Abstract
Background
Vinpocetine as a neuroprotective agent is effective in acute ischemic stroke in some randomized controlled trials (RCTs). Since the last systematic review has been published in 2008, which didn’t find conclusive evidence favoring its use, two more RCTs have also been completed.
Methods
Relevant electronic databases were searched with a suitable combination of Medical Subject Headings terms to detect publications describing RCTs exploring the safety and efficacy of vinpocetine in patients with acute ischemic stroke. The risk of bias was determined by using the Cochrane Collaboration’s tool for assessing the risk of bias in RCTs after full-text review and relevant data extraction. Higgins and Thompson’s I2 method was used to assess heterogeneity in studies. The presence of publication bias was assessed by Egger’s test. We used a random effect model when I2 was more than 50% and a fixed-effect model for other parameters.
Results
Four placebo-controlled RCTs enrolling a total of 601 and 236 patients in vinpocetine and placebo groups, respectively, were included. The number of patients with death or significant disability was lower in the vinpocetine group than that in the placebo group at both 1 and 3 months (relative risk 0.80, 95% confidence interval [CI] 0.65–0.99 and relative risk 0.67, CI 0.48–0.92, p = 0.04 and 0.02, respectively). The degree of disability in participants at 1 month and 3 months was also lower in vinpocetine group than that in the placebo group (standardized mean difference (SMD) 0.49, 95% CI 0.03–0.95 and SMD 1.22, CI 0.23–2.24, p = 0.001 and 0.04, respectively). Change in mini-mental state examination score compared with baseline at trial enrolment was also better in the vinpocetine group than in the placebo group (pooled weighted mean difference 0.92, 95% CI 0.02–1.82, p = 0.04).
Conclusions
Vinpocetine has some promising efficacy in patients with ischemic stroke when used in the acute stage in reducing the disability, but presently there is not enough evidence to suggest that it also reduces case fatality. More double-blind, placebo-controlled RCTs of adequate sample size are needed before making recommendations for the routine administration of vinpocetine for all patients with acute ischemic stroke.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12028-022-01499-y.
Keywords: Neuroprotection, Vinpocetine, Cerebrovascular events, Acute ischemic stroke
Introduction
Vascular events involving the central nervous system, especially arterial ischemic stroke, remain a significant medical and social problem all over the world because of the high incidence of mortality and disability [1]. Indian data suggest that strokes are the leading cause of disability after dementia in older people in this country, the second leading cause of death in the population over the age of 60, and the fifth leading cause of death for people between the ages of 15 and 59 years [2].
It is known that approximately 85% of strokes are ischemic (cerebral infarction) [3], and the main goal in the treatment of ischemic stroke is to restore tissue perfusion in the ischemic zone to reduce the size of the infarction by maintaining blood flow [4]. However, the use of the intravenous recombinant tissue plasminogen activator for recanalization and restoration of blood flow in the ischemic area of the brain due to the narrow time window and the risk of hemorrhagic complications is possible only for a small proportion of patients [5]. Keeping this in mind, at present, researchers are exploring neuroprotective strategies that protect the brain from ischemic damage and its progression [6]. Although several neuroprotective agents have been explored, until now none of them had sufficient evidence to be approved by the Food and Drug Administration. One of the neuroprotective drugs explored with somewhat favorable results for the treatment of stroke is vinpocetine (also Cavinton, the active ingredient of which is vinpocetine) [7, 8]. Four randomized controlled trials (RCTs) have been performed in this regard in patients with stroke [9]. The last systematic review exploring its efficacy was conducted around 13 years back and, because of the inclusion of only two RCTs, did not reach a firm conclusion to support or refute its use in patients with stroke [10]. Since then, two more RCTs have been accomplished in this regard mandating the need for an updated systematic review and meta-analysis [11].
Methods
Search Strategy
Our systematic review aimed to determine the pooled estimate of efficacy, tolerability, and safety of vinpocetine in patients with arterial ischemic stroke during the acute and early recovery phase, collated from all RCTs in the existing literature. The primary objective was to compare the degree of disability in vinpocetine and control groups of patients with acute ischemic stroke at 1 month. The secondary objectives were to compare the degree of disability in vinpocetine and control groups of patients with acute ischemic stroke at 3 months, the proportion of patients with death/significant disability (dependency in activities of daily living) at 1 and 3 months, mortality rate in both groups, change in cognition and anxiety/depression, and rheological parameters such as blood viscosity in both groups at various time points. We also compared the nature and frequency of various adverse effects in both groups, proportion of participants with at least one serious adverse event (SAE) in both groups, proportion of participants who discontinued due to any reason and particularly due to adverse effects, proportion of participants who were lost to follow-up, and all-cause mortality in both groups.
While reporting the results of the review, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement recommendations were followed [12]. The study was approved by an institutional ethics committee and/or follows the tenants of the Declaration of Helsinki.
Initially, we developed a protocol for the systematic review and attempted to register it in PROSPERO. However, logistic delays caused by the ongoing COVID-19 pandemic prevented the timely posting of the review protocol. We were unable to get the final registration number in time; hence, we went ahead with completing the systematic review. We had a predetermined suitable search strategy using relevant Medical Subject Headings terms. Thereafter, a systematic literature search was performed in electronic databases, including MEDLINE, Web of Science databases, EMBASE, and Cochrane Central Register of Controlled Trials, for all articles published until 15th August 2021. We used the following keywords: “stroke” or “ischemic stroke,” “cerebrovascular disorder” or “cerebrovascular accident” or “acute cerebral infarction,” and “vinpocetine” or “Cavinton.” We also searched using other keywords for vinpocetine, such as “kavinton,” “Rgh-4405,” “Tcv-3B,” “ethyl apovincaminate,” “vinRx,” “periwinkle,” “myrtle vincapervinc,” and “cezayirmeneksesi”. The literature search was performed in conjunction with resources from central library in our institute. We have attached an Online appendix (Online Appendix) describing the detailed specific, reproducible search strategies, explicit listing of the specific inclusion, and exclusion criteria that were used by us to determine study eligibility.
Additional relevant articles were traced by screening the bibliographies of all search items and pertinent review articles. Even abstract-only publications and relevant conference proceedings were also searched to find additional documents. When necessary, the study authors were contacted by email for additional information not mentioned in the published article. We also searched ClinicalTrials.gov and other registers of clinical trials for any ongoing or completed clinical trials with preliminary published results.
Eligibility of Studies
Inclusion Criteria
The inclusion criteria of the review were to include only RCTs exploring the safety and efficacy of vinpocetine in patients with acute ischemic stroke to ensure high quality of evidence. Among these RCTs, we only included truly randomized unconfounded clinical trials that compared the effect of vinpocetine with control in patients with acute ischemic stroke when vinpocetine was started no later than 14 days after stroke onset. Both English and non-English articles were included in the review, if it satisfied the inclusion criteria.
Exclusion Criteria
We considered a comparison of vinpocetine plus standard treatment versus standard treatment alone as acceptable, whereas randomized comparisons between vinpocetine and other standard treatments as confounded and excluded.
Studies other than RCTs were excluded from review. Even RCTs exploring/comparing other medications with vinpocetine in stroke cases or exploring vinpocetine for indications other than stroke were excluded from the review. Moreover, the studies which only explored change in rheological parameters of red blood cell (RBC) and blood and not focused on clinical parameters like disability or death were also excluded. Duplicate entries and publications enrolling repeated populations were excluded.
Study Selection, Data Extraction, and Assessment of the Risk of Bias
Initially, there was a level 1 review, in which two reviewers (PKP and AR) performed title/abstract screening against the inclusion/exclusion criteria of all search items obtained by the above search strategy. Extraction of articles for full-text review (level 2) occurred independently by two reviewers, and then extracted elements were compared to determine consensus on final extraction content. Articles selected by any of these two reviewers were subjected to full-text review. Both authors independently performed a full-text review of all the selected articles and determined whether the article is suitable for inclusion in the review. At this stage, if there was any difference of opinion regarding inclusion of any article, then it was decided by the opinion of a third independent reviewer (IKS).
Then we assessed the methodological quality of the included studies. The relevant data which were extracted after full-text review from the included articles are the following variables of study methodology and study results: study design, study period, sample population, number of patients, baseline demographic and clinical variables such as age and gender distribution, height, weight, body mass index, body temperature at presentation, time from stroke onset to hospitalization and randomization, Glasgow coma scale (GCS) at presentation, site and size of infarction, electrocardiogram abnormality, history of angina pectoris, diabetes mellitus, hypertension, baseline National Institutes of Health Stroke Scale (NIHSS) score, Modified Rankin Score (mRS) score, Barthel index score, Mini-mental state examination (MMSE) score and Hospital Anxiety and Depression Scale (HADS) score, change in these scores at various time points (at 1, 3, 6 months), number of mortality or survival with significant disability/dependency, dose, route and schedule of administration of vinpocetine and characteristics of the formulation, nature, and frequency of various adverse effects and change in rheological parameters like blood viscosity, etc.
A standardized predetermined form was used for the uniform and systematical extraction of data and, subsequently, that data were digitally transferred to a Microsoft Excel spreadsheet. Any discrepancies regarding inclusion in the review and discrepancies were resolved by consensus. Another independent author reconfirmed the accuracy and completeness of the extracted data. Every effort was made to prevent duplication of data and every case included in the final analysis was ensured not to be part of another series.
The risk of bias was determined by using either the Cochrane Collaboration’s tool for assessing the risk of bias in RCTs or the risk of bias 2 tool [13, 14]. Initially, two investigators independently determined these parameters for each included study, and subsequently, and if any disagreement occurred between them then it was settled by taking the opinion of the third investigator.
Outcome Measures
The primary efficacy outcomes were the change in the degree of disability measured by modified Rankin scale at various time points, in vinpocetine and placebo groups at 1 month. The secondary outcomes were the following: change in the degree of disability measured by modified Rankin scale at 3 months, change in the degree of neurological deficit/stroke severity measured by NIHSS score, proportion of patients with death/significant disability (dependency in activities of daily living), mortality rate in both groups, change in cognition measured by MMSE/Montreal Cognitive Assessment (MoCA) scale and anxiety/depression by Beck or HADS scale, cerebral blood flow by transcranial doppler measured in terms of mean velocity in the anterior, middle and posterior cerebral artery and basilar artery pulsatile index, rheological parameters like blood viscosity in both groups at various time points (RBC membrane elasticity measured by atomic force microscopy to determine Young’s modulus).
Various studies used individual definitions and cut-offs in the Barthel index or mRS for determining the patients with a significant disability, such as in Feigin et al. [9] defined poor outcome as death or Barthel index < 70 or modified Rankin scale 3–5. We accepted those definitions to dichotomize the study population into those with or without significant disability.
The safety outcomes were the nature and frequency of various adverse effects in both groups, proportion of participants with at least one SAE in both groups, proportion of participants who discontinued due to any reason and particularly due to adverse effects, proportion of participants who were lost to follow-up and all-cause as well as vinpocetine related mortality in both groups.
Data Synthesis and Statistical Analysis
Categorical variables were presented as frequency (percentage) and 95% CI, whereas continuous variables were presented as mean with standard deviation or median with interquartile range. The pooled estimate of various parameters was calculated with upper and lower 95% confidence intervals (CIs), whenever it seemed feasible. Various statistical analyses, including a meta-analysis of data regarding various parameters were performed using SPSS statistical software package and Revman 5.4 software. Higgins and Thompson’s I2 method and Cochran’s Q statistics with χ2 test were used to assess heterogeneity in studies. The presence of publication bias was assessed by Egger’s test. We utilized a random effect model when I2 was more than 50% and a fixed-effect model for other parameters.
Apart from the principal analysis, in which we included patients using vinpocetine through all routes, doses, or duration, but then we also tried to perform subgroup analysis for oral or intravenous route, those who received vinpocetine for < 2 weeks or > 2 weeks and those with confirmed ischemic stroke (based on imaging) or with probable ischemic stroke (no neuroimaging done). It was done because some of the probable ischemic stroke cases might be actually hemorrhagic strokes or ischemic stroke with partial hemorrhagic transformation. Vinpocetine has been proposed to have considerable effects on platelet and other hemostatic functions. Thus, it might have different effects in ischemic and hemorrhagic strokes.
Results
Results of the Search
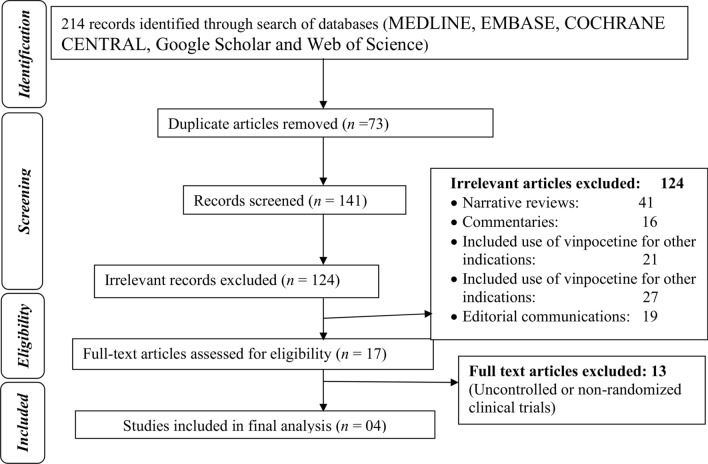
After a primary search using various combinations of keywords, a total of 214 publications were retrieved. Out of these, 73 were duplicates and hence removed. The eligibility of the remaining 141 articles was evaluated initially and 124 irrelevant articles were excluded according to the title, article type, abstract (Fig. 1). Ultimately, 17 articles were selected for full-text review, out of which four placebo-controlled RCTs were found to be eligible (one double-blind, one single-blind, and two open-label RCTs), enrolling a total of 601 and 236 patients in the vinpocetine and placebo groups, respectively [9, 11, 15, 16].
Fig. 1.
Flow diagram of the study selection process
Characteristics and Risk of Bias of Included Studies
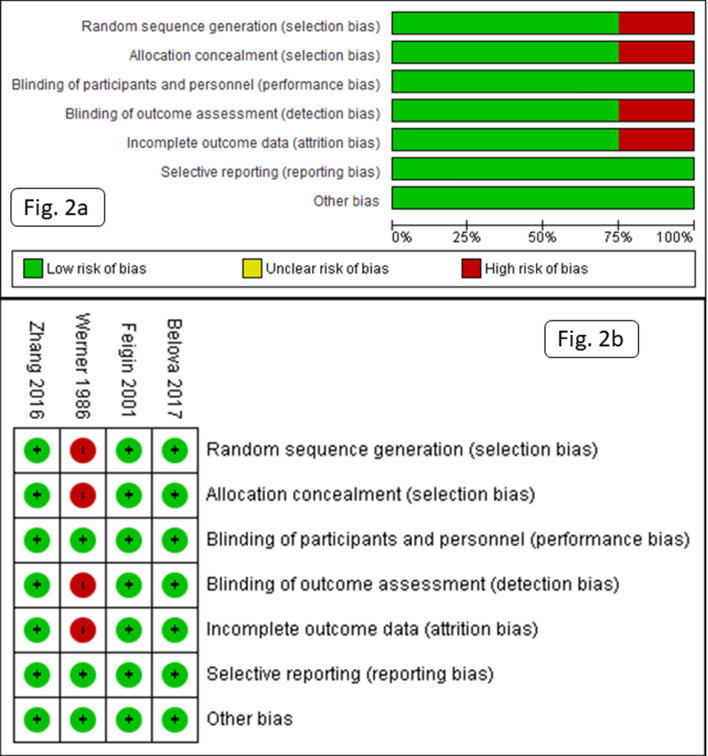
According to the risk of bias 2 tool, one RCT had a high and the other three had a low risk of bias (Fig. 2). In the RCT by Werner et al. [16], the method of randomization was not reported, neuroimaging was either not performed or the results were not reported, the follow-up duration was short, and there was no objective measurement of dependency. Outcome measures were not reported in 7/40 participants (they received some concomitant medications, which were not allowed during the study, and hence excluded from study due to protocol violation and no intention to treat analysis was performed).
Fig. 2.
Risk of bias graph (a) and risk of bias summary (b) for included clinical trials
The baseline demographic and clinical characteristics including age and sex distribution, height, weight, body mass index, body temperature at presentation, time from stroke onset to hospitalization and randomization, GCS at presentation, site and size of infarction, electrocardiography abnormality, history of angina pectoris, diabetes mellitus, hypertension, baseline NIHSS score, mRS score, Barthel index score, MMSE score, and HADS scores were comparable in both groups.
The vinpocetine group had 61% men, with an average age of 62.4 ± 11.6 years, mean time from onset of stroke to hospitalization and randomization were 28.9 ± 18.5 h and 1.9 ± 0.8 days, respectively, mean GCS at presentation, NIHSS, mRS, Barthel index, MMSE score at randomization were 13.7 ± 1.6, 11.2 ± 4.7, 2.6 ± 1.2, 65.4 ± 28.0, and 24.3 ± 6.9, respectively.
All these RCTs included patients with acute arterial ischemic stroke only. The cutoff time interval for enrolment in the study was between 24 h to 14 days in these RCTs, but all of them excluded patients with very severe arterial ischemic stroke, with high NIHSS scores (> 17 and > 25 in the study by Zhang et al. [11] and Belova et al. [15], respectively). All these RCTs excluded patients with significant coexistent other psychiatric disorders, dementia, severe, uncontrolled cardiac, renal, hepatic, or endocrinal impairment including myocardial infarction, and those with evidence of hemorrhage on neuroimaging. Table 1 shows a brief summary of included studies.
Table 1.
Summary of included clinical trials
| Author, year | Study method, sample size | Sample size | Sample characteristics | Time point for measuring key outcomes | Result for efficacy outcomes | Result for safety outcomes |
|---|---|---|---|---|---|---|
| Belova et al., 2017 | Open- label RCT | 100 and 64 in vinpocetine and control arm, respectively | Acute arterial ischemic stroke cases within 14 days of onset of stroke randomized to adjunctive Cavinton R group and basic therapy only group. Cavinton R was used intravenously for 10 days followed by Cavinton R comforte in dosagee 10 mg, 1 tablet three3 times a day during 90 days | NIHSS, Rankin, Barthel, MoCA, MMSE, Rivermead mobility index, Beck depression scale, and HADS at 1 months and 3 months after starting vinpocetine |
Higher scores on NIHSS, Rankin, Barthel, MoCA, MMSE, Rivermead mobility index were found in the vinpocetineVinpocetine group compared withto the controls. There were no differences in scores on the Beck depression scale and HADS A decrease in Young’'s modulus was found in the main group, whereashile in the control group this index remained unchanged |
There were no SAEs |
| Zhang et al., 2016 | Open- label, multicenterric RCT | 469 and 141 cases in vinpocetine and control group, respectively | Within 72 h of stroke onset, received either vinpocetine 30 mg intravenously once daily for 7 days, along with standard care or standard care alone (anti-platelets, -aspirin, clopidogrel) | MMSE, NIHSS, modified Rankin, Barthel Index at 7 days, 1 month and 3 months | MMSE, NIHSS, and Barthel IndexBI scores were significantly higher in the vinpocetine group than in the control group 3 months after treatment, indicating significantly improved cognitive skill, neurological function, and quality of life (QOL) in the vinpocetine group versus the control group | No significant difference in safety was noted between the two groups |
| Feigin et al., 2001 | Double- blind, placebo controlled RCT | 30 (15 in each group) | Within 72 h of CT‐verified acute ischemicaemic stroke, received either IV low‐molecular‐weight dextran alone (3 g in 250 ml of isotonic saline) in the control group, or in combination with 10 mg IViv vinpocetine for 5 to 7 days, followed by oral vinpocetine 3 × × 10 mg in the treatment group for 90 days | An relative risk (RR) reduction of poor outcome at 3 months follow-up was 30% (RR = 0.7; 95% confidence interval [CI] 0.1–3.4), as defined by the modified Barthel Index, and 60% as defined by the modified Rankin score (RR = 0.4, 95% CI: 0.1–1.7). The National Institute of Health (NIHS–NINDS) Stroke Scale score was marginally significantly better in the vinpocetine-treated group at 3 months of follow-up (pP = 0.05, ANOVA) | No significant adverse effects were seen | |
| Werner et al., 1986 | Double- blind, placebo controlled RCT | 40 (20 in each group) | Within 48 h of stroke onset. the participants got either placebo or 40 mg of vinpocetine in a 200 ml dextran intravenous infusion for 3three weeks | Modified Rankin scale, MMSE, self-assessment by visual analoganalogue scale, clinical global impression at 1 month | Total 8 and 12 patients had a significant disability at 1 month, but no patients died in any group. Total seven7 patients were lost to follow-up up in both groups | No significant adverse effects were observed |
ANOVA, analysis of variance, CI, confidence interval, CT, computed tomography, HADS, Hospital Anxiety and Depression Scale, IV, intravenous, MMSE, mini-mental state examination, MoCA, Montreal Cognitive Assessment, NIHSS, National Institutes of Health Stroke Scale, QOL, quality of life, RCT, randomized controlled trials, RR, relative risk, SAE, serious adverse event
Although Zhang et al. [11] used a dosage of 30 mg intravenously once daily for 7 days, Werner et al. [16] used a dosage of 40 mg intravenously once daily for 3 weeks, Feigin et al. [9] used a dosage of 10 mg intravenously once daily for 5–7 consecutive days followed by 10 mg orally three times a day for 30 days and Belova et al. [15] used a dosage of 10 mg intravenously for 10 days followed by 30 mg/day for 90 days. The overall median dosage was 30 mg/day and the median follow-up duration was 3 months (range 1–6 months).
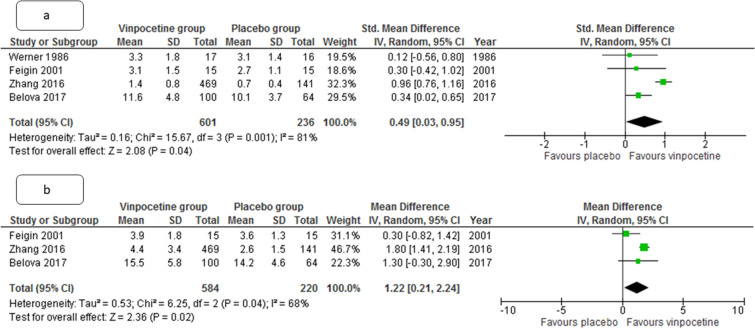
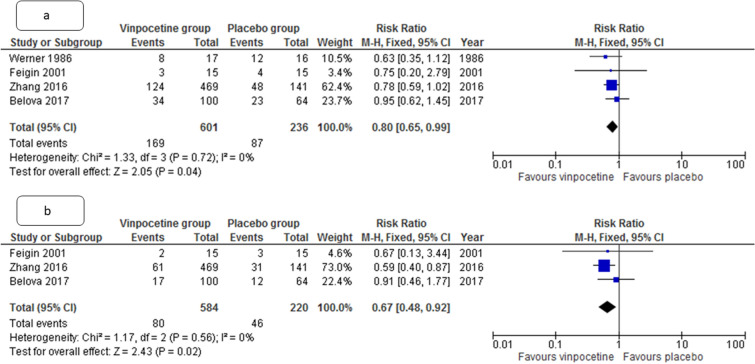
The number of patients with death or significant disability was lower in vinpocetine group, as compared to placebo group at both 1 and 3 months (relative risk [RR] 0.80, 95% CI 0.65–0.99 and RR 0.67, CI 0.48–0.92, p = 0.04 and 0.02, respectively, I2 = 0% for both). The degree of disability in participants at 1 month and 3 months was also lower in vinpocetine group than in the placebo group (standardized mean difference (SMD) 0.49, 95% CI 0.03–0.95 and std. mean difference 1.22, CI 0.23–2.24, p = 0.001 and 0.04, I2 = 84% and 68% respectively) (Figs. 3 and 4). The change in neurological deficit (stroke severity) as measured by NIHSS score was also better with vinpocetine than in the control group (SMD 1.04, CI 0.47–1.61, p = 0.03, I2 = 27%).
Fig. 3.
Metanalysis forest plot comparing pooled estimate for degree of disability at 1 month (a) and 3 months (b) in patients with acute ischemic stroke between vinpocetine and control group. CI, confidence interval, IV, inverse of variance, SD, standard deviation
Fig. 4.
Metanalysis forest plot comparing pooled estimate for the number of patients with death or significant disability at 1 month (a) and 3 months (b) in patients with acute ischemic stroke between vinpocetine and control group. CI, confidence interval, M-H, Mantel-Haenszel formula
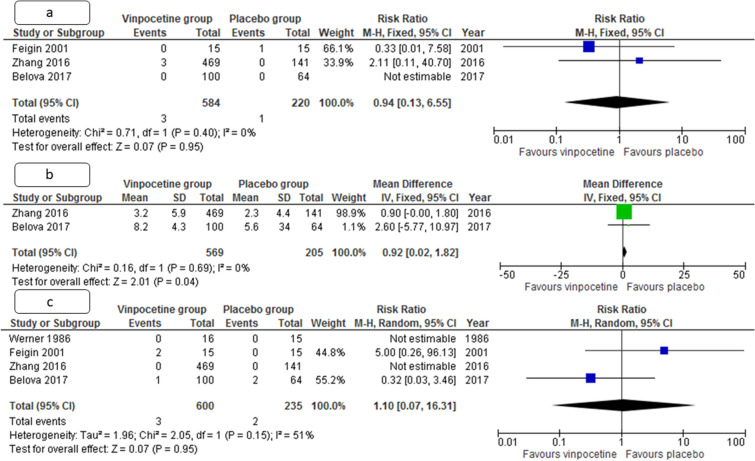
Improvement in MMSE score compared with baseline at trial enrolment was also better in the vinpocetine group, compared with the placebo group (pooled weighted mean difference 0.92, 95% CI 0.02–1.82, p = 0.04, I2 = 0%) (Fig. 5).
Fig. 5.
Metanalysis forest plot comparing pooled estimate for the number of patients with death at 3 months (a) change in cognition as measured by MMSE at 3 months (b), and the number of patients with Treatment emergent adverse events (c) in patients with acute ischemic stroke between vinpocetine and control group. CI, confidence interval, IV, inverse of variance, M-H, Mantel-Haenszel formula, SD, standard deviation
Only one study each commented on the change in anxiety, depression, rheological parameters, and cerebral blood flow. In that study, change in anxiety and depression level was not significantly different between two groups, although there was a slight trend toward more improvement in the vinpocetine group (p = 0.43, 0.29 respectively).
All-cause mortalities were also comparable in both groups (RR 0.94, CI 0.13–6.55, p = 0.95, I2 = 51%). Only one study each documented change in rheological and trans cranial doppler parameters. There was a more significant reduction in Young’s modulus of RBC membrane in vinpocetine group compared with the placebo group (60.1 vs. 1.8, p = 0.0001), suggesting a more significant increase in elasticity of RBC membrane and its deformability. There was also a significant increase in mean velocity of the anterior, middle, and posterior cerebral artery and reduction in basilar artery pulsatility index (p = 0.03, 0.01, < 0.001, and 0.02, respectively) when measured by transcranial doppler.
Vinpocetine had an excellent safety profile and only 3/660 (0.5%) had Treatment emergent adverse events (TEAEs), but some of those were seemingly unrelated to vinpocetine causally. The proportion of patients with TEAE in vinpocetine and placebo groups was comparable (RR 1.10, CI 0.07–6.31, p = 0.95, I2 = 51%). The all-cause mortality was also similar in both groups (RR 0.94, CI 0.13–6.55, p = 0.95, I2 = 0%), although this more likely indicates that the vinpocetine is unlikely to reduce mortality due to ischemic stroke. None of the mortalities were causally related to vinpocetine and no SAEs were also reported causally related to vinpocetine.
Discussion
Our systematic review showed vinpocetine reduces the degree of disability in patients with arterial ischemic stroke when used in the acute phase of the cerebral infarction and also reduces the combined likelihood of death and survival with significant disability at 1 and 3 months. It was also associated with some improvement in cognition and quality of life at 1 and 3 months. The safety and tolerability profile of vinpocetine was also excellent.
In the course of therapy, vinpocetine has also been shown to improve rheological properties of blood, causing an increase in the elasticity of the cytoplasmic membrane of erythrocytes, hence their deformability, a faster and more complete regression of neurological deficit, a more complete restoration of mobility, self-service functions, cognitive functions and social activity [17]. The fact that a more pronounced effect of therapy in the main group persisted throughout the entire observation period is the basis for long-term administration of vinpocetine to maintain positive dynamics in the health status of patients with ischemic stroke [18].
Possible pathophysiological stages that can be affected by neuroprotective agents under conditions of anaerobic glycolysis are ion imbalance, oxidative stress, excitotoxicity, neuroinflammation, and apoptosis [19]. Studies have shown that when blood passes through the distal part of an occluded vascular segment, its viscosity becomes a critical factor in determining the status of tissue perfusion. Taking into account that ischemic brain damage is a consequence of the interaction of complex pathophysiological processes, the superior advantage of drugs with pleiotropic effect and acting on several stages of the ischemic cascade becomes obvious, the use of which might lead to more meaningful improvement in neurological outcome in patients with cerebral infarction [20].
Vinpocetine is a derivative of the alkaloid vincamine [21]. It has pleiotropic effect on vascular endothelium and in the membranes of erythrocytes, which contributes to vasodilation, an increase in erythrocyte deformability, and a decrease in blood viscosity [22]. It also modulates the, vascular smooth muscle cells, macrophages and microglia by inhibiting the NF-κB signaling pathway [23]. It also increases the availability of glucose, regulates glial reactivity, and induces neuroplastic processes [24]. Researchers consider this effect of vinpocetine as an analog of ischemic preconditioning, the most powerful form of endogenous tissue protection against ischemia [25]. In patients with dementia, it has also been found to cause improvement in speech, movement, memory disorders, improvement of the quality of life [26]. But randomized trials in this regard are not so robust as in the acute stage of cerebral infarction.
In patients with stroke and its consequences, for whom swallowing dysfunction, nausea, cognitive and psychoemotional problems are common symptoms, thereby making ingestion of conventional tablets and gelatin capsules difficult. In the recent RCTs, whenever the oral route is used, the choice of medication was a mouth dispersible form of vinpocetine called Cavinton comfort. It is more suitable for patients with such a serious illness as stroke and probably associated with an increase in their adherence to treatment [27].
Previously a number of uncontrolled and controlled trials have also explored vinpocetine in patients with arterial ischemic stroke in the acute stage and some of the trials also explored in these patients during the stage of chronic cerebrovascular disease, often described in past literature as discirculatory encephalopathy. Because of various methodological limitations, we could not include those clinical trials in our review, but almost all these trials showed somewhat favorable results with vinpocetine. However, the regimen, route, and dose of vinpocetine varied across studies, and observing the studies included in the review, we could not find any obvious dose–response relationship, i.e., the trial using a higher dosage or longer duration didn’t unequivocally report better outcomes.
Vinpocetine has been shown to improve cognitive impairment also in patients with epilepsy, patients with nasopharyngeal carcinoma undergoing radiation therapy, and patients with Alzheimer’s disease, vascular dementia, and even other types of dementia [28–31]. Even one RCT showed it has a possible adjuvant antiepileptic effect when used in patients with focal epilepsy [29]. But these RCTs were often of inadequate sample size, has methodological flaws, or small duration of follow-up [29]. Because of these purposes still, vinpocetine has not been accepted by clinicians for universal routine use for any neurological disorder. Even a systematic review in patients with dementia found promising yet inconclusive evidence favoring its use in patients with dementia.
However, the evidence generated from this systematic review still seem to be insufficient to recommend universal use of vinpocetine in all cases of acute arterial ischemic stroke. The sample size of both double-blind, placebo-controlled RCTs included in our review is small and one double-blind RCT had significant drop out rate. The open labeled RCTs performed more recently, although having an adequate sample size, the open-label nature of RCT still produces some amount of bias. None of the RCTs assessed long-term outcomes at 6 month or 1 year. Dose of vinpocetine and duration of administration varied from one study to another. Some of the recent RCTs did not mention why the patient was not opted for thrombolysis or mechanical thrombectomy, which has been proven to be efficacious in acute arterial ischemic stroke cases. A phase III, multicentric double-blind, placebo-controlled RCT with adequate sample size and long-term follow-up duration showing favorable study result of moderate to large effect size, is needed before recommending universal use of this neuroprotective and vasoactive medication.
Apart from these, other limitations of our systematic review are we had to exclude many clinical trials, both controlled and uncontrolled due to methodological flaws, thereby limiting the number of patients included in our review. The dose, route of administration, and duration of follow-up, as well as the formulation of vinpocetine used were different across the studies.
In trials completed earlier to assess the effectiveness of vinpocetine in the treatment of ischemic stroke, the therapy regimen with Cavinton for infusion and Cavinton comfort was not used, which has better patient suitability. This could have been the reason behind the equivocal results in the earlier systematic review. In addition, the effect of therapy with Cavinton on the deformability of erythrocytes, which is of paramount importance for the treatment and prevention of vascular diseases, has been studied only in chronic cerebrovascular diseases, and thus we were unable to collate and meta-analyze such data about the acute stage of arterial ischemic stroke. Even in the studies with chronic cerebrovascular dysfunction, the assessment of the elasticity of the erythrocyte membrane was carried out by calculating the rigidity index, which did not allow assessing the state of an individual cell. Only one trial included in our review explored the effect of the modern therapy regimen of vinpocetine on the deformability of RBCs in the acute and early recovery periods of ischemic stroke using atomic force microscopy and showed favorable results. Similarly, some of the variables like the effect on MMSE and MOCA as well as anxiety and depression were only explored in a proportion of RCTs included in our review. Thus, for variables other than those describing death and disability, we were not able to draw firm conclusions regarding the definite clinical advantage of vinpocetine.
Despite these limitations, the results of our review suggest some promising efficacy of vinpocetine, at least in reducing disability, when used during the acute stage of cerebral infarction (arterial ischemic stroke). It should also be noted that Cavinton comfort was found to have excellent safety of the treatment, as well as the convenience of using dispersible tablets. Probably this was the cause behind high patient compliance in the RCTs, which is extremely important in the treatment of ischemic stroke. More high quality, multicentric double-blind, placebo-controlled RCTs are needed to generate more robust evidence regarding the efficacy of vinpocetine and especially to determine the optimum dose, route, formulation, and duration of administration and make an universal recommendation for its use in cases of acute arterial ischemic stroke.
Conclusions
Vinpocetine has some promising efficacy in patients with ischemic stroke when used in acute stage in reducing the disability, but presently there is not enough evidence to suggest it also reduces case fatality. More double-blind, placebo-controlled RCTs of adequate sample size are needed before making recommendations for the routine administration of vinpocetine for all patients with acute ischemic stroke.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr. Lesa Dawman for helping us in English language corrections.
Author Contributions
PKP, AR, PP, and IKS were involved in conception acquisition, analysis, or interpretation of the data. PKP and AR performed initial search and full-text review. PP and IKS performed statistical analysis. PKP, AR and PP drafted the initial draft, and IKS critically revised the article. All the authors read and approved the final version of the manuscript.
Source of Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical Approval
We confirm adherence to ethical guidelines and indicate ethical approvals (institutional review board) and use of informed consent, as appropriate.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prateek Kumar Panda and Aparna Ramachandran Contributed equally and share joint first authorship.
References
- 1.Hankey GJ. Stroke. Lancet Lond Engl. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 2.Chugh C. Acute ischemic stroke: management approach. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2019;23:S140–S146. doi: 10.5005/jp-journals-10071-23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpkins AN, Tahsili-Fahadan P, Buchwald N, De Prey J, Farooqui A, Mugge LA, et al. Adapting clinical practice of thrombolysis for acute ischemic stroke beyond 4.5 hours: a review of the literature. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2021;30:106059. doi: 10.1016/j.jstrokecerebrovasdis.2021.106059. [DOI] [PubMed] [Google Scholar]
- 4.Feske SK. Ischemic Stroke. Am J Med. 2021;134:1457–64. [DOI] [PubMed]
- 5.Imran R, Mohamed GA, Nahab F. Acute reperfusion therapies for acute Ischemic stroke. J Clin Med. 2021;10:3677. doi: 10.3390/jcm10163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaire BP. Microglia as the critical regulators of neuroprotection and functional recovery in cerebral ischemia. Cell Mol Neurobiol. 2021. 10.1007/s10571-021-01145-9. [DOI] [PMC free article] [PubMed]
- 7.Gupta S, Singh P, Sharma BM, Sharma B. Neuroprotective effects of agomelatine and vinpocetine against chronic cerebral hypoperfusion induced vascular dementia. Curr Neurovasc Res. 2015;12:240–252. doi: 10.2174/1567202612666150603130235. [DOI] [PubMed] [Google Scholar]
- 8.Nyakas C, Felszeghy K, Szabó R, Keijser JN, Luiten PGM, Szombathelyi Z, et al. Neuroprotective effects of vinpocetine and its major metabolite cis-apovincaminic acid on NMDA-induced neurotoxicity in a rat entorhinal cortex lesion model. CNS Neurosci Ther. 2009;15:89–99. doi: 10.1111/j.1755-5949.2009.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigin VL, Doronin BM, Popova TF, Gribatcheva EV, Tchervov DV. Vinpocetine treatment in acute ischaemic stroke: a pilot single-blind randomized clinical trial. Eur J Neurol. 2001;8:81–85. doi: 10.1046/j.1468-1331.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 10.Bereczki D, Fekete I. Vinpocetine for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;2008:CD000480. [DOI] [PMC free article] [PubMed]
- 11.Zhang W, Huang Y, Li Y, Tan L, Nao J, Hu H, et al. Safety and efficacy of vinpocetine as part of treatment for acute cerebral infarction: a randomized, open-label, controlled, multicenter CAVIN (Chinese Assessment for Vinpocetine in Neurology) Trial. Clin Drug Investig. 2016;36:697–704. doi: 10.1007/s40261-016-0415-x. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belova LA, Mashin VV, Proshin AN, Ovsyannicova AN. Kostishko BB [Possibilities of cavinton therapy regimen for infusions and cavinton comforte in acute and early recovery periods after ischemic stroke] Zhurnal Nevrol Psikhiatrii Im SS Korsakova. 2017;117:51–58. doi: 10.17116/jnevro20171179151-58. [DOI] [PubMed] [Google Scholar]
- 16.Werner J, Apececha M, Schaltenbrand R, Fenzl E. Clinical study to evaluate the efficacy and tolerance of vinpocetine i.v. added to standard therapy in patients suffering from an acute apoplectic insult. In: Bés A, editor. Senile dementias: early detection. John Libbey Eurotext; 1986. p. 636–41.
- 17.Zhang Y-S, Li J-D, Yan C. An update on vinpocetine: new discoveries and clinical implications. Eur J Pharmacol. 2018;819:30–34. doi: 10.1016/j.ejphar.2017.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Kuraishy HM, Al-Gareeb AI, Naji MT, Al-Mamorry F. Role of vinpocetine in ischemic stroke and poststroke outcomes: a critical review. Brain Circ. 2020;6:1–10. doi: 10.4103/bc.bc_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onwuekwe I, Ezeala-Adikaibe B. Ischemic stroke and neuroprotection. Ann Med Health Sci Res. 2012;2:186–190. doi: 10.4103/2141-9248.105669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke Off J Int Stroke Soc. 2012;7:378–385. doi: 10.1111/j.1747-4949.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Yang L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: a review of the literature. Mol Basel Switz. 2014;20:335–347. doi: 10.3390/molecules20010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooq MU, Min J, Goshgarian C, Gorelick PB. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31:759–776. doi: 10.1007/s40263-017-0459-3. [DOI] [PubMed] [Google Scholar]
- 23.Solovyeva EY, Karneev AN, Chekanov AV, Baranova OA, Choi IV. Complex application 2-ethyl-6-methyl-3-hydroxypyridine-succinate and vinpocetine in cerebrovascular disorder. Zhurnal Nevrol Psikhiatrii Im SS Korsakova. 2017;117:103–108. doi: 10.17116/jnevro201711751103-108. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Wang E, Chen F, Xiao J, Wang M. Neuroprotective phytochemicals in experimental ischemic stroke: mechanisms and potential clinical applications. Oxid Med Cell Longev. 2021;2021:6687386. doi: 10.1155/2021/6687386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu C, Li L. Pre-conditions for eliminating mitochondrial dysfunction and maintaining liver function after hepatic ischaemia reperfusion. J Cell Mol Med. 2017;21:1719–1731. doi: 10.1111/jcmm.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Yin Y, Lu Q-L, Dan Y, Xu M-S, Song G, et al. Vinpocetine in the treatment of poststroke cognitive dysfunction: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e13685. doi: 10.1097/MD.0000000000013685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Protasov KV, Dzizinskiĭ AA, Shprakh VV, Sinkevich DA, Kuklin SG, Filatova LN. Cavinton forte treatment of cerebral vascular insufficiency in patients with coronary heart disease and arterial hypertension. Zhurnal Nevrol Psikhiatrii Im SS Korsakova. 2006;106:31–34. [PubMed] [Google Scholar]
- 28.Dutov AA, Gal’tvanitsa GA, Volkova VA, Sukhanova ON, Lavrishcheva TG, Petrov AP. Cavinton in the prevention of the convulsive syndrome in children after birth injury. Zhurnal Nevropatol Psikhiatrii Im SS Korsakova Mosc Russ. 1991;91:21–22. [PubMed] [Google Scholar]
- 29.Garza-Morales S, Briceño-González E, Ceja-Moreno H, Ruiz-Sandoval JL, Góngora-Rivera F, Rodríguez-Leyva I, et al. Extended-release vinpocetine: a possible adjuvant treatment for focal onset epileptic seizures. Bol Med Hosp Infant Mex. 2019;76:215–224. doi: 10.24875/BMHIM.19000056. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Cao Y, Chen S, Shao L. Combination of vinpocetine and dexamethasone alleviates cognitive impairment in nasopharyngeal carcinoma patients following radiation injury. Pharmacology. 2021;106:37–44. doi: 10.1159/000506777. [DOI] [PubMed] [Google Scholar]
- 31.Valikovics A, Csányi A, Németh L. Study of the effects of vinpocetin on cognitive functions. Ideggyogyaszati Szle. 2012;65:115–120. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.