Abstract
Objective: The aim of this study was to describe the evolution of transfusion practices following the introduction of tranexamic acid (TXA) and ROTEM® in a trauma resuscitation unit (TRU) from a French teaching hospital (FTH).
Methods: This is a single-centre, retrospective study at a TRU from a FTH. All trauma patients aged 18 years or more and transfused with at least 4 red blood cells (RBCs) within 24 hours after trauma, from 2011 to 2016, were included. The primary objective was to analyse transfusion practices over this time period. The secondary objectives aimed at assessing differences between populations according to the fresh frozen plasma (FFP):RBC ratio applied.
Results: A total of 122 patients were included. There was a significant decrease in the proportion of patients requiring at least 4 RBCs 24 hours after trauma (9% vs. 3%, P trend < .0001) as well as a decrease in the proportion of patients with a high FFP:RBC ratio (86% vs. 62% at 6 hours, P trend = .0056 and 86% vs. 56% at 24 hours, P trend = .0047). After 2013, fibrinogen was administered to more than 70% of patients and TXA to 100% of them. The observed mortality was lower than the predicted one, irrespective of FFP:RBC ratio.
Conclusion: An important evolution of practices occurred including a decrease in the proportion of transfusions and use of high FFP:RBC ratios. The origin of these changes is multifactorial, likely including the systematic use of TXA and optimisation of the ROTEM protocol for fibrinogen administration.
Keywords : Fresh frozen plasma, tranexamic acid, transfusion, trauma induced coagulopathy, trauma injury
Introduction
Mortality from severe trauma continues to be a worldwide problem,1,2 uncontrolled haemorrhage being a major cause of these preventable deaths.3 Trauma-induced coagulopathy, which can occur early and increase mortality, is observed in one-third of patients with post-traumatic acute haemorrhage.4,5 Management of trauma-induced coagulopathy is thus a key interventional target for trauma haemorrhage care. Resuscitation, in this context, aims at managing the haemostatic conditions before, during and after damage control surgery in order to improve prognosis.5–8 During the initial management stage of patients with expected massive haemorrhage, European guidelines recommend one of the two following strategies: plasma (fresh frozen plasma [FFP] or pathogen-inactivated plasma) in a plasma–red blood cell (RBC) ratio of at least 1:2 (Grade 1B) or RBC and fibrinogen concentrate guided by viscoelastic tests (Grade 1C).9 These guidelines are somehow discordant with French ones that recommend a systematic application of FFP:RBC ratio of at least 1:2 to 1:1, reporting that a decrease in mortality should be expected with the use of high FFP:RBC ratios.10
Following the CRASH 2 study in 2010,11 a generalisation of tranexamic acid (TXA) use was observed in our trauma resuscitation unit (TRU), which coincided with an increase in the use of the ROTEM® protocol. Given the discordance of such an observation with the French guidelines in place at that time, we decided to assess our practices, with the hypothesis that although a reduction in transfusion volumes and application of high FFP:RBC ratios seemed to be occurring, this did not increase mortality.
The aim of the present study was thus to describe the evolution of transfusion practices in severely injured bleeding patients, between 2011 and 2016, in a TRU from a French teaching hospital. The primary objective was to analyse transfusion practices over time, measured by changes in blood transfusions, use of either high (≥1:2) or low (<1:2) FFP:RBC ratios, application of TXA and administration of fibrinogen according to the ROTEM protocol. Patient characteristics and mortality between high vs low FFP:RBC ratio groups were also compared as secondary objectives.
Methods
Data Source
Covering a population of 3 million inhabitants and about 25,000 km2 of rural and urban areas of the Rhône-Alpes region in France, the RESUVal system (Réseau des Urgences de la Vallée du Rhône) gathers physicians around common guidelines and actively participates in education and evaluation of professional practices. Among the 65 health institutions of the Rhône-Alpes region, 37 public and private hospitals belong to the RESUVal system, including several trauma centres. Among the evaluation tools available, the prospective observational trauma-system registry12,13 reports, since 2011, all adult patients (≥18 years old) with at least one traumatic lesion and managed by emergency medical services that are part of the RESUVal area (see Supplementary File 1). Within this system, the physician in charge of prehospital management is the first one who requests consent on eligible patients. Data for this prospective registry are collected in a structured and standardised case report form, throughout the care management pathway, from management by emergency medical services, to the emergency department or the TRU.
Study Design and Population Selection
This is a retrospective study based on data obtained from the Trauma-System registry in a single TRU from a French teaching hospital. The TRU is a dedicated resuscitation room within the intensive care unit department, which benefits from a dedicated team composed of an intensivist, the trauma leader accompanied by a resident, a visceral surgeon, an orthopaedic surgeon, a nurse, and an assistant nurse. According to both European and French guidelines,9,10 TXA was administered as early as possible, within 3 hours after injury, with a loading dose of 1 g infused over 10 minutes, followed by an intravenous infusion of 1 g over 8 hours. When indicated, ROTEM protocol (thromboelastometry, Tem International, Munich, Germany) was performed upon admission to the TRU. Fibrinogen concentrate was administered at a dose of 3 g either when indicated by the ROTEM protocol, based on FIBTEM A10 and EXTEM A15 parameters (see Supplementary File 2), or when plasma fibrinogen levels were less than 1.5-2.0 g L−1. ROTEM could be performed again if necessary. At the time of the study, FFP administration was either guided by laboratory coagulation screening parameters (prothrombine time < 50%) and/or viscoelastic evidence of a coagulation factor deficiency using ROTEM protocol (see Supplementary File 2) in accordance with European guidelines or administered probabilistically depending on the number of RBCs according to French guidelines.9,10 Massive transfusion was defined as the administration of at least 10 RBCs within 24 hours after trauma.14 Biological coagulopathy was defined as Prothrombin time ratio (PTr) > 1.2 according to Davenport’s coagulopathy definition.15
All patients managed from March 2011 to December 2016 and transfused with at least 4 RBCs within the first 24 hours after trauma were included. Cross validation was performed with the French blood services (Etablissement Français du Sang) to validate the population selected, to collect missing values if needed and to recount the number and time of RBC and FFP delivery for each patient. Data were finally completed by consulting electronic medical records to collect the date of death when the patient died at the hospital after his transfer from the TRU to another service.
Statistical Analyses
For univariate statistics, categorical data were expressed as frequencies and percentages, continuous data by median and interquartile range (IQR). Pearson’s chi-squared tests were performed for categorical data and Wilcoxon nonparametric rank sum tests for continuous data. The P-values were estimated by comparing the Z-score obtained with the reduced normal centred law, and considered significant when P-value < .05. The linear trend of frequencies of categorical variables was tested using a Cochran-Armitage test for trend (P-trend) and considered significant when P-trend < .05. For survival analysis, the Trauma Revised Injury Severity Score (TRISS) was evaluated.16 It represents the best predictive index of intrahospital at D30 mortality.17,18 Predicted mortality was evaluated using the nTRISS (ie, TRISS normalised to respiratory rate)17 and compared to observed mortality.19 The change in population characteristics over time was tested using a univariate linear regression for continuous variables (age, ISS [Injury Severity Score],20 MGAP [Mechanism Glasgow Age Pressure] score,21,22 nRTS [Normalised Revised Trauma Score]23 and nTRISS) and a univariate logistic regression for qualitative variables (mortality at 30 days) with the year of admission as explanatory variable. All statistical analyses were performed using R® 3.4.2 Software (R foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
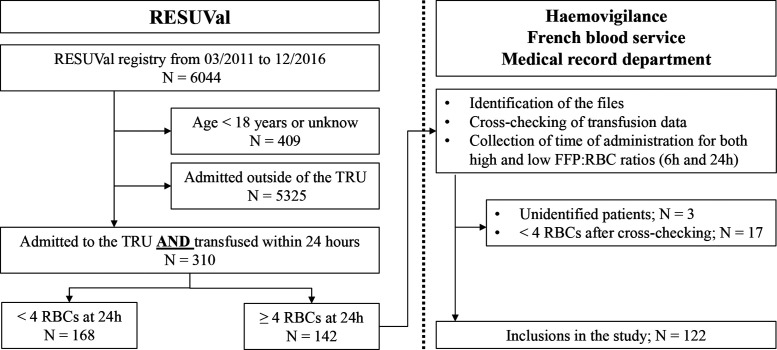
Over the 70-month study period, 122 patients were included. There were 310 patients admitted to the trauma centre and transfused within 24 hours. Of these, 185 patients were excluded because they received less than 4 RBCs at 24 hours. Another three files could not be identified (Figure 1).
Figure 1.
Study flow chart. RESUVal, emergency network of the Rhone Valley; TRU, trauma resuscitation unit; RBC, red blood cell; FFP, fresh frozen plasma.
Among the study population, 25% of patients underwent massive transfusion (≥10 RBCs at 24 hours) and 67% received more than 6 RBCs in the first 24 hours of trauma occurrence. Blunt trauma represented 84% of the studied population, and 74% of patients had biological coagulopathy on admission (Table 1).
Table 1.
Population Characteristics
| Total population | n = 122 |
|---|---|
| Direct admission from on-scene to the TRU, n (%) | 116 (95) |
| Median time between on-scene ambulance arrival and arrival to the TRU [IQR], minutes | 80 [60-118] |
| Blunt trauma, n (%) | 102 (84) |
| Road traffic accident, n (%) | 54 (44) |
| Fall, n (%) | 21 (17) |
| Aggression, n (%) | 8 (7) |
| Other/unknown, n (%) | 39 (32) |
| Median age [IQR], years | 39 [27-51] |
| Sex (male), n (%) | 71 (58) |
| Prehospital mean blood pressure < 65 mmHg, n (%) | 27 (22) |
| Prehospital Glasgow < 8, n (%) | 51 (42) |
| Prehospital SpO2 < 95%, n (%) | 26 (21) |
| Prehospital severity scale level | |
| • Level 1, n (%) | 59 (48) |
| • Level 2, n (%) | 49 (40) |
| • Level 3, n (%) | 14 (12) |
| Haemoglobin < 9 g dL–1 on-scene, n (%) | 15 (12) |
| Haemoglobin < 9 g dL–1 at TRU admission, n (%) | 43 (35) |
| Anticoagulant or antiplatelet treatment as usual treatment, n (%) | 9 (7) |
| Trauma induced coagulopathy at admission = PTr > 1.2, n (%) | 90 (74) |
| Radioembolisation, n (%) | 40 (33) |
| Surgery, n (%) | 76 (62) |
| Median MGAP [IQR], points | 22 [15-26] |
| Median nRTS [IQR], points | 6.4 [4.1-7.8] |
| Median ISS [IQR], points | 38 [25-50] |
| Median SAPS II [IQR], points | 54 [40-71] |
| RBCs ≥6 at 24 hours, n (%) | 84 (67) |
| RBCs ≥10 at 24 hours, n (%) | 30 (25) |
TRU, trauma resuscitation unit; PTr, Prothrombin time ratio; MGAP, mechanism Glasgow age pressure; nRTS, Normalised Revised Trauma Score; ISS, Injury Severity Score; SAPS II, Simplified Acute Physiology Score II; RBC, red blood cell; IQR, interquartile range.
Analysis of changes in patient characteristics showed no significant changes over the time period studied in the annual distribution of patients according to their ISS, nor their nRTS, nTRISS, age and D30 mortality. A significant decrease in the annual median MGAP score between 2011 (22 [17-27]) and 2016 (19 [13-23], P = .0353) was found.
Evolution in Transfusion Practices
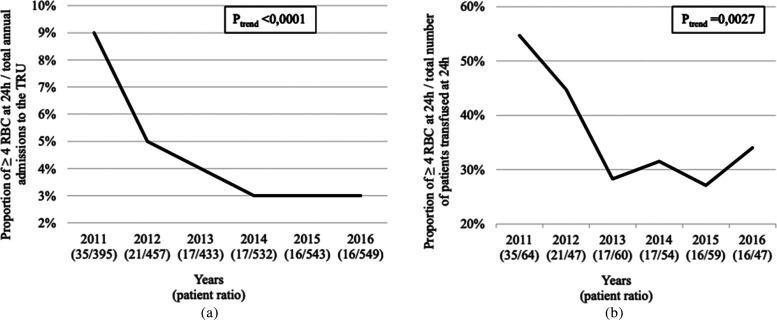
The proportion of patients transfused ≥4 RBCs within the first 24 hours of trauma significantly decreased between 2011 and 2016 when compared to either the total annual admissions to the TRU (9-3%, P trend < .0001; Figure 2A) or the total proportion of patients transfused within the first 24 hours after trauma in the TRU (55-34%, P trend = .0027; Figure 2B).
Figure 2. a,b.
Decrease in the annual proportion of patients receiving at least 4 RBCs within the first 24 hours after trauma. Proportion of patients in relation to the total annual admissions to the TRU (a) and compared to the total number of patients transfused at 24 hours in the TRU (b). For each year, patient ratio is presented between brackets. TRU, trauma resuscitation unit; RBC, red blood cell.
The proportion of a high FFP:RBC ratio significantly decreased between 2011 and 2016, at 6 hours (86-62%, P trend = .0056) and 24 hours (86-56%, P trend = .0047).
The administration of TXA within 3 hours after trauma (on-scene or hospital administration) increased significantly over the time course of the study. After 2013, TXA was administered to the entire cohort of patients.
Fibrinogen concentrate was administered in 73% (89/122) of the population. Of these, 63% (56/89) received only one supplementation of 3 g, 20% (18/89) received a second dose of 1.5-3 g and only 15% (13/89) received more than 6 g. The proportion of patients receiving fibrinogen concentrate did not change significantly over the study period. The administration of fibrinogen concentrate was significantly associated with the indication given by the ROTEM protocol used in the TRU or by plasma fibrinogen levels when ROTEM was not performed (Table 2).
Table 2.
Proportion of Respected Versus Not Respected Fibrinogen Concentrate Indication According to ROTEM Protocol *
| Fibrinogen concentrate indication | Respected | Not respected | P † |
|---|---|---|---|
| ROTEM performed (97 patients), n (%) | 77 (79) | 21 (21) | .0003 |
| ROTEM performed and plasma fibrinogen level (122 patients), n (%) | 93 (76) | 29 (24) | <.0001 |
Respected: indicated and administered or not indicated and not administered; not respected: indicated and not administered or not indicated and administered.
Cf. appendix.
P: Computed from the contingency table of fibrinogen indication and fibrinogen administration using a Pearson’s chi-squared test.
High Versus Low FFP:RBC Ratio: Patient Characteristics and Mortality
High FFP:RBC ratio groups were characterised by significantly higher proportion of massive transfusions (≥10 RBCs at 24 hours) and ≥6 RBCs administrations at 24 hours as well as higher median PTr and median INR than the low ratio groups, at both 6 and 24 hours (Table 3). There was, however, no significant difference between groups in terms of anticoagulants and antiplatelet agents administered, blunt trauma, severe cranial trauma (Glasgow score ≤ 8), administration of TXA or haemostasis procedure needed (embolisation or surgery). There was also no significant difference for the mean severity scores such as MGAP, nRTS, ISS and nTRISS. The observed mortality at D30 was significantly higher for the high ratio group at 6 hours (37% [n = 30] vs. 15% [n = 5], P = .0313); a trend towards a higher mortality was found in the high ratio group at 24 hours (33% [n = 30] vs. 19% [n = 6], P = .1842). Statistical analysis using n-TRISS revealed that in all groups (high ratio and low ratio, at 6 and 24 hours), observed mortality was significantly lower than predicted mortality (Table 4).
Table 3.
Low Versus High Ratio Group Characteristics
| Variables at 6 hours | Low ratio (N = 34) | High ratio (N = 81) | P |
|---|---|---|---|
| RBCs ≥10 at 24 hours, n (%) | 2 (6) | 26 (32) | .0059 |
| RBCs ≥6 at 24 hours, n (%) | 15 (44) | 65 (80) | .0003 |
| Median PTr [IQR], points | 1.3 [1.1-1.5] | 1.7 [1.4-2.0] | <.0001 |
| Median INR [IQR], points | 1.3 [1.2-1.5] | 1.9 [1.5-2.5] | <.0001 |
| D30 observed mortality n (%) | 5 (15) | 30 (37) | .0313 |
| Level 2 patients, n (%) | 19 (56) | 26 (32) | .0296 |
| TQ ratio > 1.2, n (%) | 20 (59) | 65 (80) | .0312 |
| Median SAPS II [IQR], points | 46 [36-63] | 60 [46-78] | .0039 |
| Variables at 24 hours | Low ratio (N = 32) | High ratio (N = 90) | P |
| RBCs ≥10 at 24 hours, n (%) | 2 (6) | 28 (31) | .0103 |
| RBCs ≥ 6 at 24 hours, n (%) | 13 (41) | 71 (79) | .0001 |
| Median PTr [IQR], points | 1.3 [1.2-1.5] | 1.6 [1.3-2.0] | .0005 |
| Median INR [IQR], points | 1.3 [1.2-1.6] | 1.8 [1.4-2.4] | .0001 |
INR, international normalised ratio; PTr, Prothrombin time ratio; SAPS II, Simplified Acute Physiology Score II; RBC, red blood cell; IQR, interquartile range.
Table 4.
Comparison of Observed Versus Predicted Mortality at D30 According to Ratio Type and Time
| Variables | Observed mortality, n (%) | Predicted mortality, n (%) | P |
|---|---|---|---|
| High ratio | |||
| 6 hours (n = 81) | 30 (37) | 38 (47) | .0036 |
| 24 hours (n = 90) | 30 (33) | 39 (43) | .0015 |
| Low ratio | |||
| 6 hours (n = 34) | 5 (15) | 10 (29) | .0031 |
| 24 hours (n = 32) | 6 (19) | 11 (34) | .0030 |
Discussion
The present study showed a sharp decline in the proportion of patients requiring at least 4 RBCs within the first 24 hours after trauma and a significant decrease in the proportion of high FFP:RBC ratio administration at 6 and 24 hours. From 2011 onwards, an increasing number of the study population received TXA, reaching 100% compliance in 2014. The proportion of patients receiving fibrinogen also appeared to increase over time, although not significantly. It seemed that good adherence to the ROTEM protocol was observed since the administration of fibrinogen concentrate was significantly associated with the indication given by the ROTEM protocol, in univariate analysis. Although observed mortality was higher in the high FFP:RBC ratio group than in the low ratio group, the proportion of observed deaths, when compared to expected deaths, was significantly lower, irrespective of FFP:RBC ratio type or time of application.
The reduction in the proportion of transfused patients and volumes administered, as well as the reduction in high FFP:RBC ratios over the study period, could be due to improvement in the overall therapeutic management strategy as depicted by the optimisation of radioembolisation techniques24 and establishment of a dedicated trauma team.25–27 Similar results have also very recently been reported by another French trauma centre.28 Of note, there was no significant change in the annual proportion of patients who underwent embolisation and/or haemostatic surgical management (data not shown). Another hypothesis is the possible decrease in patient severity over time reported in the area, due to improvement in road safety measures.29 However, patient severity in the present study was stable. Indeed the most robust ISS did not change over time, similarly to the nRTS, nTRISS, age and D30 mortality. Although there was a decrease in the median MGAP score between 2011 and 2016, this change is not clinically relevant as the scores remained within the same range19–23 of the intermediate risk group. Thus, a decrease in patient severity was not observed herein, likely reflecting a recruitment bias, since the cohort consisted only of patients admitted to the TRU and transfused ≥4 RBCs. Indeed, although there may be fewer severe patients, the more severe ones are always referred to the TRU.
Due to the study design, it is difficult to assess the impact of systematic TXA administration or the use of a ROTEM protocol on the observed changes. In the CRASH 2 population study, TXA reduced all-cause mortality by 1.5% (from 16% to 14.5%) and reduced the risk of death due to bleeding (4.9% vs. 5.7%; relative risk 0.85, 95% CI [0.76; 0.96]; P = .0077). However, the benefit of TXA on mortality is probably underestimated by the low proportion of patients transfused in this study (50% of the population).11 Moreover, another recent study showed that early prehospital administration of TXA led to clot stabilisation and a reduction in fibrinolytic activity as measured by ROTEM.30 To the best of our knowledge, no study has specifically demonstrated the benefit of TXA in reducing transfusions in traumatised patients. However, standardisation of TXA administration showed a significant benefit in the context of hip and knee arthroplasty, including reductions in perioperative haemoglobin decrement (20%), patients transfused (45%) and number of units transfused per patient (62%).31 Concerning the possible impact of using a ROTEM protocol for the administration of coagulation factors, a retrospective study with trauma patients found significantly lower observed mortality when compared to the mortality predicted by the TRISS (24.4% vs. 33.7%; P = .032).32 Another retrospective study on cardiovascular surgery patients showed that the use of a ROTEM protocol resulted in a reduction of blood product transfusions but did not influence mortality.33 Of important note, ROTEM was introduced in 2004 in the TRU but its use by the physician was improved when its relocation to the haemostasis laboratory occurred in 2010. It would have been interesting to compare the present data to those of previous years but, unfortunately, the RESUVal database did not include data prior to this period.
The proportion of observed deaths, when compared to expected deaths, was significantly lower, irrespective of FFP:RBC ratio type or time of application. These results are discordant with French guidelines10 reporting that a decrease in mortality should be expected with the use of high FFP:RBC ratios. These recommendations are largely based on the findings of the Banghu meta-analysis,34 indicating a 51% mortality reduction with the use of high FFP:RBC ratios. If this were the case, we would have expected an increase in observed mortality compared to expected mortality for the low FFP:RBC ratio group. Herein, the decrease in proportion of observed deaths was also not related to time of application for high FFP:RBC ratios. This again is not in line with the prospective, observational, multicentre, major trauma transfusion study35 that showed a better survival for patients when high FFP:RBC ratios were applied at 6 hours versus 24 hours. These results suggest an improvement in patient prognosis due to overall medical management, regardless of the FFP:RBC ratio applied. Importantly, the study population included a low proportion of haemodynamically unstable patients, few penetrating trauma cases and less anticoagulant or antiplatelet treatments than other study populations. Nevertheless, according to Davenport’s coagulopathy definition,15 the present cohort included a particularly large proportion of patients with trauma induced coagulopathy (74% of PTr ≥ 1.2 on admission).
Despite the decline in use of plasma reported here, more than half of the patients still benefitted from high ratio administration in 2016, suggesting the importance of plasma transfusion in the management of trauma patients. When indicated, the efficacy of plasma administration would certainly be improved by early application through the use, for instance, of lyophilised plasma which is now available.36
This study has a number of limitations. First, this is a retrospective study in its design, although the collection of information was done in a prospective manner using a computerised and centralised database. These data allowed us to objectively highlight the change in the transfusion practices in real-life conditions. Second, the study included a small patient population due to both the single-centre design of the study and the short period of inclusion. However, a multicentre study would not have allowed investigation of the practices of the present TRU, which was the primary objective of the current study. Moreover, including patients before 2011 would have made the analysis difficult due to the extreme diversity in clinical practices prior to that time. We could have included more patients by decreasing the transfusion volume of inclusion, but it would have been less relevant for the analysis of the FFP:RBC ratios. Third, the groups high vs low ratios were not similar. Due to the design of the study and the small number of patients, multivariate analysis, to eliminate comparability bias between groups, could not be performed. Groups were therefore compared to themselves using the n-TRISS score. Finally, adherence to the ROTEM protocol did not reach 100% over the study period. However, a 75% adherence can be considered good given that a multicentre study showed that only 24% of patients in intensive care units received fully compliant care.37 Moreover, the majority of protocol deviations occurred in 2011, at the start of protocol implementation.
Conclusion
From 2011 to 2016, an important evolution of practices occurred in the TRU including a decrease in the proportion of transfusions and use of high FFP:RBC ratios. The origin of these changes is multifactorial, likely including the systematic use of TXA and optimisation of the ROTEM protocol for fibrinogen administration.
Footnotes
Ethics Committee Approval: The Trauma-System registry received approval from the National Commission for Liberties and Data Protection (Commission Nationale de l’Informatique et des Libertés, CNIL, reference number DE-2012-059) and received approval from the Advisory Committee on the Treatment of Research Information (Comité consultatif sur le traitement de l'information en matière de recherche scientifique, CCTIRS, reference number: 15.368). This study was approved by an ethics committee and registered under the reference number 17-060 in the CNIL register.
Informed Consent: Informed consent was obtained from all partcipants who participated to this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Design - C.P., L.F., B.F., G.M., T.G., T.R.; Data collection and/or processing - C.P., L.F., C.C., M.D.; Analysis and/or interpretation - L.F., C.C., C.E.K.; Writing - C.P., L.F., G.M., F.B.; Literature review - T.G., C.C., M.D., C.E.K., T.R.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The RESUVal Network is funded by the Regional Agency for Health from the Auvergne-Rhône-Alpes region (Agence Régionale de Santé Auvergne-Rhône-Alpes).
Acknowledgements: We would like to thank Giovanna Cannas (MD) and her team for access to the haemovigilance files as well as the ICU secretaries and Véréna Landel (PhD) for her help with the manuscript preparation.
References
- 1. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global burden of disease study. Lancet Lond Engl. . 1997;349:(9064):1498–1504.. 10.1016/S0140-6736(96)07492-2 [DOI] [PubMed] [Google Scholar]
- 2. Lendrum RA, Lockey DJ. Trauma system development. Anaesthesia. . 2013;68:(Suppl. 1):30–39.. 10.1111/anae.12049 [DOI] [PubMed] [Google Scholar]
- 3. Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. . 2007;62:(1):142–146.. 10.1097/01.ta.0000251558.38388.47 [DOI] [PubMed] [Google Scholar]
- 4. Floccard B, Rugeri L, Faure A.et al. Early coagulopathy in trauma patients: An on-scene and hospital admission study. Injury. . 2012;43:(1):26–32.. 10.1016/j.injury.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 5. Maegele M, Lefering R, Yucel N.et al. Early coagulopathy in multiple injury: An analysis from the German trauma registry on 8724 patients. Injury. . 2007;38:(3):298–304.. 10.1016/j.injury.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 6. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. . 2003;54:(6):1127–1130.. 10.1097/01.TA.0000069184.82147.06 [DOI] [PubMed] [Google Scholar]
- 7. MacLeod JBA, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. . 2003;55:(1):39–44.. 10.1097/01.TA.0000075338.21177.EF [DOI] [PubMed] [Google Scholar]
- 8. Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr Opin Crit Care. . 2007;13:(6):680–685.. 10.1097/MCC.0b013e3282f1e78f [DOI] [PubMed] [Google Scholar]
- 9. Rossaint R, Bouillon B, Cerny V.et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fourth edition. Crit Care. . 2016;20:(20):100. 10.1186/s13054-016-1265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duranteau J, Asehnoune K, Pierre S, Ozier Y, Leone M, Lefrant J-Y. Recommandations sur la réanimation du choc hémorragique. Anesthésie Réanimation. . 2015;1:(1):62–74.. 10.1016/j.anrea.2014.12.007 [DOI] [Google Scholar]
- 11. Shakur H, Roberts I, Bautista R.et al. CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage: A randomised, placebo-controlled trial. Lancet. . 2010;376:(9734):23–32.. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 12. Bouzat P, David JS, Tazarourte K. French regional trauma network: The Rhone-Alpes example. Br J Anaesth. . 2015;114:(6):1004–1005.. 10.1093/bja/aev124 [DOI] [PubMed] [Google Scholar]
- 13. David JS, Bouzat P, Raux M. Evolution and organisation of trauma systems. Anaesth Crit Care Pain Med. . 2019;38:(2):161–167.. 10.1016/j.accpm.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 14. Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. . 2004;44:809–813.. 10.1111/j.1537-2995.2004.03409.x [DOI] [PubMed] [Google Scholar]
- 15. Davenport R, Manson J, De’Ath H.et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. . 2011;39:(12):2652–2658.. 10.1097/CCM.0b013e3182281af5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: The TRISS method. Trauma score and the injury severity score. J Trauma. . 1987;27:(4):370–378.. 10.1097/00005373-198704000-00005 [DOI] [PubMed] [Google Scholar]
- 17. Munter LD, Polinder S, Lansink KWW.et al. Mortality prediction models in the general trauma population: A systematic review. Injury. . 2017;48:(2):221–229.. 10.1016/j.injury.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 18. Bouzat P, Legrand R, Gillois P.et al. Prediction of intra-hospital mortality after severe trauma: Which pre-hospital score is the most accurate?. Injury. . 2016;47:(1):14–18.. 10.1016/j.injury.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 19. Flora JD. A method for comparing survival of burn patients to a standard survival curve. J Trauma Acute Care Surg. . 1978;18:(10):701. 10.1097/00005373-197810000-00003 [DOI] [PubMed] [Google Scholar]
- 20. Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. . 1974;14:(3):187–196.. 10.1097/00005373-197403000-00001 [DOI] [PubMed] [Google Scholar]
- 21. Sartorius D, Le MY, David J-S.et al. Mechanism, Glasgow coma scale, age, and arterial pressure (MGAP): A new simple prehospital triage score to predict mortality in trauma patients. Crit Care Med. . 2010;38:(3):831–837.. 10.1097/CCM.0b013e3181cc4a67 [DOI] [PubMed] [Google Scholar]
- 22. Rehn M, Perel P, Blackhall K, Lossius HM. Prognostic models for the early care of trauma patients: A systematic review. Scand J Trauma Resusc Emerg Med. . 2011;19:(19):17. 10.1186/1757-7241-19-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the trauma score. J Trauma. . 1989;29:(5):623–629.. 10.1097/00005373-198905000-00017 [DOI] [PubMed] [Google Scholar]
- 24. Chakraverty S, Zealley I, Kessel D. Damage control radiology in the severely injured patient: What the anaesthetist needs to know. Br J Anaesth. . 2014;113:(2):250–257.. 10.1093/bja/aeu203 [DOI] [PubMed] [Google Scholar]
- 25. Brindley PI. Improving teamwork in anaesthesia and critical care: Many lessons still to learn. Br J Anaesth. . 2014;112:(3):399–401.. 10.1093/bja/aet334 [DOI] [PubMed] [Google Scholar]
- 26. Howell SI. Advances in trauma care: A quiet revolution. Br J Anaesth. . 2014;113:(2):201–202.. 10.1093/bja/aeu253 [DOI] [PubMed] [Google Scholar]
- 27. Tiel Groenestege-Kreb D, van Maarseveen O, Leenen L. Trauma team. Br J Anaesth. . 2014;113:(2):258–265.. 10.1093/bja/aeu236 [DOI] [PubMed] [Google Scholar]
- 28. Guth C, Vassal O, Friggeri A.et al. Effects of modification of trauma bleeding management: A before and after study. Anaesth Crit Care Pain Med. . 2019;38:(5):469–476.. [DOI] [PubMed] [Google Scholar]
- 29. Eurostats. Deaths due to transport accidents. [Internet]. 2017. Available at https://ec.europa.eu/eurostat/documents/3217494/8309812/KS-EI-17-001-EN-N.pdf/b7df53f5-4faf-48a6-aca1-c650d40c9239.
- 30. Stein P, Studt J-D, Albrecht R.et al. The impact of prehospital tranexamic acid on blood coagulation in trauma patients. Anesth Analg. . 2018;126:(2):522–529.. 10.1213/ANE.0000000000002708 [DOI] [PubMed] [Google Scholar]
- 31. Demos HA, Lin ZX, Barfield WR.et al. Process improvement project using tranexamic acid is cost-effective in reducing blood loss and transfusions after total hip and total knee arthroplasty. J Arthroplasty. . 2017;32:(8):2375–2380.. 10.1016/j.arth.2017.02.068 [DOI] [PubMed] [Google Scholar]
- 32. Schöchl H, Nienaber U, Hofer G.et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. . 2010;14:(2):R55. 10.1186/cc8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Görlinger K, Dirkmann D, Hanke AA.et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: A retrospective, single-center cohort study. Anesthesiology. . 2011;115:(6):1179–1191.. 10.1097/ALN.0b013e31823497dd [DOI] [PubMed] [Google Scholar]
- 34. Bhangu A, Nepogodiev D, Doughty H, Bowley DM. Meta-analysis of plasma to red blood cell ratios and mortality in massive blood transfusions for trauma. Injury. . 2013;44:(12):1693–1699.. 10.1016/j.injury.2012.07.193 [DOI] [PubMed] [Google Scholar]
- 35. Holcomb JB, Junco DD, Fox EE.et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: Comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. . 2013;148:(2):127–136.. 10.1001/2013.jamasurg.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen C, Bordes J, Cungi P-J.et al. Use of French lyophilized plasma transfusion in severe trauma patients is associated with an early plasma transfusion and early transfusion ratio improvement. J Trauma Acute Care Surg. . 2018;84:(5):780–785.. 10.1097/TA.0000000000001801 [DOI] [PubMed] [Google Scholar]
- 37. Leone M, Ragonnet B, Alonso S.et al. Variable compliance with clinical practice guidelines identified in a 1-day audit at 66 French adult intensive care units. Crit Care Med. . 2012;40:(12):3189–3195.. 10.1097/CCM.0b013e31826571f2 [DOI] [PubMed] [Google Scholar]