miR-128 and Disrupts Developmental Astroglial mGluR5 Signaling. Proc. Natl. Acad. Sci. USA 117, 25092–25103. 10.1073/pnas.2014080117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D., Costain G., Butcher N. J., Warnica W., Ogura L., Alfred S. E., et al. (2014). MicroRNA Dysregulation, Gene Networks, and Risk for Schizophrenia in 22q11.2 Deletion Syndrome. Front. Neurol. 5, 238. 10.3389/fneur.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilyansky E., Rigoutsos I. (2013). The miR-17/92 Cluster: a Comprehensive Update on its Genomics, Genetics, Functions and Increasingly Important and Numerous Roles in Health and Disease. Cell Death Differ 20, 1603–1614. 10.1038/cdd.2013.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M. P., Bruse S. E., David-Rus R., Buyske S., Brzustowicz L. M. (2011). Altered MicroRNA Expression Profiles in Postmortem Brain Samples from Individuals with Schizophrenia and Bipolar Disorder. Biol. Psychiatry 69, 188–193. 10.1016/j.biopsych.2010.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty R. S., Nalavadi V. C., Gross C., Yao X., Xing L., Laur O., et al. (2011). Reversible Inhibition of PSD-95 mRNA Translation by miR-125a, FMRP Phosphorylation, and mGluR Signaling. Mol. Cel 42, 673–688. 10.1016/j.molcel.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I., Mercaldo V., Boyl P. P., Eleuteri B., Zalfa F., De Rubeis S., et al. (2008). The Fragile X Syndrome Protein Represses Activity-dependent Translation through CYFIP1, a New 4E-BP. Cell 134, 1042–1054. 10.1016/j.cell.2008.07.031 [DOI] [PubMed] [Google Scholar]
- Pinzón N., Li B., Martinez L., Sergeeva A., Presumey J., Apparailly F., et al. (2017). microRNA Target Prediction Programs Predict many False Positives. Genome Res. 27, 234–245. 10.1101/gr.205146.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Badduke C., Mercier E., Lewis S. M., Pavlidis P., Rajcan-Separovic E. (2013). miRNA and miRNA Target Genes in Copy Number Variations Occurring in Individuals with Intellectual Disability. BMC Genomics 14, 544. 10.1186/1471-2164-14-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., et al. (2019). Pathway Enrichment Analysis and Visualization of Omics Data Using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 14, 482–517. 10.1038/s41596-018-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riolo G., Cantara S., Marzocchi C., Ricci C. (2021). miRNA Targets: From Prediction Tools to Experimental Validation. MPs 4, 1. 10.3390/mps4010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S., Neale B. M., Corvin A., Walters J. T., Farh K.-H., Holmans P. A., et al. (2014). Biological Insights from 108 Schizophrenia-Associated Genetic Loci. Nature 511, 421–427. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B., Dunbar M., Shelton R. C., Dwivedi Y. (2017). Identification of MicroRNA-124-3p as a Putative Epigenetic Signature of Major Depressive Disorder. Neuropsychopharmacol 42, 864–875. 10.1038/npp.2016.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R., Koebis M., Nagai T., Shimizu K., Liao J., Wulaer B., et al. (2020). Comprehensive Analysis of a Novel Mouse Model of the 22q11.2 Deletion Syndrome: a Model with the Most Common 3.0-Mb Deletion at the Human 22q11.2 Locus. Transl Psychiatry 10, 1–13. 10.1038/s41398-020-0723-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli D. M., Beveridge N. J., Tooney P. A., Cairns M. J. (2011). Upregulation of Dicer and MicroRNA Expression in the Dorsolateral Prefrontal Cortex Brodmann Area 46 in Schizophrenia. Biol. Psychiatry 69, 180–187. 10.1016/j.biopsych.2010.09.030 [DOI] [PubMed] [Google Scholar]
- Santarelli D. M., Carroll A. P., Cairns H. M., Tooney P. A., Cairns M. J. (2019). Schizophrenia-associated MicroRNA-Gene Interactions in the Dorsolateral Prefrontal Cortex. Genomics, Proteomics & Bioinformatics 17, 623–634. 10.1016/j.gpb.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman J. D., Lorimer P. D., Rodic V., Jahromi M. S., Downie J. M., Bayerl M. G., et al. (2011). Genome Wide Copy Number Analysis of Paediatric Burkitt Lymphoma Using Formalin-Fixed Tissues Reveals a Subset with Gain of Chromosome 13q and Corresponding miRNA over Expression. Br. J. Haematol. 155, 477–486. 10.1111/j.1365-2141.2011.08883.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier C., Hwang V. J., Dandekar R., Durbin-Johnson B., Charlet-Berguerand N., Ander B. P., et al. (2014). Decreased DGCR8 Expression and miRNA Dysregulation in Individuals with 22q11.2 Deletion Syndrome. PLOS ONE 9, e103884. 10.1371/journal.pone.0103884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere L. F., Sokol N. S., Dubrovsky E. B., Berger E. M., Ambros V. (2003). Temporal Regulation of microRNA Expression in Drosophila melanogaster Mediated by Hormonal Signals and Broad-Complex Gene Activity. Dev. Biol. 259, 9–18. 10.1016/S0012-1606(03)00208-2 [DOI] [PubMed] [Google Scholar]
- Shi W., Du J., Qi Y., Liang G., Wang T., Li S., et al. (2012). Aberrant Expression of Serum miRNAs in Schizophrenia. J. Psychiatr. Res. 46, 198–204. 10.1016/j.jpsychires.2011.09.010 [DOI] [PubMed] [Google Scholar]
- Shu P., Wu C., Ruan X., Liu W., Hou L., Fu H., et al. (2019). Opposing Gradients of MicroRNA Expression Temporally Pattern Layer Formation in the Developing Neocortex. Dev. Cel 49, 764–785. 10.1016/j.devcel.2019.04.017 [DOI] [PubMed] [Google Scholar]
- Stark K. L., Xu B., Bagchi A., Lai W.-S., Liu H., Hsu R., et al. (2008). Altered Brain microRNA Biogenesis Contributes to Phenotypic Deficits in a 22q11-Deletion Mouse Model. Nat. Genet. 40, 751–760. 10.1038/ng.138 [DOI] [PubMed] [Google Scholar]
- Tan C. L., Plotkin J. L., Venø M. T., von Schimmelmann M., Feinberg P., Mann S., et al. (2013). MicroRNA-128 Governs Neuronal Excitability and Motor Behavior in Mice. Science 342, 1254–1258. 10.1126/science.1244193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.-Z., Yang J. T., Liu Q.-H., Wang Y.-R., Wang W.-S. (2019). Up‐regulated miR‐192‐5p Expression Rescues Cognitive Impairment and Restores Neural Function in Mice with Depression via the Fbln2 ‐mediated TGF‐β1 Signaling Pathway. FASEB j. 33, 606–618. 10.1096/fj.201800210RR [DOI] [PubMed] [Google Scholar]
- Tian Z., Dong Q., Wu T., Guo J. (2021). MicroRNA-20b-5p Aggravates Neuronal Apoptosis Induced by β-Amyloid via Down-Regulation of Ras Homolog Family Member C in Alzheimer's Disease. Neurosci. Lett. 742, 135542. 10.1016/j.neulet.2020.135542 [DOI] [PubMed] [Google Scholar]
- Toyoshima M., Akamatsu W., Okada Y., Ohnishi T., Balan S., Hisano Y., et al. (2016). Analysis of Induced Pluripotent Stem Cells Carrying 22q11.2 Deletion. Transl Psychiatry 6, e934. 10.1038/tp.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi V., Manikandan M., Tiwary B. K., Munirajan A. K. (2013). Insights on the Functional Impact of MicroRNAs Present in Autism-Associated Copy Number Variants. PLOS ONE 8, e56781. 10.1371/journal.pone.0056781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M. M. J., Krauskopf J., Ramaekers J. G., Kleinjans J. C. S., Prickaerts J., Briedé J. J. (2020). Circulating microRNAs as Potential Biomarkers for Psychiatric and Neurodegenerative Disorders. Prog. Neurobiol. 185, 101732. 10.1016/j.pneurobio.2019.101732 [DOI] [PubMed] [Google Scholar]
- Walsh T., McClellan J. M., McCarthy S. E., Addington A. M., Pierce S. B., Cooper G. M., et al. (2008). Rare Structural Variants Disrupt Multiple Genes in Neurodevelopmental Pathways in Schizophrenia. Science 320, 539–543. 10.1126/science.1155174 [DOI] [PubMed] [Google Scholar]
- Wang B., Howel P., Bruheim S., Ju J., Owen L. B., Fodstad O., et al. (2011). Systematic Evaluation of Three microRNA Profiling Platforms: Microarray, Beads Array, and Quantitative Real-Time PCR Array. PLOS ONE 6, e17167. 10.1371/journal.pone.0017167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnica W., Merico D., Costain G., Alfred S. E., Wei J., Marshall C. R., et al. (2015). Copy Number Variable MicroRNAs in Schizophrenia and Their Neurodevelopmental Gene Targets. Biol. Psychiatry 77, 158–166. 10.1016/j.biopsych.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen M. H., Valles A., Kirkels L. A. M. H., Mastebroek M., Olde Loohuis N., Kos A., et al. (2011). Chromosome 1p21.3 Microdeletions Comprising DPYD and MIR137 Are Associated with Intellectual Disability. J. Med. Genet. 48, 810–818. 10.1136/jmedgenet-2011-100294 [DOI] [PubMed] [Google Scholar]
- Witwer K. W., Halushka M. K. (2016). Toward the Promise of microRNAs - Enhancing Reproducibility and Rigor in microRNA Research. RNA Biol. 13, 1103–1116. 10.1080/15476286.2016.1236172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Duncan C. E., Beveridge N. J., Webster M. J., Cairns M. J., Shannon Weickert C. (2013). Expression of NPAS3 in the Human Cortex and Evidence of its Posttranscriptional Regulation by miR-17 during Development, with Implications for Schizophrenia. Schizophrenia Bull. 39, 396–406. 10.1093/schbul/sbr177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Hsu P.-K., Stark K. L., Karayiorgou M., Gogos J. A. (2013). Derepression of a Neuronal Inhibitor Due to miRNA Dysregulation in a Schizophrenia-Related Microdeletion. Cell 152, 262–275. 10.1016/j.cell.2012.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Su N., Liu L., Zhang J., Wang H., Zhang W., et al. (2018). miRBaseConverter: an R/Bioconductor Package for Converting and Retrieving miRNA Name, Accession, Sequence and Family Information in Different Versions of miRBase. BMC Bioinformatics 19, 514. 10.1186/s12859-018-2531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Lin M., Chen J., Pedrosa E., Hrabovsky A., Fourcade H. M., et al. (2015). MicroRNA Profiling of Neurons Generated Using Induced Pluripotent Stem Cells Derived from Patients with Schizophrenia and Schizoaffective Disorder, and 22q11.2 Del. PLOS ONE 10, e0132387. 10.1371/journal.pone.0132387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Yang L., Li Y.-j., Mei R., Yu H.-l., Gong Y., et al. (2018). MicroRNA-128 Protects Dopamine Neurons from Apoptosis and Upregulates the Expression of Excitatory Amino Acid Transporter 4 in Parkinson's Disease by Binding to AXIN1. Cell Physiol Biochem 51, 2275–2289. 10.1159/000495872 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.