Abstract
The default mode network (DMN) of the brain is functionally associated with a wide range of behaviors. In this study, we used functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and spectral fiber photometry to investigate the selective neuromodulatory effect of norepinephrine (NE)–releasing noradrenergic neurons in the locus coeruleus (LC) on the mouse DMN. Chemogenetic-induced tonic LC activity decreased cerebral blood volume (CBV) and glucose uptake and increased synchronous low-frequency fMRI activity within the frontal cortices of the DMN. Fiber photometry results corroborated these findings, showing that LC-NE activation induced NE release, enhanced calcium-weighted neuronal spiking, and reduced CBV in the anterior cingulate cortex. These data suggest that LC-NE alters conventional coupling between neuronal activity and CBV in the frontal DMN. We also demonstrated that chemogenetic activation of LC-NE neurons strengthened functional connectivity within the frontal DMN, and this effect was causally mediated by reduced modulatory inputs from retrosplenial and hippocampal regions to the association cortices of the DMN.
Norepinephrine from LC neurons enhances neuronal activity and connectivity in frontal DMN regions despite vasoconstriction.
INTRODUCTION
Functional magnetic resonance imaging (fMRI) has been widely used to demonstrate the presence of spatiotemporally consistent intrinsic functional brain networks during resting state. The default mode network (DMN), composed of the prefrontal, orbitofrontal, prelimbic, cingulate, retrosplenial, posterior parietal, and temporal association cortices as well as the dorsal hippocampus, is among the most robust intrinsic networks because of its highly synchronized activity in the absence of cognitive tasks or saliency (1). The DMN is vulnerable in several neurological and neuropsychiatric disorders (2), is functionally associated with a wide range of behaviors (3), integrates interoceptive and exteroceptive information from multiple brain networks (4), and maintains the brain in a semivigilant state (5). To make causal interpretations of behaviorally relevant DMN changes and design network-based interventions for disorders that afflict DMN activity, identifying the modulatory mechanisms controlling the DMN is of paramount importance.
The locus coeruleus (LC), a small nucleus within the pons, is a potential DMN modulator (6, 7). A large portion of the neuromodulator norepinephrine (NE) originates from the LC and is released in the brain regions that are considered DMN nodes (8, 9). Accumulating evidence suggests that LC-NE may be essential for DMN modulation because (i) NE receptors are prominently expressed in DMN-related brain structures (9); (ii) LC-NE can bidirectionally modulate attention reorientation in a dose-dependent manner (8); (iii) LC-NE neuron degeneration and DMN disruption are coincidently found in depression (10), traumatic brain injury (11), Parkinson’s disease (12), Alzheimer’s disease (13), and aging (14); and (iv) pharmacological treatment of pathological LC-NE levels reduces attentional lapses (15) and restores DMN integrity in attention deficit hyperactivity disorder (ADHD) patients (16). Despite these findings, the modulatory association between LC-NE and the DMN remains circumstantial because pharmacological interventions using NE-related agents inherently result in nonselective binding on dopaminergic, cholinergic, and serotonergic receptors (7, 17, 18). Furthermore, systemic administration of these agents indiscriminately targets all NE-producing neurons in the brain and sympathetic nervous system, making it difficult to determine the role of LC-NE in modulating the DMN. Although selective manipulation of LC-NE while imaging the DMN is currently impossible in humans due to technical and ethical constraints (7, 19), such studies are feasible in rodent models because structural and functional homologs of the human DMN have been identified in mice (20–28).
In this study, we used an established data-driven approach to identify DMN modules (29) and an intersectional chemogenetic strategy to selectively and reproducibly induce tonic LC-NE activity in mice (30, 31). To reveal potential confounders that could affect our interpretations of LC-NE influence on the DMN, we measured changes in several fMRI metrics, neuronal calcium activity, and glucose uptake across different spatial and temporal scales. Through modeling the signal dynamics, we revealed the circuit mechanism by which LC-NE activation modulates the DMN. Our findings should pave the way toward a better understanding of how large-scale brain networks are mediated by a specific neuromodulatory system.
RESULTS
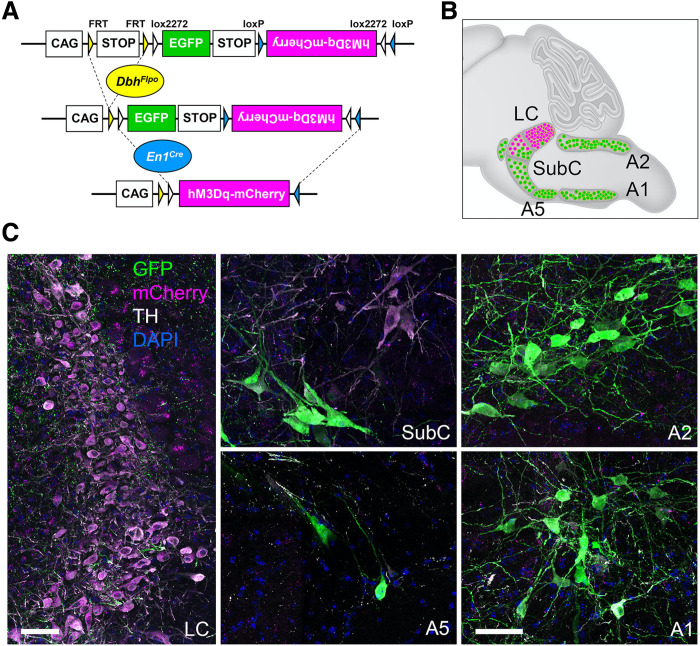
To selectively and reproducibly activate LC-NE neurons, we used an intersectional chemogenetic approach in which the excitatory G protein (heterotrimeric GTP-binding protein)–coupled receptor hM3Dq, fused to mCherry, is expressed in 99.6% of the anatomically defined LC located within the central gray and a small portion of the dorsal subcoeruleus immediately adjacent to and continuous with the LC (LC-NE/hM3Dq) (Fig. 1) (31). This population of NE neurons robustly innervates canonical DMN regions including cingulate 1 (Cg1) and retrosplenial (RSC) cortices (fig. S1) (32).
Fig. 1. Intersectional chemogenetic strategy to selectively activate LC-NE neurons.
(A) Schematic illustration of the intersectional genetic strategy. (B) A sagittal schematic diagram of the hindbrain compressed along the mediolateral axis illustrates the approximate position of NE neurons. Recombination of the RC::FL-hM3Dq allele by the noradrenergic-specific driver DbhFlpo and En1cre results in expression of the excitatory G protein–coupled receptor hM3Dq fused to mCherry in LC-NE neurons (magenta cells in schematic). Expression of DbhFlp by all remaining NE neurons results in expression of green fluorescent protein (GFP) (green neurons in schematic). (C) Immunofluorescent labeling of sections from the adult brainstem of LC-NE/hM3Dq mice reveals hM3Dq-mCherry–expressing NE neurons in the LC (magenta) and GFP-expressing NE neurons (green) in the SubC, A5, C2/A2, and C1/A1 nuclei. Scale bars, 50 μm.
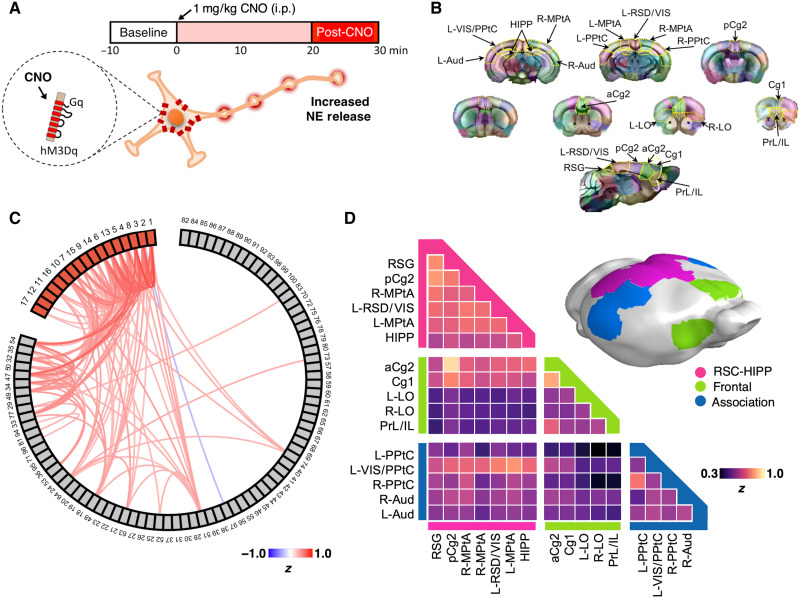
To functionally delineate the DMN and study selective NE modulatory effects, we performed in vivo cerebral blood volume (CBV)–weighted fMRI scans on LC-NE/hM3Dq mice and littermate controls (n = 9 and 12, respectively) under light isoflurane (~1%) anesthesia using a previously described isotropic echo planar imaging (EPI) protocol (33). Although medetomidine with low-dose isoflurane is considered the preferred sedative for rodent fMRI (34), we avoided its usage because it suppresses NE release (35). We collected a 10-min resting-state baseline scan before administering clozapine-n-oxide (CNO; 1 mg/kg, intraperitoneally) (Fig. 2A). CNO was selected because it was previously used for behavior studies of the same mouse line (30, 31). This protocol has been shown to activate LC-NE neurons at tonic frequency and to suppress locomotion in LC-NE/hM3Dq mice (30, 31). Subsequent comparisons were made against littermate controls to account for off-target effects of CNO and/or the back-metabolized clozapine (36). We spatially warped each imaging dataset into the Allen Mouse Common Coordinate Framework (fig. S2), functionally parcellated the baseline fMRI data from all subjects (n = 21) by performing a 100-component independent component analysis (ICA) (Fig. 2, B and C), and verified their reproducibility (fig. S3). We identified 17 DMN-related independent components (ICs) (fig. S4A) according to previous rodent DMN studies (22, 24, 25, 28, 29, 37). The areas showing significant temporal correlation associated with the 17 identified DMN ICs were reconstructed using dual regression (DR), and a one-sample two-sided t test was performed to generate the group-level maps representing the connectivity of these ICs. These maps showed high spatial similarity with an RSC seed-based connectivity map commonly used to depict DMN (fig. S4, B to D). Louvain community modularity analysis clustered the 17 DMN ICs into three distinct modules (Q = 0.10, P < 0.01; Fig. 2D): a Frontal module composed of prelimbic/infralimbic (PrL/IL), lateral orbital (LO), Cg1, and anterior cingulate 2 (aCg2) cortices; an RSC-HIPP module composed of the dorsal hippocampus (HIPP) and posterior Cg2 (pCg2), medial parietal association (MPtA), retrosplenial granular (RSG), and retrosplenial dysgranual/visual (RSD/Vis) cortices; and an Association module composed of posterior parietal (PPtC) and auditory (Aud) cortices. No significant difference among the connectivity of DMN ICs was found in the pre-CNO baseline data between LC-NE/hM3Dq and control groups (PFDR-corrected > 0.05; fig. S4E).
Fig. 2. Experimental design and identification of mouse DMN modules.
(A) CBV fMRI experimental design includes a 10-min baseline scan before CNO administration and 30 min of scans after CNO. i.p., intraperitoneally. (B) One hundred ICs were derived from group ICA of baseline scans among all subjects. Specific IC masks were determined by a winner-take-all strategy by comparing mean z values from all ICs on a voxel basis and then color-coded. DMN-related ICs were then identified according to rodent DMN topology in the literature. (C) Correlation among the 100 ICs was plotted with a threshold Fischer |z| > 0.3. DMN ICs were labeled in red. (D) Modularity analysis of DMN ICs showing that the mouse DMN is composed of Frontal (yellow), RSC-HIPP (pink), and Association modules (blue).
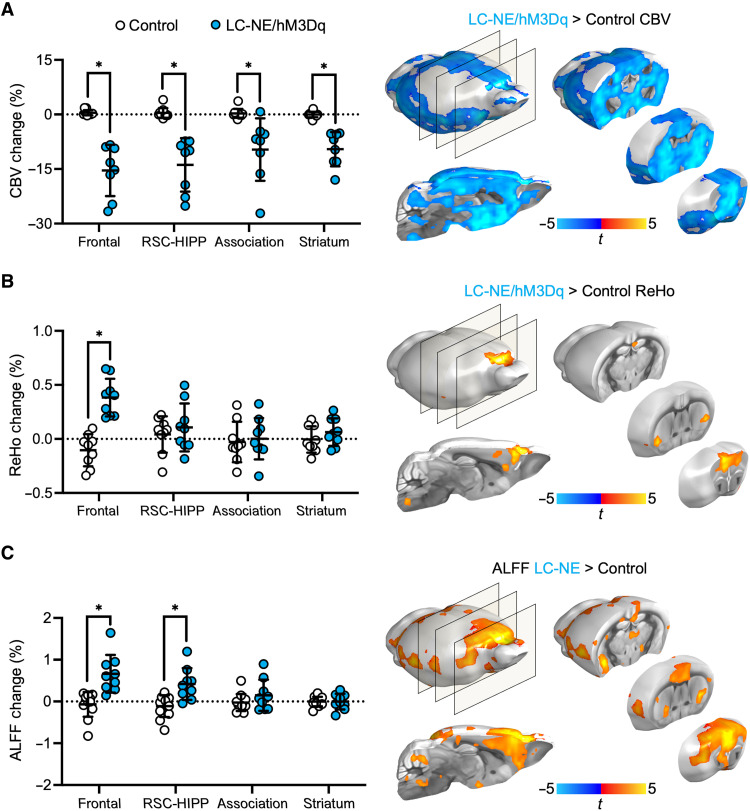
Using the Frontal, RSC-HIPP, and Association module regions of interest (one-sample two-sided t test, P < 0.0001) and striatum as a reference region due to sparse innervation from LC-NE neurons, we examined how NE release from LC modulates fMRI-derived CBV, regional homogeneity (ReHo), and amplitude of low-frequency fluctuation (ALFF) changes. We compared the 10-min pre-CNO baseline fMRI data against data acquired between 20- and 30-min post-CNO. We selected a later phase of the CNO response to avoid the transition time period that has been shown to have greater intrasubject variability and weaker behavioral effects in designer receptor exclusively activated by designer drugs induced activity kinetics as observed by fMRI (6, 38–42) and behavior studies (30, 31). CNO-evoked LC-NE activation significantly decreased CBV from pre-CNO baseline to post-CNO in all DMN modules in LC-NE/hM3Dq. These changes are significant (two-sided two-sample t test, PFDR-corrected < 0.005) compared to control mice (Fig. 3A). LC-NE activation induced robust CBV changes in the striatum despite sparse innervation from LC-NE neurons (6), possibly because of NE-induced vasoconstriction at watershed arteries upstream of the striatum (43). While CBV changes appeared less specific, ReHo (Fig. 3B) and ALFF (Fig. 3C) changes were more localized, and the increase of these signals contradicted the intuitive interpretation of CBV, suggesting a possible increase of synchronous, low-frequency activity in the Frontal and RSC-HIPP DMN modules by LC-NE. Specifically, LC-NE activation enhanced ALFF changes within 0.01- and 0.05-Hz band (unpaired t test, PFDR-corrected < 0.05; fig. S5A).
Fig. 3. CBV, ReHo, and ALFF changes in DMN modules following activation of LC-NE neurons.
(A) CBV decreased (post-CNO, baseline) significantly in LC-NE/hM3Dq following LC-NE activation across all DMN modules compared to controls. LC-NE activation significantly increased (B) ReHo change in the Frontal DMN module and (C) ALFF changes in Frontal and RSC-HIPP modules compared to controls. *PFDR-corrected < 0.005; horizontal dotted lines represent means, and error bars represent ±SD. The brain maps indicate the significant difference of CBV, ReHo, and ALFF changes between LC-NE/hM3Dq and controls (P3dClustSim-corrected < 0.05).
To validate these fMRI findings, we used spectral fiber photometry (Fig. 4A) in LC-NE/hM3Dq (n = 5) and control mice (n = 4). We virally expressed a genetically engineered NE2.1 sensor (44) and a red-shifted jRGECO1a calcium activity sensor (45) under the pan-neuronal human Synapsin-1 (hSyn) promoter in the Cg1 of the Frontal DMN module (Fig. 4B), where the effects of LC-NE activation were most robust, and intravenously administered a CY5-conjugated dextran far-red fluorescent dye to measure CBV (fig. S6). Collectively, this allowed us to simultaneously detect changes in synaptic NE release, neuronal activity–mediated calcium influx, and CBV in Cg1 (Fig. 4C). After CNO administration, we observed significant increases in NE release (unpaired t test, PFDR-corrected < 0.05; Fig. 4D), decreases in CBV (unpaired t test, PFDR-corrected < 0.05; Fig. 4E), and increases in the neuronal activity (unpaired t test, PFDR-corrected < 0.05; Fig. 4F) of LC-NE/hM3Dq mice compared to controls. Notably, the number of calcium spikes also increased significantly following CNO administration (unpaired t test, PFDR-corrected < 0.05; Fig. 4G), a hallmark of NE that tunes the signal-to-noise ratio of downstream neuronal firing (46). These findings corroborate well with our fMRI results (Fig. 3) and indicate that driving LC-NE release can concurrently induce regional vasoconstriction while enhancing neuronal excitability in Cg1. Given that fMRI does not directly measure neuronal activity, these results highlight the importance to cautiously interpret fMRI-derived DMN results when NE is involved, as inferring neuronal activity by direct fMRI signal changes in this case may be erroneous.
Fig. 4. Triple-spectral fiber photometry measuring NE release, CBV, and calcium-weighted neuronal activity in Cg1.
(A) Experimental setup of the fiber photometry system with 488-, 561-, and 640-nm lasers simultaneously used to detect NE release (NE2.1), neuronal calcium activity (jRGECO1a), and CBV (CY5-dextran dye) changes, respectively. i.v., intravenously. (B) Cg1 neurons were confirmed to be transfected to express NE2.1 (green) and jRGECO1a (red) sensors. (C) Spectral profiles of NE neuronal activity and CBV sensor emissions used to resolve signals via an established spectral unmined approach. (D to F) Respective effects of CNO on NE release, CBV, and neuronal activity in Cg1 from representative subjects and group level bar graphs. Subjects were continuously recorded for 40 min with a dose of CNO (1 mg/kg) administered via an intraperitoneal catheter at 10 min (t = 0) after scan onset. (G) CNO-induced LC-NE activation altered postsynaptic calcium spiking patterns, resulting in a significant increase in spike counts. Red dots represent the Ca2+ spikes (z score > 1.96). *P < 0.05 and **P < 0.01; horizontal lines represent means, and error bars represent ±SD.
We also examined the effect of NE on brain glucose uptake in a subset of LC-NE/hM3Dq (n = 5) and control (n = 5) mice using an established 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) protocol (47). We found that CNO-induced LC-NE activation significantly decreased glucose uptake in all three DMN modules (paired t test, PFDR-corrected < 0.001), but not in control subjects (Fig. 5 and fig. S7). Although there are mixed findings on how NE alters glucose uptake (48–53), many studies using pharmacology to enhance NE release have also shown glucose uptake suppression (48–53) but are often difficult to disambiguate. One possible mechanism governing this effect is a shift in metabolic pathway by astrocytes to preferentially use glycogen reserves (51, 52). Together, these findings suggest that additional caution needs to be considered for FDG PET data interpretation when triggering NE release.
Fig. 5. Glucose uptake changes in DMN modules following activation of LC-NE neurons.
(A) Activation of LC-NE neurons significantly decreased FDG uptake compared to saline-treated sham and littermate controls receiving CNO. (B) LC-NE activation significantly reduced standardized uptake values (SUVs) in all DMN regions. SUV changes were derived from each subject that underwent two scans (vehicle and CNO administration) following co-registration. Comparisons were made against littermate controls. *P < 0.001 and **P < 0.0001; horizontal dotted line represents the mean, and error bars represent ±SD.
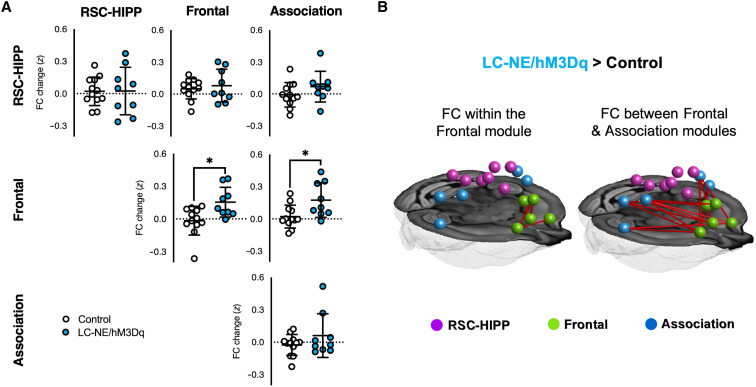
To access how selective NE release modulates DMN connectivity and network properties, we examined functional connectivity (FC) changes within and between Frontal, RSC-HIPP, and Association DMN modules in LC-NE/hM3Dq (n = 9) and control mice (n = 12). We found that CNO significantly enhanced FC within Frontal (t = 2.89, PFDR-corrected < 0.05) and between Frontal and Association modules (t = 2.63, PFDR-corrected < 0.05) of the DMN in LC-NE/hM3Dq, but not in control mice (Fig. 6, A and B). Notably, the high intramodular connectivity characterized by within-module degree (WD), which quantifies the level of node connectivity within a module, indicated that Cg1 and RSC serve as provincial hubs for the Frontal and RSC-HIPP modules of the DMN, respectively (fig. S8, A and B). The high intermodular connectivity characterized by the partition coefficient (PC), which estimates the level of interaction with nodes of other modules, indicated that the aCg2 of the Frontal module serves as a connector hub throughout the entire DMN and may control the FC between Frontal and other modules (fig. S8, A and B). Given the putative causal control of the anterior insular (AI) cortex on the Frontal module of the DMN (26, 54), we also examined the effects of LC-NE activation on their FC changes and found that CNO-evoked activation significantly enhanced anticorrelation between AI and DMN frontal module (fig. S8, C and D).
Fig. 6. FC changes among DMN modules following activation of LC-NE neurons.
(A) Within and between DMN module FC changes. (B) Network-based statistical analysis showing significant differences in FC among edges in LC-NE/hM3Dq > Control. *PFDR-corrected < 0.05; horizontal dotted lines represent means, and error bars represent ±SD.
To further unravel the causal influences on FC in DMN modules by LC-NE activation, we conducted a dynamic causal modeling (DCM) analysis and found that LC-NE activation significantly reduced effective connectivity (EC) from RSC-HIPP to the Association module (paired two-sided t test, PFDR-corrected < 0.05; Fig. 7A), whereas no change was detected in the littermate controls (fig. S9A). Coactivation pattern (CAP) (55) analyses may support these findings, as the CAP representing distinct RSC-HIPP and Association states was also suppressed following LC-NE activation (fig. S9, B to D). Together with the robust NE modulatory effects in the Frontal module, these findings prompted us to conduct a mediation analysis to determine the origin of the FC changes in the Frontal module. A moderation analysis model was constructed using the structural equation modeling method in AMOS 17.0. We first demonstrated that the reduction in EC observed from RSC-HIPP to the Association module causally manipulates the FC changes within the Frontal module (coefficient = −0.53 ± 0.13, P < 0.001; Fig. 7B). Then, when we incorporated the FC increases between the Frontal and Association modules as a mediator, we observed a full mediation effect of the reduced EC to the Frontal module FC changes, while the direct relationship between the reduced EC from RSC-HIPP to the Association module and the increased FC within the Frontal module became insignificant (coefficient = −0.26 ± 0.13, no significance). The Sobel test further indicated the significant mediation effect (Sobel z = −2.02, P < 0.05; Fig. 7B). In contrast, we did not observe the reduced EC from the RSC-HIPP to Association module being mediated by the FC increases between Frontal and Association modules (fig. S10A). Collectively, these findings indicate that LC-NE activation modulates the DMN by (i) strengthening FC within the Frontal module, (ii) strengthening FC between Frontal and Association modules, and (iii) reducing RSC-HIPP control over the Association module, which causally alters Frontal module FC, with the FC between Frontal and Association modules serving as a key mediator.
Fig. 7. DCM and mediation analysis among DMN modules.
(A) DCM analysis among DMN modules found that RSC-HIPP reduced its causal modulation to the Association module upon LC-NE activation (PFDR-corrected < 0.05; horizontal dotted lines represent means, and error bars represent ±SD). (B) Mediation analysis was performed with direct effect (a, b, and c) and with a mediator (c′). A Sobel test was also performed (red dotted line) to evaluate the significance of mediation effect (Sobel z value = −2.02, P < 0.05). *P < 0.05, **P < 0.01, and ***P < 0.001; n.s., no significance.
DISCUSSION
The mouse DMN is well documented in the literature (20–25, 28), and its structural foundation has been recently established by a pivotal study by Whitesell et al. (25). Most studies analyzing the mouse DMN using ICA or seed-based connectivity analyses report consistent DMN architectures that are homologous to the human DMN. A multicenter study compiled by Grandjean et al. (22) analyzed several resting-state mouse fMRI datasets acquired under various conditions (e.g., magnetic field strengths, coils, imaging parameters, and anesthesia protocols) and generated a group ICA atlas delineating mouse brain connectivity. The results pulled three DMN modules that include prefrontal, cingulate/retrosplenial, and temporal association areas. Similar to those findings, our data analysis revealed three modules including Frontal, RSC-HIPP, and Association modules, where the Frontal and RSC-HIPP modules included prefrontal and posterior cingulate components, respectively (Fig. 2C). Note that the involvement of the hippocampus in the mouse DMN remains disputed because of the lack of direct anatomical projections (22, 24, 25). This study includes hippocampus as part of the DMN because we followed established studies identifying the DMN constituents across rodents (29, 37) and humans (4). In addition, several unbiased hierarchical clustering analyses (24, 56), including our own (29, 33), functionally classified the hippocampus as part of the DMN and lend further support to the validity of the DMN regions used for the analysis in this study. Aside from hippocampus, note that thalamus has been recently shown to alter large-scale cortical rhythms and affects fMRI-derived functional connectivity in the cortex, including several putative DMN regions (57). Given the robust LC innervations into thalamus (32), it is crucial for future studies to examine network interactions among LC, thalamus, and DMN.
Several research groups have pioneered chemogenetic fMRI (6, 38–42) and PET (47) approaches to selectively map the influence of neurotransmitters on brain networks in rodents. Our experimental design using an intersectional chemogenetic mouse line presented a unique opportunity to selectively investigate functional DMN modulation. It is our hope that through dissemination of raw data, this work, together with a seminal study by Zerbi et al. (6), will help set the foundation for future studies examining other network systems manipulated by LC-NE.
We demonstrated that chemogenetic activation of LC-NE neurons significantly reduced CBV and glucose uptake among all three DMN modules compared to littermate controls (Figs. 3 and 5). While CBV measures may suffer from systemic effects from LC-NE activation on sympathetic outputs (58), our study used an array of multimodal techniques to help interpret the influence of LC-NE activation on DMN. We found that directional CBV changes are not a proper metric to explain network activity or connectivity changes. Future studies should incorporate central and peripheral NE levels as confounders of hemodynamic-based brain mapping techniques.
Both fMRI and fiber photometry corroborated the findings of CBV reduction in the Frontal DMN module following activation of LC-NE neurons. Concurrently, we observed robust increases in synchronous low-frequency activity as measured by ReHo, ALFF, and photometry-derived calcium activity in our experimental condition (Fig. 4, F and G). The most straightforward interpretation of the fMRI data based on well-documented neurovascular coupling rules (59) does not apply, likely because of the potent vasoconstrictive properties of LC-NE (60). It is not surprising that the neuromodulatory effect induced by NE release appears inconsistent in the literature owing to various brain states and basal firing rate examined, as well as the distinct experimental approaches used to promote/benchmark NE release (6, 61–63). The literature also shows mixed findings regarding the effects of NE on both cerebral hemodynamics and metabolism that are difficult to disambiguate because common pharmacological agents used to induce NE release suffer from differential actions on NE receptor subtypes and nonselective binding (64) that can either increase (65) or decrease perfusion (60, 66) and increase (48, 53) or decrease glucose metabolism (49, 50). One possible mechanism governing the observed changes in glucose metabolism following LC-NE stimulation could also be attributed to a dose-dependent metabolic shift in astrocytes to preferentially use glycogen reserves (51, 52). Unlike many studies that use pharmacological manipulations of NE release, our chemogenetic approach coupled with multimodal measurement of NE, neuronal activity, and CBV revealed an effect of NE in increasing synchronized low-frequency activity, strengthening neuronal firing, and decreasing CBV. This has substantial implications when using fMRI to interpret DMN neuronal activity, as “deactivation” of raw fMRI signal caused by LC-NE activation may not necessarily represent reduced DMN neuronal activity.
Recent animal and human fMRI studies support the role of NE in brain network reorganization (6, 62, 63, 67). NE release has been shown to promote topological integration within the network (68, 69). This aligns well with our findings showing enhanced ReHo and FC within the Frontal DMN module following CNO-evoked LC-NE activation. In addition, our ALFF results show that LC-NE activation enhanced low-frequency power of the Frontal DMN that was restricted to a frequency band ranging from 0.01 to 0.05 Hz—a range associated with robust changes in human DMN (70). Alterations in the power within this subfrequency band of ALFF also plays a vital role in attention reorientation during visual-motor attentional tasks (70), which has also been linked to changes in cortical NE levels (71).
LC-NE enhanced FC within the Frontal module and between Frontal and Association modules. In agreement with our finding, a seminal study virally transfecting hM3Dq via the Dbh promotor into the LC found significant FC enhancement in the Cg1, Cg2, and RSC following chemogenetic stimulation (6). Abnormally heightened FC in Frontal DMN regions has been associated with anxiety and depression (72). Anxiety-like behavioral phenotypes such as reduced locomotor activity in a novel environment and anhedonia have also been found following chemogenetic-evoked LC-NE activation in awake mice (30). Such a behavior response is similar to optogenetic-induced LC-NE neuronal activation at tonic frequencies (73). The intersectional chemogenetic strategy used in the current study has been shown to result in approximately 2 Hz of tonic firing (31), comparable to that in (74). Unlike phasic LC bursting that induces arousal by desynchronizing cortical electroencephalography (EEG) states (75), the low tonic LC firing induced by CNO as seen in our experimental condition has been shown to strengthen theta and suppress delta power (74) and does not trigger sufficient arousal activity under anesthesia (76). Together with our data showing tonically elevated NE release (Fig. 4D) and strengthened DMN connectivity (Fig. 6), these results suggest that tonic firing of LC-NE neurons shifts the brain toward a DMN-dominated state and therefore facilitates DMN-associated behaviors (77).
As we did not observe any FC decreases in the DMN and only identified strengthening FC within and between modules, our results support the functional integration theory of NE proposed by Shine (68), complemented by the results of Zerbi et al. (6), and suggest that LC-NE–induced functional integration could occur at a rather focal, subnetwork level within the DMN. In addition, we found that FC strengthened anticorrelated coupling between the Frontal DMN module and AI, a key node of the salience network (SN) that may causally suppress the DMN (fig. S8, C and D) (54). This also indicates that integration between the DMN and SN is greater following LC-NE activation under our experimental condition. This finding may suggest a putative role of tonic LC-NE activity in improving efficient cognitive control and reducing behavioral variability by fostering SN-DMN anticorrelation (78). In addition, our findings point to the possibility that tonic and phasic outputs from LC-NE neurons may preferentially drive DMN and SN, respectively. Furthermore, the enhanced anticorrelation between networks also supports the plausible mechanism by which NE-targeting pharmacological agents like atomoxetine, clonidine, and guanfacine are effective in treating ADHD patients because they strengthen the FC within the Frontal DMN module and result in improved attention (79).
Our DCM and mediation analyses show that enhanced Frontal module connectivity in the DMN was causally manipulated by reducing RSC-HIPP control of the Association module, with the connectivity between the Association and Frontal modules serving as a key mediator. These findings reveal a new understanding of how LC-NE activation controls the signaling cascades within DMN modules and achieves its control of the Frontal cortical regions, which are among the most well-studied projection targets of LC-NE because of their importance in shaping multiple behaviors (80). LC-NE neuronal loss and the subsequent depletion of cortical NE levels are widely considered to be among the first sites of neurodegeneration in Parkinson’s and Alzheimer’s diseases (81), resulting in behavioral pathologies linked to alterations in DMN such as delayed attention shifting (82), enhanced mind-wandering (83), and reduced cognitive and emotional processing of sensory information (82, 83). Optogenetic-induced LC-NE activation at lower tonic frequencies into prefrontal and orbitofrontal cortices enhances stimulus and goal-directed attention with decreased impulsivity (84). Conversely, the suppression of LC-NE activation exacerbates distractibility and impulsivity (84), similar to that observed in Parkinson’s and Alzheimer’s disease patients (83). Together, alterations within Frontal and between Frontal and RSC-HIPP DMN modules have potential to serve as early biomarkers for pathophysiological changes in LC-NE neurons. Our circuit-level findings could also pave the way toward novel targets to causally control the Frontal DMN via the RSC-HIPP module when LC neurons have degenerated such that the behavioral traits relevant to the Frontal DMN may be restored when endogenous NE is pathologically diminished.
MATERIALS AND METHODS
Animals
All animal procedures were performed in strict compliance with ethical regulations for animal research and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. En1cre (85), DbhFlpo (32), and RC::FL-hM3Dq (31) mouse colonies are maintained on a C57BL/6J background. Male and female triple-transgenic animals were generated at the National Institute of Environmental Health Sciences by crossing En1cre mice to double-transgenic DbhFlpo;RC::FL-hM3Dq mice. Single- and double-transgenic littermates served as controls essential for rigor in chemogenetic studies (86) because off-target binding of CNO or clozapine through reverse metabolism of CNO may occur (36). All animals were maintained on a 12-hour/12-hour light-dark cycle with access to food and water ad libitum.
CBV-fMRI acquisition
For fMRI studies, LC-NE (n = 9) and control mice (n = 12) were initially anesthetized using 2 to 3% isoflurane and maintained under light anesthesia (1% isoflurane) while preserving physiological homeostasis. All MRI experiments were performed on a Bruker BioSpec 9.4-T, 30-cm bore system (Bruker BioSpin Corp., Billerica, MA) with ParaVision 6.0.1 on an AVANCE II console. An RRI BFG 150/90 gradient insert (Resonance Research Inc., Billerica, MA) paired with a Copley C700 gradient amplifier (Copley Controls Corp., Canton, MA) was used for all experiments. A 72-mm-volume coil was used as the transmitter, and a quadrature mouse brain coil was used as the receiver (Bruker BioSpin Corp., Billerica, MA). Magnetic field homogeneity was optimized first by global shimming, followed by local second-order shims using a MAPSHIM protocol. All CBV-fMRI data were acquired using a two-dimensional (2D) multislice, single-shot, gradient-echo EPI sequence: TR (repetition time) = 3000 ms, TE (echo time) = 7.9 ms, bandwidth = 250 kHz, flip angle = 70°, FOV (field of view) = 19.2 mm × 19.2 mm, matrix size = 64 × 64, slice number = 26, slice thickness = 0.3 mm, resulting in an isotropic voxel size of 0.3 mm3. Subjects were continuously recorded for 40 min with a dose of CNO (1 mg/kg) administered via an intraperitoneal catheter 10 min after scan onset. CBV-weighted fMRI was achieved by a bolus dose of an in-house–developed iron oxide nanoparticle (30 mg of Fe/kg, intravenously) (87). Rectal body temperatures were continuously maintained at 37° ± 0.5°C with a temperature controller (Oakton Temp9500, Cole-Parmer, Vernon Hills, IL, USA) coupled to a circulating water bath (Haake S13, Thermo Fisher Scientific, Waltham, MA, USA) that heats the MRI mouse cradle. Respiration was monitored through a pneumatic pillow (Respiration/EEG Monitor, SA Instruments, Stony Brook, NY, USA) and maintained between 90 and 110 breaths/min through fine adjustments in the inhaled isoflurane concentration.
Physiological parameters were monitored during fMRI. The only change observed following CNO administration was an increase in respiratory rate, which rapidly recovered within a few seconds and did not follow the sustained CBV changes measured by fMRI and photometry. Moreover, these changes were observed in both LC-NE/hM3Dq and control groups, indicating that it is likely because of the well-known injection volume stress.
fMRI data analysis
Preprocessing of images
All fMRI data were corrected for slice timing and motion using Analysis of Functional NeuroImages. Brain data were isolated using a U-Net deep-learning skull stripping tool and spatially normalized to our EPI template using Advanced Normalization Tools. In addition, despiking, ICA denoising, and nuisance variable regression of the six motion parameters estimated from motion correction and the cerebrospinal fluid signal extracted using a mask of the major brain ventricles were performed. Datasets were then smoothed using a Gaussian kernel with full width at half maximum (FWHM) at 0.6 mm, detrended, and temporally filtered by applying a high-pass filter between >0.01 Hz. Datasets underwent quality control by measuring frame-wise displacement (FD), temporal signal-to-noise ratio (tSNR), and DVARS (temporal derivative of the root mean square variance over voxels). A detailed description of the preprocessing pipeline (56) can be found in Supplementary Methods.
ICA and modularity analyses
MRI data were decomposed into 100 functional components using baseline data from all subjects via a group-level ICA (FSL MELODIC). Functional modules of the identified 17 DMN components were parcellated using the Louvain community detection algorithm. Within- and between-module connectivity was then defined as the average of FC across node pairs within or between the identified DMN modules. The ICA and modularity analysis method are detailed in Supplementary Methods.
DCM and mediation analysis
We specified a DCM model with full connectivity consisting of three modules from DMN to estimate pairwise EC among the DMN modules and constructed a directed and weighted graph (representing an EC network) for each subject. We applied serial multiple mediation analysis model in AMOS 17.0 (SPSS Inc., Chicago, IL, USA) to uncover underlying functional pathways within DMN. Specifically, we first estimated the direct relationships between dependent variable (EC from RSC-HIPP module to Association module) and independent variable (FC within Frontal module). Then, in a mediation model, the FC between Association and Frontal module was added as a mediator. In this context, full mediation occurs when the relationship between the independent variable and the dependent variable is no longer significant with the inclusion of a mediator variable. Detailed data analysis is further described in Supplementary Methods.
FDG PET procedure
Mice were fasted 12 hours before undergoing 18F-FDG PET scans to reduce variability in blood glucose levels that could alter 18F-FDG uptake (47). Static PET scans were collected on the same cohort of animal over two scan sessions using LC-NE/hM3Dq (n = 5) and control (n = 6) mice to represent sham-treated baseline or CNO-treated condition. Mice were briefly anesthetized under 1 to 3% isoflurane and injected with either a saline + dimethyl sulfoxide (DMSO) vehicle or CNO dissolved in DMSO (1 mg/kg, intraperitoneally) and subsequently received an intravenous injection of ∼0.2 mCi of 18F-FDG after 5 min. Mice were recovered in their home cages for a 45-min uptake period. Mice were subsequently anesthetized with isoflurane (2%) and underwent a 10-min computed tomography (CT) and 20-min PET scan on a small-animal PET/CT scanner (Argus-2R, Sedecal, Madrid, Spain). PET data were reconstructed using the 2D ordered subset expectation maximization (OSEM) algorithm expressed in standardized uptake values (SUVs) and normalized using arm muscle uptake of 18F-FDG using PMOD (PMOD Technologies LLC, Zurich, Switzerland). Data were represented as % changes in SUV between vehicle and CNO scans.
Fiber photometry procedure
Surgical adeno-associated virus (AAV) microinjection and fiber implantation
LC-NE (n = 5) and control (n = 5) mice were microinjected with 0.3 μl of AAV5-hSyn-NE2.1 (h-N01, WZ Biosciences) and 0.5 μl of AAV9-hSyn-jRGECO1a (100854, Addgene) to the left Cg1 (Anterior-Posterior = 2.2 mm, Medial-Lateral = 0.2 mm, Dorsal-Ventral = −1.6 mm). NE2.1 (a green fluorescent NE sensor) and jRGECO1a (a red-shifted intracellular calcium sensor) were used for determining the NE release and neuronal activity, respectively. An optic fiber was implanted 0.3 mm above the injection site and imbedded to the skull using cement (C&B Metabond, S380, Parkell).
Fiber photometry recording
All recordings began at least 4 weeks after surgery. CY5-conjugated dextran fluorescent dye (20 mg/kg; R-FN-006, RuixiBio) was injected intravenously for CBV measurements. Animals were prepared and maintained under the same conditions as fMRI experiments. A spectral fiber photometry system capable of recording NE2.1, jRGECO1a, and CY5 signals was simultaneously recorded during the experiments. Detailed methods can be found in Supplementary Methods.
Immunohistology procedure
Mice were deeply anesthetized with sodium pentobarbital and transcardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were postfixed overnight by immersion in 4% PFA at 4°C. Following a rinse in PBS, brains were cryoprotected in 30% sucrose in PBS and embedded in Tissue Freezing Medium. Forty-micrometer free-floating coronal cryosections were collected in PBS and processed for immunohistochemistry according to previously published protocol (32). Briefly, free-floating sections were blocked in 5% normal goat serum in PBS with 0.1% Triton X-100 for 1 hour before incubating in primary antibody overnight at 4°C. All NE cell bodies were labeled with rabbit anti-tyrosine hydroxylase (TH) antibody (AB152, Millipore). hM3Dq-mCherry-expressing NE neurons were labeled with rat anti-mCherry primary antibody (EST202, Kerafast) and green fluorescent protein (GFP)–expressing NE neurons were labeled with chicken anti-GFP primary antibody (AB13970, Abcam). Sections were washed three times in PBS and incubated for 2 hours in Alexa Fluor 648 anti-rabbit, Alexa Fluor 568 anti-rat, and Alexa Fluor 488 anti-chicken secondary antibodies (Invitrogen). Sections were mounted onto glass slides, coverslipped with Vectashield hard-set mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (H-1500, Vector Labs), and imaged on a Zeiss LSM 880 inverted confocal microscope.
Acknowledgments
We thank the members of the UNC Center for Animal MRI and F. Crews, Z. McElligott, and B. Roth for inputs. We thank the UNC Small Animal Imaging Core Facility staff J. Frank and J. Merrill for assistance in PET data acquisition.
Funding: This research was supported by the Extramural Research Programs of U.S. National Institutes of Health, NINDS (R01NS091236), NIMH (R01MH126518, R01MH111429, and RF1MH117053), NIAAA (P60AA011605 and U01AA020023), and NICHD (P50HD103573) to Y.-Y.I.S. and the Intramural Research Program of the U.S. National Institutes of Health, National Institute of Environmental Health Sciences (ZIA-ES102805 to P.J. and 1ZIAES103310 to G.C.).
Author contributions: E.A.O., M.D., and Y.-Y.I.S. designed the study. E.A.O. and M.D. collected the imaging data. E.A.O., L.-M.H., and S.-H.L. analyzed the imaging data. J.Z. and G.C. performed the viral injections and fiber implantation. E.A.O., T.-H.H.C., and W.Z. collected and analyzed the fiber photometry data. K.G.S. processed and K.G.S. and P.J. analyzed the histology data. N.R.S., I.Y.E., and P.J. developed and shared the transgenic mice. E.A.O., L.-M.H., P.J., and Y.-Y.I.S. wrote the manuscript with input from all authors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All MRI, PET, and photometry data from this study are openly available on Mendeley Data (https://doi.org/10.17632/hxch8htz84.2).
Supplementary Materials
This PDF file includes:
Supplementary Methods
Figs. S1 to S10
References
REFERENCES AND NOTES
- 1.Raichle M. E., The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Buckner R. L., Andrews-Hanna J. R., Schacter D. L., The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Andrews-Hanna J. R., Reidler J. S., Sepulcre J., Poulin R., Buckner R. L., Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christoff K., Irving Z. C., Fox K. C. R., Spreng R. N., Andrews-Hanna J. R., Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 17, 718–731 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Mason M. F., Norton M. I., van Horn J. D., Wegner D. M., Grafton S. T., Macrae C. N., Wandering minds: The default network and stimulus-independent thought. Science 315, 393–395 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zerbi V., Floriou-Servou A., Markicevic M., Vermeiren Y., Sturman O., Privitera M., von Ziegler L., Ferrari K. D., Weber B., De Deyn P. P., Wenderoth N., Bohacek J., Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kelberman M., Keilholz S., Weinshenker D., What’s that (blue) spot on my MRI? Multimodal neuroimaging of the locus coeruleus in neurodegenerative disease. Front. Neurosci. 14, 583421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sara S. J., Bouret S., Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012). [DOI] [PubMed] [Google Scholar]
- 9.van den Brink R. L., Pfeffer T., Donner T. H., Brainstem modulation of large-scale intrinsic cortical activity correlations. Front. Hum. Neurosci. 13, 340 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu X., Zhu Q., Shen H., Liao W., Yuan F., Rumination and default mode network subsystems connectivity in first-episode, drug-naive young patients with major depressive disorder. Sci. Rep. 7, 43105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp D. J., Beckmann C. F., Greenwood R., Kinnunen K. M., Bonnelle V., de Boissezon X., Powell J. H., Counsell S. J., Patel M. C., Leech R., Default mode network functional and structural connectivity after traumatic brain injury. Brain 134, 2233–2247 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Pievani M., Filippini N., van den Heuvel M. P., Cappa S. F., Frisoni G. B., Brain connectivity in neurodegenerative diseases—Fom phenotype to proteinopathy. Nat. Rev. Neurol. 10, 620–633 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Wang P., Zhou B., Yao H., Zhan Y., Zhang Z., Cui Y., Xu K., Ma J., Wang L., An N., Zhang X., Liu Y., Jiang T., Aberrant intra- and inter-network connectivity architectures in Alzheimer’s disease and mild cognitive impairment. Sci. Rep. 5, 14824 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswal B. B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S. M., Beckmann C. F., Adelstein J. S., Buckner R. L., Colcombe S., Dogonowski A.-M., Ernst M., Fair D., Hampson M., Hoptman M. J., Hyde J. S., Kiviniemi V. J., Kötter R., Li S.-J., Lin C.-P., Lowe M. J., Mackay C., Madden D. J., Madsen K. H., Margulies D. S., Mayberg H. S., McMahon K., Monk C. S., Mostofsky S. H., Nagel B. J., Pekar J. J., Peltier S. J., Petersen S. E., Riedl V., Rombouts S. A. R. B., Rypma B., Schlaggar B. L., Schmidt S., Seidler R. D., Siegle G. J., Sorg C., Teng G.-J., Veijola J., Villringer A., Walter M., Wang L., Weng X.-C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.-F., Zhang H.-Y., Castellanos F. X., Milham M. P., Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U.S.A. 107, 4734–4739 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith A., Nutt D., Noradrenaline and attention lapses. Nature 380, 291 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Hoekzema E., Carmona S., Ramos-Quiroga J. A., Richarte Fernández V., Bosch R., Soliva J. C., Rovira M., Bulbena A., Tobeña A., Casas M., Vilarroya O., An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum. Brain Mapp. 35, 1261–1272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bymaster F. P., Katner J. S., Nelson D. L., Hemrick-Luecke S. K., Threlkeld P. G., Heiligenstein J. H., Morin S. M., Gehlert D. R., Perry K. W., Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27, 699–711 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Gobert A., Rivet J. M., Cistarelli L., Melon C., Millan M. J., Alpha2-adrenergic receptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine, and serotonin levels in the frontal cortex of freely moving rats. J. Neurochem. 69, 2616–2619 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Pais-Roldán P., Mateo C., Pan W.-J., Acland B., Kleinfeld D., Snyder L. H., Yu X., Keilholz S., Contribution of animal models toward understanding resting state functional connectivity. Neuroimage 245, 118630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stafford J. M., Jarrett B. R., Miranda-Dominguez O., Mills B. D., Cain N., Mihalas S., Lahvis G. P., Lattal K. M., Mitchell S. H., David S. V., Fryer J. D., Nigg J. T., Fair D. A., Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. U.S.A. 111, 18745–18750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandjean J., Zerbi V., Balsters J. H., Wenderoth N., Rudin M., Structural basis of large-scale functional connectivity in the mouse. J. Neurosci. 37, 8092–8101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandjean J., Canella C., Anckaerts C., Ayrancı G., Bougacha S., Bienert T., Buehlmann D., Coletta L., Gallino D., Gass N., Garin C. M., Nadkarni N. A., Hübner N. S., Karatas M., Komaki Y., Kreitz S., Mandino F., Mechling A. E., Sato C., Sauer K., Shah D., Strobelt S., Takata N., Wank I., Wu T., Yahata N., Yeow L. Y., Yee Y., Aoki I., Chakravarty M. M., Chang W. T., Dhenain M., von Elverfeldt D., Harsan L. A., Hess A., Jiang T., Keliris G. A., Lerch J. P., Meyer-Lindenberg A., Okano H., Rudin M., Sartorius A., van der Linden A., Verhoye M., Weber-Fahr W., Wenderoth N., Zerbi V., Gozzi A., Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. Neuroimage 205, 116278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrier J., Tiran E., Deffieux T., Tanter M., Lenkei Z., Functional imaging evidence for task-induced deactivation and disconnection of a major default mode network hub in the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 117, 15270–15280 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coletta L., Pagani M., Whitesell J. D., Harris J. A., Bernhardt B., Gozzi A., Network structure of the mouse brain connectome with voxel resolution. Sci. Adv. 6, eabb7187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitesell J. D., Liska A., Coletta L., Hirokawa K. E., Bohn P., Williford A., Groblewski P. A., Graddis N., Kuan L., Knox J. E., Ho A., Wakeman W., Nicovich P. R., Nguyen T. N., van Velthoven C. T. J., Garren E., Fong O., Naeemi M., Henry A. M., Dee N., Smith K. A., Levi B., Feng D., Ng L., Tasic B., Zeng H., Mihalas S., Gozzi A., Harris J. A., Regional, layer, and cell-type-specific connectivity of the mouse default mode network. Neuron 109, 545–559.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandino F., Vrooman R. M., Foo H. E., Yeow L. Y., Bolton T. A. W., Salvan P., Teoh C. L., Lee C. Y., Beauchamp A., Luo S., Bi R., Zhang J., Lim G. H. T., Low N., Sallet J., Gigg J., Lerch J. P., Mars R. B., Olivo M., Fu Y., Grandjean J., A triple-network organization for the mouse brain. Mol. Psychiatry , (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang K.-H., Nasrallah F. A., Functional networks and network perturbations in rodents. Neuroimage 163, 419–436 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Sforazzini F., Schwarz A. J., Galbusera A., Bifone A., Gozzi A., Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage 87, 403–415 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Hsu L.-M., Liang X., Gu H., Brynildsen J. K., Stark J. A., Ash J. A., Lin C. P., Lu H., Rapp P. R., Stein E. A., Yang Y., Constituents and functional implications of the rat default mode network. Proc. Natl. Acad. Sci. U.S.A. 113, E4541–E4547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.-W., Das M., Oyarzabal E. A., Cheng Q., Plummer N. W., Smith K. G., Jones G. K., Malawsky D., Yakel J. L., Shih Y.-Y. I., Jensen P., Genetic identification of a population of noradrenergic neurons implicated in attenuation of stress-related responses. Mol. Psychiatry 24, 710–725 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciolino N. R., Plummer N. W., Chen Y.-W., Alexander G. M., Robertson S. D., Dudek S. M., McElligott Z. A., Jensen P., Recombinase-dependent mouse lines for chemogenetic activation of genetically defined cell types. Cell Rep. 15, 2563–2573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson S. D., Plummer N. W., de Marchena J., Jensen P., Developmental origins of central norepinephrine neuron diversity. Nat. Neurosci. 16, 1016–1023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.-H., Broadwater M. A., Ban W., Wang T.-W. W., Kim H.-J., Dumas J. S., Vetreno R. P., Herman M. A., Morrow A. L., Besheer J., Kash T. L., Boettiger C. A., Robinson D. L., Crews F. T., Shih Y.-Y. I., An isotropic EPI database and analytical pipelines for rat brain resting-state fMRI. Neuroimage 243, 118541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paasonen J., Stenroos P., Salo R. A., Kiviniemi V., Gröhn O., Functional connectivity under six anesthesia protocols and the awake condition in rat brain. Neuroimage 172, 9–20 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Nacif-Coelho C., Correa-Sales C., Chang L. L., Maze M., Perturbation of ion channel conductance alters the hypnotic response to the α2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology 81, 1527–1534 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Gomez J. L., Bonaventura J., Lesniak W., Mathews W. B., Sysa-Shah P., Rodriguez L. A., Ellis R. J., Richie C. T., Harvey B. K., Dannals R. F., Pomper M. G., Bonci A., Michaelides M., Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu H., Zou Q., Gu H., Raichle M. E., Stein E. A., Yang Y., Rat brains also have a default mode network. Proc. Natl. Acad. Sci. U.S.A. 109, 3979–3984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu W., Ma Z., Ma Y., Dopfel D., Zhang N., Suppressing anterior cingulate cortex modulates default mode network and behavior in awake rats. Cereb. Cortex 31, 312–323 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters L. M., Hinz R., Detrez J. R., Missault S., de Vos W. H., Verhoye M., van der Linden A., Keliris G. A., Chemogenetic silencing of neurons in the mouse anterior cingulate area modulates neuronal activity and functional connectivity. Neuroimage 220, 117088 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Roelofs T. J. M., Verharen J. P. H., van Tilborg G. A. F., Boekhoudt L., van der Toorn A., de Jong J. W., Luijendijk M. C. M., Otte W. M., Adan R. A. H., Dijkhuizen R. M., A novel approach to map induced activation of neuronal networks using chemogenetics and functional neuroimaging in rats: A proof-of-concept study on the mesocorticolimbic system. Neuroimage 156, 109–118 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Giorgi A., Migliarini S., Galbusera A., Maddaloni G., Mereu M., Margiani G., Gritti M., Landi S., Trovato F., Bertozzi S. M., Armirotti A., Ratto G. M., de Luca M. A., Tonini R., Gozzi A., Pasqualetti M., Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep. 21, 910–918 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Peeters L. M., van den Berg M., Hinz R., Majumdar G., Pintelon I., Keliris G. A., Cholinergic modulation of the default mode like network in rats. iScience 23, 101455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strebel S. P., Kindler C., Bissonnette B., Tschalèr G., Deanovic D., The impact of systemic vasoconstrictors on the cerebral circulation of anesthetized patients. Anesthesiology 89, 67–72 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Feng J., Zhang C., Lischinsky J. E., Jing M., Zhou J., Wang H., Zhang Y., Dong A., Wu Z., Wu H., Chen W., Zhang P., Zou J., Hires S. A., Zhu J. J., Cui G., Lin D., Du J., Li Y., A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dana H., Mohar B., Sun Y., Narayan S., Gordus A., Hasseman J. P., Tsegaye G., Holt G. T., Hu A., Walpita D., Patel R., Macklin J. J., Bargmann C. I., Ahrens M. B., Schreiter E. R., Jayaraman V., Looger L. L., Svoboda K., Kim D. S., Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McBurney-Lin J., Lu J., Zuo Y., Yang H., Locus coeruleus-norepinephrine modulation of sensory processing and perception: A focused review. Neurosci. Biobehav. Rev. 105, 190–199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaelides M., Hurd Y. L., DREAMM: A biobehavioral imaging methodology for dynamic in vivo whole-brain mapping of cell type-specific functional networks. Neuropsychopharmacology 40, 239–240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bremner J. D., Innis R. B., Ng C. K., Staib L. H., Salomon R. M., Bronen R. A., Duncan J., Southwick S. M., Krystal J. H., Rich D., Zubal G., Dey H., Soufer R., Charney D. S., Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch. Gen. Psychiatry 54, 246–254 (1997). [DOI] [PubMed] [Google Scholar]
- 49.Justice A., Feldman S. M., Brown L. L., The nucleus locus coeruleus modulates local cerebral glucose utilization during noise stress in rats. Brain Res. 490, 73–84 (1989). [DOI] [PubMed] [Google Scholar]
- 50.French N., Lalies M. D., Nutt D. J., Pratt J. A., Idazoxan-induced reductions in cortical glucose use are accompanied by an increase in noradrenaline release: Complementary [14C]2-deoxyglucose and microdialysis studies. Neuropharmacology 34, 605–613 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Sorg O., Magistretti P. J., Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 563, 227–233 (1991). [DOI] [PubMed] [Google Scholar]
- 52.Quach T. T., Rose C., Schwartz J. C., [3H]Glycogen hydrolysis in brain slices: Responses to neurotransmitters and modulation of noradrenaline receptors. J. Neurochem. 30, 1335–1341 (1978). [DOI] [PubMed] [Google Scholar]
- 53.Cannella N., Cosa-Linan A., Roscher M., Takahashi T. T., Vogler N., Wängler B., Spanagel R., [18F]-fluorodeoxyglucose-positron emission tomography in rats with prolonged cocaine self-administration suggests potential brain biomarkers for addictive behavior. Front. Psych. 8, 218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon V., Uddin L. Q., Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 214, 655–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutierrez-Barragan D., Basson M. A., Panzeri S., Gozzi A., Infraslow state fluctuations govern spontaneous fmri network dynamics. Curr. Biol. 29, 2295–2306.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zerbi V., Grandjean J., Rudin M., Wenderoth N., Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage 123, 11–21 (2015). [DOI] [PubMed] [Google Scholar]
- 57.C. Canella, F. Rocchi, S. Noei, D. Gutierrez-Barragan, L. Coletta, A. Galbusera, S. Vassanelli, M. Pasqualetti, G. Iurilli, S. Panzeri, A. Gozzi, Cortical silencing results in paradoxical fMRI overconnectivity. bioRxiv 2020.08.05.237958 [Preprint]. 15 October 2020. 10.1101/2020.08.05.237958. [DOI]
- 58.Wood C. S., Valentino R. J., Wood S. K., Individual differences in the locus coeruleus-norepinephrine system: Relevance to stress-induced cardiovascular vulnerability. Physiol. Behav. 172, 40–48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Logothetis N. K., What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Bekar L. K., Wei H. S., Nedergaard M., The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J. Cereb. Blood Flow Metab. 32, 2135–2145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pais-Roldán P., Takahashi K., Sobczak F., Chen Y., Zhao X., Zeng H., Jiang Y., Yu X., Indexing brain state-dependent pupil dynamics with simultaneous fMRI and optical fiber calcium recording. Proc. Natl. Acad. Sci. U.S.A. 117, 6875–6882 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Brink R. L., Pfeffer T., Warren C. M., Murphy P. R., Tona K.-D., van der Wee N. J. A., Giltay E., van Noorden M. S., Rombouts S. A. R. B., Donner T. H., Nieuwenhuis S., Catecholaminergic neuromodulation shapes intrinsic MRI functional connectivity in the human brain. J. Neurosci. 36, 7865–7876 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shine J. M., van den Brink R. L., Hernaus D., Nieuwenhuis S., Poldrack R. A., Catecholaminergic manipulation alters dynamic network topology across cognitive states. Netw. Neurosci. 2, 381–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gozzi A., Lepore S., Vicentini E., Merlo-Pich E., Bifone A., Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: A fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology 38, 2120–2130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toussay X., Basu K., Lacoste B., Hamel E., Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci. 33, 3390–3401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raichle M. E., Hartman B. K., Eichling J. O., Sharpe L. G., Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc. Natl. Acad. Sci. U.S.A. 72, 3726–3730 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eldar E., Cohen J. D., Niv Y., The effects of neural gain on attention and learning. Nat. Neurosci. 16, 1146–1153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shine J. M., Neuromodulatory influences on integration and segregation in the brain. Trends Cogn. Sci. 23, 572–583 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Wainstein G., Rojas-Líbano D., Medel V., Alnæs D., Kolskår K. K., Endestad T., Laeng B., Ossandon T., Crossley N., Matar E., Shine J. M., The ascending arousal system promotes optimal performance through mesoscale network integration in a visuospatial attentional task. Netw. Neurosci. 5, 890–910 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baria A. T., Baliki M. N., Parrish T., Apkarian A. V., Anatomical and functional assemblies of brain BOLD oscillations. J. Neurosci. 31, 7910–7919 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grefkes C., Wang L. E., Eickhoff S. B., Fink G. R., Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb. Cortex 20, 783–797 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Saviola F., Pappaianni E., Monti A., Grecucci A., Jovicich J., de Pisapia N., Trait and state anxiety are mapped differently in the human brain. Sci. Rep. 10, 11112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter M. E., Yizhar O., Chikahisa S., Nguyen H., Adamantidis A., Nishino S., Deisseroth K., de Lecea L., Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vazey E. M., Aston-Jones G., Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. U.S.A. 111, 3859–3864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang M., Logothetis N. K., Eschenko O., Phasic activation of the locus coeruleus attenuates the acoustic startle response by increasing cortical arousal. Sci. Rep. 11, 1409 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross J. A., Van Bockstaele E. J., The locus coeruleus-norepinephrine system in stress and arousal: Unraveling historical, current, and future perspectives. Front. Psych. 11, 601519 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aston-Jones G., Cohen J. D., An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Kelly A. M. C., Uddin L. Q., Biswal B. B., Castellanos F. X., Milham M. P., Competition between functional brain networks mediates behavioral variability. Neuroimage 39, 527–537 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Wang M., Ramos B. P., Paspalas C. D., Shu Y., Simen A., Duque A., Vijayraghavan S., Brennan A., Dudley A., Nou E., Mazer J. A., McCormick D. A., Arnsten A. F. T., α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129, 397–410 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Chandler D. J., Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations. Brain Res. 1641, 197–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zarow C., Lyness S. A., Mortimer J. A., Chui H. C., Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 60, 337–341 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Mohan A., Roberto A. J., Mohan A., Lorenzo A., Jones K., Carney M. J., Liogier-Weyback L., Hwang S., Lapidus K. A., The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: A review. Yale J. Biol. Med. 89, 49–57 (2016). [PMC free article] [PubMed] [Google Scholar]
- 83.Peterson A. C., Li C.-S. R., Noradrenergic dysfunction in Alzheimer’s and Parkinson’s diseases—An overview of imaging studies. Front. Aging Neurosci. 10, 127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bari A., Xu S., Pignatelli M., Takeuchi D., Feng J., Li Y., Tonegawa S., Differential attentional control mechanisms by two distinct noradrenergic coeruleo-frontal cortical pathways. Proc. Natl. Acad. Sci. U.S.A. 117, 29080–29089 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimmel R. A., Turnbull D. H., Blanquet V., Wurst W., Loomis C. A., Joyner A. L., Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 14, 1377–1389 (2000). [PMC free article] [PubMed] [Google Scholar]
- 86.Roth B. L., DREADDs for neuroscientists. Neuron 89, 683–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das M., Oyarzabal E. A., Chen L., Lee S.-H., Shah N., Gerlach G., Zhang W., Chao T.-H. H., Berge N. V. D., Liu C., Donley C., Montgomery S. A., Shih Y.-Y. I., One-pot synthesis of carboxymethyl-dextran coated iron oxide nanoparticles (CION) for preclinical fMRI and MRA applications. Neuroimage 238, 118213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Power J. D., Mitra A., Laumann T. O., Snyder A. Z., Schlaggar B. L., Petersen S. E., Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu L.-M., Wang S., Ranadive P., Ban W., Chao T.-H. H., Song S., Cerri D. H., Walton L. R., Broadwater M. A., Lee S.-H., Shen D., Shih Y.-Y. I., Automatic skull stripping of rat and mouse brain MRI data using U-Net. Front. Neurosci. 14, 568614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Q., Ding S.-L., Li Y., Royall J., Feng D., Lesnar P., Graddis N., Naeemi M., Facer B., Ho A., Dolbeare T., Blanchard B., Dee N., Wakeman W., Hirokawa K. E., Szafer A., Sunkin S. M., Oh S. W., Bernard A., Phillips J. W., Hawrylycz M., Koch C., Zeng H., Harris J. A., Ng L., The allen mouse brain common coordinate framework: A 3D reference atlas. Cell 181, 936–953.e20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu T. T., Nalci A., Falahpour M., The global signal in fMRI: Nuisance or information? Neuroimage 150, 213–229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-Almagro F., Glasser M. F., Duff E. P., Fitzgibbon S., Westphal R., Carone D., Beckmann C. F., Smith S. M., Hand classification of fMRI ICA noise components. Neuroimage 154, 188–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rummel C., Verma R. K., Schöpf V., Abela E., Hauf M., Berruecos J. F. Z., Wiest R., Time course based artifact identification for independent components of resting-state FMRI. Front. Hum. Neurosci. 7, 214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt K., Bari B., Ralle M., Washington-Hughes C., Muchenditsi A., Maxey E., Lutsenko S., Localization of the locus coeruleus in the mouse brain. J. Vis. Exp. , (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zang Y.-F., He Y., Zhu C.-Z., Cao Q.-J., Sui M.-Q., Liang M., Tian L.-X., Jiang T.-Z., Wang Y.-F., Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Cordes D., Haughton V. M., Arfanakis K., Carew J. D., Turski P. A., Moritz C. H., Quigley M. A., Meyerand M. E., Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 22, 1326–1333 (2001). [PMC free article] [PubMed] [Google Scholar]
- 97.Allen E. A., Erhardt E. B., Damaraju E., Gruner W., Segall J. M., Silva R. F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A. M., Caprihan A., Turner J. A., Eichele T., Adelsheim S., Bryan A. D., Bustillo J., Clark V. P., Feldstein Ewing S. W., Filbey F., Ford C. C., Hutchison K., Jung R. E., Kiehl K. A., Kodituwakku P., Komesu Y. M., Mayer A. R., Pearlson G. D., Phillips J. P., Sadek J. R., Stevens M., Teuscher U., Thoma R. J., Calhoun V. D., A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 5, 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaefer A., Margulies D. S., Lohmann G., Gorgolewski K. J., Smallwood J., Kiebel S. J., Villringer A., Dynamic network participation of functional connectivity hubs assessed by resting-state fMRI. Front. Hum. Neurosci. 8, 195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abou-Elseoud A., Starck T., Remes J., Nikkinen J., Tervonen O., Kiviniemi V., The effect of model order selection in group PICA. Hum. Brain Mapp. 31, 1207–1216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allen E. A., Erhardt E. B., Wei Y., Eichele T., Calhoun V. D., Capturing inter-subject variability with group independent component analysis of fMRI data: A simulation study. Neuroimage 59, 4141–4159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abou Elseoud A., Littow H., Remes J., Starck T., Nikkinen J., Nissilä J., Timonen M., Tervonen O., Kiviniemi V., Group-ICA model order highlights patterns of functional brain connectivity. Front. Syst. Neurosci. 5, 37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith S. M., Fox P. T., Miller K. L., Glahn D. C., Fox P. M., Mackay C. E., Filippini N., Watkins K. E., Toro R., Laird A. R., Beckmann C. F., Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 106, 13040–13045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiviniemi V., Starck T., Remes J., Long X., Nikkinen J., Haapea M., Veijola J., Moilanen I., Isohanni M., Zang Y.-F., Tervonen O., Functional segmentation of the brain cortex using high model order group PICA. Hum. Brain Mapp. 30, 3865–3886 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.To X. V., Vegh V., Nasrallah F. A., Towards data-driven group inferences of resting-state fMRI data in rodents: Comparison of group ICA, GIG-ICA, and IVA-GL. J. Neurosci. Methods 366, 109411 (2022). [DOI] [PubMed] [Google Scholar]
- 105.Jarrahi B., Examining the influence of spatial smoothing on spatiotemporal features of intrinsic connectivity networks at low ICA model order. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 3221–3224 (2021). [DOI] [PubMed] [Google Scholar]
- 106.Zuo X.-N., Kelly C., Adelstein J. S., Klein D. F., Castellanos F. X., Milham M. P., Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage 49, 2163–2177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blondel V. D., Guillaume J.-L., Lambiotte R., Lefebvre E., Fast unfolding of communities in large networks. J. Stat. Mech. 2008, P10008 (2008). [Google Scholar]
- 108.Meilă M., Comparing clusterings—An information based distance. J. Multivar. Anal. 98, 873–895 (2007). [Google Scholar]
- 109.Zalesky A., Fornito A., Bullmore E. T., Network-based statistic: Identifying differences in brain networks. Neuroimage 53, 1197–1207 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Park H.-J., Friston K. J., Pae C., Park B., Razi A., Dynamic effective connectivity in resting state fMRI. Neuroimage 180, 594–608 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penny W. D., Stephan K. E., Daunizeau J., Rosa M. J., Friston K. J., Schofield T. M., Leff A. P., Comparing families of dynamic causal models. PLOS Comput. Biol. 6, e1000709 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boucard A., Marchand A., Noguès X., Reliability and validity of structural equation modeling applied to neuroimaging data: A simulation study. J. Neurosci. Methods 166, 278–292 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Baron R. M., Kenny D. A., The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182 (1986). [DOI] [PubMed] [Google Scholar]
- 114.Preacher K. J., Hayes A. F., Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879–891 (2008). [DOI] [PubMed] [Google Scholar]
- 115.Gutierrez-Barragan D., Singh N. A., Alvino F. G., Coletta L., Rocchi F., De Guzman E., Galbusera A., Uboldi M., Panzeri S., Gozzi A., Unique spatiotemporal fMRI dynamics in the awake mouse brain. Curr. Biol. 32, 631–644.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Perez P. D., Ma Z., Ma Z., Dopfel D., Cramer S., Tu W., Zhang N., An open database of resting-state fMRI in awake rats. Neuroimage 220, 117094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nieuwenhuis B., Haenzi B., Hilton S., Carnicer-Lombarte A., Hobo B., Verhaagen J., Fawcett J. W., Optimization of adeno-associated viral vector-mediated transduction of the corticospinal tract: Comparison of four promoters. Gene Ther. 28, 56–74 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Figs. S1 to S10
References