INTRODUCTION
Coronavirus disease 19 (COVID-19), caused by the severe acute respiratory syndrome-virus 2 (SARS-CoV-2), is a multisystem disease, but the lungs are the primary target of infection and injury. Pulmonary parenchymal involvement represents the most common cause of hospitalization for COVID-19 and may be complicated by acute respiratory distress syndrome (ARDS), refractory respiratory failure, and death.[1] Since the onset of the pandemic, concerns were raised regarding the possible chronic pulmonary consequences of COVID-19.[2,3] There is now a growing body of evidence to suggest that a substantial proportion of COVID-19 survivors, especially those who had moderate-to-severe disease, may have persistent physiologic impairments of the lung with accompanying radiologic findings even several months after recovery.[4,5,6,7,8]
Pulmonary fibrosis is a recognized long-term sequel of several coronavirus lung infections, including SARS-CoV-1 or SARS and Middle East respiratory syndrome coronavirus. Virus-induced alveolar epithelial cell lung injury, abnormal immune response, and attempts at healing are central to the process of viral-induced lung fibrogenesis. SARS survivors who were followed over 1 year after the infection showed abnormal radiological features in 28% and reduced DLCO in 24%, and the extent of radiographic abnormalities correlated with the severity of functional improvement.[9] Another 2-year follow-up study of 55 patients who survived SARS ARDS showed a reduction in DLCO in 53% of patients.[10] Approximately one-third of the survivors of SARS have been shown to have significant pulmonary fibrosis.[11] As there are similarities between SARS-CoV-1 and SARS-CoV-2, it seems likely that lung fibrosis may be a common long-term consequence of COVID-19 pneumonia.[12]
Pulmonary fibrosis is a pathological consequence of interstitial pulmonary disease characterized by persistence of fibroblasts and excessive deposition of collagen and extracellular matrix as well as the destruction of normal pulmonary architecture.[13] The progression to pulmonary fibrosis leads to a loss of pulmonary function and damage to the alveolar-capillary unit, thereby compromising oxygen delivery.[14] The SARS-CoV-2 virus may induce lung fibrosis by at least four proposed mechanisms, namely: (1) direct stimulation of transforming growth factor-beta (TGF-b), a profibrotic cytokine;[15,16] (2) COVID-19 ARDS causing lung fibrosis; (3) mechanical stretch of alveolar epithelial cells during mechanical ventilation causing a fibrotic response;[17,18] and (4) presence of excess oxygen-free radicals due to prolonged use of high oxygen causing oxidative stress-induced fibrogenic response.[19]
Definitive radiologic signs of lung fibrosis include architectural distortion, traction bronchiectasis, and honeycombing. Signs such as bands, reticulation, and perilobular opacities may represent either inflammatory or fibrotic changes. These changes may be encountered in the acute phase of COVID-19[20] and during follow-up.[4] The true burden of fibrotic involvement of the lungs following COVID-19 pneumonia is slowly emerging, but the numbers are expected to be much greater than the existing burden of idiopathic pulmonary fibrosis (IPF) and other fibrotic interstitial lung diseases (ILDs).
Should patients with post-COVID-19 fibrotic lung disease be treated with antifibrotic drugs? If so when should the treatment be initiated, which drugs are preferred and how long should the treatment be given? The scientific evidence addressing these important questions is still evolving. In the face of limited evidence available in the field and to help physicians in practice make appropriate decisions, we aimed to conduct a review of literature as well as collate the knowledge and experience of some of the eminent pulmonologists from different parts of India and make recommendations based on available scientific evidence and experience of the expert working group.
METHODS
We conducted a systematic search of several databases (PubMed, Google Scholar, and Google) using the terms: post COVID-19 lung sequelae, post COVID-19 fibrosis, COVID-19 fibrotic lung disease, post COVID-19 interstitial lung disease, COVID-19, and lung. These included original articles, review articles, editorials, letters to editor, case reports, commentaries, and viewpoints. The pooled knowledge base from this literature was then used to make a PowerPoint presentation to initiate a discussion and brainstorming by the members of the Expert Working Group (EWG). The EWG comprised 10 members who were key opinion leaders, academicians, and senior consulting pulmonologists from across India. In the first online zoom meeting, we set the agenda and goals of the meeting, made the presentation on literature search, and debated and discussed key points that were of practical relevance to the practicing physician. We then generated a list of 9 questions which had options for answers as strongly agree, mostly agree, agree, mostly disagree, completely disagree, and not answered. Individual responses of EWG members for each question were pooled together, and a draft manuscript was prepared with the review of the literature and responses to the 9 questions along with a summary and recommendation. This was shared with all members for their agreement. Each member actively contributed to fine-tuning the manuscript, and the final approved manuscript is presented herewith.
Question 1a: Is the term “Post COVID-19 Pulmonary Fibrosis” appropriate?
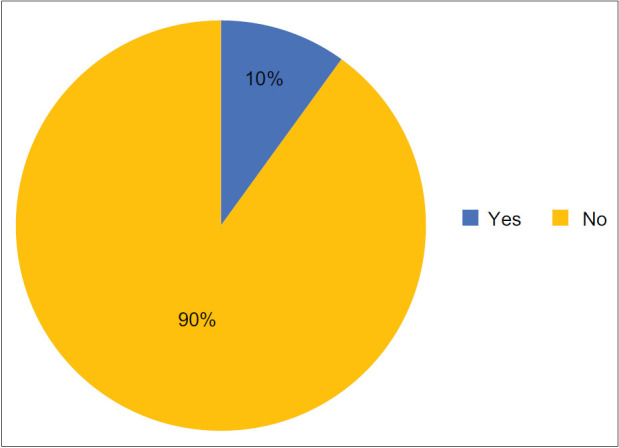
Question 1b: If not post-COVID-19 pulmonary fibrosis, what should be a better terminology?
Ninety percent of the expert working group members agreed that “Post-COVID Pulmonary Fibrosis” is not an appropriate term and should not be used.
Post-COVID-19 ILD and post-COVID pulmonary sequelae were the two names that were suggested to be the most appropriate by the EWG. The name “post-COVID-19 ILD” has also been suggested by several authors,[21,22,23,24,25] while a few have suggested post-COVID-19 pulmonary sequelae.[26] Post-COVID DPLD seems to be more technically correct because it rightly implies the involvement of parenchyma as well as other lung structures, while ILD incorrectly indicates only interstitial involvement. However, majority of the EWG members selected this because this terminology is widely used and scored higher than the term DPLD.
The term “pulmonary fibrosis” implies “irreversible” interstitial lung damage which cannot be reversed, as is classically seen in IPF. Radiological features on high-resolution computed tomography (HRCT) such as persistent reticular abnormalities, distortion of architecture, traction bronchiectasis, and honeycombing are consistent with established radiographic patterns of pulmonary fibrosis. However, it is important to emphasize that the presence of true fibrosis cannot be established solely from “fibrosis-like” imaging. The natural history of post-COVID fibrotic lung disease seems to be different from what happens in IPF, with a significant number of patients showing spontaneous complete resolution of the radiological fibrosis over a period of time.[27] Evidence from literature as well as experience of the EWG suggests that a significant number of patients with post-COVID-19 ILD resolve. There are, however, some who do not resolve, and a smaller proportion even show further progression of the lung fibrosis with time.[28] Based on collective experience, this number, however, seems to be significantly smaller compared with those who show near-complete radiological resolution.
Recommendation
The term post-COVID-19 pulmonary fibrosis should not be used and should be replaced with either post-COVID-19 ILD or post-COVID-19 pulmonary sequelae. Post-COVID-19 ILD implies that the fibrosis is not necessarily progressive and may even resolve with time.
Question 2: Based on your experience, what proportion of post-COVID-19 pneumonia patients develop post COVID-19 ILD?
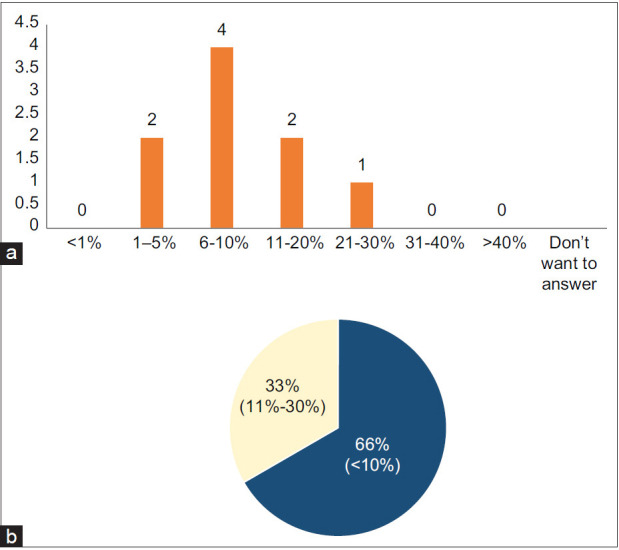
Two-thirds of the expert group members agreed that post-COVID-19 ILD develops in <10% of the SARS-CoV-2-infected patients, while one-third reported this to be between 11% and 30%. The reported proportions of post-COVID-19 ILD will likely depend on the type of COVID-19 patients that physicians treat. Physicians who predominantly treat mild-to-moderate cases will likely see less post-COVID-19 ILD, while those working in acute and postacute care settings will likely see larger numbers of cases.
It has been estimated that the risk of developing pulmonary fibrosis in patients who have recovered from moderate to severe COVID-19 is between 2% and 6%.[29] This translates to an estimated prevalence of COVID-19-induced fibrosis to be much higher than the existing prevalence of IPF.[29] Udwadia et al. have suggested that post-COVID-19 ILD is likely to be a tsunami that will follow the COVID-19 earthquake.[12]
Evidence at hospital discharge shows that nearly 50% of the survivors of COVID-19 have impaired lung diffusion capacity for carbon monoxide (DLCO) and a quarter have reduced total lung capacity, which correlates with the severity of the disease.[30] Furthermore, 55%-80% of COVID-19 patients show impaired pulmonary function and radiologic abnormalities 3 months after intensive care unit (ICU) discharge and 21% show fibrotic patterns on chest computed tomography (CT).[4,31,32] Lung sequelae were reported in 56% of the patients who experienced moderate symptoms of COVID-19 and among 71% of patients with severe symptoms 3 months after they recovered.[33] Francone et al. reported fibrotic changes on HRCT in 41% during the early phase of the disease and 53.6% during the later phase.[34] Vasarmidi[35] and Rai[36] have predicted that the prevalence of post-COVID-19 ILD may likely exceed 30% among those with severe COVID-19 pneumonia.
In a study of 62 patients, Zhou et al. reported that fibrotic changes were seen in 34% of hospitalized patients, and this was more likely to occur between 8 and 14 days after the onset of symptoms.[37] Pan et al.[38] reported fibrotic changes in 17% during the acute illness. ILD has been reported in 4.8% of patients who survived their original COVID-19 infection, and 10.8% of the patients with persistent symptoms, which included patients with mild disease not requiring oxygen therapy.[21]
Age more than 50 years, increasing severity of COVID-19 pneumonia, increased length of ICU stay and use of mechanical ventilation, smoking, and chronic alcoholism have been identified as important risk factors associated with post-COVID-19 ILD.[4,39,40,41,42]
Summary/Recommendations
The prevalence of post-COVID-19 ILD is likely to be high, although we do not know the exact numbers. In mild SARS-CoV-2 infections, the burden is likely to be minimal, while in moderate-to-severe cases, the estimated burden is expected to be higher. Increasing age, increasing severity of COVID-19, use of and increased duration of mechanical ventilation, smoking, and alcoholism are predictors of post-COVID-19 ILD.
Question 3: Is post-COVID-19 Interstitial Lung Disease a completely reversible type of fibrosis?
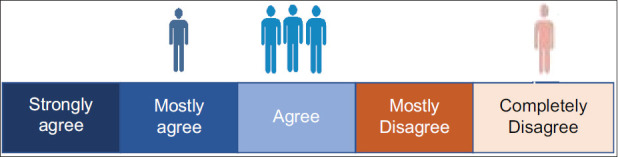
There was no consensus among the EWG members on the complete reversibility of lung fibrosis in post-COVID-19 patients although 40% of the EWG members agreed, and 60% disagreed.
Diffuse alveolar damage occurs in COVID-19-associated ARDS, which is characterized by an initial exudative phase with edema, hyaline membrane formation, and interstitial acute inflammation, followed by an organizing phase with loose organizing fibrosis mostly within the alveolar septa and type 2 pneumocyte hyperplasia.[35] The next stage is the potential fibrotic stage which can either resolve completely or progress to fibrosis.[43] The duration of the acute, critical disease seems to be a major driver of fibrotic lung disease (<1 week – 4%, between 1 and 3 weeks – 24%, and more than 3 weeks – 61%).[43]
Although reversibility of lung fibrosis in patients with COVID-19 has been observed and reported, complete reversibility does not occur in all patients. Those with mild-to-moderate disease show faster radiological resolution of interstitial lung changes observed in imaging. Cumulative absorption of fibrosis-like findings has been shown to be 24.3% in severe COVID-19 versus 52% in nonsevere COVID-19 cases.[44] Most fibrosis-like findings show a tendency to resolve rapidly and then remain stable. Parenchymal bands and bronchial dilatation were the most common fibrosis-like findings (31%–48%) in patients with severe/critical disease, and this remains relatively stable over a period.
Rapid onset of honeycombing fibrosis in a patient with COVID-19 identical to IPF is rare but has been reported.[2,45,46] Distinct from the idiopathic form of pulmonary fibrosis or other progressive ILDs, fibrosis-like changes on imaging resulting from ARDS, which is a recognized sequel of SARS-CoV-2 infection, are largely stable. However, while most patients with post-ARDS lung sequelae may recover completely, some may have lasting symptoms with or without decreased lung function.[47] Although once considered irreversible, there is now growing evidence to suggest that early lung fibrosis can be reversible if the underlying causes of injury are removed.[48]
A recent study that compared the extension of collagen deposition in COVID-19 postmortem lung samples (using cryobiopsies) and CT analysis of 10 patients with COVID-19 pneumonia who died during invasive mechanical ventilation showed that the sensitivity of CT for detecting histopathological fibrosis was 100% (66.4%–100%), but the specificity was only moderate 66.7% (41%–92.3%). Pseudofibrotic CT findings do not always correspond to increased collagen deposition.[49] Qualitative and quantitative CT analysis shows a weak correlation with collagen amount in postmortem samples. The authors observed that ground-glass opacities (GGOs), consolidation, and GGO with traction bronchiectasis had specificity ranging from 42.9% to 76% to detect samples with increased collagen deposition. This suggests that the presence of these findings on CT could be part of the evolution of the pathology, not necessarily reflecting proliferative fibrosis. The high sensitivity but moderate specificity of CT findings could explain in part the finding that lung function in severe COVID-19 survivors may return to normal or significantly improve over time.[32] Overall, these findings suggest that radiological signs of fibrosis on CT might not always be associated with increased collagen deposition, and it seems that these pseudo-fibrotic CT findings could be reversible and that the respiratory function might improve with time after recovery. It is therefore important to emphasize that presence of true fibrosis, which implies permanent changes, cannot be established solely from “fibrosis-like imaging.”
In a prospective study of adults who had been hospitalized for COVID-19, 56% had CT abnormalities after a median of 105 days post discharge. Unequivocal signs indicative of established fibrosis were present in 12, but after 1-year follow-up, 80% showed almost complete radiological resolution.[50] However, in a substantial number of patients, the lung damage may take a longer course, with some reporting to even die over the next few months from progressive pulmonary fibrosis.[51]
Summary/Recommendation
Post-COVID ILD might resolve completely in most patients, but in some, it may either remain static or progress. Most of those with mild-to-moderate disease will likely resolve completely, while those with more severe COVID-19 disease might have long-lasting post-COVID-19 ILD. In these patients, it would mostly remain stable, but in a minority, it might progress.
Question 4: Is presence of hypoxia and its increasing severity a strong predictor of future pulmonary fibrosis?

Seventy percent of the expert group members agreed that presence of hypoxia and its increasing severity increases the odds of future pulmonary fibrosis.
An increasing body of evidence indicates a distinct role for hypoxia in the dysfunction of the airway epithelium and in the responses of both innate immunity and of respiratory pathogens.[52] Interplay between hypoxia-induced inflammation and inflammation-induced hypoxic microenvironment usually aggravates morbidity in respiratory disease. Inflammation can increase the activation of hypoxia, and hypoxia-induced inflammation may be responsible for causing the systemic cytokine storm and eventual damage of the lungs.[53]
Summary
COVID-19 pneumonia with or without concomitant pulmonary vascular thromboinflammation is associated with hypoxemia in a large proportion of patients, which on its own or due to excess use of oxygen as a therapy might increase the severity and risk of developing future pulmonary fibrosis.
Question 5: For which of the following reasons would you use antifibrotic drugs in post-COVID-19 ILD?
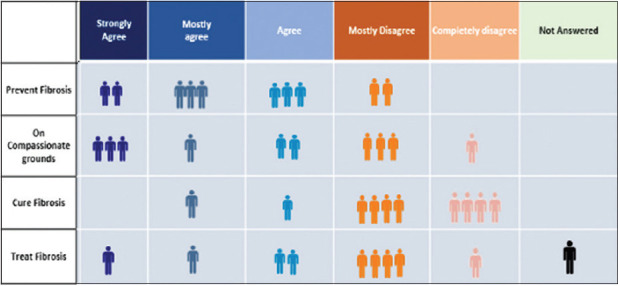
Eight out of ten expert group members agreed that they would use antifibrotic drugs for the prevention of post-COVID ILD, although two members disagreed. Sixty percent of the expert group members said that they would use antifibrotic drugs on compassionate grounds. Eighty percent of the expert group members disagreed on the use of antifibrotic drugs to “cure” post-COVID-19 ILD. There were roughly equal numbers of members who agreed as well as disagreed on using antifibrotic drugs for treating post-COVID-19 ILD.
Rather than treating fibrosis in the true sense, antifibrotics are largely used to prevent progressive fibrosis in those who have existing fibrosis. There is, however, potential for early intervention with antifibrotic agents to provide benefit for COVID-19 ILD and the possibility that these agents may prevent potential postinfection lung damage.[3,35,54] Putative benefits of antifibrotic drugs in reducing the prevalence of acute exacerbations of IPF were observed in patients already established on antifibrotic therapy.[55] George et al. argued that antifibrotic drugs may benefit patients with post-COVID-ILD with the assumptions that antifibrotic therapy has rapid onset of action, that treatment benefits observed in other forms of lung fibrosis will also be applicable to fibrosis triggered by severe viral infection, and that efficacy might depend on the combination with anti-inflammatory treatment such as systemic corticosteroids.[3] Other authors have opposed the idea of empirically using these agents.[56] Other treatments that have been proposed to be important for preventing post-COVID-19 lung fibrosis are early and aggressive use of antivirals to reduce viral load and injury, and timely use of systemic corticosteroids.[57] Because fibrosis with persistent physiological deficit has been described in patients recovering from similar coronaviruses, early treatment represents an opportunity to modify the disease course, thereby potentially preventing irreversible impairment.[21]
Summary/Recommendations
Antifibrotics might not have a role in most patients with post-COVID-19 ILD. In those with established radiological signs of fibrosis and a potential for progression, antifibrotics might be beneficial to prevent progressive fibrosis. However, currently there is no evidence for or against this strategy.
Question 6a: After seeing which of the following radiological features after a post-COVID pneumonia are you likely to start antifibrotic drugs?
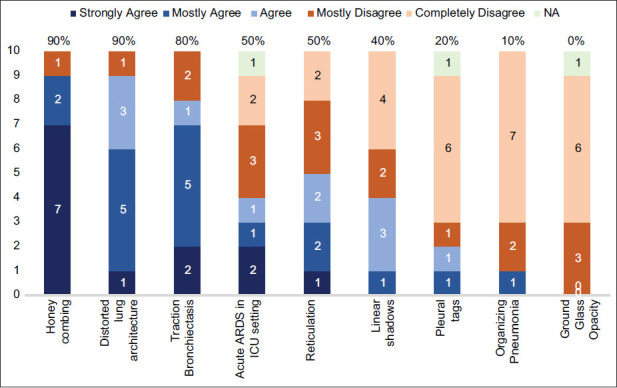
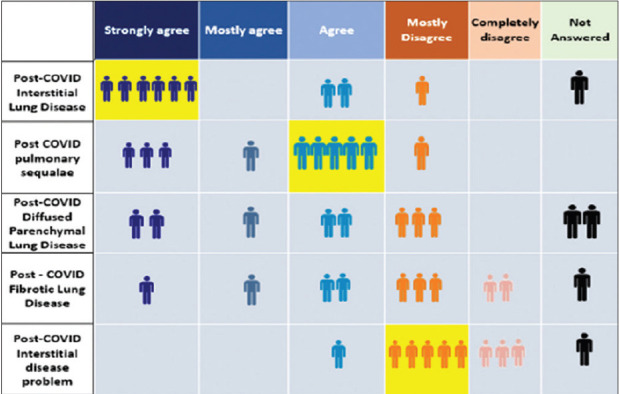
There was good consensus among the expert group members that presence of honeycombing, distorted lung architecture, and traction bronchiectasis on HRCT were reliable radiological indicators to start empiric antifibrotic drugs. Among these, the presence of honeycombing had the highest concurrence. There was consensus for the presence of ground-glass opacities, organizing pneumonia, and pleural tags as unreliable radiological markers for starting antifibrotic drugs. Most expert group members disagreed on using antifibrotic therapy for patients with acute ARDS in the ICU setting and for those with linear shadows. No consensus was reached for patients having presence of reticulation on HRCT. Although the Expert Working Group (EWG) advocated the use of antifibrotics to prevent post-COVID-19 ILD, presence of some radiological features suggestive of ILD were also indicators to start antifibrotic drugs.
Pulmonary fibrosis is often defined as architectural distortion with traction bronchiectasis or honeycombing or both.[58] The most common CT findings described in COVID-19 pneumonia are ground-glass opacities with or without consolidation, crazy paving pattern, interstitial thickening, irregular interface, and parenchymal bands with predominance of lower lobes and peripheral location.[59] It has been speculated that interstitial thickening, irregular interface, coarse reticular pattern, and parenchymal bands increase the risk of pulmonary fibrosis in patients who recovered from COVID-19.[60] Patients with <50% of radiological involvement and predominantly peripheral involvement have been shown to have a better disease outcome and lesser chronic involvement.[61]
Summary/Recommendation
Honeycombing, distorted lung architecture, and traction bronchiectasis are radiological features that may prompt considering the use of empiric antifibrotic therapy, while ground-glass opacities, organizing pneumonia, and pleural tags are not features for starting antifibrotic drugs for post-COVID-19 ILD.
Question 6b: If you agree to use antifibrotic drugs for post-COVID ILD, at what point of time are you likely to start them?
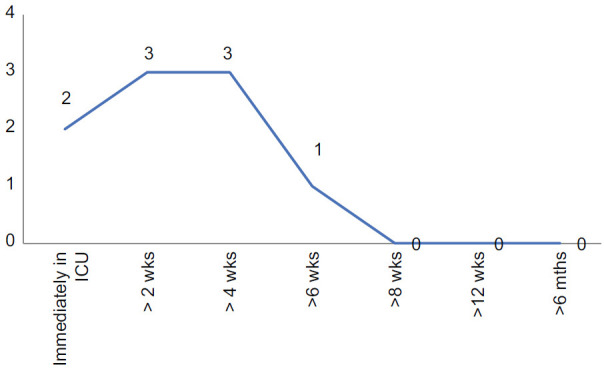
There was general consensus among the EWG that if antifibrotic drugs are used for post-COVID-19 ILD, they should be used sooner during the course of the illness. Although eight out of ten agreed that they would use these drugs within 4 weeks, there was no consensus for whether they should be started immediately in the ICU or at 2 weeks or 4 weeks. There is some incongruence with the previous question, as most experts have disagreed on starting empiric antifibrotics only based on prolonged ICU study.
In a study by Thille et al., 4% of patients had radiologic changes suggestive of lung fibrosis if ARDS disease duration was <1 week, 24% if ARDS duration was between 1 and 3 weeks, and 61% if ARDS disease duration was >3 weeks.[43] It has therefore been suggested that to be effective, any potential antifibrotic intervention should be considered within the 1st week of ARDS onset.[3] However, such a strategy would lead to significant overtreatment as a large proportion of survivors of ARDS might recover spontaneously with supportive treatment. Moreover, in the acute stage, adverse effects and drug interactions pose a significant hazard. This in the absence of a randomized controlled trial (RCT), such a practice should not be resorted to. A substantial proportion of patients who develop ARDS will experience residual long-term impairment of lung function and CT evidence of pulmonary fibrosis, with anterior reticulation being the dominant abnormality in 85% of survivors.[42] It has been proposed that some patients with post-COVID ILD with persistent symptoms and functional deficits might have persistent inflammatory ILD and might also benefit from low-dose glucocorticoids as initial therapy.
Summary/Recommendation
The correct timing of starting antifibrotic drugs in patients with post-COVID-19 ILD or those who are likely to develop post-COVID-19 ILD is still not known. The collective consensus of the expert group members favors starting antifibrotic drugs during the early course of the disease. This is at variance with the wait-and-see strategy practiced in the Western countries where antifibrotics are not approved for use in post-COVID-19 ILD. There is an urgent need for randomized, controlled trials to help find an answer to this.
Question 7: In which category of patients are you likely to consider starting antifibrotic drugs?
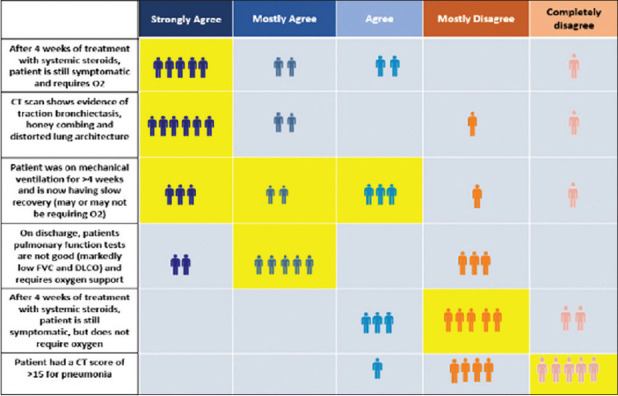
The clinical condition that had the strongest agreement among the EWG members to start antifibrotic drugs was patients who remained symptomatic and required oxygen after 4 weeks of treatment with systemic steroids. Presence of traction bronchiectasis, honecombing and distorted lung architecture on HRCT, and patients on mechanical ventilation for >4 weeks and having slow recovery had good concurrence for starting antifibrotic drugs. Majority of the Expert Working Group (EWG) members agreed that discharged patients having low FVC (Keep as is) and Diffusing lung co-efficient for carbon dioxide (DLCO) requiring oxygen support should be started on antifibrotic drugs, although one-third disagreed. Most patients having physiologic abnormalities at discharge are known to improve spontaneously over time. Seventy percent of the expert group members concurred that symptomatic patients not requiring oxygen after 4 weeks of treatment with systemic steroids and those with a lone CT score of >15 should not be started on antifibrotic drugs.
Summary/Recommendations
Presence of traction bronchiectasis, honecombing and distorted lung architecture on HRCT and patients who are symptomatic, requiring oxygen after 4 weeks of systemic steroids could be given antifibrotic therapy. Symptomatic patients not requiring oxygen and a lone high CT radiology score are not indications for starting antifibrotic therapy. Progressive decrease of lung function or worsening radiological signs of fibrosis during serial outpatient department (OPD) follow-up might also be candidates for antifibrotic drugs.
Question 8: Between Pirfenidone and Nintedanib, which antifibrotic drug are you more likely to use?
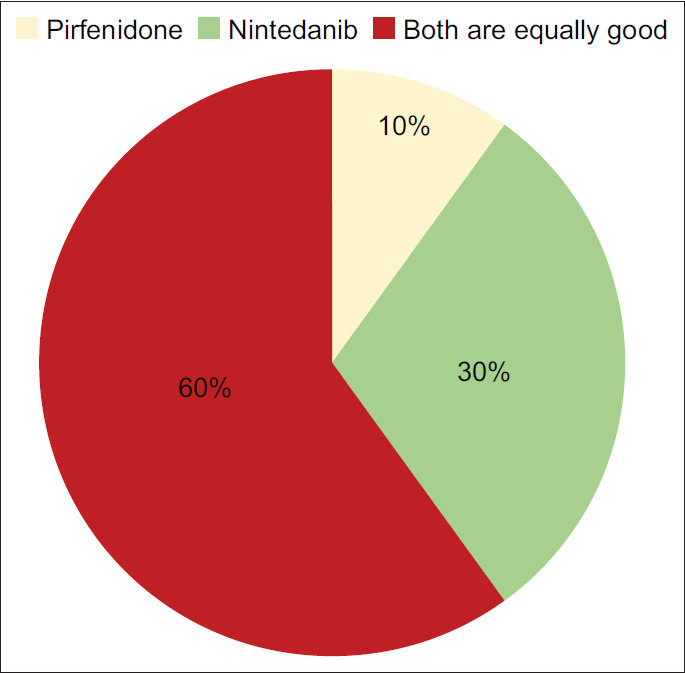
The majority of the expert group members felt that there was very little to choose between the two drugs, although one-third seemed to prefer nintedanib over pirfenidone.
There is a therapeutic rationale for the use of licensed antifibrotic drugs in acute exacerbations of IPF, including those triggered by viral infections. The available antifibrotic drugs have broad antifibrotic activity regardless of etiology, and these drugs might have a role in attenuating profibrotic pathways in SARS-CoV-2 infection.[3] Nintedanib and pirfenidone are two disease-modifying drugs that have shown promise in clinical trials in slowing down the rate of decline in pulmonary function and have been approved for the treatment of IPF, although neither of these drugs has demonstrated significant improvement in symptoms or long-term survival benefit.[62] Given the likelihood that post-ARDS fibrosis will be relatively stable with slow resolution over months to years after initial COVID-19 disease, it seems reasonable that these agents may expedite recovery in those with the most severe disease.[63]
In a nonrandomized, interventional, historical control arm study, 30 adult patients with COVID-19 who required mechanical ventilation when treated with nintedanib, showed reduction in the number of days for mechanical ventilation and a lower percentage of high attenuation areas on HRCT (38.7% vs. 25.7%; P = 0.02). However, the mortality rates did not change.[64]
Nintedanib binds to the intracellular ATP pockets of receptors stimulated by profibrotic mediators such as platelet-derived growth factor (PDGF), fibroblast growth factor, TGF-b, and vascular endothelial growth factor. This blocks profibrotic signaling and attenuates the proliferation, migration, and differentiation of fibroblasts as well as extracellular matrix component secretion.[65] Nintedanib has extremely low bioavailability at 4.7% when administered orally due to first-pass metabolism. Major side effects of nintedanib include hepatitis, diarrhea, nausea, vomiting, heart attack, stroke, bleeding problems, and gastric or intestinal perforation.
Pirfenidone is a relatively older drug which downregulates the effects of TGF-b, PDGF, connective tissue growth factor, and tumor necrosis factor-alpha. It also scavenges reactive oxygen species and downregulates angiotensin-converting enzyme-2 (ACE-II) receptor apart from having potent anti-inflammatory effects.[54]
Prospective, randomized clinical trials of these drugs in patients with post-COVID ILD are warranted. Several ongoing RCTs are investigating the role of glucocorticoids and antifibrotics agents, namely pirfenidone and nintedanib in acute COVID-19 as well as post-COVID-19 ILD (ClinicalTrials.gov identifies; NCT04657484, NCT04653831, NCT04282902, NCT04607928, NCT04856111, NCT14541680, NCT04338802, and NCT04619680). The results from these trials are eagerly awaited and might enlighten us on the benefit of glucocorticoids and antifibrotics (or the lack of it) in treating patients with post-COVID ILD. Choice of drug should be determined by factors such as cost, adverse events, and the risk of drug interactions. Pirfenidone is a lot cheaper than nintedanib, although the prices have come down significantly. Nintedanib should be given carefully in patients having an increased risk of bleeding and on anticoagulants. The adverse effects of both drugs are quite similar (pirfenidone is known to cause nausea, rashes, dyspepsia, skin photosensitivity, and elevated liver function values; nintedanib is known to cause diarrhea, nausea, elevated liver function values, and upper respiratory tract symptoms).
Summary/Recommendations
There is very little to choose between nintedanib and pirfenidone for the treatment of post-COVID-19 ILD, although both drugs are not yet licensed for this condition. A few clinical trials are currently investigating the efficacy of these drugs in post-COVID-19 ILD. We will have to wait until then to make any recommendations.
Question 9: What should be the duration of antifibrotic drugs for the management of post-COVID-19 ILD?
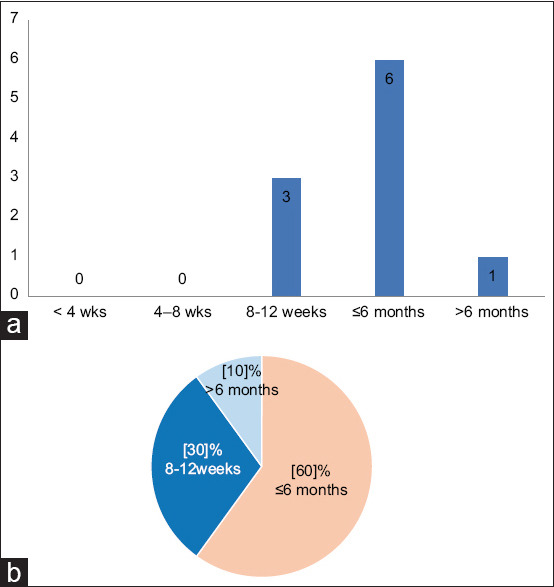
Sixty percent of the expert group members said they would use antifibrotic drugs for up to 6 months, and one-third said they would use it for between 8 and 12 weeks. There was no overall consensus on the duration of treatment with post-COVID-19 ILD.
Summary/Recommendation
In the absence of any scientific data regarding the duration of use of antifibrotic drugs in the treatment of post-COVID-19 ILD, no conclusive recommendation can be made. However, based on experience with other fibrotic lung diseases, they may be given for at least 2 months, although it may be extended for up to 6 months, but this needs to be decided on a case-to-case basis.
SUMMARY AND CONCLUSIONS
As the COVID-19 pandemic shows signs of waning after creating a global havoc for almost 2 years, it is imminent that we will now be faced with a large and growing number of patients with chronic post-COVID-19 lung sequelae. The risk factors for this condition include moderate-to-severe acute disease, age >50 years, intensive care unit stay with lengthy mechanical ventilation, chronic tobacco smoking, or chronic alcohol intake. Fibrotic lung disease has been shown to occur both during the acute stage of the disease as well as in the post-COVID 19 pneumonia stage. The interstitial lung changes that occur in post-COVID-19 patients fortunately resolve spontaneously and gradually in a large number of infected people, especially those who had the mild-to-moderate severity of the disease. However, a substantial number of patients will either have a static form of fibrotic lung disease or even a progressive type accompanied by rapid deterioration of lung function and even death.
The EWG recommended that the term post-COVID-19 pulmonary fibrosis is not appropriate because the term “fibrosis” implies a “permanent” nature, which is not the case in most patients with post-COVID-19 fibrotic lung disease. Post-COVID-19 ILD is a more appropriate terminology. Although post-COVID-19 ILD is known to resolve spontaneously in most cases, it does not happen in all patients and is therefore not a completely reversible form of the disease. The burden of post-COVID-19 ILD is estimated to be high and grow with time. The EWG recommended that antifibrotic drugs may be used to prevent pulmonary fibrosis or on compassionate grounds in a specific group of patients. Continued presence of respiratory symptoms and hypoxia, despite 2–4 weeks of treatment with systemic steroids, or presence of honeycombing, traction bronchiectasis, and distorted lung architecture were strong indications to consider the use of antifibrotic drugs. The presence of a high computed tomography score but mild symptoms and normal oxygen saturation are not indications for starting antifibrotic drugs. Although the expert group agreed that antifibrotic drugs should be used in the early course of the disease, the timing varied from immediately (ICU admission) to up to 4 weeks. There was no consensus on how long the antifibrotic drugs be given for, although most members agreed to using them for between 4 and 8 weeks, but less than 6 months, although the duration needs to be decided on a case-to-case basis. Both pirfenidone and nintedanib were equally good, although one-third of the expert group members believed nintedanib was better. Although the anti-inflammatory effects of antifibrotic drugs are known, this was not perceived to be an advantage to use them during the early course of the disease, as the evidence to support this was lacking.
As new knowledge will continue to grow in this field, the evidence base will grow stronger to help us make informed decisions on the use of antifibrotic drugs in the management of post-COVID ILD. We EWG members may be biased in their viewpoints based on personal experience over time, but until new scientific evidence emerges, the readers may find the recommendations made by the EWG useful to make appropriate decisions in their clinical practice.
Financial support and sponsorship
This project was supported by Glenmark Pharmaceuticals, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The meeting of the Expert Working Group members was supported by Glenmark Pharmaceuticals, India. However, they played no role whatsoever in the discussion that took place between the members and the content of this manuscript.
REFERENCES
- 1.Gentile F, Aimo A, Forfori F, Catapano G, Clemente A, Cademartiri F, et al. COVID-19 and risk of pulmonary fibrosis:The importance of planning ahead. Eur J Prev Cardiol. 2020;27:1442–6. doi: 10.1177/2047487320932695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD, et al. Pulmonary fibrosis secondary to COVID-19:A call to arms? Lancet Respir Med. 2020;8:750–2. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19:The potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–15. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–86. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation:A prospective study. Lancet Respir Med. 2021;9:747–54. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19:First results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–8. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–61. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–50. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome:A 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udwadia ZF, Koul PA, Richeldi L. Post-COVID lung fibrosis:The tsunami that will follow the earthquake. Lung India. 2021;38:S41–7. doi: 10.4103/lungindia.lungindia_818_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sime PJ, O’Reilly KM. Fibrosis of the lung and other tissues:New concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–19. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 14.Razzaque MS, Taguchi T. Pulmonary fibrosis:Cellular and molecular events. Pathol Int. 2003;53:133–45. doi: 10.1046/j.1440-1827.2003.01446.x. [DOI] [PubMed] [Google Scholar]
- 15.Zuo W, Zhao X, Chen YG. SARS coronavirus and lung fibrosis. In: Lal S, editor. Molecular Biology of the SARS-Coronavirus. Ch. 15. Berlin Heidelberg: Springer-Verlag; 2010. pp. 247–58. [Google Scholar]
- 16.Delpino MV, Quarleri J. SARS-CoV-2 pathogenesis:Imbalance in the renin-angiotensin system favors lung fibrosis. Front Cell Infect Microbiol. 2020;10:340. doi: 10.3389/fcimb.2020.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Pan X, Wang L, Yu G. Alveolar cells under mechanical stressed niche:Critical contributors to pulmonary fibrosis. Mol Med. 2020;26:95. doi: 10.1186/s10020-020-00223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouby JJ, Lherm T, Martin de Lassale E, Poète P, Bodin L, Finet JF, et al. Histologic aspects of pulmonary barotrauma in critically ill patients with acute respiratory failure. Intensive Care Med. 1993;19:383–9. doi: 10.1007/BF01724877. [DOI] [PubMed] [Google Scholar]
- 19.Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10:509–47. doi: 10.1002/cphy.c190017. [DOI] [PubMed] [Google Scholar]
- 20.Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, et al. Chest CT findings in cases from the cruise ship diamond princess with coronavirus disease (COVID-19) Radiol Cardiothorac Imaging. 2020;2:e200110. doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottin V, Lafitte C, Sénéchal A, Traclet J. Interstitial lung disease after COVID-19. Am J Respir Crit Care Med. 2021;203:1314–5. doi: 10.1164/rccm.202006-2466IM. [DOI] [PubMed] [Google Scholar]
- 23.Aronson KI, Podolanczuk AJ. Lungs after COVID-19:Evolving knowledge of post-COVID-19 interstitial lung disease. Ann Am Thorac Soc. 2021;18:773–4. doi: 10.1513/AnnalsATS.202102-223ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AK, Kumar OP, Bansal P, Margekar SL, Aggarwal R, Ghotekar LR, et al. Post-COVID interstitial lung disease –The looming epidemic. J Assoc Physicians India. 2021;69:11–2. [PubMed] [Google Scholar]
- 25.Wild JM, Porter JC, Molyneaux PL, George PM, Stewart I, Allen RJ, et al. Understanding the burden of interstitial lung disease post-COVID-19:The UK interstitial lung disease-long COVID study (UKILD-Long COVID) BMJ Open Respir Res. 2021;8:e001049. doi: 10.1136/bmjresp-2021-001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Türktaş H, Oğuzülgen İ K. Post-COVID-19 pulmonary sequelae:Long-term follow up and management. Tuberk Toraks. 2020;68:419–29. doi: 10.5578/tt.70353. [DOI] [PubMed] [Google Scholar]
- 27.Kong M, Yang H, Li X, Shen J, Xu X, Lv D. Evolution of chest CT manifestations of COVID-19:A longitudinal study. J Thorac Dis. 2020;12:4892–907. doi: 10.21037/jtd-20-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udwadia ZF, Pokhariyal PK, Tripathi AK, Kohli A. Fibrotic interstitial lung disease occurring as sequelae of COVID-19 pneumonia despite concomitant steroids. Lung India. 2021;38:S61–3. doi: 10.4103/lungindia.lungindia_533_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazdyrev E, Rusina P, Panova M, Novikov F, Grishagin I, Nebolsin V. Lung fibrosis after COVID-19:Treatment prospects. Pharmaceuticals. 2021;14:807. doi: 10.3390/ph14080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González J, Moncusí-Moix A, Benitez ID, Santisteve S, Monge A, Fontiveros MA, et al. Clinical consequences of COVID-19 lockdown in patients with COPD:Results of a pre-post study in Spain. Chest. 2021;160:135–8. doi: 10.1016/j.chest.2020.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truffaut L, Demey L, Bruyneel AV, Roman A, Alard S, De Vos N, et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22:29. doi: 10.1186/s12931-021-01625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Z, Tang N, Chen Y, Ma L, Wei Y, Lu Y, et al. CT features of COVID-19 patients with two consecutive negative RT-PCR tests after treatment. Sci Rep. 2020;10:11548. doi: 10.1038/s41598-020-68509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients:Correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–17. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasarmidi E, Tsitoura E, Spandidos DA, Tzanakis N, Antoniou KM. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review) Exp Ther Med. 2020;20:2557–60. doi: 10.3892/etm.2020.8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rai DK, Sharma P, Kumar R. Post COVID 19 pulmonary fibrosis. Is it real threat? Indian J Tuberc. 2021;68:330–3. doi: 10.1016/j.ijtb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–94. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 38.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV):A study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–9. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojo AS, Balogun SA, Williams OT, Ojo OS. Pulmonary fibrosis in COVID-19 survivors:Predictive factors and risk reduction strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miwa M, Nakajima M, Kaszynski RH, Hamada S, Ando H, Nakano T, et al. Abnormal pulmonary function and imaging studies in critical COVID-19 survivors at 100 days after the onset of symptoms. Respir Investig. 2021;59:614–21. doi: 10.1016/j.resinv.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58:2003677. doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome:CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 43.Thille AW, Esteban A, Fernández-Segoviano P, Rodriguez JM, Aramburu JA, Vargas-Errázuriz P, et al. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage:A prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395–401. doi: 10.1016/S2213-2600(13)70053-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Zhang C, Li X, Zhao J, An C, Peng C, et al. Thin-section computed tomography findings and longitudinal variations of the residual pulmonary sequelae after discharge in patients with COVID-19:A short-term follow-up study. Eur Radiol. 2021;31:7172–83. doi: 10.1007/s00330-021-07799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combet M, Pavot A, Savale L, Humbert M, Monnet X. Rapid onset honeycombing fibrosis in spontaneously breathing patient with COVID-19. Eur Respir J. 2020;56:2001808. doi: 10.1183/13993003.01808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letellier A, Gibelin A, Voiriot G, Fartoukh M, Djibré M. Destructive pulmonary fibrosis after severe COVID-19 pneumonia. Int J Infect Dis. 2020;100:377–8. doi: 10.1016/j.ijid.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald LT. Healing after COVID-19:Are survivors at risk for pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2021;320:L257–65. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest. 2018;128:97–107. doi: 10.1172/JCI93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ball L, Barisione E, Mastracci L, Campora M, Costa D, Robba C, et al. Extension of collagen deposition in COVID-19 post mortem lung samples and computed tomography analysis findings. Int J Mol Sci. 2021;22:7498. doi: 10.3390/ijms22147498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vijayakumar B, Tonkin J, Devaraj A, Philip KE, Orton CM, Desai SR, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 2021;211746 doi: 10.1148/radiol.2021211746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page LK, Staples KJ, Spalluto CM, Watson A, Wilkinson TM. Influence of hypoxia on the epithelial-pathogen interactions in the lung:Implications for respiratory disease. Front Immunol. 2021;12:653969. doi: 10.3389/fimmu.2021.653969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharya S, Agarwal S, Shrimali NM, Guchhait P. Interplay between hypoxia and inflammation contributes to the progression and severity of respiratory viral diseases. Mol Aspects Med. 2021;81:101000. doi: 10.1016/j.mam.2021.101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seifirad S. Pirfenidone:A novel hypothetical treatment for COVID-19. Med Hypotheses. 2020;144:110005. doi: 10.1016/j.mehy.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 56.Chaudhary S, Natt B, Bime C, Knox KS, Glassberg MK. Antifibrotics in COVID-19 lung disease:Let us stay focused. Front Med (Lausanne) 2020;7:539. doi: 10.3389/fmed.2020.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechowicz K, Drożdżal S, Machaj F, Rosik J, Szostak B, Zegan-Barańska M, et al. COVID-19:The potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. 2020;9:1917. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT:A position paper from the Fleischner Society. Lancet Respir Med. 2020;8:726–37. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kayhan S, Kocakoç E. Pulmonary fibrosis due to COVID-19 pneumonia. Korean J Radiol. 2020;21:1273–5. doi: 10.3348/kjr.2020.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21:746–55. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gulati A, Lakhani P. Interstitial lung abnormalities and pulmonary fibrosis in COVID-19 patients:A short-term follow-up case series. Clin Imaging. 2021;77:180–6. doi: 10.1016/j.clinimag.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajwah S, Higginson IJ, Ross JR, Wells AU, Birring SS, Riley J, et al. The palliative care needs for fibrotic interstitial lung disease:A qualitative study of patients, informal caregivers and health professionals. Palliat Med. 2013;27:869–76. doi: 10.1177/0269216313497226. [DOI] [PubMed] [Google Scholar]
- 63.Michalski JE, Kurche JS, Schwartz DA. From ARDS to pulmonary fibrosis:The next phase of the COVID-19 pandemic? Trans Res. doi: 10.1016/j.trsl.2021.09.001. 2021;S1931-5244(21)00243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umemura Y, Mitsuyama Y, Minami K, Nishida T, Watanabe A, Okada N, et al. Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19:An interventional study. Int J Infect Dis. 2021;108:454–60. doi: 10.1016/j.ijid.2021.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–45. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]


