Abstract
The explosive rise in angioinvasive mucormycosis (MM) in India and other parts of the world has been described as the “epidemic following the COVID-19 pandemic,” with the majority being rhino-orbital-cerebral MM. We report a case series of five COVID-19-associated pulmonary MM (CAPM) with an aggressive clinical course. Clinical and radiological clues were limited, and the initial suspicion of CAPM was the morphological appearance on bronchoscopy, which led to the diagnosis. Histopathology was consistently positive in all cases, while other microbiological and molecular tests had varying sensitivity. Most patients had a fulminant and fatal course. Also noted was dual fungal infection in 3/5 cases with coexisting multidrug resistant bacterial infection in all cases. CAPM is the hidden part of the COVID-MM epidemic and warrants a high degree of suspicion with early diagnosis and treatment.
KEY WORDS: Bronchoscopy, COVID, COVID fungal infection, COVID-19-associated pulmonary mucormycosis, mucormycosis, pulmonary mucormycosis
INTRODUCTION
COVID-19 is associated with an increased incidence of mucormycosis (MM) in adults with multisystem involvement, predominantly rhino-orbital-cerebral MM (ROC-MM).[1,2] More than 30,000 COVID-associated MM (CAM, largely ROC-CAM) cases have been reported in the current wave of COVID-19 in India.[3] We report the first case series of COVID-19-associated pulmonary MM (CAPM) detected with advanced diagnostic strategies, with a high mortality.
CASE REPORTS
Case 1
A 60-year-old diabetic female, glycosylated hemoglobin (HbA1C) 10.2 and COVID-19 infection 35 days ago treated with prolonged corticosteroids [Table 1], was referred with respiratory distress and was mechanically ventilated. Chest radiograph (CXR) showed a right lower lobe (RLL) and left upper lobe dense consolidation with right empyema-intercostal chest drainage was inserted. Bronchoscopy showed purulent, cheesy secretions, extensive necrosis of the RLL segments [Figure 1], and KOH mount of the washings suggested MM confirmed on histopathology (HP). Liposomal amphotericin B (LAmpB) was started but she expired within 48 h.
Table 1.
Clinical and laboratory details of the patients with COVID-19-associated pulmonary mucormycosis
| Age | Sex | HbA1C | Comorbidities | Time since symptom onset (days) | Steroid Dosage | Radiology- CXR/CT |
Morphology of airways | BAL KOH | Fungal culture | Fungal PCR | HPE | BAL GM | BAL COVID19 RT-PCR | Bacterial culture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | Female | 10.2 | DM/HTN | 35 | MPS 120 mg x 5 days, f/b 60 mg x 15 days | CXR: RLZ/LUZ consolidation Right empyema |
Extensive endobronchial necrosis RLL/LUL apical | Aseptate hyphae | Negative | Negative | MM | 1.75 | Negative | MDR K. pneumonia |
| 70 | Male | 8.4 | CKD | 12 | MPS 80 mg x 10 days | CXR: LUZ/LMZ dense consolidation | Thick purulent secretions, with airway necrosis | Aseptate + septate hyphae | Aspergillus fumigatus + Mucor | R. delemar | MM + Aspergillus | 1.45 | Positive | MDR Acinetobacter baumannii |
| 67 | Male | 9.9 | DM/HTN/IHD | 21 | Dexamethasone 8 mg x 21 days | CXR: Bilateral lower zone opacities | Thick purulent secretions | Aseptate hyphae + Aspergillus sporulation | Aspergillus fumigatus | Negative | MM + Aspergillus | 3.28 | Negative | MDR K. pneumonia |
| 46 | Female | 14.6 | DM | 23 | MPS 80 mg x 14 days | CXR: Left lung collapse with bronchus cut-off | Tracheal thick secretions, distal tracheal LMB mass with erosion into cartilage | Aseptate hyphae | Mucor | R. oryzae | MM | 0.57 | Negative | MDR K. pneumonia |
| 63 | Male | 9.2 | DM | 38 | Prednisolone 60 mg x 7 days | CT: LLL cavity with fungal growth | Minimal secretions | Negative | Negative | Not done | MM + Aspergillus | 2.75 | Negative | MDR K. pneumonia |
K. pneumonia: Klebsiella pneumoniae, R. delemar: Rhizopus delemar, R. oryzae: Rhizopus oryzae, DM: Diabetes mellitus, HTN: Hypertension, IHD: Ischemic heart disease, CKD: Chronic kidney disease, RUZ: Right upper zone, LUZ: Left upper zone, LMZ: Left mid zone, RMZ: Right mid zone, RLZ: Right lower zone, LLZ: Left lower zone, LLL: Left lower lobe, MDR: Multidrug resistant, LMB: Left main bronchus, GM: Galactomannan, MM: Mucormycosis, HbA1C: Hemoglobin A1C, CXR: Chest radiograph, RLL: Right lower lobe, LUL: Left upper lobe, BAL: Bronchoalveolar lavage, RT-PCR: Reverse transcription polymerase chain reaction, CT: Computerized tomography, KOH: Potassium Hydroxide
Figure 1.
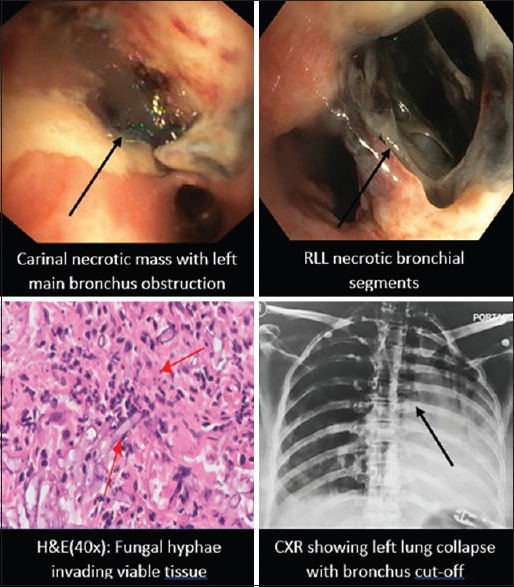
Bronchoscopic, radiological, and histopathological images of COVID-19-associated pulmonary mucormycosis
Case 2
A 70-year-old male with chronic kidney disease (CKD) had COVID-19 infection for 12 days treated with intravenous steroids, presented with respiratory failure, and was mechanically ventilated. CXR showed bilateral lower zone dense consolidations. Bronchoscopy showed thick purulent secretions with mucus plugging and microbiology and HP confirmed Aspergillus fumigatus and Mucor spp. Bronchoalveolar lavage (BAL) panfungal polymerase chain reaction (PCR) grew Rhizopus delemar. He was started on LAmpB, went into progressive septic shock, and expired after 3 days.
Case 3
A 67-year-old diabetic male with HbA1C 9.9 and COVID-19 infection 21 days ago treated with steroids was referred for worsening respiratory distress and ventilated. CXR showed bilateral lower zone patchy opacities. Bronchoscopy showed purulent secretions with mucosal ulcerations and microbiology grew Aspergillus flavus, while endobronchial biopsy showed dual infection with Aspergillus and Mucor spp. After initial improvement, he worsened rapidly and expired 4 days later.
Case 4
A 46-year-old female uncontrolled diabetic with HbA1C 14.2 with COVID-19 23 days prior treated with high dose steroids was referred for respiratory distress with left lung collapse and was ventilated. Bronchoscopy showed purulent tracheal secretions and a necrotic mass at distal trachea/carina eroding the posterior tracheal wall [Figure 1]. Cryoadhesion was used to remove the mass. KOH mount showed aseptate hyphae, BAL fungal PCR grew Rhizopus oryzae, and HP showed angio-invasive Mucor spp. [Figure 1]. She was initiated on LAmpB but expired 48 h later.
Case 5
A 63-year-old male diabetic treated for severe COVID pneumonia 3 weeks ago presented with a left tension pneumothorax, and a chest drain was inserted. Computed tomography (CT) chest showed diffuse fibrosis and a left LL cavity with a fungal ball [Figure 1]. BAL and transbronchial biopsy confirmed Aspergillus and Mucor dual infection. L-Amp B was started and he stabilized.
All patients had additional multidrug-resistant (MDR) bacterial infection. Table 1 summarizes the demographic, radiological, and clinical details of the patients.
DISCUSSION
This case series describes critical CAPM as an important subset of the CAM epidemic in India. CAPM appears to be difficult to suspect, is often diagnosed late, and has limited treatment options with a bad outcome.
The main risk factors postulated for the CAM epidemic in India are extensive corticosteroid usage and uncontrolled DM.[1,2,4,5] In our series, 4/5 patients were uncontrolled diabetics with a mean HbA1c of 10.46%. One patient had CKD, and all patients received high dose (equivalent of methylprednisolone >80 mg/day) and long-term corticosteroids – median steroid treatment was for 21 days (range 9–31 days).
The diagnostic tools for CAPM include radiology, followed by appropriate microbiology and HP. CXR findings are nonspecific and described CT findings for CAPM include the “reverse halo” sign, nodular infiltrates, dense consolidation, cavitation, and pleural effusion.[6] However, CT scan is not always feasible in these unstable ventilated patients, and in addition, CT findings have a low specificity. Significant challenges are there with microbiological tests, due to varying sensitivity, specificity, and turnaround time, with treatment and outcome implications. Guidelines recommend demonstration of aseptate fungal hyphae on direct microscopy, culture, and HP. PCR-based tests have a moderate recommendation due to nonstandardization, despite high sensitivity.[6] Bronchoscopy, though challenging, is useful to obtain BAL/biopsy samples, as sick patients are often unable to expectorate.[7]
In our series, in 4/5 patients, the most common CXR finding was patchy dense consolidation, with 1 empyema. One patient had a cavity with COVID fibrosis with the differential of CAPM or COVID-associated pulmonary aspergillosis (CAPA). None of these patients were able to produce sputum. CT could not be done in 4/5 patients, and CAPM was therefore not suspected. Bronchoscopy done in 4/5 patients for worsening infiltrates revealed unsuspected CAPM on visual inspection of necrotic/mass lesions.
KOH mount of the BAL/biopsy was positive with a rapid turnaround time. HP was consistently positive in all cases, fungal culture was positive in 3/5 cases, and fungal PCR was positive in 2 cases. Bronchoscopy was the pivotal test for diagnosis and sample acquisition in this cohort. Bronchoscopically visualized necrosis with anatomical obliteration was paradoxically an adverse prognostic factor in 4/5 patients, as none of them were candidates for surgery. Dual infection with Aspergillus and Mucor spp. was also seen in 3/5 patients.
Historically, the prognosis of pulmonary MM is poor, with mortality as high as 87%.[8] Early diagnosis and therapy with appropriate antifungals (amphotericin B, LAmpB) and aggressive surgery (debridement and debulking) can significantly reduce mortality.[9] These concepts have been extended to CAPM management,[10] with significant limitations mentioned below.
All patients were initiated on LAmp B but could not be operated due to instability, extensive disease, and fibrosis– this feature seems to be unique to CAPM. The fulminant course led to demise within 4 days of diagnosis in 4/5 patients. Dual infection with MDR bacterial infection was another concern which may have added to mortality.
CONCLUSION
This series highlights that CAPM is infrequently reported, may co-exist with CAPA, and is the worrisome subset of the CAM epidemic. It presents enhanced challenges from both aspects – diagnosis and therapy. CAPM needs a higher index of suspicion, and often bronchoscopy is needed for diagnosis. Visual necrosis appears to be an adverse factor with imminent mortality, and dual fungal with MDR bacterial co-infection has can further worsen outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–59. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg D, Muthu V, Sehgal I, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM):Case Report and Systematic Review of Literature. Mycopathologia. 2021;186:289–98. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Last accessed on 2021 Jul 21]. Available from: https://www.deccanherald.com/national/in-the-wake-of-india-s-covid-crisis-a-black-fungus-epidemic-follows-999682.html .
- 4.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19:A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15:102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, Kaur H, Xess I, Michael J, Savio J, Rudramurthy S, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clinical Microbiology and Infection. 2020;26:944.e9–944.e15. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et al. European society for clinical microbiology and infectious diseases (ESCMID) and European confederation of medical mycology (ECMM): Joint clinical guidelines for the diagnosis and management of mucormycosis. 2013 doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 7.Al-Abbadi MA, Russo K, Wilkinson EJ. Pulmonary mucormycosis diagnosed by bronchoalveolar lavage:A case report and review of the literature. Pediatr Pulmonol. 1997;23:222–5. doi: 10.1002/(sici)1099-0496(199703)23:3<222::aid-ppul9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis:A review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 9.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 10.Sun HY, Aguado JM, Bonatti H, Forrest G, Gupta KL, Safdar N, et al. Pulmonary zygomycosis in solid organ transplant recipients in the current era. Am J Transplant. 2009;9:2166–71. doi: 10.1111/j.1600-6143.2009.02754.x. [DOI] [PubMed] [Google Scholar]


