Abstract
Background:
Indian data on treatment outcomes and survival in advanced non-small cell lung cancer (NSCLC) remain scarce.
Materials and Methods:
A retrospective review of 537 advanced NSCLC patients treated at a tertiary care facility in North India from January 2008 to March 2018 was done to assess treatment response and survival in terms of objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS).
Results:
Median age of enrolled patients was 60 years (range: 26–89 years). The majority were males (78.2%) and smokers (66.5%). Adenocarcinoma (51.2%) was the most common pathological type. Most patients had good performance status (PS) (the Eastern Cooperative Oncology Group [ECOG] 0 or 1 in 55.7%) and received conventional chemotherapy (86.6%). ORR and DCR after 3–4 months of first-line treatment were 55.2% and 71.75%, respectively (n = 223). Never smokers had better ORR as well as DCR compared to chronic smokers whereas treatment with tyrosine kinase inhibitors achieved significantly better ORR, and patients with good PS had better DCR compared to those with poor PS. Median PFS (n = 455) was 7.0 months (95% confidence interval [CI]: 3.7–14.0) and median OS was 11.7 months (95% CI: 5.5–29.9 months). Good PS and nonsmoking status were independent predictors of better PFS on multivariate analysis. For OS, good PS, nonsmoking behavior, and treatment with epidermal growth factor receptor inhibitors were independent predictors.
Conclusion:
In advanced NSCLC, never-smokers, and patients with good baseline ECOG have favorable treatment and survival outcomes. Treatment with targeted therapy results in better ORR and OS but did not affect PFS.
KEY WORDS: India, lung cancer, overall survival, progression-free survival, response rate
INTRODUCTION
Lung cancer is a leading cause of mortality in India and continues to pose challenges to survival and treatment outcomes despite several therapeutic advancements. Majority of lung cancer is diagnosed at an advanced stage and causes significant morbidity and mortality.[1,2] However, information regarding the impact of treatment on response and survival is scant from India with most studies focusing on long-term survival. The predictors of short-term response rates are not clearly elucidated.[3,4,5]
Hence, the present study analyzed various treatment and survival responses in advanced non-small cell lung cancer (NSCLC) patients and attempted to identify relevant prognostic factors.
MATERIALS AND METHODS
The study participants included patients with pathologically (biopsy or cytology) proven lung cancer between January 1, 2008, and March 31, 2018, at the Department of Pulmonary, Critical Care and Sleep Medicine, All India Institute of Medical Sciences, New Delhi, India. Prior approval for the study was obtained from the Institutional Ethics Committee.
The clinical and treatment details were recorded in a predesigned structured pro forma. With regard to smoking, subjects were classified as current smokers (patients who were actively smoking or quit smoking in the past 1 year at the time of treatment), reformed smokers (patients who quit smoking for more than a year), and never smokers (who had smoked <100 cigarettes in their lifetime). Smoking index was defined as the product of the number of cigarettes smoked and the number of years of smoking. In cases where the disease was diagnosed at another health center, the relevant slides/blocks were re-evaluated by our pathologist; and if any discrepancy or inconclusive results were found, re-sampling was done to achieve the final diagnosis.
Based on morphology and immunohistochemistry (IHC), lung tumors were divided as (1) non-small cell lung carcinoma (NSCLC, including squamous cell carcinoma [SCC], adenocarcinoma [ADC], and non-small cell cancer-not otherwise specified [NSCLC-NOS]) and (2) small cell lung cancer.
Tissue samples of patients (primarily ADC) were tested for epidermal growth factor receptor (EGFR) mutations from 2012 onward and anaplastic lymphoma kinase (ALK) rearrangements from 2014 onward. Tissue EGFR mutations were performed using Qiagen ARMS scorpion PCR assay and ALK rearrangements tested using fluorescence in situ hybridization (FISH) method or IHC. Lung cancer staging was done using either computed tomography (CT) scan of the chest and upper abdomen, positron emission tomogram scan, bone scan, and magnetic resonance imaging/contrast-enhanced CT brain. The American Joint Committee on Cancer 7th edition TNM staging system was used for staging of lung cancer patients.[6]
Patients were treated as per department treatment policy with a multidisciplinary approach. For chemotherapy, a doublet regimen consisting of a platinum-based drug was administered at 3 weekly intervals, and for targeted therapy, gefitinib and crizotinib were primarily used. Radiotherapy was given with palliative intent wherever indicated. Patients who were unfit for chemotherapy and whose EGFR mutation/ALK rearrangement status was negative or unknown were offered empirical targeted therapy on compassionate grounds after discussion with the patient and family.
Radiological assessment of response was done after 4 cycles of chemotherapy or after 3–4 months of targeted therapy, using the RECIST 1.1 criteria.[7] Response was categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease. Based on this, the objective response rate (ORR) and disease control rate (DCR) were calculated. Objective response rate included patients with CR or PR following first-line treatment and DCR included patients with CR, PR, or SD. Subsequently, treatment was modified if required and further response assessment was done every 3–4 monthly and earlier if clinically indicated.
Overall survival (OS) was calculated from the date of treatment to the date of death or date of last known follow-up (either clinic visit or telephonically), and progression-free survival (PFS) was calculated from the date of start of treatment to the date of disease progression, documented after response assessment. Various factors affecting OS, PFS, ORR, and DCR were evaluated.
In this study, only NSCLC patients were included who had TNM Stage 4 disease at the time of diagnosis and had at least 1 follow-up visit after the start of treatment. Patients were considered on continuous follow-up if the last visit occurred within 1 month of data censoring (July 31, 2018). In a case where the last visit was more than 1 month ago, attempts were made to contact the patient or relatives by telephone for their current status. Patients were censored at the date they were last known to be alive, i.e., date of the last follow-up either in person or telephonically.
Statistical analysis
Data were entered on a predesigned pro forma, recorded on an STATA 14.0, Texas USA, licensed to AIIMS, New Delhi, Department of Biostatistics. Quantitative variables were checked for approximate normality. Variables following normal distribution were expressed as mean +/- standard deviation (SD) and variables that followed skewed distribution were expressed as median (range or interquartile range). Categorical variables were expressed as frequency (%). Association between two categorical variables was compared by the Chi-square test. Comparison of quantitative variables was done by independent t-test and Mann–Whitney U-test, following normal and skewed distribution, respectively. Quantitative variables among more than two groups were compared by one-way ANOVA followed by Bonferroni multiple comparison test and Kruskal–Wallis H-test followed by Dunn test for multiple comparisons, following normal and skewed distribution, respectively. Time-to-event data were compared by log-rank test and median survival was estimated and represented as Kaplan–Meier survival curve. Univariate and multivariable Cox-regression method was used to find independently associated variables with OS and PFS. Those variables found statistically significant in univariate analysis and clinically relevant were included in stepwise multiple cox regression model with a probability of entry 0.05 and probability of removal 0.10. A P < 0.05 was considered statistically significant.
RESULTS
During the study period, 1862 patients were registered in our clinic, of which treatment details were known for 1013 patients. Small cell cancer and miscellaneous varieties of lung cancer were diagnosed in 188 patients, hence were excluded. Out of the remaining 825 patients, only 537 patients with metastatic disease were included for final analysis. Table 1 shows the demographic and baseline characters of the patients included in the study.
Table 1.
Demographic and baseline characteristics of advanced metastatic non-small cell lung cancer (n=537)
| Variable | Sub-group | n (%) |
|---|---|---|
| Age (years) | <60 | 266 (49.5) |
| >60 | 271 (50.5) | |
| Sex | Male | 420 (78.2) |
| Female | 117 (21.8) | |
| Education level (n=442) | Illiterate | 105 (23.8) |
| Primary level | 113 (25.5) | |
| Secondary level | 102 (23.0) | |
| Higher secondary | 48 (10.9) | |
| Graduation | 52 (11.8) | |
| Postgraduation | 22 (5.0) | |
| Smoking status (n=516) | Never smoker | 173 (33.5) |
| Current smokers | 189 (36.6) | |
| Reformed smokers | 154 (29.9) | |
| Diagnostic modality (n=518) | Flexible bronchoscopy | 232 (44.8) |
| CT-guided FNAC/biopsy (lung) | 121 (23.3) | |
| USG-guided FNAC/biopsy (lung) | 48 (9.3) | |
| Thoracentesis/pleural biopsy | 55 (10.6) | |
| Peripheral lymph node sampling | 27 (5.2) | |
| EBUS | 19 (3.7) | |
| Lung biopsy (surgical) | 1 (0.2) | |
| Others | 15 (2.9) | |
| Pathological type | SCC | 154 (28.7) |
| ADC | 275 (51.2) | |
| NSCLC (NOS) | 108 (20.1) | |
| ECOG (n=454) | 0 | 17 (3.7) |
| 1 | 236 (52.0) | |
| 2 | 148 (32.6) | |
| 3 | 40 (8.8) | |
| 4 | 13 (2.9) | |
| EGFR mutations (n=169) | Positive | 46 (27.2) |
| Negative | 123 (72.8) | |
| ALK mutations (n=116) | Positive | 17 (14.7) |
| Negative | 99 (85.3) | |
| Treatment (first line) | Conventional chemotherapy | 465 (86.6) |
| Type of conventional chemotherapy (n=386) | Paclitaxel plus carboplatin/cisplatin | 295 (76.4) |
| Gemcitabine plus carboplatin/cisplatin | 27 (7.0) | |
| Pemetrexed plus carboplatin/cisplatin | 49 (12.7) | |
| Etoposide plus carboplatin/cisplatin | 6 (1.6) | |
| Others | 9 (2.3) | |
| Type of EGFR inhibitors (including compassionate based TKI) (n=55) | Gefitinib | 44 (80) |
| Erlotinib | 11 (20) | |
| Type of ALK inhibitors (n=9) | Crizotinib | 9 (100) |
FNAC: Fine-needle aspiration cytology, EBUS: Endobronchial ultrasound, ECOG: Eastern Cooperative oncology group, EGFR: Epidermal growth factor receptor, ALK: Anaplastic lymphoma receptor tyrosine kinase, TKI: Tyrosine kinase inhibitors, CT: Computed tomography, USG: Ultrasonography, SCC: Squamous cell carcinoma, ADC: Adenocarcinoma, NSCLC: Non-small cell lung cancer, NOS: Not otherwise specified
The majority of patients were males (78.2%) with a mean (SD) age of 58 (11.2) years and median age of 60 years (range: 26–89 years). Smokers comprised 66.5% of all subjects, of whom 36.6% were current smokers and 29.9% were reformed smokers; the median smoking index was 500 (range: 2–2700). Flexible bronchoscopy was the most common diagnostic modality (44.8%), followed by CT or ultrasound-guided interventions (32.6%), thoracocentesis/pleural biopsy (10.6%), peripheral lymph node fine-needle aspiration/biopsy (5.2%), and endobronchial ultrasound (3.7%). More than half of patients (55.7%) had good performance status (PS), i.e., the Eastern Cooperative Oncology Group (ECOG) 0 or 1. Mutations in EGFR and rearrangements in the ALK gene were detected in 27.2% and 14.7% of ADC, respectively. Chemotherapy was the most common first-line treatment modality (86.6%), followed by targeted therapy. Sixty-three patients (11.6%) received EGFR inhibitors, of which 23 patients received compassionate-based tyrosine kinase inhibitors (TKI), while, nine patients (1.7%) received ALK inhibitors. These nine patients were out of the 17 patients who had ALK relocation. The reason for not receiving ALK inhibitors in the remainder is primarily financial constraints. Platinum-based doublet with paclitaxel was the most common chemotherapy regimen (76.4%), followed by pemetrexed (12.7%) and gemcitabine (7.0%). The most common EGFR inhibitor used was gefitinib (80%) followed by erlotinib (20%) as first-line management and crizotinib (100%) was the most commonly used ALK inhibitor.
Table 2 depicts various factors at baseline and after 3–4 months of treatment that affected overall response rate (ORR) and DCR. Among patients in whom data was available (n = 223), ORR and DCR were 55.2% and 71.7%, respectively. Factors that affected ORR included smoking status (67.1% for never smoker vs. 47.2% for smokers, p = 0.005) and type of treatment (50.8% for chemotherapy, 76.7% for EGFR inhibitors, and 75.0% for ALK inhibitors). Factors affecting DCR were baseline PS (75.0% for ECOG 0/1 and 61.2% for ECOG 2/3/4) and smoking status (85.4% for never smokers compared to 63.8% for smokers).
Table 2.
Factors affecting overall response rate and disease control rate after treatment of non-small cell lung cancer patients
| Factor/variable | Subgroup | n | ORR (%) | P | DCR (%) | P |
|---|---|---|---|---|---|---|
| Age (years) | ≤60 | 122 | 54.9 | 0.937 | 68.8 | 0.291 |
| >60 | 101 | 55.4 | 75.2 | |||
| Sex | Male | 166 | 53.1 | 0.272 | 68.7 | 0.082 |
| Female | 57 | 61.4 | 80.7 | |||
| Education | Up to primary level | 84 | 51.2 | 0.289 | 65.5 | 0.121 |
| Above primary level | 107 | 58.9 | 75.7 | |||
| Pathology | SCC | 55 | 56.4 | 0.274 | 70.9 | 0.316 |
| ADC | 131 | 58.0 | 74.8 | |||
| NSCLC (NOS) | 37 | 43.2 | 62.2 | |||
| ECOG | 0,1 | 140 | 56.4 | 0.442 | 75.0 | 0.041 |
| 2,3,4 | 67 | 50.7 | 61.2 | |||
| Smoking | Never smoker | 82 | 67.1 | 0.005 | 85.4 | 0.001 |
| Chronic smoker | 127 | 47.2 | 63.8 | |||
| Treatment | Chemotherapy | 183 | 50.8 | 0.023 | 69.4 | 0.177 |
| EGFR inhibitors | 30 | 76.7 | 86.7 | |||
| ALK inhibitors | 8 | 75.0 | 75.0 | |||
| Compassionate based oral TKI therapy | 2 | 50.0 | 50.0 |
ORR: Overall response rate, DCR: Disease control rate, SCC: Squamous cell carcinoma, ADC: Adenocarcinoma, NSCLC: Non-small cell lung cancer, NOS: Not otherwise specified, ECOG: Eastern Cooperative oncology group, EGFR: Epidermal growth factor receptor, ALK: Anaplastic lymphoma receptor tyrosine kinase, TKI: Tyrosine kinase inhibitors
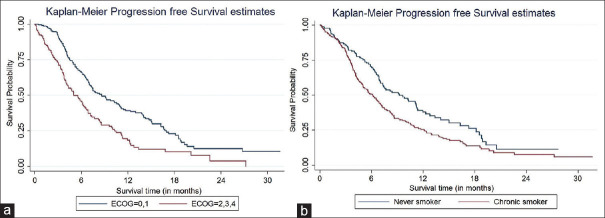
Table 3 depicts various factors affecting the PFS on univariate and multivariate analysis. For patients in whom relevant data were available (455 patients), the median PFS was 7.0 months (95% confidence interval [CI]: 3.7–14.0). On univariate analysis, factors that significantly affected PFS included gender, PS, and smoking status. On multivariate analysis, only the performance and smoking status affected PFS [Figure 1a and b].
Table 3.
Factors affecting progression-free survival after treatment
| Group | Subgroup | n | Median PFS (months) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95%CI) | P | HR (95% CI) | P | ||||
| Age (years) | ≤60 | 225 | 7.4 | 1.0 | 0.131 | ||
| >60 | 230 | 6.5 | 1.2 (0.9-1.5) | ||||
| Sex | Male | 350 | 6.7 | 1.0 | 0.05 | 0.8 (0.6-1.8) | 0.331 |
| Female | 105 | 6.8 | 0.7 (0.6-1.0) | ||||
| Education | Up to primary level | 179 | 6.1 | 1.0 | 0.293 | ||
| Above primary level | 196 | 7.3 | 0.9 (0.7-1.1) | ||||
| NSCLC pathology | SCC | 125 | 6.8 | 1.0 | |||
| ADC | 244 | 7.3 | 0.8 (0.6-1.1) | 0.196 | |||
| NSCLC (NOS) | 86 | 4.7 | 1.0 (0.7-1.5) | 0.805 | |||
| ECOG | 0,1 | 220 | 8.6 | 1.0 | <0.001 | 1.9 (1.5-2.4) | <0.001 |
| 2,3,4 | 173 | 5.0 | 1.8 (1.4-2.4) | ||||
| Smoking | Never smoker | 149 | 9.2 | 1.0 | 0.002 | 1.4 (1.1-1.9) | 0.014 |
| Chronic smoker | 288 | 6.1 | 1.5 (1.2-1.9) | ||||
| Treatment | Chemotherapy | 391 | 6.9 | 1.0 | |||
| EGFR inhibitors | 39 | 9.6 | 0.9 (0.6-1.3) | 0.502 | |||
| ALK inhibitors | 9 | 11.2 | 0.6 (0.2-1.5) | 0.251 | |||
| Compassionate based oral TKI therapy | 16 | 3.7 | 1.7 (1.0-2.9) | 0.067 | |||
PFS: Progression-free survival, NSCLC: Non-small cell lung cancer, NOS: Not otherwise specified, ECOG: Eastern cooperative oncology group, EGFR: Epidermal growth factor receptor, ALK: Anaplastic lymphoma receptor tyrosine kinase, TKI’s: Tyrosine kinase inhibitors, HR: Hazard ratio, CI: Confidence interval, SCC: Squamous cell carcinoma, ADC: Adenocarcinoma
Figure 1.
(a) Comparison of progression-free survival based on performance status. (b) Comparison of progression-free survival based on smoking status
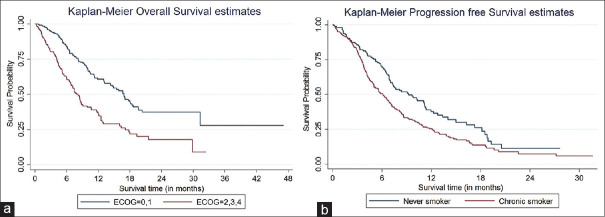
The OS of patients in our study was 11.7 months (95% CI: 5.5–29.9). Factors that affected median OS on the univariate analysis included age, sex, education level, NSCLC pathology, PS, smoking status, and type of treatment [Table 4]. However, on multivariate analysis, only the PS i.e. ECOG, type of treatment, and smoking status were the independent predictive factors for OS [Figure 2a and b]. Among patients in whom data beyond first-line treatment was available, 156 patients received maintenance treatment, 220 patients received second-line treatment, and 173 patients received third-line treatment. The patients who received targeted therapy as first-line were more likely to receive maintenance treatment and second-line treatment compared to chemotherapy group (68.1% vs. 35.4%, p = 0.004 and 69.0% vs. 44.3%, p = 0.014, respectively), but there were no significant differences for receiving third-line treatment (14.3% vs. 12.9%, P = 0.863).
Table 4.
Factors affecting overall survival
| Group | Subgroup | n | Median OS (months) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95%CI) | P | HR (95%CI) | P | ||||
| Age (years) | ≤60 | 266 | 13.2 | 1.0 | 0.037 | 1.1 (0.8-1.5) | 0.416 |
| >60 | 270 | 10.0 | 1.3 (1.0-1.6) | ||||
| Sex | Male | 419 | 10.5 | 1.0 | 0.049 | 1.1 (0.7-1.6) | 0.786 |
| Female | 117 | 16.3 | 0.7 (0.5-1.0) | ||||
| Education | Up to primary level | 217 | 9.1 | 1.0 | 0.032 | 0.9 (0.6-1.2) | 0.466 |
| Above primary level | 224 | 13.2 | 0.7 (0.6-0.9) | ||||
| NSCLC pathology | SCC | 153 | 9.9 | 1.0 | 0.9 (0.8-1.2) | 0.910 | |
| ADC | 275 | 14.5 | 0.6 (0.5-0.9) | 0.004 | |||
| NSCLC (NOS) | 108 | 9.3 | 1.1 (0.8-1.6) | 0.477 | |||
| ECOG | 0,1 | 253 | 16.9 | 1.0 | <0.001 | 2.4 (1.8-3.3) | <0.001 |
| 2,3,4 | 200 | 7.9 | 2.2 (1.7-2.9) | ||||
| Smoking | Never smoker | 173 | 17.1 | 1.0 | <0.001 | 1.6 (1.2-2.3) | 0.004 |
| Chronic smoker | 342 | 9.6 | 1.8 (1.4-2.4) | ||||
| Treatment | Chemotherapy | 464 | 10.5 | 1.0 | |||
| EGFR inhibitors | 40 | NR | 0.3 (0.2-0.6) | <0.001 | 0.4 (0.2-0.9) | 0.017 | |
| ALK inhibitors | 9 | NR | 0.1 (0.0-0.9) | 0.048 | 0.2 (0.1-1.8) | 0.172 | |
| Compassionate based treatment | 23 | 4.7 | 1.8 (1.1-3.2) | 0.030 | 2.0 (1.0-4.2) | 0.054 | |
OS: Overall survival, NSCLC: Non-small cell lung cancer, NOS: Not otherwise specified, ECOG: Eastern cooperative oncology group, EGFR: Epidermal growth factor receptor, ALK: Anaplastic lymphoma receptor tyrosine kinase, TKI’s: Tyrosine kinase inhibitors, HR: Hazard ratio, CI: Confidence interval, SCC: Squamous cell carcinoma, ADC: Adenocarcinoma, NR: Not reached
Figure 2.
(a) Comparison of overall survival based on performance status. (b) Comparison of overall survival based on smoking status
DISCUSSION
The present study comprehensively analyses the treatment response and survival outcomes after first-line management of advanced NSCLC. While several prior studies have reported survival outcomes, emphasis on ORR and DCR has been lacking. These are equally important clinical outcomes especially for assessing short-term prognosis and response to a particular treatment. They are also useful parameters to compare the efficacy of different treatment regimens. The ORR and DCR after 3–4 months of first-line treatment were 55.2% and 71.7%, respectively. Factors that significantly affected ORR included a never-smoking status and administration of targeted therapy, whereas a better baseline PS and never-smoker status significantly influenced DCR. On multivariate analysis, PS, i.e. ECOG, type of treatment, and smoking status were the independent factors that affected OS.
The majority of affected patients in our cohort were males. This parallels the data reported from other Indian studies where the male/female ratio was around 4:1.[2] The median age of our patients was 60 years that is slightly higher than other studies from North India which reported median age of around 55 years.[8,9] However, most of these studies have included small cell carcinoma and this could be a possible reason for variation in the reported median age.
Educational status has an important bearing on treatment and outcomes. In our study, patients receiving primary education or less constituted almost half of the affected cohort. It has been previously reported that incidence rates of lung cancer range from 166.6/100,000 in patients who were nongraduates, to 57.6/100,000 in college graduates. The same study also showed that lack of education influenced smoking habits and their inability to quit.[10] It has also been shown that persons with lower educational status had a lesser likelihood of undergoing definite investigations and disease-specific treatment, thereby translating into higher mortality.[11]
Among patients with NSCLC, ADC constituted the most common histology. This is similar to the data reported globally as well in India and indicates a change in the histology from squamous to ADC.[9,12] Better characterization of morphology leading to less reporting as NSCLC-NOS may be responsible for this change.[1,13]
Most of our patients had good PS (ECOG ≤2). This is in congruence to the study from South India where 89% of the patients had a good PS.[2] Similarly, another study by Malik et al.[9] hreported that more than 70% of patients had good performance status PS ≤2. This is important because assessment of PS helps clinical decision-making toward definite treatment which in turn may help in improving survival. However, a review of a large database has found that the prevalence of poor PS in lung cancer patients has been quite high, and there is a high rate of discordance between the PS assessed by health-care providers and that reported by the patients themselves. This data is retrospective in nature and also does not mention the presence of other underlying comorbidities which could have probably contributed to the poor PS.[14]
Among ADC, the proportion of patients tested for EGFR and ALK mutations was around 61% and 42%, respectively. The rates of positivity of EGFR and ALK among these patients were 27.2% and 14.7%, respectively. The incidence of EGFR positivity is lower than that of other Indian studies, where the reported incidence varies from 31% to 46%.[2,15] On the other hand, our cohort had higher ALK positivity than that of other studies, which ranges from 2.7% to 6%.[2,16,17] We have used either IHC or FISH for the detection of ALK and this might be the probable reason for higher positive ALK rearrangements in the present study.
Conventional chemotherapy using platinum-based doublet was the most commonly prescribed agent in our patients. Among ALK-positive patients, approximately 50% received targeted therapy, primarily due to cost constraints since most patients are not covered by insurance.
OS and PFS are usually the preferred outcome measure reported in most cancer studies. However, these usually need a long follow-up duration and posttreatment evaluation, which are often not available. In this context, ORR and DCR are useful indices of response to therapy after a finite predefined period. A systematic review of 44 RCTs involving more than 20,000 patients with NSCLC found that these response rates could be potentially useful surrogates to OS, although they are not strong enough to replace it as a primary endpoint.[18] Hence, we analyzed and reported both response and survival end-points. The ORR and DCR after 3–4 months of first-line treatment was 55.2% and 71.7%, respectively. Nonsmokers, subjects with better PS, and those who were initiated on TKIs had better responses to therapy. Prior knowledge of these prognostic factors may help clinicians to counsel patients, plan appropriate management, and anticipate therapeutic responses accordingly.
The median PFS and OS in our study were 7.0 months and 11.7 months, respectively. Similar PFS was reported in a large series from southern India which had patients predominantly in Stage 4.[2] However, a study from north India by Malik et al. reported a PFS of 7.8 months.[9] This is because the definition of PFS used in the above-mentioned study included patients from presentation to the hospital, whereas our study as well that by Murali et al.[2] calculated PFS from the start of treatment initiation. The OS in our study was longer than that reported by Murali et al. (7.6 months), probably because many patients in their cohort did not receive second-line chemotherapy. However, the OS is similar to that reported in multiple international studies. Recently, a real-world survival analysis from a German cohort reported a median OS of 11.5 months.[19]
The factors which consistently influenced survival in our study were better PS and nonsmoking status. One of the reasons for this is could be the fact that PS is an important factor for initially assessing suitability for administering definitive therapy.[20] Hence, patients with better PS are more likely to receive definite therapy, thereby translating into improved survival. It has been noted in a study from the Western part of India that the survival of patients with poor PS was poor.[5]
Smoking is not only one of the most common causes of bronchogenic carcinoma but also adversely affects survival. Bryant and Cerfolio.[21] reported that smokers with early-stage NSCLC have worse survival compared to nonsmokers. This study also found that the survival drops with an increase in the smoking burden. Patients with 20–40 pack years had a 5-year survival rate of 48%, which declined to 35% in patients who smoked more than 40 pack years. An Indian study has also found a negative impact of smoking on OS.[3] The adverse impact of smoking on survival is irrespective of gender, age, stage of disease, and histology. Possible reasons for the negative association are reduced local and systemic immunity, increase in systemic inflammation, upregulation of proto-oncogenes, and a down-regulation of tumor suppressor genes due to the tobacco constituents.[21]
The age of our subjects influenced OS on univariate analysis, a finding commensurate with previous reports. Tas et al.[22] reported that response to therapy, rate of mortality, and median survival were lower in patients older than 60 years. It is likely that elderly patients have co-morbidities or suffer from toxic effects of chemotherapy leading to a poor PS thereby mandating discontinuation of therapy.
In general, it has been reported that males have poorer outcomes in comparison to females, seen more so in ADC.[23] The ECOG trial found that the median survival time for women was 9.2 months compared to males (7.3 months).[24] Our study also found a higher OS in women compared to males. We postulate that lower rates of smoking, higher incidence of ADC, EGFR mutation positivity, and suitability for targeted therapy are possible reasons for this finding.
The influence of histology on survival has yielded conflicting results. Our study found the OS to be highest in ADC, followed by SCC and NSCLC-NOS subtypes. This is in sync with multiple global studies.[25,26,27] Wang et al. reported that this survival benefit in patients of ADC is maintained across all stages of the disease. However, contrasting results favoring a higher survival in patients of SCC have also been reported.[28,29,30,31]
The type of therapy administered has an important bearing on survival outcomes. While conventional chemotherapy using platinum-based doublet has remained the sheet anchor of lung cancer treatment for many years, the recent development of targeted and immunotherapy has revolutionized lung cancer management. TKIs are associated with better PFS compared to chemotherapy, but OS is similar.[32,33,34,35] Interestingly, in the present study, we found a better OS in patients receiving targeted therapy, although no difference in PFS was observed. An explanation for this may be that a higher proportion of our patients on targeted therapy as first-line treatment received maintenance and second-line therapy, thereby prolonging their OS. The lesser adverse effect profile and better PS following targeted therapy may be one of the reasons for getting higher subsequent lines of therapy, although this needs to be confirmed in further studies.
The main criticisms of our study could be its retrospective nature, lack of homogeneity in management, as well as missing data. However, the strengths include a large database of advanced NSCLC patients, reporting of ORR and DCR which are useful surrogates to survival outcomes and interpretation in a real-world scenario. Hence, these results could be useful in clinical decision-making and early prognostication.
CONCLUSIONS
Among a large cohort of metastatic NSCLC, those who were lifetime nonsmokers and had good baseline PS had better survival outcomes. First-line treatment with targeted therapy resulted in a better overall response rate and OS but did not affect PFS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mohan A, Garg A, Gupta A, Sahu S, Choudhari C, Vashistha V, et al. Clinical profile of lung cancer in North India:A 10-year analysis of 1862 patients from a tertiary care center. Lung India. 2020;37:190–7. doi: 10.4103/lungindia.lungindia_333_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murali AN, Radhakrishnan V, Ganesan TS, Rajendranath R, Ganesan P, Selvaluxmy G, et al. Outcomes in lung cancer:9-year experience from a tertiary cancer center in India. J Glob Oncol. 2017;3:459–68. doi: 10.1200/JGO.2016.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahesh PA, Archana S, Jayaraj BS, Patil S, Chaya SK, Shashidhar HP, et al. Factors affecting 30-month survival in lung cancer patients. Indian J Med Res. 2012;136:614–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Julka PK, Sharma DN, Madan R, Mallick S, Benson R, Kunhi PH, et al. Patterns of care and survival among small cell lung cancer patients:Experience from a tertiary center in India. J Egypt Natl Canc Inst. 2017;29:47–51. doi: 10.1016/j.jnci.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Dixit R, Sharma S. Pattern and determinants of survival among lung cancer patients in Western part of India. Chest. 2005;128 336S-a. [Google Scholar]
- 6.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project:Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours:Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Behera D, Balamugesh T. Lung cancer in India. Indian J Chest Dis Allied Sci. 2004;46:269–81. [PubMed] [Google Scholar]
- 9.Malik PS, Sharma MC, Mohanti BK, Shukla NK, Deo S, Mohan A, et al. Clinico-pathological profile of lung cancer at AIIMS:A changing paradigm in India. Asian Pac J Cancer Prev. 2013;14:489–94. doi: 10.7314/apjcp.2013.14.1.489. [DOI] [PubMed] [Google Scholar]
- 10.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 11.Willén L, Berglund A, Bergström S, Bergqvist M, Öjdahl-Bodén A, Wagenius G, et al. Educational level and management and outcomes in non-small cell lung cancer. A nationwide population-based study. Lung Cancer. 2019;131:40–6. doi: 10.1016/j.lungcan.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy A, Vijayalakshmi R, Gadigi V, Ranganathan R, Sagar TG. The relevance of “Nonsmoking-associated lung cancer”in India:A single-centre experience. Indian J Cancer. 2012;49:82–8. doi: 10.4103/0019-509X.98928. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilenbaum RC, Cashy J, Hensing TA, Young S, Cella D. Prevalence of poor performance status in lung cancer patients:Implications for research. J Thorac Oncol. 2008;3:125–9. doi: 10.1097/JTO.0b013e3181622c17. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt VR, D’Souza SP, Smith LM, Cushman-Vokoun AM, Noronha V, Verma V, et al. Epidermal growth factor receptor mutational status and brain metastases in non-small-cell lung cancer. J Glob Oncol. 2017;3:208–17. doi: 10.1200/JGO.2016.003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai SS, Shah AS, Prabhash K, Jambhekar NA. A year of anaplastic large cell kinase testing for lung carcinoma:Pathological and technical perspectives. Indian J Cancer. 2013;50:80–6. doi: 10.4103/0019-509X.117007. [DOI] [PubMed] [Google Scholar]
- 17.Doval D, Prabhash K, Patil S, Chaturvedi H, Goswami C, Vaid A, et al. Clinical and epidemiological study of EGFR mutations and EML4-ALK fusion genes among Indian patients with adenocarcinoma of the lung. Onco Targets Ther. 2015;8:117–23. doi: 10.2147/OTT.S74820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima K, Horita N, Nagai K, Manabe S, Murakami S, Ota E, et al. Progression-free survival, response rate, and disease control rate as predictors of overall survival in phase III randomized controlled trials evaluating the first-line chemotherapy for advanced, locally advanced, and recurrent non-small cell lung carcinoma. J Thorac Oncol. 2016;11:1574–85. doi: 10.1016/j.jtho.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Hardtstock F, Myers D, Li T, Cizova D, Maywald U, Wilke T, et al. Real-world treatment and survival of patients with advanced non-small cell lung cancer:A German retrospective data analysis. BMC Cancer. 2020;20:260. doi: 10.1186/s12885-020-06738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabchi S, Kassouf E, Florescu M, Tehfe M, Blais N. Factors influencing treatment selection and survival in advanced lung cancer. Curr Oncol. 2017;24:e115–22. doi: 10.3747/co.24.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant A, Cerfolio RJ. Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest. 2007;132:185–92. doi: 10.1378/chest.07-0442. [DOI] [PubMed] [Google Scholar]
- 22.Tas F, Ciftci R, Kilic L, Karabulut S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett. 2013;6:1507–13. doi: 10.3892/ol.2013.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radkiewicz C, Dickman PW, Johansson AL, Wagenius G, Edgren G, Lambe M. Sex and survival in non-small cell lung cancer:A nationwide cohort study. PLoS One. 2019;14:e0219206. doi: 10.1371/journal.pone.0219206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, Belani CP, et al. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 2006;1:441–6. [PubMed] [Google Scholar]
- 25.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, et al. AJapanese lung cancer registry study:Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 26.Foeglé J, Hédelin G, Lebitasy MP, Purohit A, Velten M, Quoix E. Specific features of non-small cell lung cancer in women:A retrospective study of 1738 cases diagnosed in Bas-Rhin between 1982 and 1997. J Thorac Oncol. 2007;2:466–74. doi: 10.1097/01.JTO.0000275340.39960.25. [DOI] [PubMed] [Google Scholar]
- 27.Lopez Guerra JL, Gomez DR, Lin SH, Levy LB, Zhuang Y, Komaki R, et al. Risk factors for local and regional recurrence in patients with resected N0-N1 non-small-cell lung cancer, with implications for patient selection for adjuvant radiation therapy. Ann Oncol. 2013;24:67–74. doi: 10.1093/annonc/mds274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P, et al. The International Association for the Study of Lung Cancer Staging Project:Prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 29.Pfannschmidt J, Muley T, Bülzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer:Experiences in 2083 patients. Lung Cancer. 2007;55:371–7. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Wang BY, Huang JY, Chen HC, Lin CH, Lin SH, Hung WH, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. 2020;146:43–52. doi: 10.1007/s00432-019-03079-8. [DOI] [PubMed] [Google Scholar]
- 31.Strand TE, Rostad H, Møller B, Norstein J. Survival after resection for primary lung cancer:A population based study of 3211 resected patients. Thorax. 2006;61:710–5. doi: 10.1136/thx.2005.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 33.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC):A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 34.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 35.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802):A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]