Abstract
Compared to MICs (more than 800 μg/ml) of (−)-epigallocatechin gallate (EGCg) against Escherchia coli, MICs of EGCg against methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA) were 100 μg/ml or less. Furthermore, less than 25 μg EGCg per ml obviously reversed the high level resistance of MRSA to all types of tested β-lactams, including benzylpenicillin, oxacillin, methicillin, ampicillin, and cephalexin. EGCg also induced a supersusceptibility to β-lactams in MSSA which does not express mecA, encoding penicillin-binding protein 2′ (PBP2′). The fractional inhibitory concentration (FIC) indices of the tested β-lactams against 25 isolates of MRSA were from 0.126 to 0.625 in combination with 6.25, 12.5 or 25 μg of EGCg per ml. However, no synergism was observed between EGCg and ampicillin against E. coli. EGCg largely reduced the tolerance of MRSA and MSSA to high ionic strength and low osmotic pressure in their external atmosphere, indicating damage of the cell wall. Unlike dextran and lipopolysaccharide, peptidoglycan from S. aureus blocked both the antibacterial activity of EGCg and the synergism between EGCg and oxacillin, suggesting a direct binding of EGCg with peptidoglycan on the cell wall. EGCg showed a synergistic effect with dl-cycloserine (an inhibitor of cell wall synthesis unrelated to PBP2′) but additive or indifferent effect with inhibitors of protein and nuclear acid synthesis. EGCg did not suppress either PBP2′ mRNA expression or PBP2′ production, as confirmed by reverse transcription-PCR and a semiquantitative PBP2′ latex agglutination assay, indicating an irrelevance between the synergy and PBP2′ production. In summary, both EGCg and β-lactams directly or indirectly attack the same site, peptidoglycan on the cell wall. EGCg synergizes the activity of β-lactams against MRSA owing to interference with the integrity of the cell wall through direct binding to peptidoglycan.
β-Lactams function by covalently combining with penicillin-binding proteins (PBPs) and inactivating their transpeptidase and carboxypeptidase activities that are responsible for catalyzing the final transpeptidation step of bacterial cell wall biosynthesis (3, 4). Although all strains of Staphylococcus aureus have four PBPs (PBP1 to PBP4), only methicillin-resistant S. aureus (MRSA) expresses a specific PBP (PBP2′ or PBP2a) from the mecA gene. PBP2′ takes over the biosynthetic functions of normal PBPs in the presence of inhibitory concentrations of β-lactams because PBP2′ has a decreased binding affinity to β-lactams (7, 19). MRSA has become a serious problem since MRSA has already made its way into the community, not just as a primarily nosocomial pathogen (1, 8). Increasing reports of vancomycin-resistant MRSA and antibiotic-resistant tuberculosis as well as other bacteria indicate the worst situation that humans have faced in the battle against bacterial infections since Fleming's great finding. Therefore, concerted efforts have again been made to find antimicrobial materials from natural products and traditional medicines.
Tea (Camellia sinensis), a universally popular beverage, is consumed every day by billions of people worldwide. In traditional Chinese medicine, tea was considered a panacea with antipyretic, antidotal, antidiarrheal, and diuretic effects, indicating an anti-infectious activity according to our modern concept. Until 1989, there was rather limited experimental evidence of the antimicrobial activity of tea, as reviewed by Hamilton-Miller (5, 6). A series of experiments in our laboratory has demonstrated the antimicrobial effects of tea. Especially we found that epigallocatechin gallate (EGCg) in tea catechins is mainly responsible for the antimicrobial activity (9, 17, 21). Tea and EGCg have bactericidal activity against MRSA and methicillin-susceptible S. aureus (MSSA) (22). Surprisingly, we found that less than 1/4 MIC of EGCg reversed the resistance of all tested clinical isolates of MRSA to β-lactams (20). Other two groups using an aqueous extract of tea also confirmed the synergistic effect (25, 26). However, the mechanism of the synergy between EGCg and β-lactams is still unclear. We hypothesized that direct effects of EGCg on bacterial cell wall might be responsible for the synergy between β-lactams and EGCg against MRSA.
MATERIALS AND METHODS
EGCg, antibiotics and other reagents.
EGCg was extracted from green tea as previously reported (12). The purity of EGCg was 98%, as confirmed by high-performance liquid chromatography. The following materials were purchased from commercial sources: oxacillin, methicillin, dl-cycloserine, minocycline, and ofloxacin (Sigma, St. Louis, Mo.); ampicillin (Banyu Pharmaceutical Co., Tokyo, Japan); peptidoglycan purified from S. aureus (Fluka Chemika AG, Buchs, Switzerland); lipopolysaccharide (LPS) purified from Escherichia coli O26:B6 (Difco, Detroit, Mich.); and benzylpenicillin, cephalexin, and dextran (molecular weight, 100,000 to 200,000) purified from Leuconostoc dextranicum (Wako Pure Chemical Industries, Osaka, Japan).
Bacterial strains and media.
Twenty-five clinical isolates of MRSA were from specimens submitted for routine cultures to the clinical microbiology laboratories of two hospitals, Fujigaoka Hospital (F strains) and Hatanodai Hospital (H strains), at Showa University. A screening test for MRSA was performed with 6 μg of oxacillin per ml on Mueller-Hinton (MH) agar supplemented with 4% NaCl. MSSA (ATCC 25923 and FDA 209p), Staphylococcus epidermidis ATCC 1228, E. coli (ATCC 25922, ODL 931 and C600), Salmonella enterica serovar Typhi (clinical isolate 1), S. enterica serovar Typhimurium TSA 2121, S. enterica serovar Enteritidis 87-350, Klebsiella pneumoniae (IID 5207 and ST 101), and Proteus mirabilis were used. All strains were maintained on MH agar plates, and antimicrobial assays were carried out in MH broth (MHB, Becton Dickinson, Cockeysville, Md.).
Antimicrobial assay.
Bacteria (3 × 106) were inoculated into 3 ml of MHB containing different concentrations of EGCg and/or antibiotics, and then the cultures were incubated stationarily at 37°C for 24 h. Growth of the bacteria was examined as a function of turbidity (optical density [OD] at 600 nm). The lowest concentration of the twofold serially diluted antibiotic or EGCg in which no growth occurred was defined as the MIC. MICs were also confirmed by counting the viable cells in the tubes near the MICs. The interactions between EGCg and antibiotics were tested by the checkerboard method (18). Twofold dilutions of one antibiotic were tested in combination with twofold dilutions of EGCg. Synergy between β-lactams and EGCg was evaluated as a fractional inhibitory concentration (FIC) index. The FIC was calculated as the MIC of an antibiotic or EGCg in combination divided by the MIC of the antibiotic or EGCg alone, and the FIC index was obtained by adding the FICs. If the FIC index was ≤0.5, the combination was defined as synergy.
Effects of EGCg on the tolerance of MRSA and MSSA to high ionic strength and low osmotic pressures.
To assess the tolerance of MRSA and MSSA to high ionic strength, the bacteria (3 × 106) were cultured in 3 ml of MHB containing different concentrations of NaCl and EGCg at 37°C for 24 h. To assess the tolerance of the bacteria to low osmotic pressure, the bacteria (104 cells/ml) were incubated in water containing various concentrations of EGCg at 37°C for 0, 4, 8, 12, and 24 h, and then viability was confirmed by culturing the cells on MH agar plates for additional 48 h.
RT-PCR analysis of mecA gene expression.
The expression of PBP2′ mRNA was detected by reverse transcription-PCR (RT-PCR). Total RNA from strains F-74, F-98, F-68, and FDA 209p cultured in MHB with or without 25 μg of EGCg per ml for 24 h was purified with TRIZOL (GIBCO BRL Life Technologies, Grand Island, N.Y.) according to the manufacturer's instructions. RT-PCR was performed using a RT-PCR kit (Stratagene, La Jolla, Calif.) with 30 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 62°C, and extension for 30 s at 72°C. PCR primers for the mecA gene (449F [5′-AAA CTA CGG TAA CAT TGA TCG CAA C-3′] and 761R [5′-CTT GTA CCC AAT TTT GAT CCA TTT G-3′]) and primers specific to S. aureus 16S rRNA (387F [5′-CGA AAG CCT GAC GGA GCA AC-3′] and 914R [5′-AAC CTT GCG GTC GTA CTC CC-3′]) were designed as described previously (10). PCR products were analyzed on 1.2% agarose gels and visualized by ethidium bromide staining. A 313-bp fragment of the mecA gene and a 528-bp fragment of the 16S rRNA gene should be amplified from MRSA. The 528-bp fragment only should be amplified from MSSA.
Latex agglutination assay of PBP2′.
An MRSA screening kit (Denka Seiken Co. Ltd., Tokyo, Japan) was used. Latex agglutination assay was performed with a modification of the manufacturer's instructions and a previous report (16). Briefly, the bacterial strains were cultured in MHB with or without EGCg (12.5 to 50 μg/ml) for 24 h. After three washes with saline by centrifugation, the bacterial pellets were suspended in 0.1 M NaOH to a final concentration of 109 bacteria per 200 μl and boiled for 3 min to release PBP2′ from the bacterial cells. pH was adjusted with 50 μl of 0.5 M KH2PO4 before a 5-min centrifugation at 1,500 × g. For a semiquantitative estimation of PBP2′ production, 50 μl of the original or two- to seven-times-diluted supernatants was placed on a slide and then mixed with 25 μl of anti-PBP2′ monoclonal antibody-sensitized latex. The intensity of agglutination and times of agglutination appearance were observed and scored between + and +++.
Presentation of data.
All experiments were performed three times or more, and the data presented are from one of those experiments.
RESULTS
Dose-dependent inhibition of MRSA and MSSA growth by EGCg.
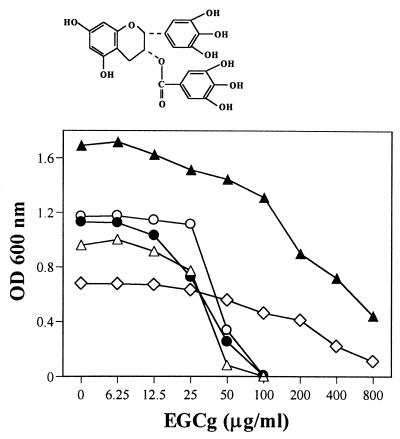
The chemical structure of EGCg is shown in Fig. 1. EGCg dose-dependently inhibited the growth of MRSA and MSSA (Fig. 1). Although the growth rates of the strains were different, the MICs of EGCg for these strains were 100 μg/ml or less. As controls in the same culture condition, S. epidermidis showed the same susceptibility to EGCg (MIC, 100 μg/ml). However, gram-negative bacilli tested were not as susceptible as S. aureus to EGCg. The MICs were more than 800 μg/ml for E. coli (ATCC 25922, ODL 931, and C600), S. enterica serovar Typhimurium TSA 2121, S. enterica serovar Enteritidis 87-350, K. pneumoniae (IID 5207 and ST 101), and P. mirabilis and more than 400 μg/ml for S. enterica serovar Typhi 1.
FIG. 1.
Chemical structure and antibacterial activities of EGCg against MRSA, MSSA, S. epidermidis, E. coli, and S. enttrica serovar Typhimurium. Bacteria (3 × 106 cells) were inoculated into 3 ml of MHB containing different concentrations of EGCg. The cells were then incubated stationarily at 37°C for 24 h. Growth of bacteria was determined by OD at 600 nm. ○, MRSA F-74; ●, MSSA ATCC 25923; ▵, S. epidermidis ATCC 1228; ▴, E. coli ATCC 25922; ◊, S. enterica serovar Typhimurium TAS 2121.
Synergy between EGCg and β-lactams against MRSA and MSSA.
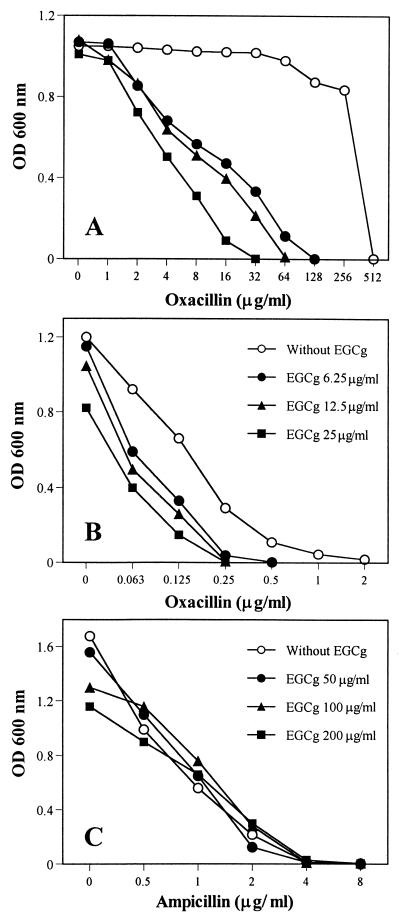
Low concentrations of EGCg (less than 1/4 MIC) reversed the high-level resistance of F-74 to oxacillin, as shown in Fig. 2A. At the same time, EGCg induced a supersusceptibility of MSSA ATCC 25923 against oxacillin (Fig. 2B). The synergy against MRSA and MSSA was confirmed in the combinations between EGCg and all types of tested β-lactams, including benzylpenicillin, ampicillin, oxacillin, methicillin, and cephalexin. As summarized in Table 1, FIC indices of benzylpenicillin and oxacillin were from 0.126 to 0.625 in combination with 6.25, 12.5, or 25 μg of EGCg per ml against 25 isolates of MRSA and two strains of MSSA. However, no synergy was observed between EGCg and ampicillin, the modified broad-spectrum penicillin, against E. coli ATCC 25922 (Fig. 3C) and other gram-negative bacilli tested (data not shown).
FIG. 2.
Synergistic anti-MRSA (A) and anti-MSSA (B) effects and indifferent anti-E. coli (C) effect between EGCg and β-lactams. MRSA F-74 and MSSA ATCC 25923 were cultured in MHB containing EGCg and oxacillin. E. coli ATCC 25922 was cultured in MHB containing EGCg and ampicillin. Symbols in panel B apply also to panel A.
TABLE 1.
MICs and FIC indices of penicillin and oxacillin in combination with EGCg against 25 isolates of MRSA and two strains of MSSA
| Strain | Penicillin
|
Oxacillin
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml)
|
FIC index
|
MIC (μg/ml)
|
FIC index
|
|||||||||||
| A | B | C | D | B | C | D | a | b | c | d | b | c | d | |
| F-8 | 64 | 32 | 16 | 8 | 0.563 | 0.375 | 0.375 | 256 | 16 | 8 | 4 | 0.126 | 0.156 | 0.266 |
| F-10 | 128 | 32 | 32 | 4 | 0.313 | 0.375 | 0.281 | 128 | 32 | 16 | 8 | 0.313 | 0.25 | 0.313 |
| F-34 | 64 | 16 | 16 | 4 | 0.313 | 0.375 | 0.313 | 1,024 | 512 | 256 | 128 | 0.563 | 0.375 | 0.375 |
| F-36 | 16 | 4 | 2 | 0.5 | 0.313 | 0.25 | 0.281 | 256 | 16 | 16 | 4 | 0.126 | 0.188 | 0.266 |
| F-38 | 64 | 32 | 16 | 8 | 0.563 | 0.375 | 0.375 | 1,024 | 512 | 256 | 128 | 0.563 | 0.375 | 0.375 |
| F-41 | 16 | 4 | 1 | 0.5 | 0.313 | 0.188 | 0.281 | 256 | 32 | 16 | 8 | 0.188 | 0.188 | 0.281 |
| F-47 | 64 | 32 | 16 | 4 | 0.563 | 0.375 | 0.313 | 1,024 | 512 | 256 | 128 | 0.563 | 0.375 | 0.375 |
| F-49 | 64 | 16 | 16 | 8 | 0.313 | 0.375 | 0.375 | 512 | 128 | 64 | 32 | 0.313 | 0.25 | 0.313 |
| F-56 | 64 | 32 | 32 | 8 | 0.563 | 0.625 | 0.375 | 1,024 | 512 | 256 | 128 | 0.563 | 0.375 | 0.375 |
| F-61 | 64 | 32 | 16 | 2 | 0.563 | 0.375 | 0.281 | 1,024 | 512 | 256 | 32 | 0.563 | 0.25 | 0.281 |
| F-68 | 64 | 32 | 32 | 16 | 0.563 | 0.625 | 0.5 | 512 | 256 | 128 | 64 | 0.563 | 0.375 | 0.375 |
| F-69 | 64 | 32 | 32 | 8 | 0.563 | 0.625 | 0.375 | 1,024 | 512 | 256 | 128 | 0.563 | 0.375 | 0.375 |
| F-74 | 64 | 32 | 32 | 16 | 0.563 | 0.625 | 0.5 | 512 | 128 | 64 | 32 | 0.313 | 0.25 | 0.313 |
| F-87 | 256 | 32 | 32 | 16 | 0.188 | 0.25 | 0.313 | 256 | 32 | 16 | 4 | 0.188 | 0.188 | 0.266 |
| F-94 | 64 | 32 | 32 | 8 | 0.563 | 0.625 | 0.375 | 512 | 128 | 128 | 8 | 0.313 | 0.375 | 0.266 |
| F-96 | 64 | 32 | 32 | 8 | 0.563 | 0.625 | 0.375 | 512 | 128 | 64 | 32 | 0.313 | 0.25 | 0.313 |
| F-98 | 256 | 32 | 32 | 8 | 0.188 | 0.25 | 0.281 | 64 | 8 | 2 | 2 | 0.188 | 0.156 | 0.281 |
| F-100 | 32 | 8 | 8 | 2 | 0.313 | 0.375 | 0.313 | 512 | 128 | 128 | 8 | 0.313 | 0.375 | 0.266 |
| H-5 | 64 | 16 | 16 | 8 | 0.313 | 0.375 | 0.375 | 256 | 32 | 16 | 4 | 0.188 | 0.188 | 0.266 |
| H-9 | 64 | 32 | 16 | 8 | 0.563 | 0.375 | 0.375 | 256 | 32 | 16 | 4 | 0.188 | 0.188 | 0.266 |
| H-13 | 64 | 16 | 16 | 8 | 0.313 | 0.375 | 0.375 | 256 | 32 | 16 | 4 | 0.188 | 0.188 | 0.266 |
| H-15 | 64 | 16 | 16 | 8 | 0.313 | 0.375 | 0.375 | 256 | 64 | 32 | 4 | 0.313 | 0.25 | 0.266 |
| H-20 | 128 | 32 | 32 | 16 | 0.313 | 0.375 | 0.375 | 512 | 128 | 64 | 8 | 0.313 | 0.25 | 0.266 |
| H-29 | 64 | 32 | 32 | 8 | 0.563 | 0.625 | 0.375 | 256 | 64 | 32 | 4 | 0.313 | 0.25 | 0.266 |
| H-49 | 64 | 32 | 16 | 8 | 0.563 | 0.375 | 0.375 | 512 | 256 | 128 | 32 | 0.563 | 0.375 | 0.313 |
| 25923 | 0.125 | 0.0625 | 0.0625 | 0.0156 | 0.563 | 0.625 | 0.375 | 2 | 0.5 | 0.25 | 0.25 | 0.313 | 0.25 | 0.375 |
| 209p | 0.25 | 0.125 | 0.0625 | 0.0156 | 0.563 | 0.375 | 0.313 | 2 | 1 | 1 | 0.25 | 0.563 | 0.625 | 0.375 |
A and a, without EGCg; B to D and b to d, EGCg at 6.25, 12.5, and 25 μg/ml, respectively.
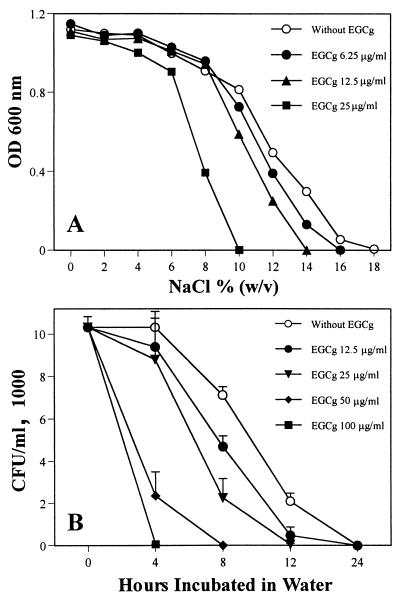
FIG. 3.
Reduction of tolerance of MRSA and MSSA to high ionic strength and low osmotic pressure in the presence of EGCg. (A) MRSA F-74 cells (3 × 106) were cultured in 3 ml of MHB containing different concentrations of EGCg and NaCl at 37°C for 24 h. Growth of bacteria was determined by OD at 600 nm. (B) F-74 cells (104/ml) were incubated in water with various concentrations of EGCg at 37°C for 0, 4, 8, 12, and 24 h. Viable cell numbers were then determined by culturing the cells on MH agar plates for additional 48 h.
Reduction of tolerance of MRSA and MSSA to high ionic strength and low osmotic pressure in the presence of EGCg.
Two strains of MRSA and two strains of MSSA were cultured in MHB containing different concentrations of NaCl. EGCg at 6.25, 12.5, and 25 μg/ml largely reduced the tolerance of both MRSA and MSSA to high concentrations of NaCl in their external atmosphere. Figure 3A shows that the highest ionic strength which F-74 was able to tolerate decreased from 18% to 10% NaCl in the presence of EGCg at 25 μg/ml. Furthermore, the bacteria were incubated in water containing various concentrations of EGCg. Despite of the nongrowing condition, EGCg greatly reduced the tolerance of the bacteria to low osmotic pressure. Figure 3B shows that F-74 cells could not survive longer than 4 h in water in the presence of 100 μg of EGCg per ml. The above results are possibly a result of direct damage of cell wall by EGCg.
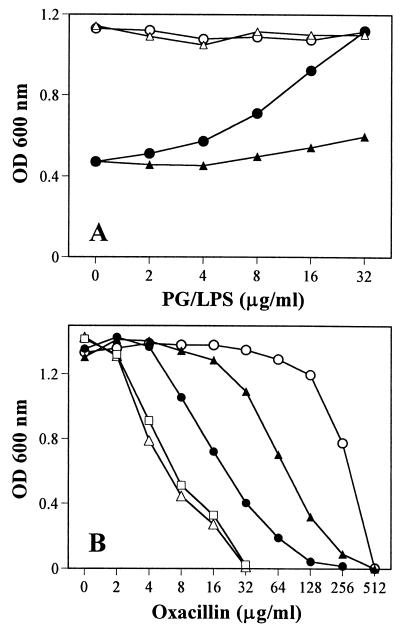
Direct binding of EGCg with peptidoglycan.
EGCg may directly bind to the cell wall and interfere with its integrity. This was confirmed by adding peptidoglycan of S. aureus into MHB containing EGCg alone or MHB containing both EGCg and oxacillin. As controls, we added the LPS and dextran to the cultures. Peptidoglycan at 32 μg/ml completely blocked the antibacterial activity of EGCg at 50 μg/ml (Fig. 4A). LPS at 32 μg/ml did not show the blocking effect but showed some effect at concentrations of more than 100 μg/ml. Dextran at concentrations of more than 1,024 μg/ml did not show any blocking effect. When peptidoglycan at 32 or 16 μg/ml was added to the cultures with EGCg (25 μg/ml) and various concentrations of oxacillin, peptidoglycan apparently blocked the synergistic effect between EGCg and oxacillin, but the same amount of LPS did not. The MIC of oxacillin against MRSA F-74 dropped from 512 to 32 μg/ml in combination with 25 μg of EGCg per ml but was reversed to 256 and 512 μg/ml by peptidoglycan at 16 and 32 μg/ml, respectively (Fig. 4B). Peptidoglycan or LPS alone showed no effect on the activity of oxacillin (data not shown). These results suggest the direct binding of EGCg with peptidoglycan.
FIG. 4.
Direct binding of EGCg with peptidoglycan (PG) of the cell wall. PG from S. aureus was added to MHB containing EGCg only (A) or EGCg plus oxacillin (B). The same amount of LPS was used as a control. MRSA F-74 cells were inoculated and cultured at 37°C for 24 h. (A) ○, PG alone; ●, EGCg (50 μg/ml) plus PG; ▵, LPS alone; ▴, EGCg (50 μg/ml) plus LPS. (B) ○, oxacillin alone; ▵, plus EGCg (25 μg/ml); ●, plus EGCg (25 μg/ml) and PG (16 μg/ml); ▴, plus EGCg (25 μg/ml) and PG (32 μg/ml); □, plus EGCg (25 μg/ml) and LPS (32 μg/ml).
Synergistic, additive, and indifferent effects between EGCg and other antibiotics.
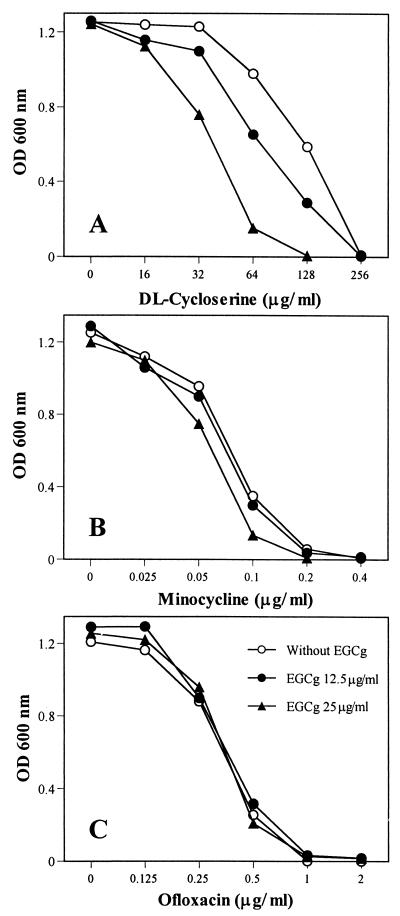
It seems that EGCg and β-lactams synergistically inhibit the growth of MRSA mainly because they directly or indirectly attack the same site, the cell wall. If this hypothesis is correct, EGCg should also show a synergistic effect when used together with non-β-lactam inhibitors of cell wall biosynthesis. We used dl-cycloserine to confirm this hypothesis. EGCg did synergize the activity of dl-cycloserine (Fig. 5A). Furthermore, when minocycline (an inhibitor of protein synthesis) and ofloxacin (an inhibitor of nuclear acid synthesis) were used, an additive or indifferent effect was observed between EGCg and minocycline or ofloxacin (Fig. 5B and C). These results were also confirmed with other 12 antibiotics acting as inhibitors of protein and nuclear acid synthesis against eight strains of MRSA (data not shown).
FIG. 5.
Effects of EGCg in combination with dl-cycloserine (A), minocycline (B), or ofloxacin (C) on MRSA F-74 growth. Symbols in panel C apply to all panels.
No inhibition of mecA gene expression and PBP2′ synthesis by EGCg.
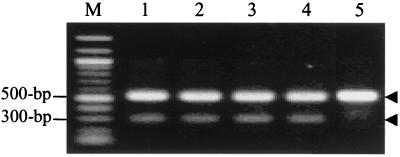
To clarify if PBP2′ is involved in the synergism, we performed RT-PCR using MRSA treated with EGCg. Although EGCg at 12.5 and even 6.25 μg/ml was able to reverse the resistance of F-74 to oxacillin, EGCg at 25 μg/ml did not suppress PBP2′ mRNA expression (Fig. 6). On the other hand, oxacillin at 4 μg/ml showed some induction of PBP2′ mRNA expression (Fig. 6, lanes 3 and 4). Furthermore, untreated bacteria and bacteria treated with 25 μg of EGCg per ml showed no differences either in the intensity of agglutination or in the times of agglutination appearance when PBP2′ was detected semiquantitatively by a latex agglutination assay.
FIG. 6.
RT-PCR analysis of PBP2′ mRNA expression. Lane M, 100-bp DNA ladder (molecular weight marker); lane 1, MRSA F-74 not treated with EGCg; lane 2, F-74 treated with EGCg (25 μg/ml); lane 3, F-74 treated with oxacillin (4 μg/ml); lane 4, F-74 treated with EGCg (25 μg/ml) and oxacillin (4 μg/ml); lane 5, MSSA FDA 209p as a control. Arrowheads: expected size (528 bp) of S. aureus-specific 16S rRNA (top) and expected size (313 bp) of PBP2′ gene (bottom).
DISCUSSION
About 10 to 15% of the dry weight of green tea is made up of catechins, including (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, and EGCg. EGCg, the main constituent (60%) in tea catechins, has the strongest bactericidal activity. Interestingly, low concentrations of EGCg showed synergistic activity with β-lactams against MRSA (Table 1). This effect of EGCg, however, has no direct relation with PBP2′ synthesis or production because it is not specific to MRSA. First, EGCg dose-dependently inhibited the growth of both MRSA and MSSA (Fig. 1) and also largely reduced their tolerance to high ionic strength and low osmotic pressure in their external atmosphere (Fig. 3). Second, EGCg not only reversed the high-level resistance of MRSA to oxacillin (Fig. 2A) but also induced a supersusceptibility of MSSA to oxacillin (Fig. 2B), although MSSA does not express the mecA gene. Third, EGCg in combination with dl-cycloserine also showed a synergistic effect against MRSA. dl-Cycloserine, a structural analogue of d-alanine, competitively inhibits both alanine racemase and d-alanyl-d-alanine synthetase, resulting in the accumulation of mucopeptide precursors lacking the terminal d-alanyl-d-alanine residue and thus inhibiting bacterial growth (24). This inhibition of peptidoglycan synthesis by dl-cycloserine has nothing to do with PBP2′ expression. Fourth, although 12.5 and even 6.25 μg of EGCg per ml was sufficient to reverse the resistance of F-74 to oxacillin (Fig. 2A), 25 to 50 μg of EGCg per ml did not suppress either PBP2′ mRNA expression or PBP2′ production, as confirmed by RT-PCR (Fig. 6) and PBP2′ latex agglutination assay.
The internal osmotic pressure of most bacteria ranges from 5 to 20 atm as a result of solute concentration via active transport. Cells are protected from lysis in low osmotic atmosphere owing to the high-tensile-strength cell wall. Any damage of the cell wall will certainly decrease the tolerance of the cells to high ionic strength and low osmotic pressure.
The cell wall in gram-positive bacteria is composed of 30 to 50 sheets of peptidoglycan external to the cell membrane and plays an essential role not only in osmotic protection but also in cell division, as well as serving as a primer for its biosynthesis (13). In gram-negative bacteria, however, the peptidoglycan layer is thin (one or two sheets) and is overlaid by an outer membrane composed mainly of LPS. The structural differences between gram-positive and gram-negative bacteria and the low affinity between EGCg and LPS may be the main factor for the different susceptibilities to EGCg and to EGCg–β-lactam combination.
It becomes clear that direct effects of EGCg on the bacterial cell wall are responsible for the synergistic anti-MRSA activity. Although staphylococci are relatively resistant to both high concentrations of sodium chloride and low osmotic pressure in their external atmosphere, EGCg greatly reduced the tolerance, as shown in Fig. 3. These results indicate damage of the cell wall by EGCg. The blocking of the antibacterial activity of EGCg and the synergistic effect between EGCg and oxacillin by peptidoglycan from S. aureus demonstrated the direct binding of EGCg with peptidoglycan (Fig. 4). EGCg may synergize the activity of β-lactams mainly because both EGCg and β-lactams directly or indirectly attack the same site, peptidoglycan on the cell wall. The EGCg-induced damage of the bacterial cell wall and the possible interference with its biosynthesis through direct binding with peptidoglycan may be the major reasons for the synergism against MRSA. The synergistic effect in the EGCg-cycloserine combination (Fig. 5A) and the additive or indifferent effects in EGCg-minocycline (Fig. 5B) and EGCg-ofloxacin (Fig. 5C) combinations all strongly support this explanation.
From a clinical standpoint, our data indicate a possible use of EGCg together with β-lactams to treat MRSA-infected patients, especially those with topical or digestive tract infections. Usually, the EGCg concentration in tea beverage is 2 to 3 mg/ml. Compared to that, 6.25, 12.5 or 25 μg/ml is a low concentration. The safe consumption of tea for thousands of years suggests a low toxicity of tea and EGCg. However, it is hard to predict synergistic effects in vivo just on the basis of the presented in vitro evidence, because it is difficult to estimate the in vivo concentration of EGCg, especially the concentration of free (active) EGCg, after drinking tea or taking EGCg capsules. EGCg is absorbed through the digestive tract and distributed to many organs of animals and humans (2, 11, 14, 15, 23). EGCg (5.6 μg/ml) was detected in blood plasma of rats given EGCg at 500 mg/kg of body weight (14). EGCg (2 μg/ml) was detected in human blood plasma 3 h after the subjects took a 525-mg EGCg capsule (15). Therefore, further in vivo experiments are needed to confirm the possibility that β-lactams in combination with EGCg may be effective against MRSA infections.
ACKNOWLEDGMENT
This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Chambers H F. Penicillin-binding protein-mediated resistance in pneumococci and staphylococci. J Infect Dis. 1999;179(Suppl. 2):S353–S359. doi: 10.1086/513854. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Lee M J, Li H, Yang C S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 3.de Lencastre H, de Jonge B L, Matthews P R, Tomasz A. Molecular aspects of methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1994;33:7–24. doi: 10.1093/jac/33.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Ghuysen J M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton-Miller J M T. Antimicrobial properties of tea (Camellia sinensis L.) Antimicrob Agents Chemother. 1995;39:2375–2377. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton-Miller J M T. Microbiological properties of tea infusions. In: Clifford M N, Clifford J, Schubert R, Spiro M, editors. Chemical and biological properties of tea infusions. Frankfurt am Main, Germany: Deutscher Medizinischer Imformationsdienst; 1997. pp. 63–75. [Google Scholar]
- 7.Hartman B J, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold B C, Immergluck L C, Maranan M C, Lauderdal D S, Gaskin R E, Boyle-Vavra S, Leitch C D, Daum R S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 9.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 10.Kohner P, Uhl J, Kolbert C, Persing D, Cockerill F. Comparison of susceptibility testing methods with mecA gene analysis for determining oxacillin (methicillin) resistance in clinical isolates of Staphylococcus aureus and coagulase-negative Staphylococcus spp. J Clin Microbiol. 1999;37:2952–2961. doi: 10.1128/jcm.37.9.2952-2961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M J, Wang Z Y, Li H, Chen L, Sun Y, Gobbo S, Balentine D A, Yang C S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–399. [PubMed] [Google Scholar]
- 12.Matsuzaki T, Hara Y. Antioxidative activity of tea leaf catechins. Nippon Nogeikagaku Kaisi. 1985;59:129–134. . (In Japanese.) [Google Scholar]
- 13.Morse S A. Cell structure. In: Jawetz E, Melnick J L, Adelberg E A, Brooks G F, Butel J S, Ornston L N, editors. Medical microbiology. 20th ed. Norwalk, Conn: Appleton & Lange; 1995. pp. 7–34. [Google Scholar]
- 14.Nakagawa K, Miyazawa T. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate in rats. J Nutr Sci Vitaminol (Tokyo) 1997;43:679–684. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Okuda S, Miyazawa T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci Biotechnol Biochem. 1997;61:1981–1985. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- 16.Nakatomi Y, Sugiyama J. A rapid latex agglutination assay for the detection of penicillin-binding protein 2′. Microbiol Immunol. 1998;42:739–743. doi: 10.1111/j.1348-0421.1998.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir Res. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- 18.Norden C W, Wentzel H, Keleti E. Comparison of techniques for measurement of in vitro antibiotic synergism. J Infect Dis. 1979;140:629–633. doi: 10.1093/infdis/140.4.629. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds P E, Brown D F J. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. FEBS Lett. 1985;192:28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi O, Cai Z, Toda M, Hara Y, Shimamura T. Appearance of antibacterial activity of oxacillin against methicillin resistant Staphylococcus aureus (MRSA) in the presence of catechin. J Jpn Assoc Infect Dis. 1995;69:1126–1134. doi: 10.11150/kansenshogakuzasshi1970.69.1126. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 21.Toda M, Okubo S, Hiyoshi R, Shimamura T. The bactericidal activity of tea and coffee. Lett Appl Microbiol. 1989;8:123–125. [Google Scholar]
- 22.Toda M, Okubo S, Hara Y, Shimamura T. Antibacterial and bactericidal activities of tea extract and catechins against methicillin resistant Staphylococcus aureus. Jpn J Bacteriol. 1991;46:839–845. doi: 10.3412/jsb.46.839. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 23.Unno T, Kondo K, Itakura H, Takeo T. Analysis of (−)-epigallocatechin gallate in human serum obtained after ingesting green tea. Biosci Biotechnol Biochem. 1996;60:2066–2068. doi: 10.1271/bbb.60.2066. [DOI] [PubMed] [Google Scholar]
- 24.Willett H P. Antimicrobial agents. In: Joklik W K, Willett H P, Amos D B, Wilfert C M, editors. Zinsser microbiology. 20th ed. Norwalk, Conn: Appleton & Lange; 1992. pp. 153–187. [Google Scholar]
- 25.Yam T S, Hamilton-Miller J M T, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2′ synthesis, and β-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 1998;42:211–216. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki K. Enhancing effect of Japanese green tea extract on the growth-inhibitory activity of antibiotics against isolated MRSA strains. Jpn J Chemother. 1996;44:477–482. . (In Japanese.) [Google Scholar]