Abstract
Methionine γ-lyase, the enzyme which catalyzes the single-step conversion of methionine to α-ketobutyrate, ammonia, and methanethiol, is highly active in many anaerobic pathogenic microorganisms but has no counterpart in mammals. This study tested the hypothesis that this pathogen-specific enzyme can be exploited as a drug target by prodrugs that are exclusively activated by it. Trifluoromethionine was confirmed as such a prodrug and shown to be highly toxic in vitro to the anaerobic protozoan parasite Trichomonas vaginalis, to anaerobic bacteria containing methionine γ-lyase, and to Escherichia coli expressing the trichomonad gene. The compound also has exceptional activity against the parasite growing in vivo, with a single dose preventing lesion formation in five of the six mice challenged. These findings suggest that trifluoromethionine represents a lead compound for a novel class of anti-infective drugs with potential as chemotherapeutic agents against a range of prokaryotic and eukaryotic anaerobic pathogens.
Infections caused by anaerobic pathogens, both bacterial and protozoal, are common and present major problems. Treatment can be difficult, as many drugs have poor activity and drug resistance is an increasing problem (3). Frontline drugs for the majority of these infections are the 5-nitroimidazoles, typically metronidazole (4). Three additional groups of drugs, the carbapenems, chloramphenicol, and combinations of β-lactam drugs (penicillins and cephalosporins) with a β-lactamase inhibitor, are used clinically for treating the bacterial pathogens. These drugs usually show good efficacy, but resistance is a constant threat (3). Thus, there is a need to discover novel antianaerobe drugs.
Metronidazole is one of the most successful drugs currently in clinical use against anaerobic microorganisms. This drug has almost total specificity for microorganisms that contain the enzyme pyruvate:ferredoxin oxidoreductase, which is involved in the reductive activation of the drug. Metronidazole thus provides an excellent example of how drug activation by an enzyme in a pathogen but not its host can result in sufficient selective toxicity for the compound to be clinically useful (4). A similar approach has recently yielded novel drug candidates for the treatment of tuberculosis (9).
The pyridoxal-5′-phosphate-dependent enzyme methionine γ-lyase catalyzes the breakdown of methionine by an α,γ elimination reaction to yield α-ketobutyrate, ammonia, and methanethiol (5, 8). The enzyme characteristically occurs in anaerobic microorganisms. Many of these are important pathogens, including bacteria causing botulism (Clostridium botulinum), colitis (Clostridium difficile), tooth decay (Porphyromonas species), and postoperative intra-abdominal infections (Bacteroides species) (3). There are also widespread anaerobic parasites of humans. These include Entamoeba histolytica, which causes amoebiasis, and Trichomonas vaginalis, which causes the sexually transmitted disease trichomoniasis. The latter is highly prevalent in women and appears to be a risk factor for human immunodeficiency virus infections. A new class of antianaerobe chemotherapeutic agents would have important applications, especially for diseases such as trichomoniasis, for which 5-nitroimidazoles are currently the only effective drugs.
Methionine γ-lyase has no counterpart in mammals and so appears to be a good drug target. Our aim was to exploit this difference in biochemistry between host and pathogen. We hypothesized that the fluorine-substitution-containing analogue of methionine, trifluoromethionine (TFMET), may be a substrate for methionine γ-lyase, which would catalyze an α,γ elimination reaction to yield α-ketobutyrate, ammonia, and trifluoromethanethiol (CF3SH). This last compound is unstable under physiological conditions and nonenzymatically breaks down to carbonothionic difluoride (CSF2), a potent cross-linker of primary amine groups (1, 7). CSF2 would be highly toxic to the pathogen in which it is produced, whereas toxicity to a mammalian host should be minimal, as activation of the prodrug would not occur in the absence of methionine γ-lyase and pathogen-generated CSF2 would be bound by pathogen material before it encountered any host molecules. We report here that TFMET indeed acts in this way, is efficacious in vitro and in vivo against microorganisms containing methionine γ-lyase, and so represents a lead compound for a novel class of drugs against a range of anaerobic pathogens.
MATERIALS AND METHODS
T. vaginalis G3 was grown, the parasite's methionine γ-lyase was assayed and purified, and the reaction products were analyzed by high-pressure liquid chromatography as described previously (5). The parasite's susceptibility to TFMET in vitro and in vivo was determined using methods described previously (2, 10). Generation of the Escherichia coli strain expressing T. vaginalis MGL1 has been described previously (6). Briefly, plasmid pGL14 (the MGL1 gene in pQE60) was transformed into M15[pREP4] E. coli (Qiagen). The sensitivity of the transformed cells to TFMET was assessed as follows. The cells were grown overnight in Luria-Bertani medium (LB) containing 100 μg of ampicillin and 25 μg of kanamycin/ml at 37°C. Cells were diluted 1:100 into fresh medium and incubated at 37°C for 15 min. Isopropylthiogalactoside (IPTG) was then added to a final concentration of 0.1 mM, and the cells were incubated for a further 30 min at 37°C to allow induction of recombinant MGL1 (6). TFMET (stock solution of 10 mg/ml in water) was added to a final concentration of 2.5 to 50 μg/ml. Samples were removed at 0, 2, and 4 h, and the optical density at 600nm was determined. The number of viable E. coli organisms surviving after 6 h of incubation with 50 μg of TFMET/ml was determined by spreading cells on LB plates containing 100 μg of ampicillin and 25 μg of kanamycin/ml and incubating overnight at 37°C. Percent survival was calculated using the number of viable cells at time zero as determined above. TFMET was a gift from Mike Riscoe (Medical Research Service, Veterans Administration Medical Center, Portland, Oreg.).
RESULTS
We used the protozoan parasite T. vaginalis as our model anaerobic pathogen because it contains high activity of methionine γ-lyase (5). TFMET was shown to be a substrate for methionine γ-lyase purified from T. vaginalis and underwent α,γ elimination to yield α-ketobutyrate at a rate of 3.3 μmol/min/mg of protein, compared with a rate of 0.50 μmol/min/mg of protein for methionine. Trifluoromethanethiol, which would also be a product of this reaction, could not be detected using the assay previously used to measure methanethiol (12), presumably due to its rapid nonenzymatic decomposition to carbonothionic difluoride (1). The apparent Km for TFMET was 0.32 ± 0.6 mM, and that for methionine 4.3 ± 1.1 mM. To demonstrate that just the one enzymatic activity was responsible for the α,γ elimination of both methionine and TFMET, the enzyme was purified from T. vaginalis. The ratio of the activity towards the two substrates was found to remain effectively constant throughout the whole procedure (data not shown), providing good evidence that methionine γ-lyase was responsible for the catabolism of TFMET.
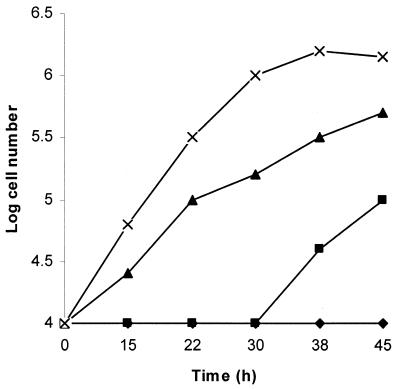
TFMET has good activity against T. vaginalis in culture. At concentrations of 5 μg/ml or more, all the cells were killed within 24 h. In order to provide evidence that the high toxicity of TFMET towards T. vaginalis was due to the presence of methionine γ-lyase activity, we measured the efficacy of the compound in the presence of propargylglycine. This compound has been shown previously to inhibit completely the methionine γ-lyase activity while still allowing the parasite to grow (11). Addition of propargylglycine at the start of the experiment (Fig. 1) greatly reduced the antitrichomonal effect of TFMET but did not eliminate it entirely, there being a lag period before the parasites grew. It seemed likely that this reflected the time it took for all of the parasite's methionine γ-lyase to be inhibited. To test this, the experiment was repeated using parasites already grown in the presence of propargylglycine for 24 h. Under these circumstances the parasite grew in the presence of TFMET without a lag period, thus showing the direct correlation between the presence of active methionine γ-lyase and susceptibility to the compound.
FIG. 1.
Inhibition of growth of T. vaginalis by TFMET is abolished by propargylglycine. ×, control; ▴, 0.01 mM propargylglycine; ▪, 0.01 mM propargylglycine and 5 μg of TFMET/ml; ♦, 5 μg of TFMET/ml.
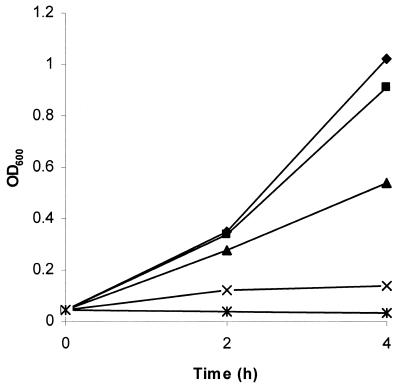
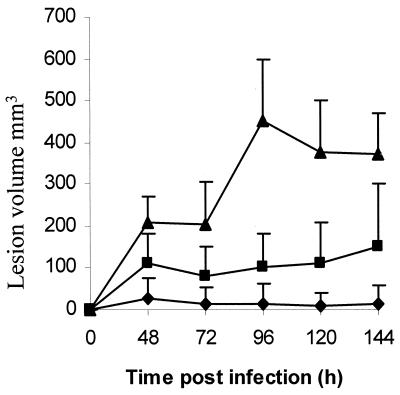
To confirm that susceptibility to TFMET was mediated by methionine γ-lyase, the MGL1 gene encoding the enzyme of T. vaginalis was introduced into E. coli on the pQE60 plasmid (6). Expression of methionine γ-lyase was induced by the addition of IPTG to the E. coli culture, and the susceptibilities to TFMET of the parent and transformed bacterial lines were compared. Neither line was affected by 10 μg of TFMET/ml during 4 h of growth in the absence of IPTG, but the addition of 0.1 mM IPTG made the methionine γ-lyase-containing line susceptible to the prodrug such that growth was almost totally abolished at 10 μg/ml (Fig. 2). Moreover, TFMET killed the methionine γ-lyase-containing E. coli, with the viable cell count being reduced to 0.013% of the starting number by 6 h in the presence of 50 μg of TFMET/ml. This demonstrates that the activated prodrug is highly toxic and can exert its effect rapidly. The efficacy of TFMET in vivo was studied using mice infected subcutaneously with T. vaginalis. A marked inhibitory effect was observed using 40 mg of TFMET/kg of body weight administered intravenously as a single dose 2 h after inoculation of the parasites (Fig. 3). Five of the six mice treated produced no lesions at all. Lesions appeared when 12 mg of the drug/kg was used, but with much lower volumes than the controls.
FIG. 2.
Growth of E. coli expressing T. vaginalis methionine γ-lyase is inhibited by TFMET. E. coli M15[pREP4] transformed with pGL14 containing T. vaginalis MGL1 was grown in LB supplemented with 0.1 mM IPTG and then exposed to 0 (♦), 2.5 (▪), 5 (▴), 10 (×), and 50 (∗) μg of TFMET/ml.
FIG. 3.
A single dose of TFMET administered to mice by intravenous injection inhibits the production of subcutaneous lesions by T. vaginalis. ▴, controls (no drug); ▪, 12 mg of TFMET/kg; ♦, 40 mg of TFMET/kg. Data are means plus standard deviations for each group of six mice.
DISCUSSION
The results confirm that methionine γ-lyase can be targeted by prodrugs to generate compounds toxic to anaerobic bacteria and protozoa containing the enzyme. The metabolism of TFMET by the enzyme purified from T. vaginalis together with the efficacy of the compound against not only the parasite in vitro and in vivo but also E. coli expressing the trichomonad gene are all consistent with the postulated mode of action. TFMET is also similarly active in vitro against the bacteria Pseudomonas putida and Clostridium pasteurianum (data not shown), both of which contain high activity of methionine γ-lyase (8). In contrast, no effect was observed on the growth of Giardia lamblia (another anaerobic parasitic protozoon) even at concentrations as high as 100 μg/ml. This correlates with the apparent absence of methionine γ-lyase activity from this species. Similarly, TFMET at 100 μg/ml did not inhibit the growth in vitro of mouse myeloma cells.
The high efficacy of a single dose of TFMET in the in vivo model for trichomoniasis is impressive. This murine model for infections of T. vaginalis is the standard one for testing putative antitrichomonal drugs, despite not resembling the natural infection closely. Metronidazole is used as the positive drug control for this method, but to eliminate infections completely, three doses of 15 mg/kg during the first 24 h of infection are required (2).
The data presented in this paper suggest that TFMET represents a lead compound for a novel class of anti-infective drugs with high potential as chemotherapeutic agents against a range of prokaryotic and eukaryotic anaerobic pathogens. Importantly, just as with the 5-nitroimidazoles such as metronidazole, the compound's activity traverses the prokaryotic-eukaryotic boundary. It should be possible to develop compounds that are activated by methionine γ-lyase similarly to TFMET and yet are sufficiently different from methionine so that they will have no antimetabolite activity and toxicity in mammals. We have recently solved the structure of methionine γ-lyase of T. vaginalis (G. Goodall, J. C. Mottram, G. H. Coombs, and A. Lapthorne, submitted for publication), which will facilitate the design of favorable TFMET analogues with potential as novel antianaerobe drugs.
ACKNOWLEDGMENTS
J.C.M. is a UK MRC Senior Research Fellow.
REFERENCES
- 1.Alston T A, Bright H J. Conversion of trifluoromethionine to a cross linking agent by γ-cystathionase. Biochem Pharmacol. 1983;32:947–950. doi: 10.1016/0006-2952(83)90608-1. [DOI] [PubMed] [Google Scholar]
- 2.Bremner A F, Coombs G H, North M J. Antitrichomonal activity of α-difluoromethylornithine. J Antimicrob Chemother. 1987;20:405–411. doi: 10.1093/jac/20.3.405. [DOI] [PubMed] [Google Scholar]
- 3.Finegold S M, Wexler H M. Present status of therapy for anaerobic infections. Clin Infect Dis. 1996;23(Suppl. 1):S9–S14. doi: 10.1093/clinids/23.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 4.Freeman C D, Klutman N E, Lamp K C. Metronidazole—a therapeutic review and update. Drugs. 1997;54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lockwood B C, Coombs G H. Purification and characterisation of methionine γ-lyase from Trichomonas vaginalis. Biochem J. 1991;279:675–682. doi: 10.1042/bj2790675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKie A E, Edlind T, Walker J, Mottram J C, Coombs G H. The primitive protozoon Trichomonas vaginalis contains two methionine-γ-lyase genes that encode members of the γ-family of pyridoxal-5′-phosphate-dependent enzymes. J Biol Chem. 1998;273:5549–5556. doi: 10.1074/jbc.273.10.5549. [DOI] [PubMed] [Google Scholar]
- 7.Riscoe M K, Ferro A J, Fitchen J H. Methionine recycling as a target for antiprotozoal drug development. Parasitol Today. 1989;5:330–333. doi: 10.1016/0169-4758(89)90128-2. [DOI] [PubMed] [Google Scholar]
- 8.Soda K. Microbial sulphur amino acids—an overview. Methods Enzymol. 1987;143:453–459. doi: 10.1016/0076-6879(87)43080-2. [DOI] [PubMed] [Google Scholar]
- 9.Stover C K, Warrener P, VanDevanter D R, Sherman D R, Arain T M, Langhorne M H, Anderson S W, Towell J A, Yuan Y, McMurray D N, Kreiswirth B N, Barry C E, Baker W R. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 10.Thong K-W, Coombs G H. The effects of inhibitors of sulphur-containing amino acid metabolism on the growth of Trichomonas vaginalis in vitro. J Antimicrob Chemother. 1987;19:429–437. doi: 10.1093/jac/19.4.429. [DOI] [PubMed] [Google Scholar]
- 11.Thong K-W, Coombs G H. Trichomonas species: homocysteine desulphurase and serine sulphydrase activities. Exp Parasitol. 1987;63:143–151. doi: 10.1016/0014-4894(87)90155-x. [DOI] [PubMed] [Google Scholar]
- 12.Thong K-W, Coombs G H, Sanderson B E. l-Methionine metabolism in trichomonads. Mol Biochem Parasitol. 1987;23:223–231. doi: 10.1016/0166-6851(87)90029-6. [DOI] [PubMed] [Google Scholar]