Abstract
Background
Concerns have been raised regarding the risks of SARS-CoV-2 breakthrough infections in vaccinated patients with immune-mediated inflammatory diseases treated with immunosuppressants, but clinical data on breakthrough infections are still scarce. The primary objective of this study was to compare the incidence and severity of SARS-CoV-2 breakthrough infections between patients with immune-mediated inflammatory diseases using immunosuppressants, and controls (patients with immune-mediated inflammatory diseases not taking immunosuppressants and healthy controls) who had received full COVID-19 vaccinations. The secondary objective was to explore determinants of breakthrough infections of the delta (B.1.617.2) variant of SARS-CoV-2, including humoral immune responses after vaccination.
Methods
In this substudy, we pooled data collected in two large ongoing prospective multicentre cohort studies conducted in the Netherlands (Target to-B! [T2B!] study and Amsterdam Rheumatology Center COVID [ARC-COVID] study). Both studies recruited adult patients (age ≥18 years) with immune-mediated inflammatory diseases and healthy controls. We sourced clinical data from standardised electronic case record forms, digital questionnaires, and medical files. We only included individuals who were vaccinated against SARS-CoV-2. For T2B!, participants were recruited between Feb 2 and Aug 1, 2021, and for ARC-COVID, participants were recruited between April 26, 2020, and March 1, 2021. In this study we assessed data on breakthrough infections collected between July 1 and Dec 15, 2021, a period in which the delta SARS-CoV-2 variant was the dominant variant in the Netherlands. We defined a SARS-CoV-2 breakthrough infection as a PCR-confirmed or antigen test-confirmed SARS-CoV-2 infection that occurred at least 14 days after vaccination. All breakthrough infections during this period were assumed to be due to the delta variant due to its dominance during the study period. We analysed post-vaccination serum samples for anti-receptor binding domain (RBD) antibodies to assess the humoral vaccination response (T2B! study only) and anti-nucleocapsid antibodies to identify asymptomatic breakthrough infections (ARC-COVID study only). We used multivariable logistic regression analyses to explore potential clinical and humoral determinants associated with the odds of breakthrough infections. The T2B! study is registered with the Dutch Trial Register, Trial ID NL8900, and the ARC-COVID study is registered with Dutch Trial Register, trial ID NL8513.
Findings
We included 3207 patients with immune-mediated inflammatory diseases who receive immunosuppressants, and 1807 controls (985 patients with immune-mediated inflammatory disease not on immunosuppressants and 822 healthy controls). Among patients receiving immunosuppressants, mean age was 53 years (SD 14), 2042 (64%) of 3207 were female and 1165 (36%) were male; among patients not receiving immunosuppressants, mean age was 54 years (SD 14), 598 (61%) of 985 were female and 387 (39%) were male; and among healthy controls, mean age was 57 years (SD 13), 549 (67%) of 822 were female and 273 (33%) were male. The cumulative incidence of PCR-test or antigen-test confirmed SARS-CoV-2 breakthrough infections was similar in patients on immunosuppressants (148 of 3207; 4·6% [95% CI 3·9–5·4]), patients not on immunosuppressants (52 of 985; 5·3% [95% CI 4·0–6·9]), and healthy controls (33 of 822; 4·0% [95% CI 2·8–5·6]). There was no difference in the odds of breakthrough infection for patients with immune-mediate inflammatory disease on immunosuppressants versus combined controls (ie, patients not on immunosuppressants and healthy controls; adjusted odds ratio 0·88 [95% CI 0·66–1·18]). Seroconversion after vaccination (odds ratio 0·58 [95% CI 0·34–0·98]; T2B! cohort only) and SARS-CoV-2 infection before vaccination (0·34 [0·18–0·56]) were associated with a lower odds of breakthrough infections.
Interpretation
The incidence and severity of SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases on immunosuppressants was similar to that in controls. However, caution might still be warranted for those on anti-CD20 therapy and those with traditional risk factors.
Funding
ZonMw (the Netherlands Organization for Health Research and Development) and Reade foundation.
Introduction
Vaccination against COVID-19 might be less effective in protecting patients with immune-mediated inflammatory diseases because the use of some immunosuppressants, most notably anti-CD20 therapy, methotrexate, S1P receptor modulators, and mycophenolate mofetil, reduces humoral or cellular immune responses, or both.1, 2, 3, 4, 5 Therefore, concerns have been raised regarding the potential risk of SARS-CoV-2 breakthrough infections in patients who have been vaccinated against COVID-19 and who are treated with immunosuppressants; however, data on SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases who are on immunosuppressants are scarce. A registry study found a higher incidence of SARS-CoV-2 breakthrough infections in patients who were immunocompromised, including some with immune-mediated inflammatory diseases, than in the general population.6 However, severity of disease due to SARS-CoV-2 breakthrough infections and confounding risk factors for breakthrough infections were not compared between patients and controls. Other studies in healthy individuals observed associations between reduced humoral responses after SARS-CoV-2 vaccination and an increased incidence of SARS-CoV-2 breakthrough infections and possibly more severe disease,7, 8, 9 but there is a paucity of data from patients with immune-mediated inflammatory diseases.
We compared the incidence and severity of SARS-CoV-2 breakthrough infections between patients with immune-mediated inflammatory diseases treated with immunosuppressants and controls (ie, patients not on immunosuppressants and healthy controls), and investigated determinants for the occurrence of SARS-CoV-2 breakthrough infections, including humoral immune responses after vaccination.
Research in context.
Evidence before this study
We searched PubMed and Google Scholar on Feb 1, 2022, for studies published since Jan 1, 2020, in English that describe the occurrence of SARS-CoV-2 breakthrough infections in patients receiving immunosuppressive drugs, using the terms “SARS-CoV-2 breakthrough infections”, “immunosuppressive agents”, and “autoimmune diseases”, and variations on these. Data from studies on SARS-CoV-2 infections before vaccination suggest that patients with immune-mediated inflammatory diseases were more frequently admitted to hospital when infected with SARS-CoV-2 than were members of the general population, most notably patients receiving anti-CD20 therapy. Because treatment-specific blunted humoral or cellular immune responses after SARS-CoV-2 vaccination have been found in several studies, assessment of whether patients receiving immunosuppressants are at increased risk of severe SARS-CoV-2 breakthrough infections is essential.
Added value of this study
To our knowledge, this is the first prospective study to compare the incidence of SARS-CoV-2 breakthrough infections between patients with immune-mediated inflammatory diseases treated with immunosuppressants and patients not receiving immunosuppressants and healthy controls. A unique aspect of our study is that we combined clinical and serological data collection. This approach enabled us to cross-sectionally estimate the incidence of asymptomatic COVID-19 cases using serological analyses that measured antibodies against the nucleocapsid protein of SARS-CoV-2. We found that breakthrough infections in patients with immune-mediated inflammatory diseases treated with immunosuppressants are mostly mild and similar to those of controls, with the exception of patients treated with anti-CD20 therapy. Post-vaccination humoral data showed that good humoral immune responses after COVID-19 vaccination are associated with a reduced risk of SARS-CoV-2 breakthrough infections, in both immunosuppressed patients and controls.
Implications of all the available evidence
Our data will help formulate recommendations on SARS-CoV-2 vaccinations in patients with immune-mediated inflammatory diseases treated with immunosuppressants. Traditional risk factors such as older age, male sex, and the presence of comorbidities have a critical contribution to the disease course of COVID-19. Integrating other risk factors should become standard practice when discussing treatment options, SARS-CoV-2 vaccination strategies, and adherence to infection prevention measures with patients.
Methods
Study design
This is a prespecified substudy using pooled data from two ongoing prospective multicentre cohort studies in the Netherlands, the Target to-B! (T2B!) study and Amsterdam Rheumatology Center COVID (ARC-COVID) study, to assess SARS-CoV-2 breakthrough infections.
The T2B! study is a prospective, multiple-arm, multicentre study that includes patients with different immune-mediated inflammatory diseases using specific immunosuppressants, or combinations or these immunosuppressants, treated at outpatients clinics at seven participating academic and non-academic hospitals or at one rheumatology treatment centre in the Netherlands, and patients from the ARC-COVID study (appendix p 3).5, 10 Patients with immune-mediated inflammatory diseases not on immunosuppressants and healthy controls were recruited for comparisons. Patients with immune-mediated inflammatory diseases and healthy controls who had SARS-CoV-2 infection before vaccination were actively recruited either from the ARC-COVID study or via active recruitment on the basis of known history of a previous SARS-CoV-2 infection before vaccination. All participants needed to be at aged at least 18 years. Participants were recruited between Feb 2 and Aug 1, 2021. Data on breakthrough infections were collected between week 27 (from July 1) and week 50 (up to Dec 15) of 2021. During this time, the SARS-CoV-2 delta (B.1.617.2) variant was dominant in the Netherlands, with two infection waves: from week 27 to week 31 (July 7 to Aug 2) and from week 41 to week 50 (Oct 11 to Dec 15), 2021. The primary objective of the T2B! study was to investigate humoral and cellular immunity after SARS-CoV-2 vaccination in patients being treated with predefined types of immunosuppressants. The research protocol of this study was approved by the medical ethical committee of the Amsterdam UMC (T2B-COVID study: 2020.194). All participants provided written informed consent.
The ARC-COVID study is a prospective cohort study with the primary objective to compare the disease severity of primary SARS-CoV-2 infections (ie, those occurring before vaccination) between patients with rheumatic immune-mediated inflammatory diseases and healthy controls.10 All adult patients (aged ≥18 years) with chronic inflammatory diseases from the Amsterdam Rheumatology and Immunology Center in Amsterdam, the Netherlands, were invited to participate between April 26, 2020, and March 1, 2021. All patients were asked (but not obliged) to recruit their own control participant of the same sex, of similar age (difference of <5 years), who did not have a chronic inflammatory disease. At the start of the Dutch SARS-CoV-2 vaccination programme (from Jan 6, 2021), the objectives of the ARC-COVID study were expanded to include comparisons of clinical efficacy and immunogenicity of SARS-CoV-2 vaccinations between patients and healthy controls (ie, SARS-CoV-2 breakthrough infections and humoral immunity).1, 10 The research protocol of this study was approved by the medical ethical committee of the Amsterdam UMC (ARC-COVID study: 2020.169). All participants provided written informed consent.
Additional details of both study protocols are in the appendix (p 3). Some data from these studies have been published elsewhere.1, 4, 5, 10, 11, 12, 13, 14, 15
Participants
In this substudy, we included patients with immune-mediated inflammatory diseases receiving immunosuppressants and a control group that consisted of patients with immune-mediated inflammatory diseases not on immunosuppressants and healthy controls (hereafter referred to as combined controls). Patients not on immunosuppressants and healthy controls were combined into one control group because we did not observe differences in seroconversion rates between both groups in previously published results.1, 5 All participants could only be included once, such that participants who participated in both the T2B! study and ARC-COVID study were only included in our analysis as participating in the T2B! study. Only participants who were vaccinated against SARS-CoV-2 were included. Individuals were considered as being vaccinated after having received two doses of BNT162b2 (Pfizer–BioNtech), CX-024414 (mRNA-1273; Moderna), or ChAdOx1 nCoV-19 (Oxford–AstraZeneca), or after one dose of Ad.26.COV2.S (Janssen, Johnsen & Johnsen). Individuals who had a SARS-CoV-2 infection before or within 90 days after first vaccination and who had at least one dose of either BNT162b2 or CX-024414 were also considered as being vaccinated. Participants were excluded if their last follow-up date was before Nov 16, 2021, and if no breakthrough infection had occurred before that date. Full inclusion and exclusion criteria for the different cohorts are in the appendix (p 3).
Procedures
The following vaccines were used in the primary vaccination campaign in the Netherlands: ChAdOx1 nCoV-19, BNT162b2, CX-024414, and Ad.26.COV2.S vaccines. Vaccinations started on Jan 6, 2021. For healthy individuals with a previous SARS-CoV-2 infection, a second vaccination was optional. In September, 2021, a third vaccination was offered to specific susceptible populations as part of the primary vaccination schedule, including patients being treated with anti-CD20 therapy, S1P receptor modulators, and mycophenolate mofetil combination therapies. Distribution of SARS-CoV-2 booster vaccinations in the Dutch population started on Nov 18, 2021. All people, including those who received a third vaccination as part of their primary vaccination schedule, were eligible to receive a booster vaccination at least 3 months after their last dose of SARS-CoV-2 vaccine. Third and booster vaccinations were given either with BNT162b2 or CX-024414.
In the T2B! study, self-completed questionnaires and clinical files were used to register demographic data; immune-mediated inflammatory disease diagnoses; start and stop dates for all immunosuppressants used since Jan 1, 2021, or Jan 1, 2020, for treatments with long-term effects (eg, anti-CD20 therapies or cyclophosphamide); and dates of potential SARS-CoV-2 infections. Clinical data on SARS-CoV-2 breakthrough infections were collected via questionnaires that were sent to each participant every 2 months after first vaccination for the entire follow-up period (ie, at least 1 year after vaccination). If participants indicated a positive PCR or antigen test, they were contacted by a researcher to verify the data and to ascertain the disease severity score (using the WHO COVID-19 Clinical Progression scale16). Hospital discharge data were used when applicable. As part of the primary objective of the T2B! study, serum samples were collected from all participants via venipuncture or a finger prick blood test kit sent to the participant at home. Serum samples were collected before vaccination (ie, baseline) and 28 days after first and second SARS-CoV-2 vaccination (when applicable).
In the ARC-COVID study, online questionnaires were sent to participants to collect demographic data and clinical data on SARS-CoV-2 breakthrough infections as described previously.1, 10 Demographic data were collected at enrolment (ie, baseline) and included age, sex, height, bodyweight, and autoimmune disease type. Questionnaires to collect data on SARS-CoV-2 breakthrough infections were sent to participants at three fixed timepoints: on April 26, Aug 24, and Dec 10, 2021. Data collected included vaccination dates, vaccine type, information on COVID-19 symptoms, and admissions to hospital due to COVID-19. Hospital discharge data were used to verify the COVID-19 disease severity score of participants who had been admitted to hospital. For this substudy, serum samples from participants of the ARC-COVID study were collected cross-sectionally (ie, at one timepoint) between Oct 1 and Dec 15, 2021, via self-test at home or at the study centre.
Serum samples from both studies were sent to and stored at a central laboratory (Sanquin, Amsterdam, Netherlands). All serological assays for both cohorts were done by the same central laboratory. In serum samples collected in the T2B! study, the concentration of SARS-CoV-2 antibodies in serum samples was measured using an in-house anti-receptor binding domain (RBD) IgG ELISA, as described previously.17, 18 Additionally, we identified participants with previous SARS-CoV-2 infection using a semi-quantitative total antibody RBD-antibody bridging ELISA on baseline samples before vaccination. We used this assay because the sensitivity of the semi-quantitative RBD-antibody ELISA is higher than anti-RBD IgG ELISA in very low antibody ranges (98·1% sensitivity and 99·5% specificity).17, 18
In serum samples collected in the ARC-COVID study, we additionally used a semi-quantitative total antibody nucleocapsid-antibody bridging ELISA, as described previously,17, 18 but using a truncated version of the nucleocapsid protein to enhance specificity to detect asymptomatic infections with SARS-CoV-2 (specificity >99% and sensitivity 95%).17, 18
Outcomes
Outcome definitions were harmonised between both studies. The primary outcome was incidence of SARS-CoV-2 breakthrough infections with the SARS-CoV-2 delta variant. The primary objective was to compare the cumulative incidence of SARS-CoV-2 breakthrough infections with the SARS-CoV-2 delta variant between patients with immune-mediated inflammatory diseases on immunosuppressants and combined controls. Other objectives were to explore associations between the humoral immune response after SARS-CoV-2 vaccination and breakthrough infections, and to compare the severity of breakthrough infections between patients with immune-mediated inflammatory diseases on immunosuppressants and combined controls.
A SARS-CoV-2 breakthrough infection was defined as a PCR-confirmed or antigen-confirmed SARS-CoV-2 infection that occurred at least 14 days after vaccination, and that was detected between July 1 and Dec 14, 2021. All infections during this period were assumed to be due to the delta variant because it was the dominant variant during the study period. A SARS-CoV-2 infection before vaccination was defined as a self-reported positive COVID-19 PCR test or evidence of anti-RBD SARS-CoV-2 antibodies before the first COVID-19 vaccination. Disease severity of SARS-CoV-2 breakthrough infections was categorised using the WHO COVID-19 Clinical Progression Scale.16 A WHO score of 1 indicates asymptomatic infection, a score of 2 indicates mild disease without need for assistance, a score of 3 indicates mild disease with need for assistance (ie, could not care for themselves in daily life due to the severity of their symptoms) but no hospitalisation; we classified participants with a score of 1–3 as being ambulatory. A WHO score of 4 or higher indicated severe disease—ie, admission to hospital (hospitalisation) or death. A score of 4 indicates hospitalisation without oxygen supplementation; 5 indicates hospitalisation with oxygen supplementation via mask or nasal prongs; 6 indicates admission to an intensive care unit (ICU) and oxygen by non-invasive ventilation or high-flow ventilation; 7 indicates admission to an ICU with mechanical ventilation, with partial pressure of oxygen (pO2) to fraction of inspired oxygen (FiO2) ratio of ≥150 or peripheral blood oxygen saturation (SpO2) to FIO2 ratio of ≥200; 8 indicates mechanical ventilation with pO2 to FIO2 ratio of <150 or SpO2 to FIO2 ratio of <200 or use of vasopressors; 9 indicates mechanical ventilation with a SpO2 to FIO2 ratio of <150 and vasopressors, dialysis, or extracorporeal membrane oxygenation; and 10 indicates the patient has died. Participants had to score the severity of their symptoms (mild: annoying but not limiting daily activities; moderate: limiting daily activities; or severe: unable to execute daily activities). Participants received a COVID-19 disease severity score of 3 when they rated at least one of their symptoms as severe.
In participants of the T2B! study, seroconversion after vaccination was defined as an anti-RBD IgG response of 4·0 arbitrary units per mL (AU/mL) or higher. In participants of the ARC-COVID study, participants were defined as having additional asymptomatic SARS-CoV-2 infections if they had detectable nucleocapsid antibodies without a previously confirmed COVID-19 diagnosis (PCR-confirmed or antigen-confirmed diagnosis at any time during the pandemic, or detectable RBD antibodies before SARS-CoV-2 vaccination). For these analyses, we only used samples collected between Oct 1 and Dec 31, 2021. Definitions of active treatment and types of immunosuppressants used as monotherapy or as part of combination therapy are in the appendix (p 3).
Statistical analysis
Because the study of breakthrough infections was a secondary objective for both T2B! and ARC-COVID, no sample size calculation for this substudy was done. Sample size calculation for the primary outcomes of the studies have been described previously.5, 10
We present characteristics of patients with immune-mediated inflammatory diseases treated with immunosuppressants and combined controls group as mean (SD), median (IQR), or frequencies with corresponding proportions, depending on the type and distribution of the data. We calculated 95% CIs for the cumulative incidence of breakthrough infections in patients with immunosuppressants, combined controls, and both control groups separately. We used publicly available epidemiological data from the National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu [RIVM]) to investigate changes in the incidence of PCR-confirmed breakthrough infections in the general Dutch population during follow-up.19 Participants contributed to person-time at risk from 14 days after vaccination (third and booster doses not included) to the date of breakthrough infection, death, or data cutoff (Dec 15, 2021). We used univariable and multivariable logistic regression analyses to investigate whether use of immunosuppressants or humoral responses after vaccination were associated with SARS-CoV-2 breakthrough infections. We adjusted multivariable models for age, sex, cardiovascular disease, diabetes, chronic pulmonary disease, obesity, previous SARS-CoV-2 infection before first vaccination, and vaccine type, on the basis of pre-existing literature that identified these variables as potential risk factors.20 Initially, we used Cox regression analyses to assess associations with SARS-CoV-2 breakthrough infections, but the proportional hazards assumption was not met (appendix p 16). Therefore, we analysed the association between humoral responses after vaccination and breakthrough infections in two separate multivariable logistic regression models: as seroconversion (yes vs no) in all participants of the T2B! study, and as anti-RBD titre in participants who seroconverted (IgG titre ≥4·0 AU/mL). Because anti-RBD titres did not show a linear relationship with breakthrough infections (appendix p 18), titres were grouped into quartiles.
We did sensitivity analyses to assess the effect of combining patients with immune-mediated inflammatory diseases not on immunosuppressants and healthy controls into one control group, and to account for the potential effect of previous SARS-CoV-2 infection before vaccination. We investigated risk factors for severe breakthrough infections (ie, WHO COVID-19 Clinical Progression Scale score of 4 or higher) using descriptive statistics. We investigated risk factors for severe breakthrough infections (ie, WHO COVID-19 Clinical Progression Scale score of 4 or higher) using descriptive statistics, and we calculated 95% CIs for rates of hospitalisation (WHO score of 4 or higher) of patients on immunosuppressants and combined controls. We did a post-hoc Fisher's exact test to compare the risk of severe SARS-CoV-2 breakthrough infections between patients treated with anti-CD20 therapy and patients receiving other immunosuppressants. We did not do any analyses to investigate effect modification. We also did the following additional post-hoc analyses: subgroup analyses to investigate effects of different immunosuppressants on the risk of breakthrough infections, and logistic regression models adjusted for time since vaccination. We only did complete case analyses, and variables with missing data are shown in the appendix (p 4). We did no correction for multiple testing.
We used R (version 4.1.0) and SPSS (version 27.0) for all analyses. We considered p value of less than 0·05 to be significant. The T2B! study is registered with the Dutch Trial Register, trial ID NL8900, and the ARC-COVID study is registered with Dutch Trial Register, trial ID NL8513.
Role of the funding source
The funders of the studies (T2B! and ARC-COVID) had no role in study design, data collection, data analyses, data interpretation, or writing of the report.
Results
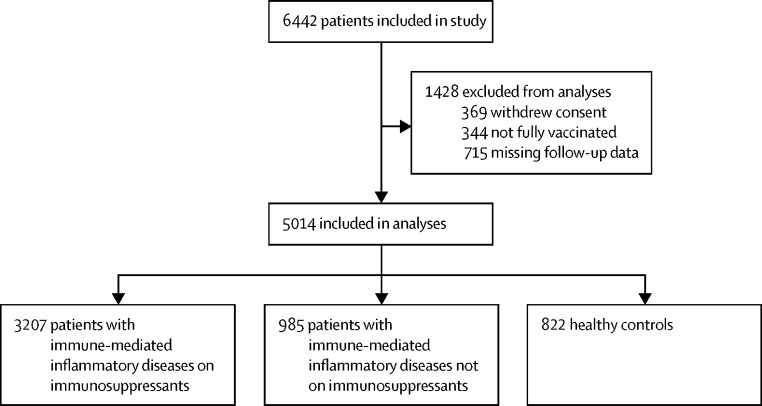
Of 3233 individuals in the T2B! study and 3212 in the ARC-COVID study, we included 5014 participants in the analysis for this substudy; 3207 patients with immune-mediated inflammatory diseases on immunosuppressants, and 1807 combined controls (985 patients with immune-mediated inflammatory diseases not on immunosuppressants and 822 healthy controls; figure 1 ; appendix p 17). Among patients receiving immunosuppressants, mean age was 53 years (SD 14), 2042 (64%) of 3207 were female and 1165 (36%) were male; among patients not receiving immunosuppressants, mean age was 54 years (SD 14), 598 (61%) of 985 were female and 387 (39%) were male; and among healthy controls, mean age was 57 years (SD 13), 549 (67%) of 822 were female and 273 (33%) were male (table 1 ; appendix pp 5–6). Although race and ethnicity data were collected in ARC-COVID, they were not collected in T2B!, so they are not reported here. Participants in the ARC-COVID cohort were older than those in the T2B! cohort (59 years [SD 13] vs 49 years [13]; appendix pp 5–6). Patients with immune-mediated inflammatory diseases on immunosuppressants most frequently used methotrexate (992 [31%] of 3207 overall, and 442 [14%] as monotherapy) and TNF inhibitors (929 [29%] overall, and 523 [16%] as monotherapy). Age and sex distribution were similar in participants with and without a breakthrough infection (appendix pp 7–8).
Figure 1.
Study profile
The number of participants included from the T2B! and ARC-COVID studies are reported separately in the appendix (p 17).
Table 1.
Baseline characteristics of all participants of both cohorts (T2B! and ARC-COVID) combined
| Patients with immune-mediate inflammatory diseases on immunosuppressants (n= 3207) | Patients with immune-mediate inflammatory diseases not on immunosuppressants (n=985) | Healthy controls (n=822) | ||
|---|---|---|---|---|
| SARS-CoV-2 breakthrough infection | ||||
| Cumulative incidence | 148 (5%) | 52 (5%) | 33 (4%) | |
| Incidence rate, events per 1000 person-months | 8·0 | 9·2 | 6·6 | |
| Time at risk, days | 172 (157–193) | 174 (154–195) | 182 (165–201) | |
| Demographic characteristics | ||||
| Age, years | 53 (14) | 54 (14) | 57 (13) | |
| Sex | ||||
| Female | 2042 (64%) | 598 (61%) | 549 (67%) | |
| Male | 1165 (36%) | 387 (39%) | 273 (33%) | |
| Clinical characteristics | ||||
| Comorbidities | ||||
| Cardiovascular disease | 347 (11%) | 110 (11%) | 60 (7%) | |
| Chronic pulmonary disease | 293 (9%) | 93 (9%) | 42 (5%) | |
| Diabetes | 161 (5%) | 49 (5%) | 25 (3%) | |
| Obesity | 518 (16%) | 155 (16%) | 85 (10%) | |
| SARS-CoV-2 infection before first vaccination | ||||
| Total confirmed COVID-19 diagnoses | 402 (13%) | 126 (13%) | 130 (16%) | |
| PCR-confirmed diagnosis | 79 (2%) | 48 (5%) | 4 (<1%) | |
| Serological confirmed diagnosis | 171 (5%) | 25 (3%) | 58 (7%) | |
| PCR and serological confirmed | 152 (5%) | 53 (5%) | 68 (8%) | |
| Immune-mediated inflammatory disease | ||||
| Rheumatic disease* | 1989 (62%) | 542 (55%) | NA | |
| Neurological† | 492 (15%) | 183 (19%) | NA | |
| Gastroenterological‡ | 484 (15%) | 121 (12%) | NA | |
| Dermatological§ | 242 (8%) | 139 (14%) | NA | |
| Immunosuppressants¶ | ||||
| Methotrexate | 992 (31%) | NA | NA | |
| Monotherapy | 442 (14%) | NA | NA | |
| TNF inhibitor | 929 (29%) | NA | NA | |
| Monotherapy | 523 (16%) | NA | NA | |
| Anti-CD20 | 266 (8%) | NA | NA | |
| Monotherapy | 170 (5%) | NA | NA | |
| Mycophenolate mofetil | 105 (3%) | NA | NA | |
| Monotherapy | 35 (1%) | NA | NA | |
| S1P receptor modulator | 66 (2%) | NA | NA | |
| Monotherapy | 66 (2%) | NA | NA | |
| Other immunosuppressants‖ | 432 (13%) | NA | NA | |
| Monotherapy | 957 (30%) | NA | NA | |
| COVID-19 vaccine type | ||||
| ChAdOx1 nCoV-19 (Oxford–AstraZeneca) | 469 (15%) | 132 (13%) | 172 (21%) | |
| BNT162b2 (Pfizer–BioNTech) | 2019 (63%) | 598 (61%) | 446 (54%) | |
| CX-024414 (mRNA-1273; Moderna) | 563 (18%) | 203 (21%) | 147 (18%) | |
| Ad.26.COV2.S (Janssen) | 65 (2%) | 26 (3%) | 40 (5%) | |
| Combination of vaccines | 91 (3%) | 26 (3%) | 17 (2%) | |
| Additional vaccine dose during follow-up | 957 (30%) | 166 (17%) | 142 (17%) | |
| Additional vaccine dose received before breakthrough infection | 16 (<1%) | 2 (<1%) | 2 (<1%) | |
| Post-vaccination antibody response | ||||
| n | 1656 | 474 | 174 | |
| Seroconversion | 1427/1656 (86%) | 461/474 (97%) | 172/174 (99%) | |
| IgG titre, all groups | 85 (19–199) | 157 (65–278) | 227 (138–357) | |
| Poor responders** | 3 (<1–25) | .. | .. | |
| Other immunosuppressants | 110 (47–223) | .. | .. | |
Data are mean (SD), median (IQR), or n (%) or n/N (%), unless otherwise stated. NA=not applicable. T2B!=Target to-B!
Includes rheumatoid arthritis, spondylarthritis, systemic lupus erythematosus, Sjögren's syndrome, vasculitis (small, medium, and large vessel vasculitis and other forms of vasculitis except giant cell arteritis), and other rheumatological disease (eg, giant-cell arteritis, polymyalgia rheumatica, and others).
Includes multiple sclerosis and neuromyelitis optica spectrum disorder, inflammatory neuropathies, myopathies (eg, chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, and inflammatory myositis), and myasthenia gravis.
Includes Crohn's disease, ulcerative colitis, autoimmune hepatitis, and other inflammatory bowel disorders (eg, autoimmune sclerosing cholangitis).
Includes atopic dermatitis, psoriasis, pemphigus, and others (eg, vitiligo, pemphigus, and others).
Patients could be treated with multiple immunosuppressants.
Other immunosuppressants include abatacept, belimumab, calcineurin inhibitors, cladribine, corticosteroids, dihydroorotate dehydrogenase (known as DHODH) inhibitors, dimethyl fumarate, dupilumab, eculizumab, glatiramer, hydroxychloroquine, IL-17A antagonists, IL-23 antagonists, immunoglobulin, interferon beta, JAK inhibitors, natalizumab, omalizumab, purine antagonists, tocilizumab, ustekinumab, vedolizumab, cyclophosphamide, anakinra, sarilumab, sulfasalazine, leflunomide, and azathioprine.
Anti-CD20 therapy, S1P receptor modulators, and mycophenolate mofetil.
The incidence of SARS-CoV-2 breakthrough infections in patients on immunosuppressants versus combined controls, based on positive PCR or antigen test results, over time compared with development of the COVID-19 pandemic in the Netherlands is shown in figure 2 . Because we did not observe differences in seroconversion rates between both groups in previously published results,1, 5 patients not on immunosuppressants and healthy controls were combined into one control group. Sensitivity analyses confirmed that the risk of breakthrough infection was similar in both patients not on immunosuppressants and in healthy controls (appendix pp 9–10). SARS-CoV-2 breakthrough infections were detected in 148 (4·6% [95% CI 3·9–5·4]) of 3207 patients with immune-mediated inflammatory diseases on immunosuppressants (8·0 per 1000 person-months) and in 85 (4·7%) of 1807 combined controls (8·0 per 1000 person-months; in 52 [5·3%; 95% CI 4·0–6·9] of 985 patients not on immunosuppressants and in 33 [4·0%; 2·8–5·6] of 825 healthy controls). Among 148 patients on immunosuppressants who had breakthrough infections, 16 (11%) were on anti-CD20 therapy (vs 250 [8%] of 3059 who did not have breakthrough infections), three (2%) were on mycophenolate mofetil (vs 102 [3%] who did not have breakthrough infections), and five (3%) were on S1P receptor modulators (vs 61 [2%] who did not have breakthrough infections; appendix pp 7–8).
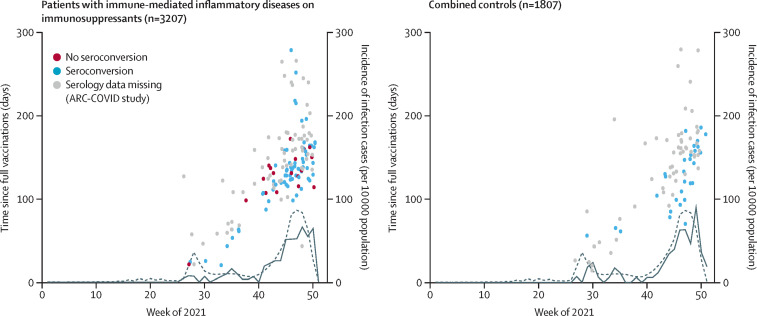
Figure 2.
Incidence of SARS-CoV-2 breakthrough infections versus time since full vaccination and the occurrence of infection waves in the Netherlands, 2021
The dotted line shows total incidence of SARS-CoV-2 infections among the Dutch population, with the solid line showing the incidence of breakthrough infections among patients on immunosuppressants and combined controls. Publicly available epidemiological data from the National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu [RIVM]) were used to study changes in the incidence of PCR-confirmed breakthrough infections in the Netherlands during follow-up. Incidence rates are displayed as cases per 10 000 population. Seroconversion was measured at 28 days after full vaccination in participants of the T2B! study only. Participants of the ARC-COVID study are all depicted with grey dots due to missing serology. Combined controls includes patients with immune-mediated inflammatory diseases not on immunosuppressants and healthy controls.
Case descriptions of breakthrough cases treated with anti-CD20 therapy are in the appendix (p 11). The cumulative incidence of breakthrough infections for patients on immunosuppression versus combined controls is in the appendix (p 16). The incidence of breakthrough infections over time in patients on immunosuppressants was similar to that of combined controls (figure 2). Most breakthrough infections (196 [84%] of 233) occurred at least 3 months after vaccination, and these infections mostly coincided with the two infection waves of the delta variant in the Netherlands. The number of asymptomatic breakthrough infections that were additionally identified serologically in the ARC-COVID cohort was similar in patients receiving immunosuppressants and combined controls: nucleocapsid antibodies were detected in 129 (21%) of 628 patients and 121 (21%) of 571 controls, and 55 (9%) of 628 patients and 56 (10%) of 571 controls had no previously confirmed COVID-19 diagnosis.
Use of immunosuppressants was not associated with SARS-CoV-2 breakthrough infections (adjusted odds ratio [OR] 0·88 [95% CI 0·66–1·18]; table 2 ). Sensitivity analyses showed that effect estimates remained similar when patients with immune-mediated inflammatory diseases with immunosuppressants were separately compared with patients not on immunosuppressants and healthy controls (appendix pp 9–10). Post-hoc subgroup analyses did not show differences between different types of immunosuppressants (appendix p 12). Seroconversion after vaccination was associated with a lower odds of SARS-CoV-2 breakthrough infections in the T2B! cohort (OR 0·58 [95% CI 0·34–0·98]) and SARS-CoV-2 infection before vaccination was associated with lower odds of breakthrough infection in the combined cohort (OR 0·34 [0·18–0·56]). In participants of the T2B! cohort with seroconversion, there was no association between the concentration of anti-RBD titres divided in quartiles and SARS-CoV-2 breakthrough infections (table 2; appendix p 18). Sensitivity analyses showed that results remained similar when patients with a SARS-CoV-2 infection before vaccination were excluded from analyses, and when analyses were adjusted for time since vaccination (post hoc; appendix p 9–10). Participants with and without seroconversion showed similar trends between time of breakthrough infection and time after vaccination (figure 2).
Table 2.
Univariable and multivariable analyses of potential determinants for SARS-CoV-2 breakthrough infections
| Odds ratio (95% CI) | p value | ||||
|---|---|---|---|---|---|
| Clinical determinants analyses (combined cohort) | |||||
| Univariable model (N=5014; 235 events) | |||||
| Combined controls | 1·00 (ref) | .. | |||
| Patients with immune-mediated inflammatory diseases on immunosuppressants | 0·98 (0·75–1·29) | 0·89 | |||
| Multivariable model (N=4906; 228 events)*† | |||||
| Combined controls | 1·00 (ref) | .. | |||
| Patients with immune-mediated inflammatory diseases on immunosuppressants | 0·88 (0·66–1·18) | 0·54 | |||
| Covariables | |||||
| Age | 0·98 (0·96–0·99) | <0·0001 | |||
| Sex | |||||
| Male | 1·00 (ref) | .. | |||
| Female | 0·94 (0·71–1·25) | 0·69 | |||
| Obesity | 1·02 (0·77–1·34) | 0·90 | |||
| Cardiovascular disease | 0·98 (0·60–1·53) | 0·93 | |||
| Pulmonary disease | 1·04 (0·63–1·64) | 0·87 | |||
| Diabetes | 2·06 (1·18–3·39) | 0·0071 | |||
| SARS-CoV-2 infection before first vaccination | |||||
| No previous infection | 1·00 (ref) | .. | |||
| Previous SARS-CoV-2 infection | 0·34 (0·18–0·56) | 0·0010 | |||
| Vaccine type | |||||
| ChAdOx1 nCoV-19 (Oxford–AstraZeneca) | 1·00 (ref) | .. | |||
| BNT162b2 (Pfizer–BioNTech) | 0·94 (0·64–1·41) | 0·74 | |||
| CX-024414 (mRNA-1273; Moderna) | 0·79 (0·49–1·30) | 0·38 | |||
| Ad.26.COV2.S (Janssen) | 0·40 (0·09–1·15) | 0·13 | |||
| Combination of vaccines | 0·28 (0·02–1·35) | 0·22 | |||
| Humoral determinants analyses (T2B! cohort only) | |||||
| Multivariable model (N=2225; 108 events)‡ | |||||
| No seroconversion after full vaccination | 1·00 (ref) | .. | |||
| Seroconversion after full vaccination | 0·58 (0·34–0·98) | 0·044 | |||
| Multivariable model (N=1983; 90 events)‡ | |||||
| Anti-RBD titre in AU/mL | |||||
| 4·000 to <53·025 | 0·97 (0·50–1·88) | 0·93 | |||
| 53·025 to <126·250 | 1·55 (0·88–2·73) | 0·13 | |||
| 126·250 to <249·750§ | 1·00 (ref) | .. | |||
| ≥249·750 | 0·57 (0·28–1·16) | 0·12 | |||
Clinical determinants were studied in the combined cohort; humoral determinants were studied in the T2B! cohort only. The number of observations analysed in each model is shown (numbers varying due to selection for the model and/or missing data). AU=arbitrary units. RBD=receptor binding domain. T2B!=Target to-B!
Only the presented variables were included in the model.
Variables included were age, sex, obesity, cardiovascular disease, pulmonary disease, diabetes, SARS-CoV-2 infection before first vaccination, and vaccine type.
Variables included were age, sex, obesity, cardiovascular disease, pulmonary disease, diabetes, and vaccine type.
Third quartile was chosen as reference group on the basis of median antibody titres observed in controls with seroconversion (181 AU/mL [IQR 84–299]).
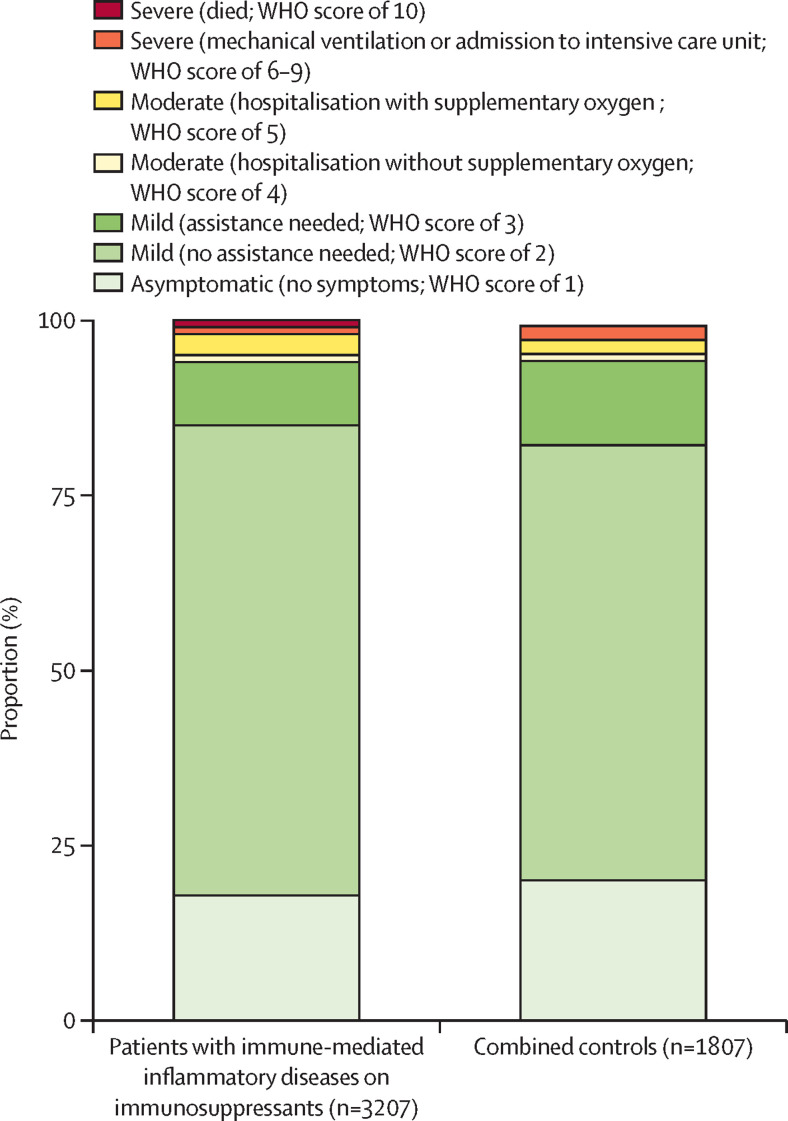
Participants with a SARS-CoV-2 breakthrough infection requiring ambulatory care only were classified as follows; asymptomatic (27 [18%] of 148 patients on immunosuppressants vs 17 [20%] of 85 combined controls), mild disease without need for assistance (99 [67%] vs 53 [62%]), or mild disease with need for assistance (14 [9%] vs ten [12%]; figure 3 ; appendix pp 13, 19). Three patients with immune-mediated inflammatory diseases on immunosuppressants (who had a WHO score of 2, 5, and 7) and two individuals in the combined control cohort (who had a WHO score of 5 and 7) were treated with recombinant anti-SARS-CoV-2 monoclonal antibodies. Hospitalisation due to COVID-19 was required in eight (5·4% [95% CI 2·5–10·7]) of 148 patients on immunosuppressants, and five (5·9% [2·1–13·7]) of 85 combined controls. Of the eight patients on immunosuppressants who were hospitalised, two did not need oxygen, four needed supplementary oxygen, and two were admitted to the intensive-care unit (ICU) for mechanical ventilation, of whom one died. Of the five individuals in the combined control cohort who were hospitalised, one did not need oxygen, two needed supplementary oxygen, and two were admitted to the ICU for mechanical ventilation; none died. Participants with breakthrough infections (including patients with and without immunosuppressants and healthy controls) who were admitted to hospital (ie, WHO score of 4–10) were on average older (mean age 60 years [SD 11] vs 51 years [SD 14]) and more frequently male (nine [69%] of 13 vs 78 [35%] of 220), obese (five [38%] vs 33 [15%]), had diabetes (three [23%] vs 15 [7%]), and had cardiovascular diseases (five [38%] vs 17 [8%]) than those who were ambulatory (WHO score of 1–3). These data were consistent across groups (table 3 ). In post-hoc analyses, rates of hospitalisation (WHO score of 4 or higher) were higher among patients with immune-mediated inflammatory diseases using anti-CD20 therapy than among those using other immunosuppressants (three [19%] of 16 patients using anti-CD20 therapy who had a breakthrough infection vs five [4%] of 132 patients using other immunosuppressants who had a breakthrough infection; p=0·041). Individual case descriptions of participants with breakthrough infections who were admitted to hospital are shown in the appendix (p 14). Individual case descriptions of participants with a breakthrough infection after receiving an additional vaccine dose are in the appendix (p 15).
Figure 3.
Severity of breakthrough infection in patients with immune-mediated inflammatory diseases on immunosuppressants and combined controls, for both cohorts combined
Severity of SARS-CoV-2 breakthrough infections categorised using the WHO COVID-19 Clinical Progression Scale.16 Combined controls includes patients with immune-mediated inflammatory diseases not on immunosuppressants and healthy controls.
Table 3.
Characteristics of patients with immune-mediated inflammatory disorder on and not on immunosuppressants compared with controls who had a SARS-CoV-2 breakthrough infection, by severity of breakthrough infection
|
Patients with immune-mediated inflammatory disease on immunosuppressants (n=148) |
Patients with immune-mediated inflammatory disease patients not on immunosuppressants (n=52) |
Healthy controls (n=33) |
|||||
|---|---|---|---|---|---|---|---|
| Ambulatory care (n=140) | Hospitalised or deceased (n=8) | Ambulatory care (n=49) | Hospitalised or deceased (n=3) | Ambulatory care (n=31) | Hospitalised or deceased (n=2) | ||
| Demographic characteristics | |||||||
| Age, years | 49 (13) | 60 (10) | 50 (15) | 57 (17) | 56 (12) | 62 (5) | |
| Sex | |||||||
| Female | 87 (62%) | 3 (38%) | 31 (63%) | 1 (33%) | 24 (77%) | 0 | |
| Male | 53 (38%) | 5 (63%) | 18 (37%) | 2 (67%) | 7 (23%) | 2 (100%) | |
| Clinical characteristics | |||||||
| Comorbidities | |||||||
| Cardiovascular disease | 12 (9%) | 2 (25%) | 3 (6%) | 2 (67%) | 2 (6%) | 1 (50%) | |
| Chronic pulmonary disease | 10 (7%) | 2 (25%) | 4 (8%) | 1 (33%) | 3 (10%) | 1 (50%) | |
| Diabetes | 9 (6%) | 1 (13%) | 5 (10%) | 1 (33%) | 1 (3%) | 1 (50%) | |
| Obesity | 25 (18%) | 2 (25%) | 5 (10%) | 2 (67%) | 3 (10%) | 1 (50%) | |
| SARS-CoV-2 infection before first vaccination | |||||||
| Total confirmed COVID-19 diagnoses | 10 (7%) | 0 | 3 (6%) | 0 | 2 (6%) | 0 | |
| PCR confirmed | 4 (3%) | 0 | 0 | 0 | 0 | 0 | |
| Serologically confirmed | 6 (4%) | 0 | 3 (6%) | 0 | 2 (6%) | 0 | |
| PCR and serologically confirmed | 0 | 0 | 0 | 0 | 0 | 0 | |
| Immunosuppressants* | |||||||
| Methotrexate | 35 (25%) | 2 (25%) | NA | NA | NA | NA | |
| Monotherapy | 15 (11%) | 1 (13%) | NA | NA | NA | NA | |
| TNF inhibitor | 48 (34%) | 1 (13%) | NA | NA | NA | NA | |
| Monotherapy | 29 (21%) | 1 (13%) | NA | NA | NA | NA | |
| Anti-CD20 | 13 (9%) | 3 (38%) | NA | NA | NA | NA | |
| Monotherapy | 9 (6%) | 3 (38%) | NA | NA | NA | NA | |
| Mycophenolate mofetil | 3 (2%) | 0 | NA | NA | NA | NA | |
| Monotherapy | 0 | 0 | NA | NA | NA | NA | |
| S1P receptor modulator | 5 (4%) | 0 | NA | NA | NA | NA | |
| Monotherapy | 5 (4%) | 0 | NA | NA | NA | NA | |
| Other immunosuppressants† | 70 (50%) | 3 (38%) | NA | NA | NA | NA | |
| Monotherapy | 40 (29%) | 2 (25%) | NA | NA | NA | NA | |
| COVID-19 vaccine types | |||||||
| ChAdOx1 nCoV-19 (Oxford–AstraZeneca) | 18 (13%) | 1 (13%) | 5 (10%) | 1 (33%) | 10 (32%) | 1 (50%) | |
| BNT162b2 (Pfizer–BioNTech) | 94 (67%) | 5 (63%) | 32 (65%) | 2 (67%) | 18 (58%) | 1 (50%) | |
| CX-024414 (mRNA-1273; Moderna) | 25 (18%) | 2 (25%) | 10 (20%) | 0 | 3 (10%) | 0 | |
| Ad.26.COV2.S (Janssen) | 1 (1%) | 0 | 2 (4%) | 0 | 0 | 0 | |
| Combination of vaccines | 3 (2%) | 0 | 0 | 0 | 0 | 0 | |
| Time since full vaccination, days | 137 (115–162) | 124 (110–172) | 137 (89–166) | 139 (NA) | 155 (119–173) | 64 (NA) | |
| Additional vaccine dose during follow-up | 21 (15%) | 2 (25%) | 2 (4%) | 0 | 2 (6%) | 1 (50%) | |
| Additional vaccine received prior to breakthrough | 15 (11%) | 1 (13%) | 1 (2%) | 0 | 1 (3%) | 0 | |
Data are n (%), mean (SD), or median (IQR). Patients were defined as being ambulatory if they had a WHO COVID-19 Clinical Progression Scale score of 1–3, and being hospitalised or having died if they had a WHO score of 4–10. NA=not applicable.
Patients could be treated with multiple immunosuppressants.
Other immunosuppressants include abatacept, belimumab, calcineurin inhibitors, cladribine, corticosteroids, dihydroorotate dehydrogenase (known as DHODH) inhibitors, dimethyl fumarate, dupilumab, eculizumab, glatiramer, hydroxychloroquine, IL-17A antagonists, IL-23 antagonists, immunoglobulin, interferon beta, JAK inhibitors, natalizumab, omalizumab, purine antagonists, tocilizumab, ustekinumab, vedolizumab, cyclophosphamide, anakinra, sarilumab, sulfasalazine, leflunomide, and azathioprine.
Discussion
In this study, we observed that the incidence of SARS-CoV-2 breakthrough infections was similar in patients with immune-mediated inflammatory diseases receiving immunosuppressants and controls. Our explorative analyses suggest that no seroconversion after SARS-CoV-2 vaccination was associated with an increased risk of breakthrough infections in all participants. The severity of breakthrough infections was similar between most patients using immunosuppressants and combined controls, and infections were mostly mild or asymptomatic. Hospital admissions were uncommon and primarily seen in people with traditional risk factors for severe COVID-19, such as older age and comorbidities, and in patients receiving anti-CD20 therapy, although these results should be interpreted with caution due to small sample sizes.
To our knowledge, this is the first large prospective study comparing SARS-CoV-2 breakthrough infections during infection waves of the SARS-CoV-2 delta variant between vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants and controls, and the first study that combined clinical and serological data collection. The similar incidence of breakthrough infections in patients using immunosuppressants and controls in this study is in contrast with a large retrospective registry study conducted in the USA, in which the incidence of SARS-CoV-2 breakthrough infections was significantly higher in patients who were immunocompromised than in the general population.6 However, that US study included other patient groups in addition to patients with immune-mediated inflammatory diseases, such as patients with haematological diseases and HIV, and data were derived from national health registers. Our finding of a protective effect of seroconversion after SARS-CoV-2 vaccination is in line with findings from a vaccination trial,21 and an observational study in healthy individuals.7 Additionally, infection with SARS-CoV-2 before vaccination was associated with a reduced incidence of breakthrough infection. Studies in healthy individuals have shown that hybrid immunity (ie, immune responses developing after vaccination in individuals previously infected with SARS-CoV-2) is associated with increased protection against COVID-19 compared with standard vaccination responses, probably due to increased breadth of humoral and cellular repertoires.22 Our findings suggest that such mechanisms are also present in patients with immune-mediated inflammatory diseases who are on immunosuppressants.
Our combined cohort included a large group of patients with immune-mediated inflammatory diseases on potent immunosuppressants such as anti-CD20 therapy, which is a probable cause for the relatively large proportion of patients who did not seroconvert upon vaccination.1, 5 The severity of SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases on immunosuppressants was generally mild, although in post-hoc analyses we found that patients on anti-CD20 therapy were more frequently admitted to hospital than were those receiving other immunosuppressants. Anti-CD20 therapy is known to considerably reduce humoral responses, but not cellular immune responses, after SARS-CoV-2 vaccination,23 and data from studies on primary SARS-CoV-2 infections showed that these patients were at increased risk of severe primary COVID-19.24, 25 Additionally, in line with our own data, a case series of 12 patients with SARS-CoV-2 breakthrough infections and rheumatic immune-mediated inflammatory diseases suggested that anti-CD20 therapy might also increase the risk of severe SARS-CoV-2 breakthrough infections after vaccination.26 However, notably, participants who were hospitalised due to a breakthrough infection in our cohort, including those treated with anti-CD20 therapy, were considerably older and had more comorbidities than were those who were not hospitalised. Therefore, clustering of risk factors, but not the presence of an individual risk factor, might result in a high risk for severe COVID-19 disease.27 However, the risks of COVID-19 will vary with upcoming variants of SARS-CoV-2, which means that variants with decreasing virulence, such as the omicron (B.1.1.529) variant, are associated with decreased risks of severe COVID-19 in general.28 Therefore, the implications of absence of SARS-CoV-2 antibodies after SARS-CoV-2 vaccination or treatment with anti-CD20 therapy should be weighed in light of the presence of other risk factors for severe COVID-19 disease, and the changing field of the pandemic burden and SARS-CoV-2 variants when informing patients and making clinical decisions.29
Strengths of this study include the prospective follow-up of two large, well defined, cohorts of patients with immune-mediated inflammatory diseases receiving immunosuppressants and controls, and the combination of clinical and serological data collection. The inclusion of simultaneously enrolled control participants allowed us to also study traditional risk factors, such as age and comorbidities. Additionally, because patients with a broad variety of immune-mediated inflammatory diseases and immunosuppressants were included in the study, our results are applicable to a large patient population. Another strength is that we estimated the number of undetected asymptomatic cases in our study. Previous studies stressed that it was unknown how undetected asymptomatic cases affected reported comparisons of incidence and disease severity between patients who are immunosuppressed and controls.6 For example, patients receiving immunosuppressants might be more likely to seek medical care than controls, which could result in a higher detection rate of asymptomatic cases.30 Our results suggest that an important proportion of asymptomatic breakthrough cases remain undetected, but that this proportion is similar among patients and controls. This finding implies that estimates of relative risk for severe SARS-CoV-2 breakthrough infections are unaffected by asymptomatic cases, whereas estimates of absolute risk could be overestimated.
The most important limitation of our study is that, despite the large number of participants studied, only a small proportion of individuals who had SARS-CoV-2 breakthrough infections developed severe disease that required admission to hospital. Subsequently, we were not able to do detailed analyses of determinants of disease severity—in particular, analyses to investigate associations between the concentration of antibody titres and protection against COVID-19, and our results of these analyses should be interpreted with caution. Second, this study was largely conducted when the delta variant of SARS-CoV-2 was dominant, so we were unable to provide data for the currently dominant omicron variant. Healthy individuals are more susceptible to the omicron variant than to the delta variant, but their disease is less severe;28 whether this trend in susceptibility and severity also applies to patients with immune-mediated inflammatory diseases treated with immunosuppressants is not yet known. Third, previous studies have shown that protective immunity after SARS-CoV-2 vaccination wanes over time, particularly from 3 months after vaccination.31 However, antibody quality (ie, antibody diversity and affinity) increases with time,32 which might compensate for decreasing titres in persistence of protective immunity, but these processes might be impaired by immunosuppressant treatments such as TNF inhibitors.33 In our study, breakthrough infections mostly occurred at least 90 days after vaccination, but this timepoint coincided with the largest SARS-CoV-2 infection wave seen in the Netherlands to date, and so should be interpreted with caution. The incidence of SARS-CoV-2 infection in the Dutch population was low during the months preceding this infection wave, so, accordingly, only a small number of COVID-19 cases were reported during this period. Therefore, the small number of cases within 90 days after vaccination in both patients on immunosuppressants and controls precluded detailed analyses, and we were unable to assess whether protective immunity waned more rapidly in patients with immune-mediated inflammatory diseases on immunosuppressants than in controls. Fourth, the incidence and severity of breakthrough infections might have been selectively influenced by the Dutch vaccination campaign, during which patients with immune-mediated inflammatory diseases treated with anti-CD20 therapies, mycophenolate mofetil, and S1P receptor modulators received a third vaccination whereas other patients with immune-mediated inflammatory diseases did not. Although added humoral and cellular effects of additional vaccinations in these patients seem low at best,5, 23 a clinical effect on protection might exist, which could have led to an overestimation of our results. Additionally, these data were only collected in the Netherlands, and so further studies are needed internationally to confirm these findings elsewhere. Finally, we did not account for the extent to which people adhered to infection prevention measures, which might have differed between patients receiving immunosuppressants and controls,15 especially because participants were aware of their antibody titres. Therefore, we cannot exclude the possibility that the absence of increased risk of breakthrough infections in patients with immunosuppressants in our study is due to stricter adherence to preventive measures rather than effective immune responses after SARS-CoV-2 vaccination.
In summary, we found that the incidence of SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases on immunosuppressants was similar to that of controls, and that infections were mostly mild. A SARS-CoV-2 infection before vaccination and the presence of a humoral response after SARS-CoV-2 vaccination were associated with reduced odds of breakthrough infection. Anti-CD20 therapy might increase patients' susceptibility to severe SARS-CoV-2 breakthrough infections, but traditional risk factors also continue to have a crucial contribution to the disease course of COVID-19. Therefore, our data suggest that most patients with immune-mediated inflammatory diseases should not necessarily be seen as being at high risk for severe COVID-19, although caution might still be warranted in patients who do not seroconvert after vaccination. Additionally, we believe that integrating other risk factors (eg, comorbidities and age) should become standard practice when discussing treatment options, SARS-CoV-2 vaccination strategies, and adherence to infection prevention measures with patients.
Data sharing
Aggregated deidentified data and code for reproducing the results of this analysis can be shared upon reasonable request. Requests should be made to the corresponding author.
Declaration of interests
FE and TWK report (governmental) grants from ZonMw (the Netherlands Organization for Health Research and Development) to study immune responses after SARS-CoV-2 vaccination in autoimmune diseases. FE also reports grants from Prinses Beatrix Spierfonds, CSL Behring, Kedrion, Terumo BCT, Grifols, Takeda Pharmaceutical Company, and Guillain-Barré Syndrome-Chronic Inflammatory Demyelinating Polyneuropathy (GBS-CIDP) Foundation; consulting fees from UCB Pharma and CSl Behring; and honoraria from Grifols. AJvdK reports grants from CSL Behring and participation on an advisory board for Argen-X. MLö reports a grant from Galapagos NV not related to this study, and honoraria from Bristol Myers Squibb, Pfizer, Takeda, and Tillotts. PIS is involved in clinical trials with Regeneron, Sanofi, Leopharma, Lilly, AbbVie, Boerhinger, Celgene, Janssen, and UCB, which manufacture drugs used for the treatment of conditions, including psoriasis and atopic dermatitis, for which financial compensation is paid to their department or hospital, and is a chief investigator of the TREAT NL registry taskforce and SECURE-AD registry. MWB is a secretary for the Dutch Experimental Dermatology Board; head of the pigmentary disorders group within the Dutch Dermatology Board; and reports honoraria from Pfizer, Sanofi, Novartis, and Fondation René Touraine. JKi has consulting relationships with Merck Serono, Biogen Idec, Teva, Genzyme, Sanofi, Roche, and Novartis; Amsterdam UMC, location VUmc, MS Center Amsterdam has received financial support for research activities from Merck, Celgene, Biogen, GlaxoSmithKline, Immunic, Roche, Teva, Sanofi, Genzyme, and Novartis. BH reports unpaid positions as a medical adviser for several patient groups, a board position for European Reference Network for rare skin diseases (ERN-Skin), and associate editor for The British Journal of Dermatology; reports grants from AbbVIe, Akari Therapeutics, Celgene, and Novartis; consulting fees from UCB Pharma, Novartis, and Janssen; and honoraria from AbbVie. JJGMV reports consulting fees from Argen-X, Alexion, and NMD Pharma, and is a co-inventor on a patent applications based on MuSK-related research (patent number 9574015). DJH reports grants from AbbVie, AstraZeneca, Janssen, LEO Pharma, and UCB; honoraria from AbbVie, Galderma, Janssen, Lilly, Pfizer, Sanofi, and UCB; and a paid position on an advisory board for Biomarkers in Atopic Dermatitis and Psoriasis (BIOMAP IMI). PAvD has participated on an advisory board for Octapharma. PvP reports grants from Alexion Pharma and GSK, and participation on advisory boards for GSK and Vifor Pharma. GRAMD reports consulting fees from AbbVie, Agomab, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, GSK, Gossamerbio, Pfizer, Immunic, Johnson & Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus Laboratories, Prometheus Biosciences, Progenity, and Protagonist; honoraria from AbbVie, Arena, Galapagos NV, Gilead, Pfizer, Bristol Myers Squibb, and Takeda; and participation on advisory boards for AbbVie, Seres Health, Galapagos NV, and AstraZeneca. RBT reports honoraria from Sobi and Norgine and participation on an advisory board for Norgine. HSG is a board member of the Dutch Society of Clinical Neurophysiology (unpaid), reports grants from Prinses Beatrix Spierfonds, and has received speaker fees from Shire/Takeda. KAHZ reports paid data safety monitoring board positions for Torrent and Foresee. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The T2B! study was supported by ZonMw (project number 10430012010009). The ARC-COVID study was supported by ZonMw (project number: 10430022010020) and Reade foundation. We thank the funders of the study, and the T2B! partners, including the patient groups, and Health Holland for the support in this study. This collaboration project is financed by the PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public-private partnerships and co-financing by health foundations that are part of the SGF.
Contributors
LB, EWS, LW, FE, and GW wrote the first draft of the manuscript; all other authors revised the manuscript for important intellectual content. LB, EWS, and LW did the statistical analyses and had full access to and verified the underlying data. TR, MS, SK, JKe, AB, and OC did the serological assays, and all other authors contributed in data acquisition. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
T2B! immunity against SARS-CoV-2 study group:
Rivka de Jongh, Carolien van de Sandt, Lisan Kuijper, Mariel Duurland, Ruth Hagen, Jet van den Dijssel, Christine Kreher, Amelie Bos, Viriginia Palomares Cabeza, Veronique Konijn, George Elias, Juan Vallejo, Marrit van Gils, Tom Ashhurst, Sergey Nejentsev, and Elham Mirfazeli
Supplementary Material
References
- 1.Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kempen ZLE, Wieske L, Stalman EW, et al. Longitudinal humoral response after SARS-CoV-2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult Scler Relat Disord. 2022;57 doi: 10.1016/j.msard.2021.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4:e338–e350. doi: 10.1016/S2665-9913(22)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte LF, Gálvez NMS, Iturriaga C, et al. Immune profile and clinical outcome of breakthrough cases after vaccination with an inactivated SARS-CoV-2 vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.742914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, Maeda K, Matsuda K, et al. COVID-19 breakthrough infection and post-vaccination neutralizing antibody among healthcare workers in a referral hospital in Tokyo: a case-control matching study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1048. published online Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boekel L, Hooijberg F, Vogelzang EH, et al. Antibody development and disease severity of COVID-19 in non-immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boekel L, Hooijberg F, Besten YR, et al. COVID-19 vaccine acceptance over time in patients with immune-mediated inflammatory rheumatic diseases. Lancet Rheumatol. 2022;4:e310–e313. doi: 10.1016/S2665-9913(22)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boekel L, Hooijberg F, van Kempen ZLE, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3:e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boekel L, Hooijberg F, Vogelzang EH, et al. Spinning straw into gold: description of a disruptive rheumatology research platform inspired by the COVID-19 pandemic. Arthritis Res Ther. 2021;23:207. doi: 10.1186/s13075-021-02574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boekel L, Kummer LY, van Dam KPJ, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3:e542–e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooijberg F, Boekel L, Vogelzang EH, et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2:e583–e585. doi: 10.1016/S2665-9913(20)30286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenhuis M, van Mierlo G, Derksen NI, et al. Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors. Clin Transl Immunology. 2021;10 doi: 10.1002/cti2.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelzang EH, Loeff FC, Derksen NIL, et al. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and non-hospitalized patients with COVID-19. J Immunol. 2020;205:3491–3499. doi: 10.4049/jimmunol.2000767. [DOI] [PubMed] [Google Scholar]
- 19.Dutch National Institute for Public Health and the Environment Development SARS-CoV-2 in graphs. https://www.rivm.nl/coronavirus-covid-19/grafieken
- 20.Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020 doi: 10.1159/000512592. published online Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro Dopico X, Ols S, Loré K, Karlsson Hedestam GB. Immunity to SARS-CoV-2 induced by infection or vaccination. J Intern Med. 2022;291:32–50. doi: 10.1111/joim.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotty S. Hybrid immunity. Science. 2021;372:1392–1393. [Google Scholar]
- 23.Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–e187. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen KM, Bates BA, Rashidi ES, et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4:e33–e41. doi: 10.1016/S2665-9913(21)00325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook C, Patel NJ, D'Silva KM, et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:289–291. doi: 10.1136/annrheumdis-2021-221326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M, Gao Y, Zhang Y, Shi S, Chen Y, Tian J. The association between severe or dead COVID-19 and autoimmune diseases: a systematic review and meta-analysis. J Infect. 2020;81:e93–e95. doi: 10.1016/j.jinf.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boekel L, Wolbink GJ. Rituximab during the COVID-19 pandemic: time to discuss treatment options with patients. Lancet Rheumatol. 2021;4:e154–e155. doi: 10.1016/S2665-9913(21)00418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accorsi EK, Qiu X, Rumpler E, et al. How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur J Epidemiol. 2021;36:179–196. doi: 10.1007/s10654-021-00727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim W, Zhou JQ, Horvath SC, et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature. 2022;604:141–145. doi: 10.1038/s41586-022-04527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen RE, Gorman MJ, Zhu DY, et al. Reduced antibody activity against SARS-CoV-2 B.1.617.2 delta virus in serum of mRNA-vaccinated individuals receiving tumor necrosis factor-α inhibitors. Med (NY) 2021;2:1327–1341. doi: 10.1016/j.medj.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregated deidentified data and code for reproducing the results of this analysis can be shared upon reasonable request. Requests should be made to the corresponding author.