Abstract
Cataplexy in the narcoleptic canine has been shown to increase after systemic administration of cholinergic agonists. Furthermore, the number of cholinergic receptors in the pontine reticular formation of narcoleptic canines is significantly elevated. In the present study we have investigated the effects of cholinergic drugs administered directly into the pontine reticular formation on cataplexy, as defined by brief episodes of hypotonia induced by emotions, in narcoleptic canines. Carbachol and atropine were perfused through microdialysis probes implanted bilaterally in the pontine reticular formation of freely moving, narcoleptic and control Doberman pinschers. Cataplexy was quantified using the Food-Elicited Cataplexy Test, and analysed using recordings of electroencephalogram, electrooculogram and electromyogram. Cataplexy was characterized by a desynchronized electroencephalogram and a drop in electromyogram and electrooculogram activity. In narcoleptic canines, both unilateral and bilateral carbachol (10−5 to 10−3 M) produced a dose-dependent increase in cataplexy, which resulted in complete muscle tone suppression at the highest concentration. In control canines, neither bilateral nor unilateral carbachol (10−5 to 10−3 M) produced cataplexy, although bilateral carbachol, did produce muscle atonia at the highest dose (10−3). The increase in cataplexy after bilateral carbachol (10−4 M) was rapidly reversed when the perfusion medium was switched to one containing atropine (10−4 M). Bilateral atropine (10−3 to 10−2 M) alone did not produce any significant effects on cataplexy in narcoleptic canines; however, bilateral atropine (10−2 M) did reduce the increase in cataplexy produced by systemic administration of physostigmine (0.05 mg/kg, i.v.).
These findings demonstrate that cataplexy in narcoleptic canines can be stimulated by applying cholinergic agonists directly into the pontine reticular formation. The ability of atropine to inhibit locally and systemically stimulated cataplexy indicates that the pontine reticular formation is a critical component in cholinergic stimulation of cataplexy. Therefore, it is suggested that the pontine reticular formation plays a significant role in the cholinergic regulation of narcolepsy.
Narcolepsy is an incurable sleep disorder characterized by excessive daytime sleepiness, fragmented night-time sleep and pathological manifestations of rapid eye movement (REM) sleep such as sudden episodes of muscle atonia called cataplexy, sleep paralysis and hypnagogic hallucinations (see Ref. 17). Canine narcolepsy is an autosomal recessive, genetically transmitted model of the human disease with many symptoms paralleling those found in humans. Similar to the human condition, narcoleptic canines exhibit excessive daytime sleepiness, fragmented night-time sleep and cataplexy.5,37 Cataplexy, a particularly debilitating symptom of narcolepsy, is elicited by emotional stimulation such as laughter, excitement and anger.19 In canine narcolepsy, cataplexy is often induced by the excitation produced by the presentation of food, and consequently cataplexy may be measured using a Food-Elicited Cataplexy Test (FECT).5 This test is a useful tool for investigating the severity of the disease and has been established as a standardized method for testing the efficacy of various pharmacological compounds on cataplexy.36 Previous studies have identified two classes of drugs which modify cataplexy after systemic administration, monoaminergic and cholinergic, and have shown that indirect agonists such as amphetamine and physostigmine reduce and exacerbate cataplexy, respectively.36,37 These findings are consistent with clinical studies on the therapeutic value of monoaminergic drugs on narcoleptic patients,18,20 while the effects of cholinergic drugs on narcoleptic patients remains unclear.14,19
The cholinergic effects on cataplexy appear to be central in nature, since the anticholinesterase physostigmine enhances cataplexy, whereas neostigmine, which does not penetrate the blood-brain barrier, has no effect on cataplexy.11 It is likely that this effect is mediated via muscarinic receptors since the muscarinic agonist arecoline and the muscarinic antagonists atropine and scopolamine increase and decrease cataplexy, respectively,” whereas nicotine and nicotinic antagonists have no effect on cataplexy.” In binding studies it has been shown that muscarinic receptor levels are increased in the pontine reticular formation (PRF) of narcoleptic canines.9,31 Thus, these studies suggest that muscarinic receptors in the PRF may be involved in the cholinergic regulation of cataplexy in the narcoleptic canine. Therefore, in the present study we have investigated the effects of cholinergic drugs infused directly into the PRF on cataplexy in narcoleptic canines. This was achieved using in vivo microdialysis probes implanted bilaterally into the PRF of freely moving, narcoleptic Doberman pinschers and measuring cataplexy with the FECT test. The effects of carbachol and atropine applied locally through the microdialysis probes, as well as the ability of local atropine to modulate the effects of systemically applied physostigmine were tested.
EXPERIMENTAL PROCEDURES
All studies were performed on adult Doberman pinschers, which included five narcoleptic (three male and two female) two control (both male) and one heterozygous narcoleptic (female) canine. All animals were bred at the Stanford University narcoleptic dog colony. Heterozygous narcolep tic canines contain one copy of the narcolepsy transmitting gene locus, canarc-1, but do not express the symptoms of narcolepsy.37,38 Because the heterozygous canine showed no differences from the control canines in the present study, it was included in the control group. The animals were kept under a 12/12-h light-dark schedule with food and water available ad libitum.
Surgery
All canines were anaesthetized with a mixture of air and isofluorane (2%) and placed on a Kopf stereotaxic frame. For sleep/cataplexy recording, they were implanted with screw electrodes in the skull over the mid frontal and lateral parietal cortex for recording of electroencephalogram (EEG) and in the orbit of the frontal bone for the recording of electroculogram (EOG). Stranded stainless steel wires were inserted into the dorsal neck musculature for recording the electromyogram (EMG). The electrodes were soldered to a 14-pin electrical plug. Custom made guide cannula bundles, consisting of two 20-mm cannulae soldered to a nut stack, were lowered into position over the cortical dura. Four guides, each separated by 2 mm along the rostrocaudal axis, were positioned over the PRF bilaterally [lateral (L): 3.2 and anterior (A): 1.0–7.0 from stereotaxic zero, according to Lim el al.33], such that a row of four guide cannulae was positioned over the rostrocaudal extent of the PRF, all at the same laterality. In some animals it was necessary to place the cannulae slightly more lateral (up to 0.7 mm) in order to avoid placing the cannulae over the mid-saggital sinus. In the present study only the guide cannulae over A 3.0 and A 5.0 were used. Guide cannulae were also placed over other structures though these were not used in this study. The recording electrodes, electrical plug and guide cannulae were cemented to the skull using dental acrylic. The animals were allowed at least three weeks to recover from surgery.
One day prior to experimentation the animals were anaesthetized with a mixture of air and isofluorane (2%), and microdialysis probes (70 mm shaft with 5 mm membrane, CM A/10, CMA/Microdialysis, Stockholm, Sweden) were lowered bilaterally into the PRF [L: 3.2, A: 3.0 or 5.0, ventral (V): 39.0 from stereotaxic zero, according to Lim el al.33] (Fig. 1a, b) and anchored in place using a microdrive device which screwed into the nut stack. The left and right probes were always implanted at the same coordinate on the anterior-posterior axis. Each probe was tested for in vitro recovery of 10−7 M acetylcholine before implantation and the relative recovery found was: 25 + 2%, n = 10. After completing the experiment the probes were removed and the animals returned to their cages. Histological verification of the probe placements was performed on seven of the eight animals in this study, using 2% Neutral Red (four narcoleptic, one control) or FluoroGold (one heterozygous and one control), which was injected (1.0 /ul) through the same guide cannulae and at the same depth coordinate as the dialysis probes.
Fig. 1.
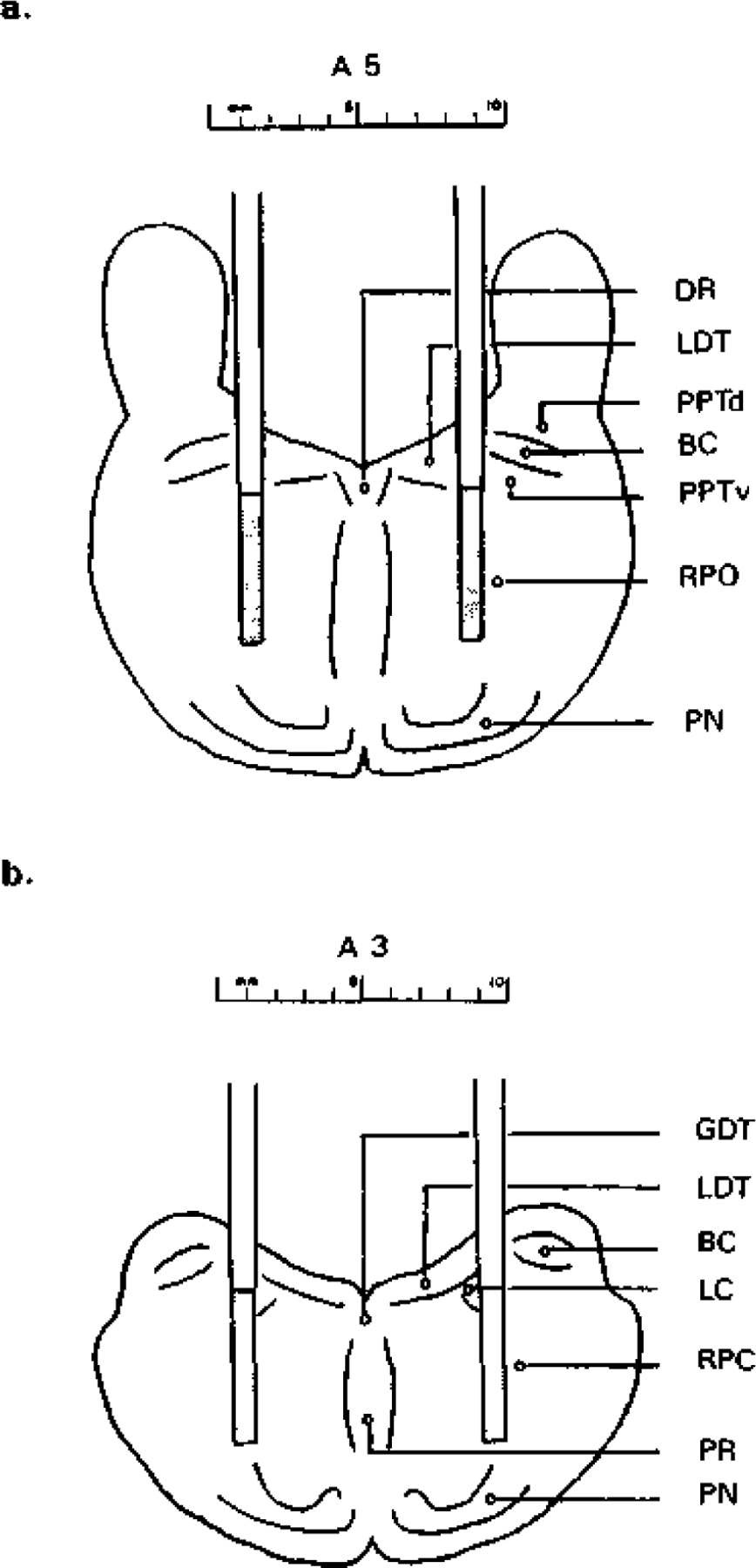
Schematic diagrams illustrating the positions of the microdialysis probes in the pontine reticular formation. In a and b the locations of the probes when implanted bilaterally through the guide cannulae positioned at A 5.0 and A 3.0, respectively, are schematically represented, the dialysis membrane at the tip of each probe is indicated by the dotted area. Schematic figures are based on anatomical data presented in the dog atlas33 and by Isaacson and Tanaka.24 BC, brachium conjunctivum; DR, dorsal raphe nucleus; GDT, dorsal nucleus of Gudden; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; PN, pontine nulcei; PPTd, pedunculopontine tegmental nucleus dorsalis; PPTv, pedunculopontine tegmental nucleus ventralis; PR, pontine raphe nucleus; RPC, nucleus reticularis pontis caudalis; RPO, nucleus reticularis pontis oralis.
Testing procedure
All canines were given at least five days of habituation to the experimental chamber before testing. The chamber is a small room of approximately 3 × 3 m separated from the recording room by a door with one-way mirror glass. The recording cable and the dialysis lines were insulated and wrapped around a 3-m tether which was attached to a shoulder harness. The probes were perfused at 2.0 /ul/min with artificial cerebrospinal fluid (CSF) (125mM NaCl, 0.5 mM NaH2P04, 2.5 mM Na2HPO4, 2 mM CaCl2, 1 mM MgCl2, pH 7.4) using a Harvard Pump. The perfusion medium contained 1 /uM of the acetylcholine esterase inhibitor neostigmine in order to obtain measurable amounts of acetylcholine (see Ref. 46). Cataplexy was measured using the FECT,5 combined with recordings of EEG, EOG and EMG. In each FECT, the subject ate ten small bites of wet dog food which were lined up on the floor in a semi circle over a distance of approximately 3 m and cataplectic attacks were scored when the animal stopped forward motion and the hind quarters were lowered towards the floor, thus initiating either a partial or complete attack. Time required to eat all ten bites of food and successfully move away was also recorded.
Experiments were performed on days 1–5, beginning approximately 18 h after microdialysis probe implantation, between the hours of circadian time 3 and 8. Initially, the animals were tested for baseline cataplexy using two consecutive FECT trials, and then were attached to the microdialysis and recording lines for a control CSF perfusion period of 60 min (bilateral perfusion). At the end of the 60-min control perfusion period baseline cataplexy levels were measured again using four consecutive FECT trials performed over a period of 20 min. After completion of the four FECT trials, the perfusion medium was switched from CSF to CSF plus drug using a manual liquid switch (CMA/110, CMA Microdialysis). Carbachol was tested at three concentrations (10−5 to 10−3 M, pH 7), both bilaterally and unilaterally, by increasing the concentration of carbachol over the course of an experiment: 10−5 M was perfused for the first hour, 10−4 M for the second hour and 10−3 M for the third hour. Cataplexy was measured by two consecutive FECT trials during min 20–30 and min 50–60 of each carbachol concentration period. Control levels of cataplexy were tested under the same schedule in narcoleptic canines receiving no drug infusion. The animals were awake by both behavioral and EEG criteria at the start of all FECT trials. The carbachol experiments were performed during the first two days after implantation, bilateral carbachol on day one and unilateral carbachol (left or right side chosen randomly) on day two. Animals with a positive response to carbachol treatment were used for further investigation. Atropine was tested bilaterally at two concentrations (10−3 to 10−2 M, pH 7) in a manner similar to the carbachol testing during day three after implantation. The ability of atropine to reverse the cataplexy enhancing effects of carbachol was tested by switching from bilateral carbachol to bilateral atropine: carbachol (10−4 M) was perfused for 1 h, followed by a 20 min CSF washout period, and then atropine (10−4M) was perfused for 1 h. Basal cataplexy was measured as described above and drug induced cataplexy was measured during min 20–30 and min 50–60 of each drug perfusion period. The ability of atropine to reverse the cataplexy enhancing effects of systemically applied physostigmine was tested by injecting physostigmine (0.05 mg/kg, i.v.) into an animal which was receiving bilateral atropine (10−2 M) perfusion simultaneously. The atropine perfusion started 1 h before the physostigmine injection. Basal cataplexy was measured as described above and drug induced cataplexy was measured during min 20–30 and min 50–60 of each drug treatment period.
Drugs
Carbamylcholine chloride (carbachol), neostigmine (Sigma, St Louis, MO) and atropine sulfate (Calbiochem, San Diego, CA) were dissolved directly into CSF and tested for pH before local administration. Physostigmine (Calbiochem) was dissolved in 0.9% NaCl and administered intravenously in a 1 mg/ml volume.
Statistics
All results are presented as mean + S.E.M. Control and drug induced levels of cataplexy were analysed with one-way ANOVA, followed by post hoc Fisher PLSD tests. Comparison between drug treatment groups as well as between narcoleptic and control groups was tested across the time variable using two-way ANOVA.
RESULTS
Basal FECT-induced cataplectic attacks in the narcoleptic canines ranged from partial, in which the animal could remain partially upright though a clear loss of muscle tone was indicated by the EMG, to complete, in which the animal would go down on all four limbs and remain atonic for up to one minute. During cataplexy the EEG signal remained desynchronized and the EOG was mostly quiet. No rapid eye movement was observed. A typical baseline cataplectic attack, consisting of a short, complete attack, is shown in Fig. 2a. Baseline levels of FECT induced cataplexy were tested on all five narcoleptic dogs over a 3.5-h control period with no drug treatment (Table 1). While a good deal of individual variability existed, the FECT scores were relatively stable over this control period averaging between 2.4 and 3.0 cataplectic attacks per. FECT (F = 0.126, d.f. = 5, P = 0.986) and elapsing over a time of 47–63 s per FECT (F = 0.315, d.f. = 5, P = 0.902). The possibility that neostigmine (10−6M) in the perfusion medium could modify the level of cataplexy was tested by comparing the average preFECT scores with the ensuing baseline FECT scores. No significant difference was found in number of attacks (F = 0.045, d.f. = 1, P = 833) nor elapsed time (F = 0.174, d.f. = 1, P = 0.678). The control canines and the heterozygous canine did not exhibit cataplexy under baseline conditions and normally completed the FECT within 30–40 s.
Fig. 2.
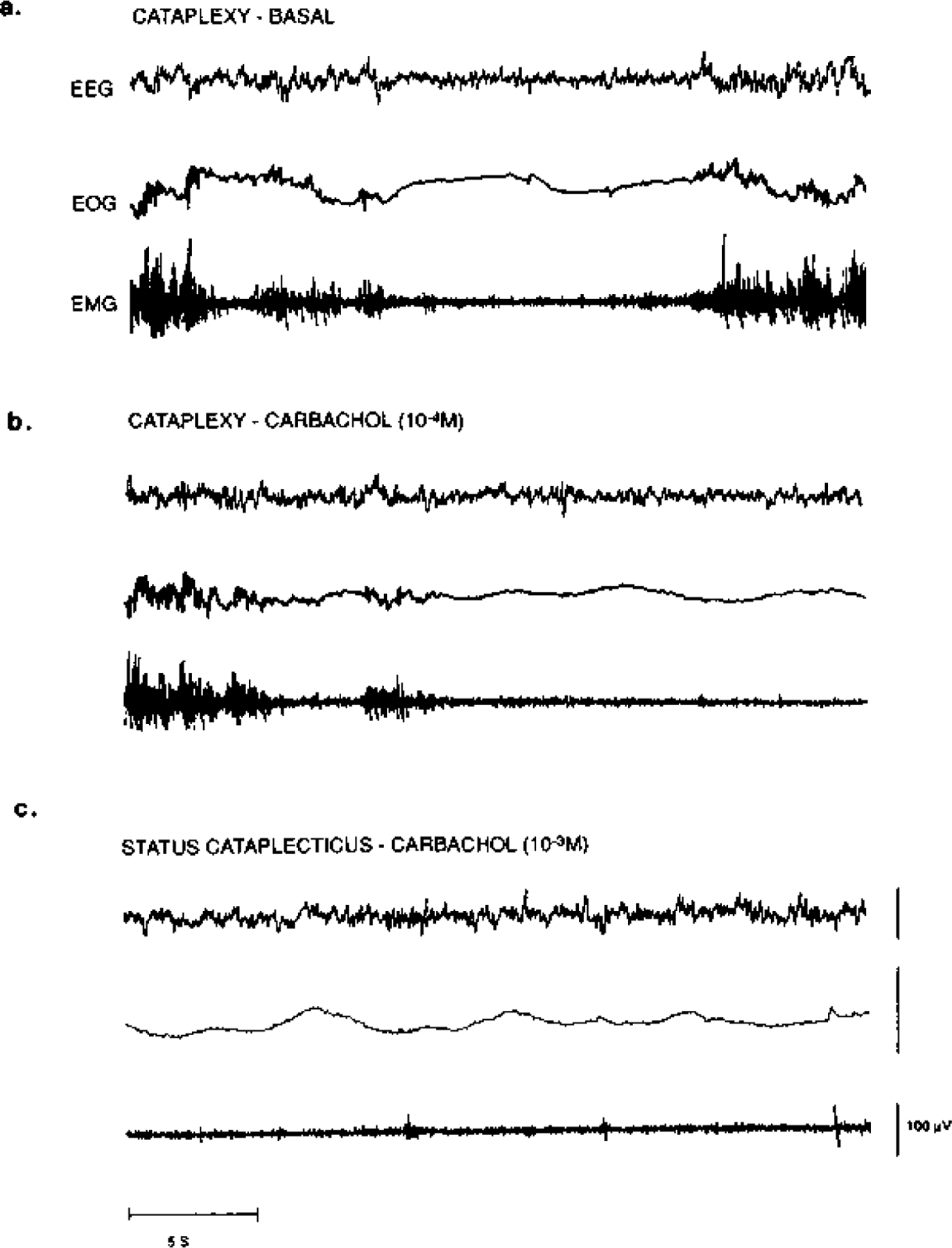
Polygraph recording of cataplexy in a narcoleptic canine during (a) baseline, (b) bilateral 10−4 M carbachol and (c) bilateral 10−3 M carbachol conditions. All recordings were taken from the same animal on the same day. EEG, frontal cortex electroencephalogram.
Table 1.
Cataplexy in five narcoleptic canines peffused bilaterally with cerebrospinal fluid in the pontine reticular formation. Cataplexy was measured using the same schedule as drug perfused animals. The mean number of cataplectic attacks and elapsed time for two FECT per test period is shown
| FECT induced cateplexy: control levels | ||||||
|---|---|---|---|---|---|---|
| Time (min) | −20 | −10 | 20 | 50 | 80 | 110 |
| Attacks M= 5 | 2.8 + 0.6 | 2.9 + 0.4 | 2.4 + 0.4 | 2.8 + 0.5 | 2.6 + 0.4 | 3.0 + 0.6 |
| Elapsed time (s) | 63 + 11 | 47 + 9 | 52 ±8 | 47 + 5 | 61 + 13 | 52 + 8 |
The effects of carbachol perfusion in the PRF on cataplexy in narcoleptic canines are shown in Fig. 3a, b. At low concentrations, bilateral carbachol (10−5 to 10~4 M) produced a dose dependent increase in FECT-induced cataplexy in the narcoleptic canines, which was evident in both the number of attacks (F = 5.539, d.f. = 5, P = 0.0003) and elapsed time (F = 7.068, d.f. = 5, P= 0.0001). In general, complete cataplectic attacks were more prevalent and would last longer during carbachol perfusion, and as with basal cataplexy, the cataplectic attacks were associated with a desynchronized EEG and a decrease in EMG and EOG activity (Fig. 2b). Unilateral carbachol (10−5 to 10−4M) also produced an increase in cataplexy in the narcoleptic canines, evident in the number of attacks (F = 5.384, d.f. = 5, P = 0.0007) and elapsed time (F = 5.607, d.f. = 5, P = 0.0005), but this effect was weaker than bilateral carbachol and was not observed at the lowest concentration. There were no significant differences in the carbachol effect whether the probes were placed at A 3.0 or A 5.0 (cataplectic attacks: F = 1.379, d.f. = 5, P = 0.246; elapsed time: F = 0.338, d.f. = 5, t = 0.888), or perfused unilaterally on either left or right side (cataplectic attacks: F = 0.827, d.f. = 5, t= 0.539; elapsed time: F= 1.877, d.f. = 5, t = 0.126). Furthermore, these concentrations of carbachol (10−5 to 10 −4M), both unilateral and bilateral, did not produce a significant decrease in basal muscle tone in the narcoleptic canines as noted by behavioral observation and EMG analysis. At the highest concentration, both bilateral and unilateral carbachol (10−3 M) produced complete muscle atonia in the narcoleptic canines, which was defined as status cataplecticus. During status cataplecticus there was a predominance of desynchronized EEG, a decrease in EOG activity, and EMG activity was strongly suppressed, similar to that observed during basal cataplexy (Fig. 2c). During status cataplecticus the animals were unable to complete a FECT during the 10-min testing period; therefore, for the purpose of presentation in Figs 3 and 5 status cataplecticus was defined as a FECT with 15 cataplectic attacks which elapsed over a 600-s (10-min) period.
Fig. 3.
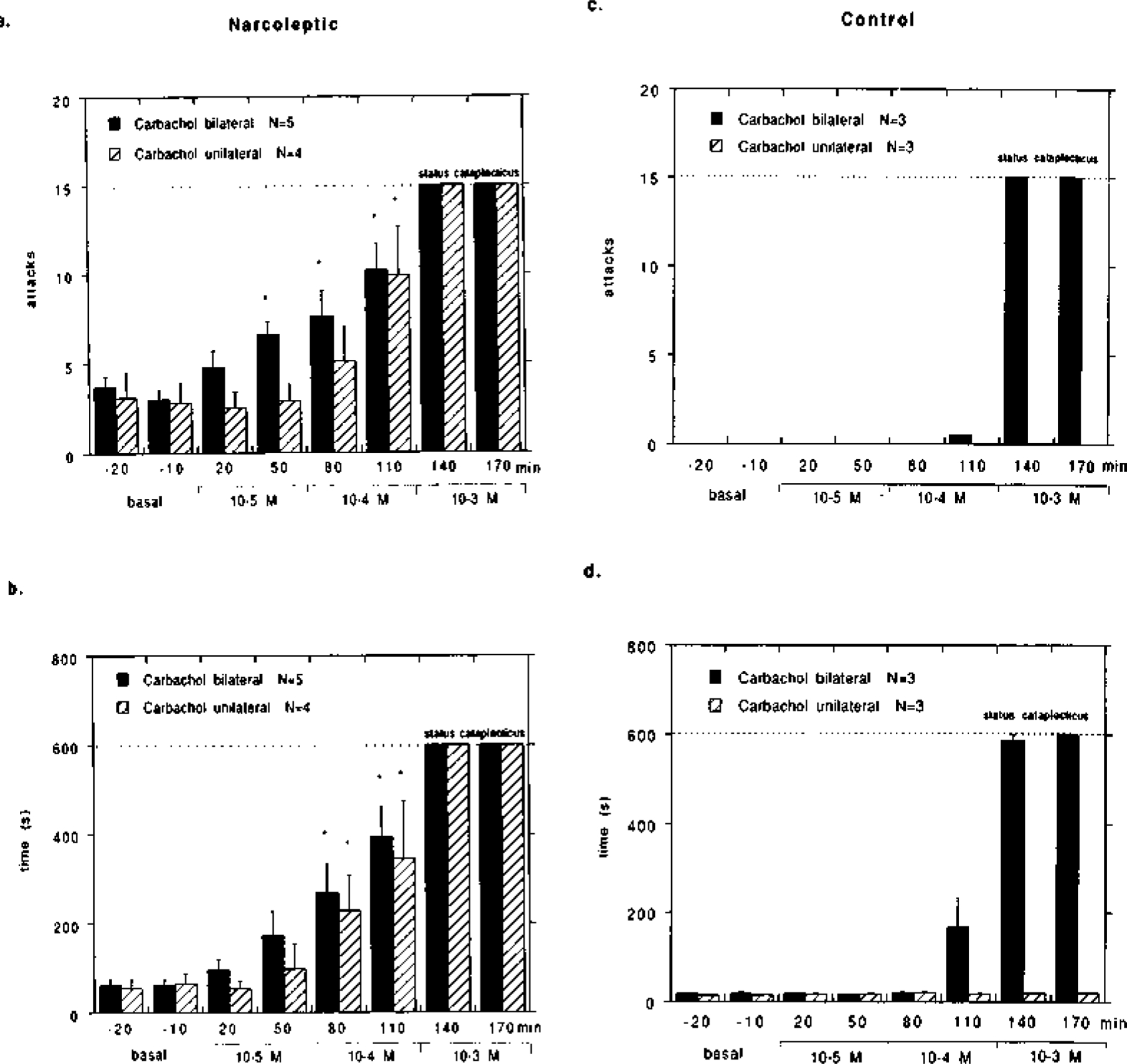
Effect of carbachol (10-s to 10−3 M) perfusion in the pontine reticular formation on cataplexy in (a, b) narcoleptic and (c, d) control canines. Carbachol was mixed into artificial cerebrospinal fluid and perfused through microdialysis probes at the indicated concentrations over the course of a four hour experiment: none during the first hour, 10−5 M during the second hour, 10−4 M during the third hour and 10−3 M during the fourth hour. Five narcoleptic canines were tested bilaterally, and four narcoleptic canines were tested unilaterally. Two control and one heterozygous canine (control group) were tested both bilaterally and unilaterally. The mean number of cataplectic attacks (a, c) and elapsed time (b, d) for two FECTs per test period is shown. For the purpose of figure presentation, status cataplecticus (carbachol atonia) was arbitrarily designated as 15 attacks elapsed over 600 s. Because of the arbitrary, non-variable score for status cataplecticus, statistical analysis was performed on the scores prior to this state. Each carbachol time-point prior to status cataplecticus was compared with the basal time-points using a Fisher PLSD post hoc test; *P < 0.05 satisfactory for comparison with either basal time-point.
Fig. 5.
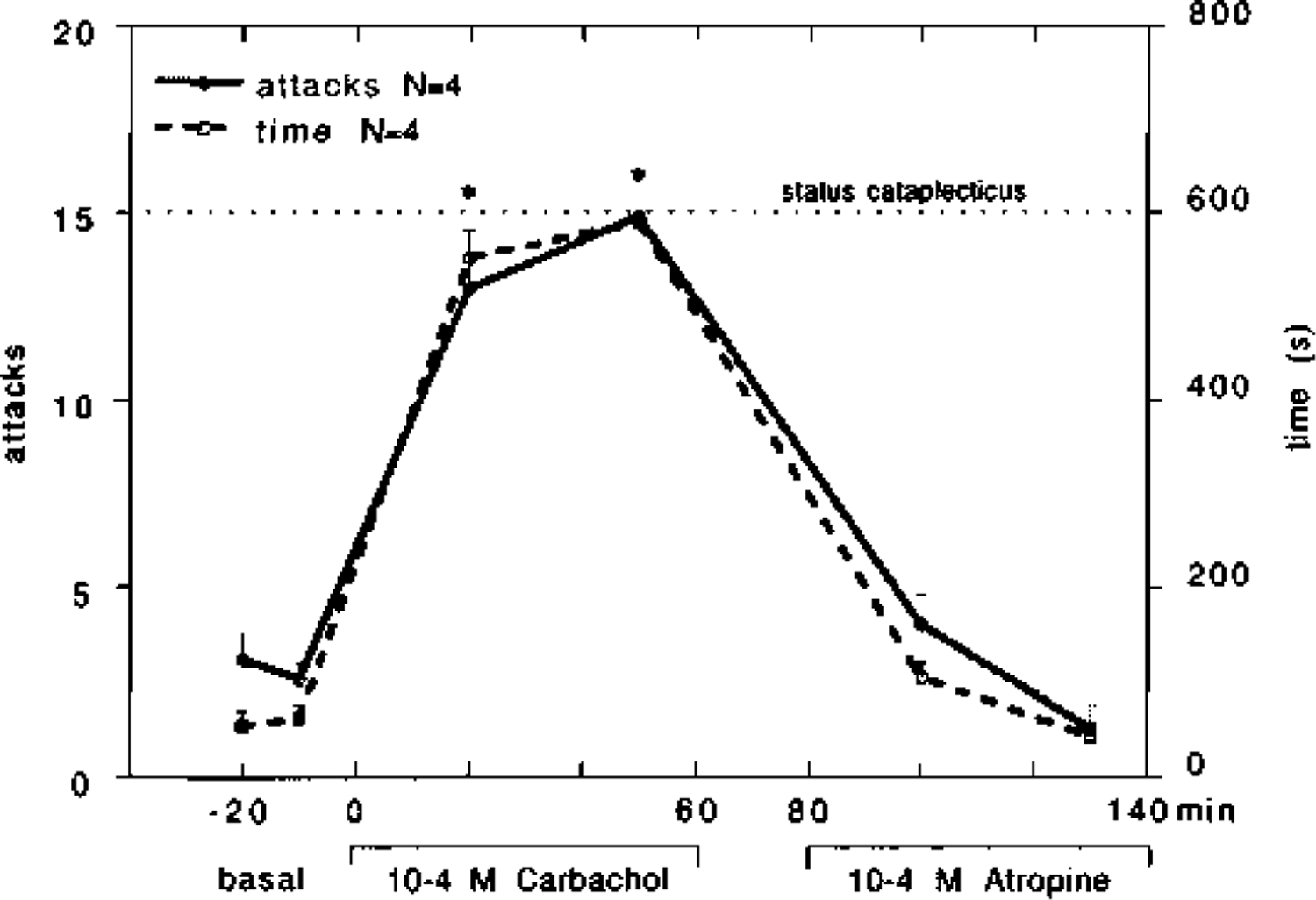
Effect of perfusion in the pontine reticular formation of four narcoleptic canines with bilateral carbachol (10−4M) for 60min, followed by bilateral cerebrospinal fluid for 20min and then bilateral atropine (10−4M) for 60min on cataplexy. The mean number of cataplectic attacks and elapsed time for two FECTs per test period is shown. For the purpose of figure presentation, status cataplecticus (carbachol atonia) was arbitrarily designated as 15 attacks elapsed over 600 s. This level of cataplexy was obtained in two of the four canines tested. Each drug time-point was compared with the basal time-points using a Fisher PLSD post hoc test; *P < 0.05 satisfactory for comparison with either basal time-point.
The control and the heterozygous canines all responded similarly to carbachol, and are therefore presented together as the control group. The effects of carbachol perfusion in the PRF on the control group are shown in Fig. 3c, d. At low concentrations, bilateral carbachol (10−5 to 10−4M) did not induce cataplexy in the control group, though a moderate increase in elapsed time occurred after 10−4 M carbachol (F = 3.350, d.f. = 5, P = 0.016). No decrease in basal muscle tone, as noted by behavioral observation and EMG analysis, was noted at these concentrations of carbachol. At the highest concentration, bilateral carbachol (10−3 M) produced complete muscle tone suppression, similar to status cataplecticus, during which there was a predominance of desynchronized EEG, while EOG and EMG activity was strongly suppressed. Unilateral carbachol (10~5 to 10−3 M) did not induce cataplexy and did not effect elapsed time (F = 0.198, d.f. = 7, P = 0.975) in the control group. In addition, EMG activity was not significantly reduced at any concentration of unilateral carbachol.
The effects of bilateral atropine perfusion in the PRF of narcoleptic canines are shown in Fig. 4. High concentrations of atropine (10−3 to 10−2 M) produced a slight reduction in FECT-induced cataplexy in some animals; however, statistical analysis revealed that this effect was not significant based on the number of attacks (F = 0.820, d.f. = 7, P = 0.447) nor elapsed time (F = 0.422, d.f. = 7. P = 0.657).
Fig. 4.
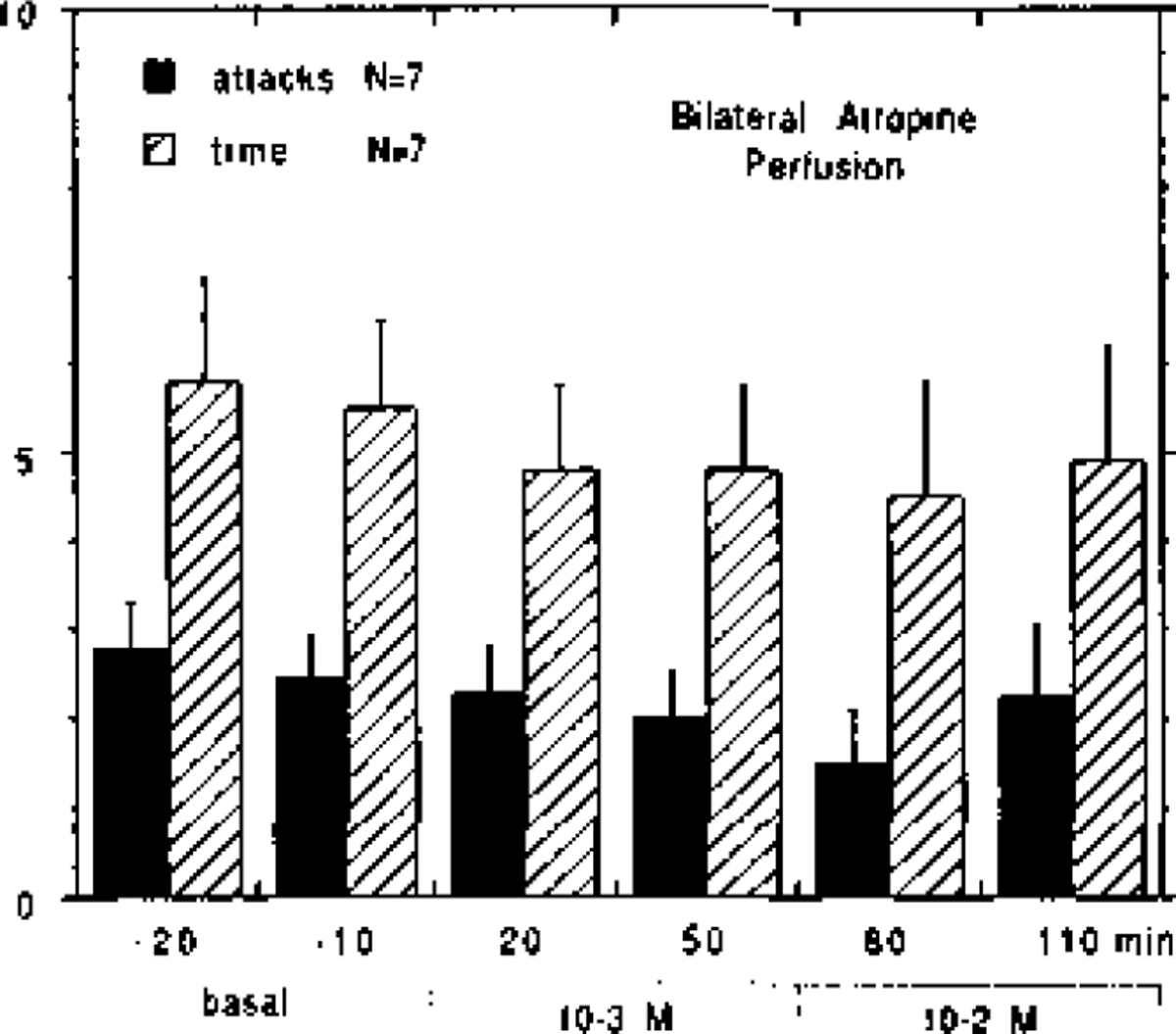
Effect of bilateral atropine (10−3 to 10−2 M) perfusion in the pontine reticular formation on cataplexy in five narcoleptic canines. In two canines, atropine was tested twice, each time at different anterior-posterior coordinates. The mean number of cataplectic attacks and elapsed time for two FECTs per test-period is shown.
The increase in cataplexy after bilateral carbachol perfusion in the narcoleptic canines was rapidly reversed when followed by bilateral atropine perfusion (Fig. 5). Bilateral carbachol (10−4M) perfusion produced a strong increase in cataplexy that reached status cataplecticus in two of four animals. This high level of cataplexy was maintained for over 1 h after switching the perfusion medium to one containing CSF only, but rapidly returned to basal levels when the perfusion medium was switched to bilateral atropine (10−4M). This reversal to basal levels was evident both in number of cataplectic attacks (F = 0.108, d.f. = 3, P= 0.743) and elapsed time (F = 0.809, d.f. = 3,P= 0.376). Two-way analysis of variance revealed that both treatment groups, carbachol followed by CSF and carbachol followed by atropine, were significantly different from one another in number of attacks (F = 7.798, d.f. = 3, P = 0.0001) and elapsed time (F = 8.450, d.f. = 3, P = 0.0001).
Bilateral perfusion with atropine significantly reduced the cataplexy enhancing effects of systemically applied physostigmine in narcoleptic canines (Fig. 6a, b). In animals with no pretreatment, physostigmine (0.05 mg/kg, i.v.) produced a robust increase in cataplexy, evident in number of cataplectic attacks (F = 7.918, d.f. = 3, P = 0.0003) and elapsed time (F = 3.738, d.f. = 3, P= 0.0195) (Fig. 6a). In animals receiving 1 h of bilateral atropine (10−2) perfusion as pretreatment, physostigmine (0.05 mg/ kg, i.v.) did not produce a significant increase in cataplexy over baseline levels, as seen in the number of cataplectic attacks (F = 0.218, d.f. = 3, P = 0.644) and elapsed time (F = 0.476, d.f. = 3, P = 0.496). Both non-pretreated and atropine pre-treated animals showed ataxia and muscular discoordination for up to 40min after physostigmine administration. Two-way analysis of variance revealed that atropine pretreated animals responded to physostigmine differently than non-pretreated animals in number of cataplectic attacks (F = 2.935, d.f. = 3, P = 0.040). However, these groups did not differ in regards to elapsed FECT time (F = 0.275, d.f. = 3, P = 0.843). Histological analysis revealed that the microdialy-sis probes were located within the PRF. When implanted at A 5.0 the ventral and central portions of the dialysis membrane were located in the nucleus reticularis pontis oralis (RPO), close to its border with the nucleus reticularis pontis caudalis (RPC) (Fig. 1a). When implanted at A 3.0 the ventral and central portions of the dialysis membrane were located in the RPC (Figs 1b, 7a-c). The most dorsal portions of membrane had contact with the dorsal pontine tegmentum. Some variability was noted in the rostrality of the probe tracts when implanted at either A 5.0 or A 3.0, varying no more than 1.5 mm from the correct positioning as judged according to the canine brain atlas.33 However, more variability was noted in the laterality of the probe tracts, which ranged between 2.0 and 5.0 mm lateral from midline, and the left and right sides were not always positioned asymmetrically. This may have been due to the considerable variability in brain size in the animals that were studied and/or the need to place some guide cannulae slightly more lateral because of the midsaggital sinus (see above). In general, all probe tracts were found in the PRF but ranged from the middle to the lateral parts of the structure. In Fig. 7a-c cannula tracts in the left and right PRF after implantation in an animal at A 3.0 are shown. Figure 7a and b are from the same animal. In Fig. 7a the ventral-most 5 mm of the tract on the left side and a dorsal portion of the tract on the right side can be seen. In Fig. 7b ventral portions, approximately 6 mm, of the tract on the right side are apparent. The tract in Fig. 7b, 0.45 mm caudal from the tract in Fig. 7a, was the most lateral tract observed in all animals examined. A moderate level of heavy staining seen at the tip of the tract on the right side is probably due to non-specific staining of damaged tissue which occurred when implanting the injection cannula. In Fig. 7c tracts in both the left and right side of the PRF of another animal are shown in a section under higher magnification. The tract on the left side is at the ventral-most level that was seen for both left and right tracts in all sections examined. The tracts in Fig. 7c were the most medial observed in all animals examined.
Fig. 6.
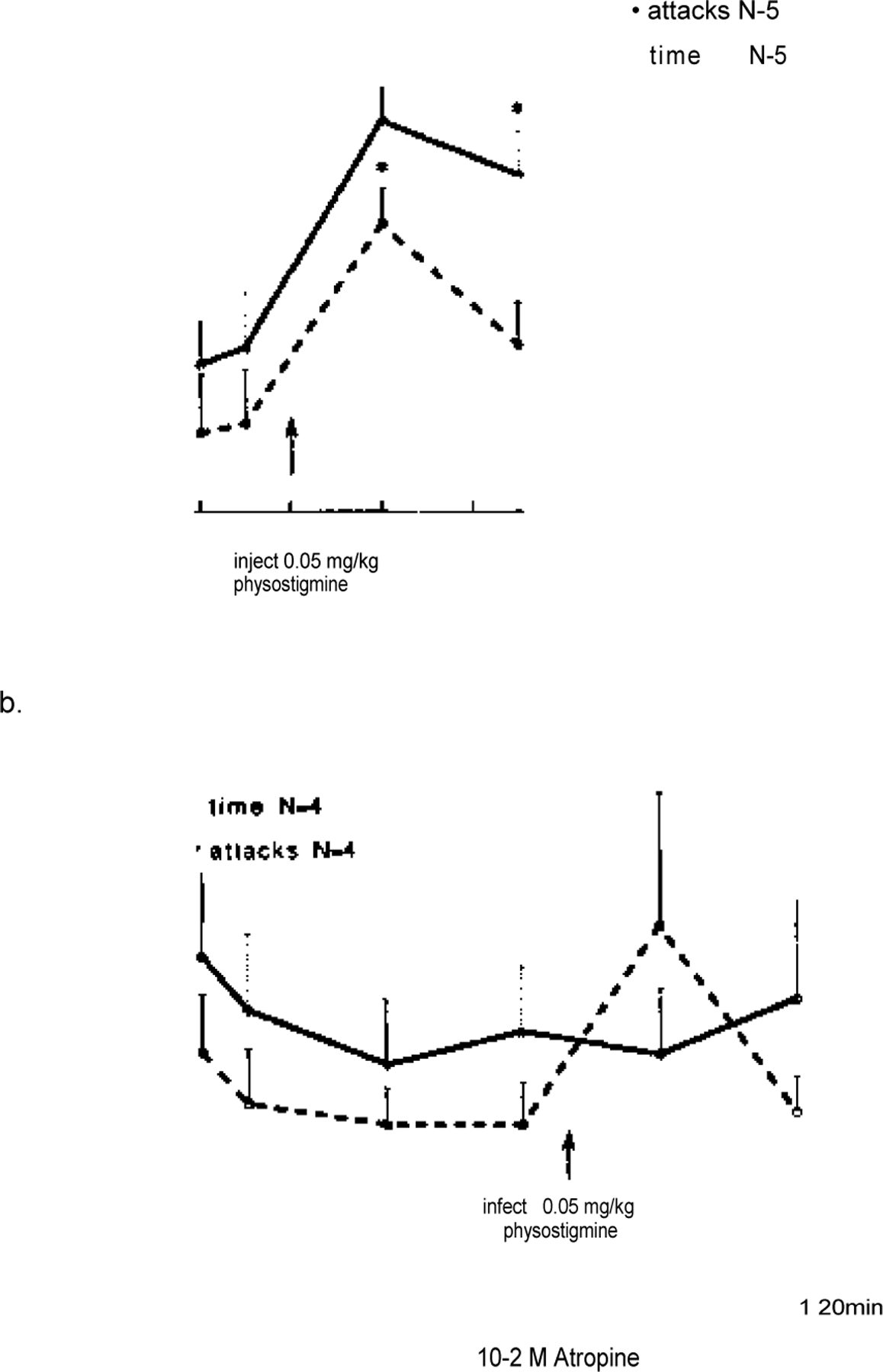
Effect of an intravenous injection of physostigmine (0.05 mg/kg) on cataplexy in narcoleptic canines. In a, five animals were tested without any perfusion in the PRF. In b, four of these animals received 60 min of bilateral atropine (10−2 M) perfusion in the PRF prior to physostigmine injection and continued to receive atropine perfusion for the next 60 min. The mean number of cataplectic attacks and elapsed time for two FECTs per test period is shown. Each drug time-point was compared with the basal time points using a Fisher PLSD post hoc test; *P < 0.05 satisfactory for comparison with either basal time-point.
Fig. 7.
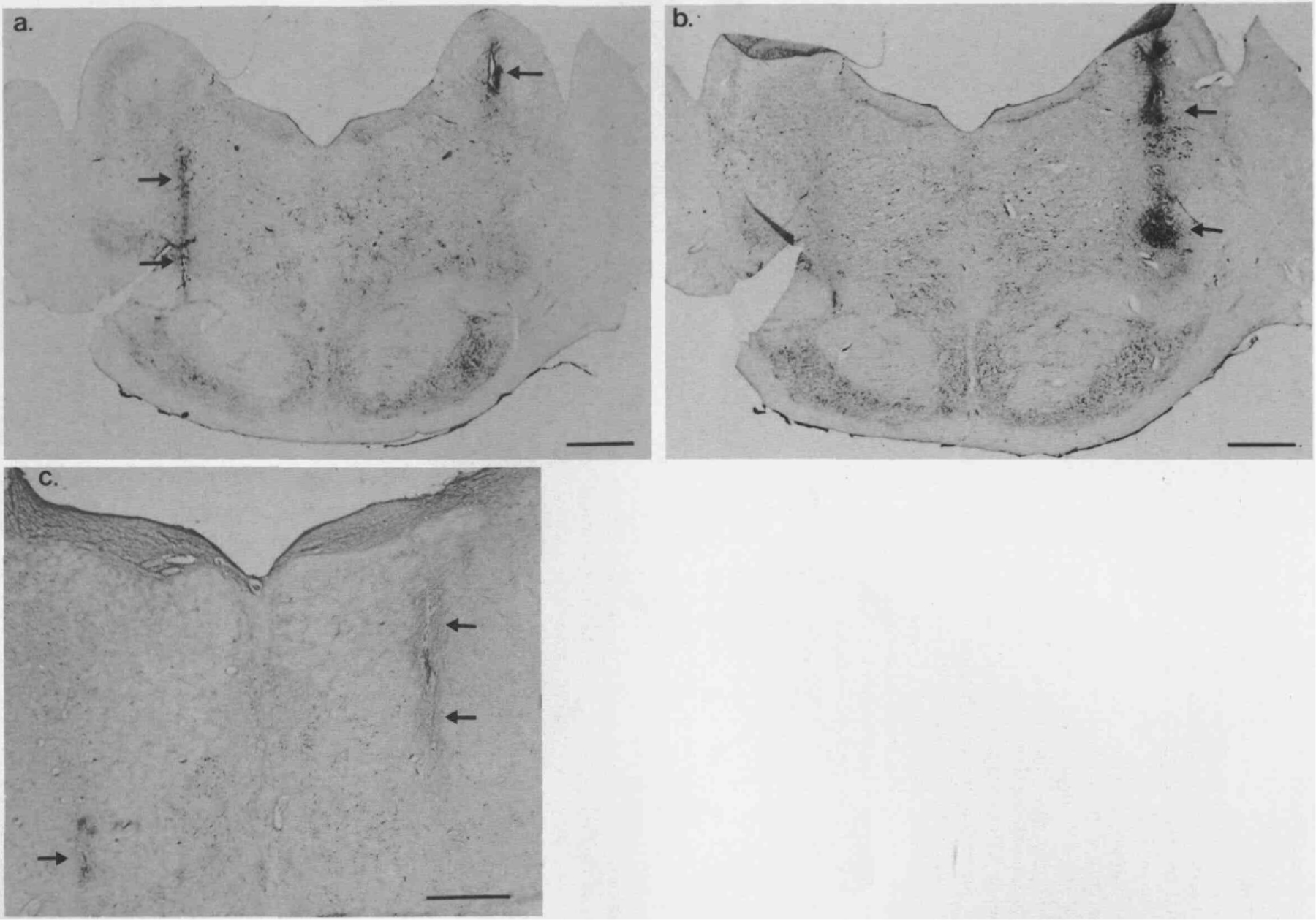
Photomicrographs showing the positions of the microdialysis probes in the pontine reticular formation. The pontine sections were stained with thionin after bilateral cannulation through the guide cannula positioned at A 3.0 with injection cannulae for the injection of FluoroGold (immunocytochemistry not shown) at coordinates: A 3.0, L + 3.2 and −3.2, V 39.0. Panels a and b were from the same animal and c was from a second animal. Black arrows indicate the injection cannula tracts on the right and left sides in the RPC. The scale bars in a and b indicate 2 mm and the bar in c indicates 1 mm. In a the ventral most aspects of the implantation tract on the left, extending approximately 5 mm along the dorsoventral axis, and part of the tract on the right are shown. In b the ventral portions of the implantation tract on the right, extending approximately 6 mm along the dorsoventral axis, are shown. The sections in a and b are 450 /um apart. In c portions of both tracts in the left and right sides are shown under higher magnification. When the microdialysis probes were implanted in the RPC of these canines, at the same coordinates as the dye injection cannulae, the 5-mm dialysis membranes were located in approximately the same positions as the tracts.
DISCUSSION
The present study on the effects of cholinergic drugs infused locally within the PRF on cataplexy in narcoleptic, control and heterozygous Doberman pinschers confirms and extends previous findings on the effects of microinjections of cholinergic compounds into the PRF of cats,3,13,29,40,50 showing that high doses of carbachol produce complete muscle tone suppression and an increase in desynchronized EEG activity. The ability of low concentrations of carbachol to enhance cataplexy in the narcoleptic canines, without producing significant, tonic motor inhibition, suggests that cholinergic mechanisms in the PRF may also be involved in the regulation of cataplexy in the narcoleptic canine.
Microdialysis as a method for drug infusion
The use of microdialysis probes to deliver cholinergic drugs directly into the PRF via the perfusion medium is a relatively novel method for local drug administration. While this method has been used previously to investigate the neuropharmacology of specific brain nuclei,22,44,45,55 there are few studies reporting the modulation of behavioral states from drug infusion via a microdialysis probe.55,58 In the present studies microdialysis probes were used to study the PRF for two major reasons, to deliver drugs directly into the PRF and to assay acetylcholine levels in the PRF during pharmacological and behavioral treatment, as described in the accompanying paper.46 Furthermore, as a drug infusion method microdialysis enables one to continuously administer any combination of drugs to a specific location without introducing an exogenous volume of fluid, thus reducing tissue trauma at the site of interest.
The delivery of a drug via a microdialysis probe is mediated by its passive diffusion across the dialysis membrane. Since this process is equal in either direction,2 the concentration of a drug passing outwards across the membrane may be estimated using the calculated recovery rate of that drug passing inwards across the membrane. In the present study the technology necessary for the measurement of carbachol or atropine was not available; therefore, we chose to measure the recovery rate of acetylcholine, which is nearly identical to carbachol in structure and molecular weight. Atropine, though a larger and more complex structure than carbachol, should cross the membrane at nearly the same rate because of the wide dynamic range of the dialysis membranes used in this study.30 The in vitro recovery rate for acetylcholine was 25%. Since in vivo recovery rates are suggested to be approximately 50% lower than in vitro recovery rates,1,7,34,56 it may be suggested that the concentrations of carbachol and atropine passing across the brain into the PRF were 12% of their perfusate concentrations. Based on this conversion factor it was estimated that the concentration of carbachol and atropine (10−5 to 10−2M in the perfusion medium) immediately outside the dialysis membrane in the PRF was 1.2–120, and 12–1200 /uM, respectively. These concentrations of carbachol and atropine are well beyond their reported binding affinities for muscarinic receptors in the CNS.8,23 Considering that carbachol concentrations were applied continuously at 2 /ul/min for up to 180 min, it is possible to estimate a total dose of carbachol applied in the PRF during a dose-response study. These calculated values were: 0.14 nmol, 1.54 nmol and 15.9 nmol after 60, 120 and 180 min, respectively. The lower two doses, which produced a significant increase in cataplexy, are also well below those shown to produce an increase in REM sleep in cats (5–42 nmol).3,29,50 Thus, in the present study relatively low doses of carbachol were administered into the PRF.
The diffusion of a drug administered via a microdialysis probe into the surrounding tissue is dependent on tissue tortuosity and interstitial clearance mechanisms such as microvasculature, metabolism and intra/extracellular exchange.6,7,10,41 The response time necessary for drug concentrations in the surrounding tissue to reach equilibrium is hypothesized to be relatively short,27,41 and the concentration gradient in the surrounding tissue is suggested to decrease exponentially with distance from the membrane surface.41 These characteristics are consistent with immunohistochemical studies showing the spread of dopamine perfused into the striatum58 or Bromophenol Blue perfused into the preoptic area,43 via a microdialysis probe. Based on these studies it can be suggested that the concentrations of carbachol and atropine infused via the dialysis probes decreased rapidly within a short distance from the membrane, such that at a radial distance of 1 mm from the membrane the concentration was probably less than 5% of its original level at the surface of the membrane. Though it is possible that higher concentrations of carbachol and atropine achieved a greater effective spread in the PRF, these differences become less of a factor at greater distances from the probe.7 Therefore, the effective levels of carbachol and atropine infused into the PRF via microdialysis probe in this study were probably limited to an area of 0.5–1.0 mm from the surface of the membrane. Furthermore, since the bilateral probes were greater than 6 mm apart, it is unlikely that there was any effective bilateral interaction of drugs.
Neurochemical and functional properties of the perfusion site
Histological analysis revealed that the dialysis probes were placed within the PRF, in the RPO when implanted at A 5.0 and in the RPC when implanted at A In the cat and the rat, the PRF is heavily innervated by cholinergic projections from the laterodorsal tegmental nucleus and the pedunculopontine tegmental nucleus.25,26,39,51 Furthermore, this area is a well-established site for cholinergic stimulated REM sleep and muscle atonia.3,29,40,50 Histological analysis also revealed that dorsal regions of the dialysis probe were in contact with the dorsal pontine tegmentum. The dorsal pontine tegmentum includes the peri-locus coeruleus pars-alpha (subcoeruleus), which has been shown to mediate REM sleep muscle atonia,21,47,48,52 and parts of the pedunculopontine tegmental nucleus and laterodorsal tegmental nucleus, which send cholinergic projections to the PRF, medulla, superior colliculus and thalamus26,42,49,57 and are involved in the generation of REM sleep.53 Thus, in the present study carbachol and atropine perfusion into the PRF via microdialysis probes effected a relatively broad area of the brainstem involved in the regulation of REM sleep and muscle atonia.
Cholinergic stimulation of cataplexy in the pontine reticular formation
The artificial CSF used in this study included a low concentration of neostigmine (10−6M) in order to obtain measurable amounts of acetylcholine in the perfusate (see Ref. 46). Since it has been shown in cats that REM sleep may be enhanced by injections neostigmine into the PRF4 control tests for the effect of neostigmine on cataplexy in the narcoleptic canines were necessary. It was found that neostigmine had no effect on cataplexy. Indeed, the levels of neostigmine used in this study (approximately 0.12 nmol/h) were significantly lower than those used by Baghdoyan and colleagues in cats (6–60 nmol).4
Low concentrations of carbachol (10 −5 to 10−4 M) produced a significant increase in FECT-induced cataplexy in the narcoleptic canines, evident after either unilateral or bilateral perfusion in the PRF, though the bilateral effect was more robust (Fig. 3a, b). Cataplectic attacks increased in frequency and severity with increased concentration of carbachol, and the time required to complete each FECT was more than doubled. Indeed, full attacks lasting up to one minute were often observed during 10−4 M carbachol perfusion. Nevertheless, a general suppression of muscle tone was not observed at these levels of carbachol, the animal’s behavior was otherwise completely normal. In the control and heterozygous canines, no cataplexy and no decrease in muscle tone was observed at these levels of carbachol (10−5 to 10−4M). These findings indicate that cataplexy in canine narcolepsy may be regulated by cholinergic mechanisms in the PRF, and are consistent with previous studies reporting enhanced muscarinic binding in the nucleus reticularis gigantocellularis and the RPC of narcoleptic canines.9,31 Such an upregulation of muscarinic receptors in the PRF could account for the highly sensitive carbachol response seen in our study of narcoleptic canines. In vitro and in vivo studies on cellular activity in the PRF of rats12,15 and cats16 have shown that carbachol administration can cause either depolarization15 or hyperpolarization12,15,16 of pontine neurons. Furthermore, unit recording studies have found that most cells in the PRF reduce activity during cataplexy in narcoleptic canines.54 We hypothesize that the perfusion of carbachol into the PRF of narcoleptic canines produces a decrease in the activity of PRF neurons, similar to the reduction in unit activity seen during spontaneous cataplexy,54 and thereby enhances the incidence of cataplectic behavior.
During episodes of carbachol enhanced cataplexy in the narcoleptic canines, the EEG was desynchronized and EMG activity was greatly suppressed, similar to that seen during basal cataplexy. This is similar to EEG and EMG recordings associated with REM sleep in narcoleptic canines.30,35 However, unlike REM sleep, there were no eye movements observed during cataplexy, in either basal or carbachol-stimulated conditions, in our sample of narcoleptic canines. A similar lack of eye movement during cataplexy has been reported more recently.54 These electrographic characteristics are similar to the first stage of cataplexy in narcoleptic canines as described by Kushida and colleagues, with the one difference being their observation of continued visual tracking during the onset of cataplexy.32 Thus, in the present study cataplexy simply appeared as strong muscle tone inhibition.
The highest concentration of carbachol (10−3 M) tested in this study produced a complete suppression of muscle tone, defined as status cataplecticus. This was seen with both bilateral and unilateral perfusion of carbachol in the narcoleptic canines, but only after bilateral perfusion of carbachol in the control group. During this condition desynchronized EEG activity was predominant, which is consistent with previous studies on the effects of carbachol adminstration in the cat PRF.3,50 This carbachol-induced atonia possibly represents cholinergic stimulation of the dorso-lateral pons, an area which has been shown to be a low threshold site for cholinergic-mediated muscle atonia in cats.3,29,40 It is also possible that this carbachol-induced atonia was mediated by cholinergic stimulation of the subcoereleus, although it is not clear whether muscle atonia regulation in this region is stimulated by cholinergic mechanisms.
Atropine modulation of cataplexy in the pontine reticular formation
Bilateral perfusion with high concentrations of atropine (10 −3 to 10 −2 M) in the PRF did not produce a significant decrease in cataplexy in the narcoleptic canines, though a slight reduction in some animals was observed. This suggests that cholinergic regulation of cataplexy could also be mediated by areas outside the PRF. However, it is also likely that this reflects the limited diffusion of atropine in the PRF when administered via a microdialysis probe, which would suggest that a cholinergic blockade needs to cover a broader area in order to be effective. Nevertheless, atropine perfusion rapidly reversed the cataplexy enhancing effects of locally perfused carbachol and partially suppressed the cataplexy enhancing effects of intravenously applied physostigmine. The ability of atropine to reverse the effects of carbachol indicates that the carbachol effect is mediated via muscarinic receptors. The ability of atropine to inhibit the effects of physostigmine suggests that the PRF has a central role in the cholinergic regulation of cataplexy, perhaps as a point of convergence for cholinergic pathways regulating cataplexy in narcoleptic canines. It should be noted that atropine selectively reduced the incidence of cataplectic attacks induced by physostigmine but not the time required to complete a FECT test. Indeed, the tremors, muscular discoordination and ataxia which normally accompany the physostigmine effect was still observed. Thus, this test revealed a distinction between the inhibition of muscle coordination and the occurrence of cataplectic attacks.
CONCLUSION
The present study demonstrates that cataplexy in the narcoleptic canine can be stimulated by local administration of carbachol into the PRF. This response was highly potent and mediated by muscarinic receptors. In addition, local administration of atropine significantly reduced the increase in cataplexy produced by systemically administered physostigmine. These findings suggest that cholin-ergic stimulation of cataplexy in narcoleptic canines is mediated by, or passes through, the PRF. However, when atropine was perfused alone in the PRF there was no significant reduction in cataplexy, leaving open the possibility that other cholinergic pathways may be involved in the regulation of cataplexy. Nevertheless, the present findings provide clear evidence that the PRF plays a significant role in the cholinergic regulation of cataplexy in canine narcolepsy.
Acknowledgements—
We wish to thank Jeff Shelton for technical assistance and Pam Hyde for administrative support. This study was supported by grants NS23724, NS27710 and NS15184 from NIH to Dr Dement and NS14610 to Dr Siegel from VA Medical Services.
Abbreviations—
- A
anterior
- CSF
cerebrospinal fluid
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- FECT
Food-Elicited Cataplexy Test
- PRF
pontine reticular formation
- REM
rapid eye movement
- RPC
nucleus reticulatis pontis caudalis
- RPO
nucleus reticularis pontis oralis
REFERENCES
- 1.Alexander GM, Grothusen JR and Schwartzman RJ (1988) Flow dependent changes in the effective surface area of microdialysis probes. Life Sci 43, 595–601. [DOI] [PubMed] [Google Scholar]
- 2.Amberg G and Lindefors N (1989) Intracerebral Microdialysis: II. Mathematical studies of diffusion kinetics. J. Pharmac. Meth 22, 157 183. [DOI] [PubMed] [Google Scholar]
- 3.Armatruda T, Black D, McKenna T, McCarley RW and Hobson JA (1975) Sleep cycle control and cholinergic mechanisms: differential effects of carbachol injections at pontine brainstem sites. Brain Res 98, 501–515. [DOI] [PubMed] [Google Scholar]
- 4.Baghdoyan HA, Monaco AP, Rodrigo-Angulo ML, Assens F, McCarley RW and Hobson JA (1984) Microinjection of neostigmine into the pontine reticular formation of cats enhances desynchronized sleep signs. J. Pharmac. cxp. Ther 231, 173–180. [PubMed] [Google Scholar]
- 5.Baker TL and Dement WC (1985) Canine narcolepsy-cataplexy syndrome: evidence for an inherited monoaminergic-cholincrgic imbalance. In Brain Mechanisms in Sleep (eds McGinty DJ, Drucker-Collin R, Morrison A and Parmengiani P), pp. 199–233. Raven, New York. [Google Scholar]
- 6.Bcnvcniste H (1989) Brain Microdialysis. J. Neurochem 52, 1667–1679. [DOI] [PubMed] [Google Scholar]
- 7.Benveniste H, Hansen AJ and Ottosen NS (1989) Determination of brain interstitial concentrations by microdialysis. J. Neurochem 52, 1741–1750. [DOI] [PubMed] [Google Scholar]
- 8.S.Birdsall NJM, Burgen ASV and Hulme EC (1978) The binding of agonists to brain muscarinic receptors. Molec. Pharmac 14, 723 736. [PubMed] [Google Scholar]
- 9.Boehme RE, Baker TL, Mefford IN, Barchas JD, Dement WC and Ciaranello RD (1984) Narcolepsy: cholinergic receptor changes in an animal model. Life Sci 34, 1825–1828. [DOI] [PubMed] [Google Scholar]
- 10.Bungay PM, Morrison PF and Dedrick RL (1990) Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci 46, 105–119. [DOI] [PubMed] [Google Scholar]
- 11.Delashaw JB, Foutz AS, Guilleminault C and Dement WC (1979) Cholinergic mechanisms and cataplexy in dogs. Expl Neurol 66, 745–757. [DOI] [PubMed] [Google Scholar]
- 12.Egan TM, North RA (1986) Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature 319, 405–407. [DOI] [PubMed] [Google Scholar]
- 13.George R, Haslett WL and Jenden DJ (1964) A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int. J. Neuropharmac 3, 541–552. [DOI] [PubMed] [Google Scholar]
- 14.Gillin JC, Horowitz D and Wyatt RJ (1976) Pharmacological studies of narcolepsy involving serotonin, acetylcholine and monoamine oxidase in narcolepsy. In Narcolepsy (eds Guilleminault C, Passuoant P and Dement WC), pp. 125–143. Spectrum, New York. [Google Scholar]
- 15.Greene RW and Carpenter DO (1985) Actions of neurotransmitters on pontine reticular formation neurons of the cat. J. Neurophysiol 54, 520–531. [DOI] [PubMed] [Google Scholar]
- 16.Greene RW, Gerber U and McCarley RW (1989) Cholinergic activation of medial pontine reticular formation neurons in vitro. Brain Res 476, 154–159. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Passuoant P and Dement WC (1976) Narcolepsy Spectrum, New York. [Google Scholar]
- 18.Guilleminault C, Carskadon M and Dement WC (1974) On the treatment of REM narcolepsy. Archs Neurol 30, 90–93. [DOI] [PubMed] [Google Scholar]
- 19.Guilleminault C, Wilson R and Dement WC (1974) A study on cataplexy. Archs Neurol 31, 255–261. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C, Mignot E, Aldrich M, Quera-Salva M-A, Tiberge M and Partinen M (1988) Prazosin is contraindicated in patients with narcolepsy. Lancet 609, 511–512. [DOI] [PubMed] [Google Scholar]
- 21.Henley K and Morrison R (1974) A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Ada neurobiol. exp 34, 215–232. [PubMed] [Google Scholar]
- 22.Hernandez L, Lee F and Hoebel BG (1987) Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: Increase in extracellular dopamine and serotonin. Brain Res. Bull 19, 623–628. [DOI] [PubMed] [Google Scholar]
- 23.Hulme EC, Birdsall NJM, Burgen ASV and Mehta P (1978) The binding of antagonists to brain muscarinic receptors. Molec. Pharmac 14, 737–750. [PubMed] [Google Scholar]
- 24.Isaacson LG and Tanaka D (1986) Cholinergic and non-cholinergic projections from the canine pontomesencephalic tegmentum (Ch5 area) to the caudal intralaminar nuclei. Expl Brain Res 62, 179–188. [DOI] [PubMed] [Google Scholar]
- 25.Jones BE (1990) Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation. J. comp. Neurol 295, 485–514. [DOI] [PubMed] [Google Scholar]
- 26.Jones BE and Webster HH (1988) Neurotoxic lesions of the dorsolateral popntomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Res 451, 13–32. [DOI] [PubMed] [Google Scholar]
- 27.Juhasz G, Tarcali J, Pungor K and Pungor E (1989) Electrochemical calibration of in vivo brain dialysis samplers. J. Neurosci. Meth 29, 131–137. [DOI] [PubMed] [Google Scholar]
- 28.Kaitin KI, Kilduff TS and Dement WC (1986) Sleep fragmentation in canine narcolepsy. Sleep 9, 116–119. [DOI] [PubMed] [Google Scholar]
- 29.Katayama Y, DeWitt DS, Becker DP and Hayes RL (1984) Behavioral evidence for cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Res 296, 241–262. [DOI] [PubMed] [Google Scholar]
- 30.Kendrick KM (1991) Microdialysis measurements of in vivo neuropeptide release. J. Neurosci. Meth 34, 35–46. [DOI] [PubMed] [Google Scholar]
- 31.KilduffT S, Bowersox S, Kaitin KI, Baker TL, Ciaranello RD and Dement WC (1986) Muscarinic cholinergic receptors and the canine model of narcolepsy. Sleep 9, 102–106. [DOI] [PubMed] [Google Scholar]
- 32.Kushida CA, Baker TL and Dement WC (1985) Electroencephalographic correlates of cataplectic attacks in narcoleptic canines. Electroenceph. din. Neurophysiol 61, 61–70. [DOI] [PubMed] [Google Scholar]
- 33.Lim RKS, Liu C-N and Moffitt RL (1960) A Stereotaxic Atlas of the Dog’s Brain Thomas Books, Springfield. [Google Scholar]
- 34.Lindefors N, Amberg G and Ungerstedt U (1989) Intracerebral microdialysis: I. Experimental studies of diffusion kinetics. J. Pharmac. Meth 22, 141–156. [DOI] [PubMed] [Google Scholar]
- 35.Lucas EA, Foutz AS, Dement WC and Mitler MM (1979) Sleep cycle organization in narcoleptic and normal dogs. Physiol. Behav 23, 737–743. [DOI] [PubMed] [Google Scholar]
- 36.Mignot E, Nishino S, Guilleminault C and Dement WC (1990) Noradrenergic and cholinergic control of cataplexy: pharmacological evidence. In Endogenous Sleep Factors (eds Krueger JM and Inoue S), pp. 203–215. SPB Academic Publishing, The Hague. [Google Scholar]
- 37.Mignot E, Guilleminault C, Dement WC Grumet FC (1992) Genetically determined animal models of narcolepsy, a disorder of REM sleep. In Genetically Determined Animal Models of Neurobehavioral Dysfunction (ed. Driscol P), pp. 90–110. Birkhauser Boston, Cambridge (U.S.A.). [Google Scholar]
- 38.Mignot E, Nishino S, Sharp LH, Arrigoni J, Siegel MJ, Reid MS, Edgar DM, Ciaranello RD and Dement WC (1992) Heterozygocity at the canarc-1 locus can confer susceptibility for narcolepsy: Induction of cataplexy in heterozygous asymptomatic dogs after administration of a combination of drugs acting on monoaminergic and cholinergic systems. J. Neurosci 13, 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K and McCarley RW (1988) Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res 451, 397–402. [DOI] [PubMed] [Google Scholar]
- 40.Mitler M and Dement WC (1974) Cataplectic-like behavior in cats after microinjection of carbachol in pontine reticular formation. Brain Res 68, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison PF, Bungay PM, Hsiao JK, Ball BA, Mefford IN and Dedrick RL (1991) Quantitative Microdialysis: Analysis of transients and application to pharmacokinetics in brain. J. Neurochem 57, 103–119. [DOI] [PubMed] [Google Scholar]
- 42.Pare DY, Smith Y, Parent A and Steriade M (1988) Projections of brainstem core cholinergic and non-cholinergic neurons to intralaminar and reticular thalamic nuclei. Neuroscience 25, 69–86. [DOI] [PubMed] [Google Scholar]
- 43.Quan N, Xin L and Blatteis CM (1991) Microdialysis of norepinephrine into preoptic area of guinea pigs: characteristics of hypothermic effect. Am. J. Physiol 261, R378–R385. [DOI] [PubMed] [Google Scholar]
- 44.Reid MS, Herrera-Marschitz M, Kehr J and Ungerstedt U (1990a) Striatal dopamine and glutamate release: effects of intranigral injections of substance P. Acta physiol. Scand 140, 527–537. [DOI] [PubMed] [Google Scholar]
- 45.Reid MS, O’Connor WT, Herrera-Marschitz M and Ungerstedt U (1990) The effects of intranigral GABA and dynorphin A injections on striatal dopamine and GABA release: evidence that dopamine provides inhibitory regulation of striatal GABA neurons via D2 receptors. Brain Res 519, 255–260. [DOI] [PubMed] [Google Scholar]
- 46.Rei MS., Siege JM., Demen WC. and Migno E. (199. 2) Cholinergic mechanisms in canine narcolepsy—II. Acetylcholine release in the pontine reticular formation is enhanced during cataplexy. Neuroscience 59, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sastre JP, Sakai K and Jouvet M (1978) Bilateral lesions of the dorsolateral pontine tegmentum. II. Effect upon muscle atonia. Sleep Res 1, 44–49. [Google Scholar]
- 48.Sastre JP, Sakai K and Jouvet M (1979) Persistance du sommeil chez le chat apres destruction de l’aire gigantocellulaire du tegmentum pontique par l’acide kainique. C. R. Soc. Biol 289, 959–964. [PubMed] [Google Scholar]
- 49.Semba K, Reiner PB and Fibiger HC (1990) Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38, 643–654. [DOI] [PubMed] [Google Scholar]
- 50.Shiromani PJ and Fishbein W (1986) Continuous pontine cholinergic microinfusion via mini-pump induces sustained alterations in rapid eye movement (REM) sleep. Pharmac. Biocem. Behav 25, 1253–1261. [DOI] [PubMed] [Google Scholar]
- 51.Shiromani PJ, Armstrong DM and Gillin JC (1988) Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci Lett 95, 19–23. [DOI] [PubMed] [Google Scholar]
- 52.Shouse MN and Siegel JM (1992) Pontine regulation of REM sleep components in cats: Integrity of the pedunculopontine tegmentum is important for phasic events but unnecessary for atonia during REM sleep. Brain Res 571, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel JM (1989) Brainstem mechanisms generating REM sleep. In Principles and Practice of Sleep Medicine (eds Kryger MH, Roth T and Dement WC), pp. 104–120. W. B. Saunders Co., New York. [Google Scholar]
- 54.Siegel JM, Nienhuis R, Fahringer HM, Chui C, Dement WC, Mignot E and Lufkin R (1992) Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J. Neurosci 12, 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smullin DH, Skilling SR and Larson AA (1990) Interactions between substance P, calcitonin gene-related peptide, taurine and excitatory amino acids in the spinal cord. Pain 42, 93–101. [DOI] [PubMed] [Google Scholar]
- 56.Stahle L, Segersvard S and Ungerstedt U (1991) A comparison between three methods for estimation of extracellular concentrations of exogenous and endogenous compounds by microdialysis. J. Pharmac. Meth 25, 41–52. [DOI] [PubMed] [Google Scholar]
- 57.Steriade M, Pare D, Parent A and Smith Y (1988) Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience 25, 47–67. [DOI] [PubMed] [Google Scholar]
- 58.Stromberg I, Herrera-Marschitz M, Ungerstedt U, Ebendal T and Larson O (1985) Chronic implants of chromaffin tissue into the dopamine-denervated striatum. Effects of NGF on graft survival, fiber growth and rotational behavior. Expl Brain Res 60, 335–349. [DOI] [PubMed] [Google Scholar]


