Sir,
Bronchial arteries are the culprit for the majority of patients presenting with hemoptysis in tuberculosis. Ruptured bronchial artery pseudoaneurysm secondary to pulmonary tuberculosis causing massive hemothorax is rarely seen. Clinical diagnosis is often difficult as patient symptoms can mimic a wide variety of diseases with high suspicion of malignancy. However, imaging tools such as contrast-enhanced computed tomography (CECT) chest and computed tomography angiography aid in the diagnosis. Interventional radiology is considered to be a key diagnostic as well as therapeutic procedure as an urgent endovascular approach is most commonly preferred for its nonsurgical management. This report describes a rare case of a 60-year-old male smoker with a history of pulmonary tuberculosis presented with sudden onset left-sided chest pain, hemoptysis, and shortness of breath with substantially low hemoglobulin levels. CECT chest revealed a pseudoaneurysm of a left bronchial artery with large mediastinal hematoma which was further confirmed on digital angiography and the patient was managed with endovascular embolization.
A 60-year-old male smoker with a past medical history of pulmonary tuberculosis presented with sudden onset left-sided chest pain, hemoptysis, and shortness of breath for 1 day. The patient also had an episode of loss of consciousness following hemoptysis of ~100 ml. General examination findings of the patient on admission revealed tachycardia, hypotension, and reduced SpO2 (88%). Routine blood investigations revealed low hemoglobulin (6.6 g/dl). Chest examination revealed decreased air entry on the left side with crepitations. The sputum culture demonstrated acid-fast bacilli. The patient was transfused whole blood and was stabilized. For diagnosis, CT angiography was performed and scanogram revealed a large mediastinal mass on the left side with areas of bronchiectasis in the retrocardiac region [Figure 1a]. Noncontrast CT (NCCT) chest revealed a large mixed density lobulated lesion in the anterior mediastinum with areas of hemorrhage [Figure 1b]. CT angiogram revealed hypertrophied left bronchial artery measuring 3.5 mm at the origin arising from descending thoracic aorta at T4-T5 IVD level with the presence of large fusiform multilobulated aneurysmal outpouching of size ~4 cm × 1.8 cm. The aneurysm was narrow necked and was pointing anterolaterally toward the left side with a large perianeurysmal hematoma of size ~11.5 cm × 10 cm × 19. 2 cm causing mild mediastinal shift to the right side. The hematoma was extending along the anterior mediastinum and into the left side, causing mass effect over the adjacent mediastinal structures was extending inferiorly up to the left hemidiaphragm and superiorly extending up to the arch of the aorta. CT findings were suggestive of a ruptured large bronchial artery pseudoaneurysm with associated hematoma [Figure 1c and d]. Bilateral lung parenchyma showed emphysematous changes with areas of cystic bronchiectasis in the left lower lobe with areas of consolidation. There were areas of ground-glass attenuation in the left upper lobe, right middle, and lower lobe with interlobar septal thickening giving a crazy-paving appearance which was suggestive of pulmonary hemorrhage [Figure 2a and b].
Figure 1.
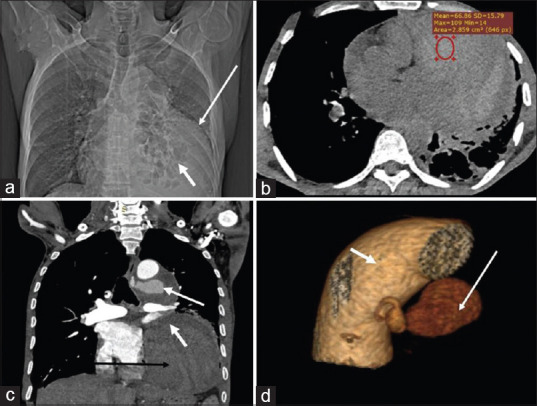
(a) Scannogram shows a large lobulated lesion in left lower and mid lung zones with broad base towards mediastinum, silhouetting left heart border, left hemidiaphragm, and descending thoracic aorta (large white arrow). Cystic bronchiectasis (small white arrow).(b) Axial noncontrast computed tomography scan through the chest shows large mediastinal hematoma (HU ~ 66) (red circle) along the anterior and left para mediastinum. (c) Computed tomography aortogram. Coronal image depicts contrast-filled pseudoaneurysm (large white arrow) with hematoma causing mass effect over left inferior pulmonary vein (small white arrow) and left ventricle (large black arrow). (d) Volume rendered image showing bronchial artery pseudoaneurysm (large white arrow) arising from the descending thoracic aorta.(small white arrow)
Figure 2.
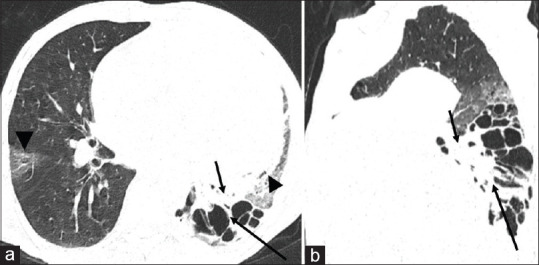
(a and b) Axial and sagittal reformatted computed tomography chest (lung window) show cystic bronchiectasis in left lower lobe (black arrow in a and b) poster with surrounding consolidation (small black arrow) and areas of ground-glass opacities in lingular lobe and right middle lobe (black arrowhead) likely due to pulmonary hemorrhage
A transcatheter bronchial angiogram with embolization for bronchial artery pseudoaneurysm was performed. Access was gained through the right common femoral artery, and with the help of a diagnostic angiographic catheter (Shepherd catheter 5 Fr), the left bronchial artery was cannulated. A selective angiogram revealed a pseudoaneurysm arising from the left bronchial artery [Figure 3a]. Using a 2.1 Fr microcatheter, pseudoaneurysm was reached embolized using glue mixed with lipiodol (1:7 ratio). Postembolization angiogram revealed complete thrombosis of the pseudoaneurysm sac [Figure 3a and b]. No immediate complication was noted. Hemostasis was achieved at the groin by manual compression, and the patient was discharged after 2 days, with antitubercular treatment. The patient was followed up telephonically, however, follow-up imaging could not be obtained due to the COVID pandemic and later, it came to know that the patient succumbed to coinfection with COVID-19 pneumonia.
Figure 3.
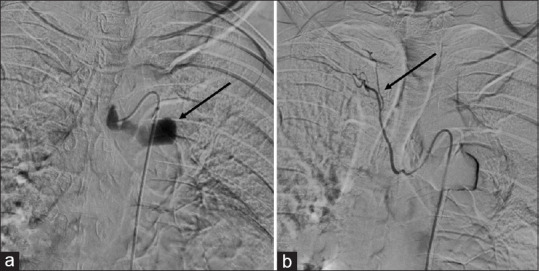
(a) Digital subtraction angiography spot image of the selective left bronchial artery using microcatheter shows pseudoaneurysm (black arrow). (b) Final fluoroscopic spot image after glue embolization of aneurysmal sac, showing glue cast within the pseudoaneurysm sac in the left bronchial artery with the patent distal flow
DISCUSSION
Bronchial artery aneurysm is either primary in which the cause is unknown while the secondary aneurysm can be associated with inflammatory lung diseases, bronchiectasis, atherosclerosis, bronchitis, and systemic vascular abnormalities such as Osler–Weber–Rendu syndrome. By definition, a pseudoaneurysm is the hematoma outside the arterial wall due to injury to the wall and contains either tunica adventitia or media or contained by surrounding tissue. The hematoma must communicate through the artery through the defect in the arterial wall. Various causes of pseudoaneurysm include trauma, infections, vasculitis, connective tissue disorder, malignancies, coagulopathies, rupture of an aneurysm, and iatrogenic.[1] In our patient, the probable etiology may be likely due to spontaneous rupture of a bronchial artery aneurysm. Bronchial artery aneurysms, and pseudoaneurysms can be either asymptomatic and are detected incidentally or present with varied symptoms due to mass effect or rupture that can mimic other medical pathologies, the index case simulates mediastinal mass on the radiograph. Patients can present with hemoptysis[2,3] or hematemesis when there is a rupture into the bronchus or esophagus, respectively. Chest pain occurs if the pseudoaneurysm ruptures into the mediastinum and can mimic aortic dissection. Ruptured aneurysm with large mediastinal hematoma compresses the mediastinal structures and causes dysphagia or superior vena cava syndrome due to compression of esophagus or superior vena cava, respectively.[4] In our case, the HRCT chest done from outside raises the suspicion of mediastinal mass due to large mediastinal hematoma and referred to our institute. Noncontrast and CT angiography revealed large mediastinal hematoma due to rupture of pseudoaneurysm of the left branchial artery. CT angiography plays a significant role in the diagnosis as well as in providing a roadmap for angiography. Digital Subtraction Angiography (DSA) is the gold standard for the diagnosis of pseudoaneurysms because of its ability to assess real-time contrast filling. According to Habib et al., DSA has the highest sensitivity (100%), followed by CT (67%).[5] Any diagnosed visceral artery aneurysm or pseudoaneurysm whether symptomatic or asymptomatic should be managed electively as the risk of mortality is considerably high after the rupture of an aneurysm.[6] The risk of rupture of an aneurysm is not dependent on the size of the lesion. Risk of rupture of a pseudoaneurysm is higher than aneurysm as the vessel wall is already disrupted.
All visceral artery aneurysms and pseudoaneurysms are treated either by an endovascular approach or an open surgical repair, however, an endovascular approach is desired due to its much less invasive nature. Postoperative pain and wound complications are particularly less with reduced hospital stay and improved quality of life.[7] Embolization is done using various embolizing agents or by stenting depending upon the presence or absence of collateral circulation, respectively.[8,9] The index patient was managed with transcatheter bronchial artery embolization using glue. Complications of this procedure are rare and include local complications such as groin hematomas, pseudoaneurysms, and arterial thrombosis. Other methods for endovascular embolization include coiling or covered stent. Complications of coil embolization are recanalization due to revascularization or collateral circulation or migration of the coil.
To conclude, ruptured nontraumatic bronchial artery pseudoaneurysm secondary to pulmonary tuberculosis causing massive hemothorax is rarely seen and suspicion should be made after exclusion of other causes of hemothorax. CECT thorax can aid in the detection of pathology, however, DSA is the quintessential technique both for diagnosis and management. Endovascular embolization under DSA is preferred over surgical management, and early management is crucial for preventing mortality and morbidity in these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kaufman C, Kabutey NK, Sgroi M, Kim D. Bronchial artery pseudoaneurysm with symptomatic mediastinal hematoma. Clin Imaging. 2014;38:536–9. doi: 10.1016/j.clinimag.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Kalina M, Giberson F. Hemoptysis secondary to pulmonary artery pseudoaneurysm after necrotizing pneumonia. Ann Thorac Surg. 2007;84:1386–7. doi: 10.1016/j.athoracsur.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Kalangos A, Khatchatourian G, Panos A, Faidutti B. Ruptured mediastinal bronchial artery aneurysm:A dilemma of diagnosis and therapeutic approach. J Thorac Cardiovasc Surg. 1997;114:853–6. doi: 10.1016/S0022-5223(97)70094-1. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann V, Ysebaert D, De Schepper A, Colpaert C, Jorens P. Acute superior vena cava obstruction after rupture of a bronchial artery aneurysm. Chest. 1996;110:1356–8. doi: 10.1378/chest.110.5.1356. [DOI] [PubMed] [Google Scholar]
- 5.Habib N, Hassan S, Abdou R, Torbey E, Alkaied H, Maniatis T, et al. Gastroduodenal artery aneurysm, diagnosis, clinical presentation and management:A concise review. Ann Surg Innov Res. 2013;7:4. doi: 10.1186/1750-1164-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordova AC, Sumpio BE. Visceral artery aneurysms and pseudoaneurysms –Should they all be managed by endovascular techniques? Ann Vasc Dis. 2013;6:687–93. doi: 10.3400/avd.ra.13-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang YK, Hsieh HC, Tsai FC, Chang SH, Lu MS, Ko PJ. Visceral artery aneurysm:Risk factor analysis and therapeutic opinion. Eur J Vasc Endovasc Surg. 2007;33:293–301. doi: 10.1016/j.ejvs.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga A, Okushiba S, Ohno K, Kitashiro S, Kawarada Y, Shitinohe T, et al. Mediastinal bronchial artery aneurysm with hematemesis. Dis Esophagus. 2003;16:328–31. doi: 10.1111/j.1442-2050.2003.00360.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Wood DE. Bronchial artery aneurysm refractory to transcatheter embolization. Ann Thorac Surg. 2008;86:306–8. doi: 10.1016/j.athoracsur.2008.01.033. [DOI] [PubMed] [Google Scholar]


