Abstract
Introduction
Early mobilization of patients in the postoperative period of cardiac surgery who are hospitalized in the intensive care unit (ICU) is a practice that has a positive impact.
Methods
This is a systematic review of studies published until September 2020 in the Medical Literature Analysis and Retrieval System Online (or MEDLINE®), Embase, Physiotherapy Evidence Database (or PEDro), Scientific Electronic Library Online (or SciELO), and Latin American and Caribbean Health Sciences Literature (or LILACS) databases. Randomized clinical trials describing mobilization protocols performed early in ICU patients after cardiac surgery were included.
Results
According to the eligibility criteria, only 14 of the 1,128 articles found were included in the analysis. Early mobilization protocols were initiated in the immediate postoperative period or first postoperative day. The resources and technics used were progressive mobilization, cycle ergometer, early bed activities, walking protocols, resistance exercise, and virtual reality. Intensity of the mobilization activities was determined using the Borg scale and heart rate.
Conclusion
Early mobilization protocols are generalist (not individual), and low-intensity exercises are used, through progressive mobilization, with two daily physical therapy sessions, during 10 to 30 minutes.
Keywords: Cardiac Surgical Procedures, Early Ambulation, Resistance Training, Intensive Care Units, Postoperative Period
Abbreviations, Acronyms & Symbols
| 6MWT | = 6-minute walk test | METs | = Metabolic equivalent of task |
| CABG | = Coronary artery bypass grafting | MIP | = Maximal inspiratory pressure |
| CPAP | = Continuous positive airway pressure | MRC | = Medical Research Council |
| HF | = High frequency | NR | = Not reported |
| HR | = Heart rate | POD | = Postoperative day |
| HRV | = Heart rate variability | RR | = R-R intervals |
| ICU | = Intensive care unit | SpO2 | = Saturation of peripheral oxygen |
| IMT | = Inspiratory muscle training | VO2 | = Oxygen uptake |
| LF | = Low frequency |
INTRODUCTION
Cardiac surgery is an option to treat patients with cardiovascular disease, aiming minimize symptoms, optimize cardiac function, and increase survival. Because it is an invasive procedure, it implies numerous functional and systemic consequences in the postoperative period[1-3].
Complications resulting from the surgical procedure may be caused by physiological changes, comorbidities, and previous risk factors. In addition, intraoperative conditions such as mechanical ventilation, cardiopulmonary bypass, surgical time, and anesthesia determine longer hospital stay with negative outcomes[4].
Conditions acquired during hospitalization due to immobilization, such as loss of strength and muscle mass, reduction of functional capacity, and physical deconditioning, are common and directly associated with greater disability and need for prolonged rehabilitation[5].
Early mobilization of patients in the postoperative period of cardiac surgery who are hospitalized in the intensive care unit (ICU) is a practice that has a positive impact on cardiovascular conditioning and ventilatory mechanics, consequently implying improvement of functional capacity, shorter hospitalization time, and lower mortality rate, besides contributing to the prevention of ICU-acquired weakness and to the improvement of muscle strength[4,6].
Exercises are essential for a quick postoperative recovery. Protocols and resources to assist in cardiovascular rehabilitation have been frequently applied[2]. Cycle ergometer, neuromuscular electrical stimulation, virtual reality through video games, and protocols of active and resisted mobilization with levels of progression have been increasingly used, presenting positive results in functional capacity[7,8] and motivation[9] of patients undergoing cardiac surgery.
Thus, considering the various therapeutic proposals, this review aims to describe the prescription of early mobilization in patients undergoing cardiac surgery.
METHODS
This is a systematic review following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (or PRISMA) Statement[10] and registered in the International Prospective Register of Systematic Reviews (or PROSPERO) (CRD42020197787).
Eligibility Criteria
Randomized clinical trials describing early mobilization protocols applied to patients following cardiac surgery were included. Early mobilization has been considered as any mobilization activity that has been carried out as soon as possible during the ICU stay, such as turning, sitting, and orthostatism; passive, assisted, or active exercises; marching on the spot; walking; resistance or aerobic exercise; cycle ergometer; or virtual reality games. The year of publication, as well as the language, were not considered as exclusion criteria.
Search Strategy
The search was conducted in the Medical Literature Analysis and Retrieval System Online (or MEDLINE®) via PubMed®, Embase, Physiotherapy Evidence Database (or PEDro), Latin American and Caribbean Health Sciences Literature (or LILACS), and Scientific Electronic Library Online (or SciELO) databases.
The search strategy comprised the keywords and synonyms for “early mobilization”, and for the study population we searched for “adults undergoing cardiac surgery hospitalized in an intensive care unit”, also including interventions as “marching on the spot”, “walking”, “resistance or aerobic exercise”, “cycle ergometer”, or “virtual reality games”. The search was carried out using terms of Medical Subject Headings (or MeSH) and synonyms, without restriction of date and language, until the period of June 2020, being updated in September 2020. PubMed®'s complete search strategy is described in Table 1. Two studies were manually added after the analysis of Kanejima et al.[11] study.
Table 1.
Search strategy used in PubMed®.
| #1 | (“Intensive care” OR “Critical care” OR “Intensive care unit” OR “Critical illness” OR “Care, Critical” OR “Care, Intensive” OR “Surgical Intensive Care” OR “Care, Surgical Intensive” OR “Intensive Care, Surgical”) |
| #2 | (“Early Ambulation” OR “Accelerated Ambulation” OR “Ambulation, Accelerated” OR “Ambulation, Early” OR “Early Mobilization” OR “Mobilization, Early” OR “Exercise” OR “Physical Activity” OR “Activities, Physical” OR “Activity, Physical” OR “Physical Activities” OR “Exercise, Physical” OR “Exercises, Physical” OR “Physical Exercise” OR “Physical Exercises” OR “Acute Exercise” OR “Acute Exercises” OR “Exercise, Acute” OR “Exercises, Acute” OR “Exercise, Isometric” OR “Exercises, Isometric” OR “Isometric Exercises” OR “Isometric Exercise” OR “Exercise, Aerobic” OR “Aerobic Exercise” OR “Aerobic Exercises” OR “Exercises, Aerobic” OR “Exercise Training” OR “Exercise Trainings” OR “Training, Exercise” OR “Trainings, Exercise” OR “Motion Therapy, Continuous Passive” OR “Movement Therapy, Continuous Passive” OR “Passive Movement Therapy, Continuous” OR “Continuous Passive Motion Therapy” OR “Passive Motion Therapy, Continuous” OR “Continuous Passive Movement Therapy” OR “CPM Therapy” OR “CPM Therapies” OR “Therapies, CPM” OR “Therapy, CPM” OR “Resistance Training”) |
| #3 | (“Procedure, Cardiac Surgical” OR “Procedures, Cardiac Surgical” OR “Surgical Procedure, Cardiac” OR “Surgical Procedures, Cardiac” OR “Surgical Procedures, Heart” OR “Cardiac Surgical Procedure” OR “Heart Surgical Procedures” OR “Procedure, Heart Surgical” OR “Procedures, Heart Surgical” OR “Surgical Procedure, Heart” OR “Heart Surgical Procedure” OR “Cardiac Surgery”) |
| #4 | #1 AND #2 AND #3 AND |
Two researchers performed the initial search independently through the evaluation of titles and abstracts. Subsequently, the reviewers assessed the full texts for the independent verification of inclusion and exclusion criteria. In cases of disagreement, a third evaluator was consulted.
Data extraction was performed using a standardized Excel® spreadsheet, with the following information: first author, year of publication, country, number of patients in the study, sample, objective of the study, and, finally, description of the protocol of early mobilization (type of intervention, intensity, frequency, duration, and progression). In cases of incomplete or absent data, the corresponding authors were contacted. Data analysis was performed descriptively.
The assessment of the risk of bias in randomized controlled clinical trials followed the recommendations of the Cochrane Collaboration, using these items: random sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessment), incomplete outcome data, selective reporting, and other sources of bias[12].
RESULTS
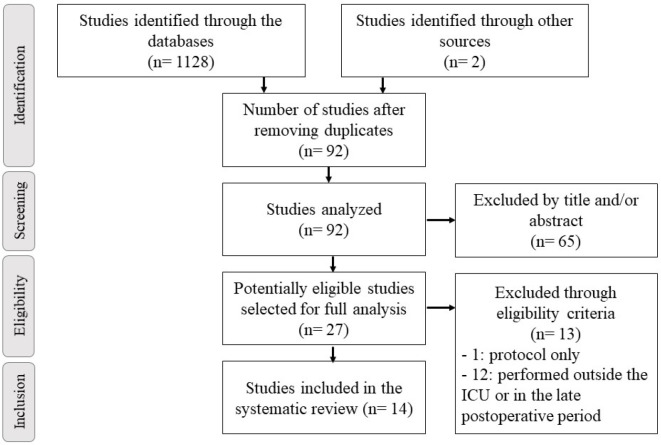
The search identified 1,128 studies, but only 14 controlled and randomized clinical trials, totaling 1,170 patients included in this systematic review (Figure 1). Most studies included only coronary artery bypass grafting procedures. Mean age of patients was 58,67±4,5 years. Study characteristics are summarized in Table 2.
Fig. 1.
Flowchart of studies included in this systematic review. ICU=intensive care unit
Table 2.
Characteristics of the included studies.
| Author | Sample | Age (years) | Sample characteristic | Objective |
|---|---|---|---|---|
| Borges et al.[37], 2016 | 34 | 62.7±15.6 | Adults undergoing CABG | To evaluate whether the addition of aerobic exercise during hospitalization improves lung function, respiratory muscle strength, and functional capacity |
| Cacau et al.[7], 2013 | 60 | 50.6±2.5 | Adults under 75 years, undergoing CABG or valve surgeries | To evaluate the use of virtual reality in functional rehabilitation |
| Herdy et al.[31], 2008 | 56 | 59.5±9.5 | Adults in the preoperative period of CABG | To evaluate the effects of cardiopulmonary rehabilitation before and after surgery in postoperative outcomes |
| Hirschhorn et al.[18], 2007 | 88 | 62.9±8.9 | Adults undergoing CABG | To assess whether a supervised walking program with or without musculoskeletal or respiratory exercises can improve walking capacity and other outcomes |
| Hojskov et al.[19], 2019 | 310 | 65±8.8 | Adults undergoing CABG | To assess the impact of phases 1 and 2 of cardiovascular rehabilitation on functionality, physical and mental function, anxiety, depression, sleep, pain, and quality of life |
| Gama Lordello et al.[9], 2020 | 228 | 57.7±13 | Adults undergoing CABG and/or valve surgeries | To evaluate the effect of early use of cycle ergometer, compared with conventional therapy, on in-hospital mobility |
| Mendes et al.[35], 2010 | 47 | 59±8.5 | Adults undergoing CABG | To determine whether a short exercise protocol during in-hospital cardiac rehabilitation can improve cardiac autonomic regulation |
| Pantoni et al.[14], 2016 | 27 | 57.85±7.3 | Adults undergoing CABG | To evaluate the effectiveness of CPAP on the first day of walking |
| Silva et al.[15], 2017 | 19 | 52±17 | Adult undergoing elective cardiac surgery (corrections of congenital heart diseases, CABG, valve surgeries, and/or associated surgical procedures) | To check the cardiorespiratory repercussions of early sitting out of bed and its effects on muscle strength, functional capacity, and pulmonary function |
| Stein et al.[16], 2009 | 20 | 63.5 ± 6.5 | Adults undergoing CABG | To evaluate the effect of a cardiopulmonary rehabilitation program on inspiratory muscle strength and its possible association with maximum and submaximal functional capacity |
| Tariq et al.[17], 2017 | 174 | 51.9±13.8 | Adults undergoing CABG or valve surgeries | To determine the effect of physical activities (≤ 3 METs) in the immediate postoperative period on respiratory and hemodynamic parameters |
| Ximenes et al.[2], 2015 | 34 | 60.9±6.8 | Adults undergoing CABG | To evaluate the effects of early resistance exercise |
| Windmoller et al.[23], 2020 | 31 | 60±7 | Adults undergoing CABG | To evaluate the effectiveness of cycle ergometer with CPAP |
| Zanini et al.[22], 2019 | 40 | 58.5±6.25 | Adults undergoing CABG | To evaluate the effect of different rehabilitation protocols on pulmonary function and functional capacity |
CABG=coronary artery bypass grafting; CPAP=continuous positive airway pressure; METs=metabolic equivalent of task
Age data were expressed by mean ± standard deviation
Early mobilization protocols were initiated in the immediate postoperative period or first postoperative day. Different resources and techniques were used to mobilize patients submitted to cardiac surgery: progressive mobilization (four studies), cycle ergometer (three studies), out of bed activities (two studies), walking protocols (three studies), resistance exercise (one study), and virtual reality (one study). The description of the mobilization protocols is shown in Table 3.
Table 3.
Early mobilization protocols in patients undergoing cardiac surgery.
| Authors | Mode | Intensity | Frequency | Duration | Progression |
|---|---|---|---|---|---|
| Borges et al.[37], 2016 | Active and assisted exercises and progressive walking + active cycle ergometer | “As much as possible” | ICU: twice daily | 1st and 2nd POD: 10 minutes | Time |
| Ward: once daily | From the 3rd POD: 20 minutes | ||||
| Cacau et al.[7], 2013 | Metabolic exercises and mobilization using virtual reality | NR | NR | NR | Progressive METs |
| Herdy et al.[31], 2008 | Progressive exercises (passive, walking, and climbing stairs) | NR | Once daily | NR | 2-4 METs |
| Hirschhorn et al.[18], 2007 | 1st POD: marching on the spot (3 × 1 minute) and sitting out of bed | Borg: 3 a 4/10 | Twice daily | Variable, depending on the patient’s condition | Increased walking distance and time |
| 2nd POD: assisted walking (300 meters in the morning and 5 minutes in the afternoon) | |||||
| 3rd POD: assisted walking (at least 5 minutes in the morning and afternoon) | |||||
| 4th POD until discharge: supervised walking with increments of 2.5 minutes, as tolerated, up to 10 minutes | |||||
| Hojskov et al.[19], 2019 | 1st to 7th POD: walking, shoulder and neck mobilization, and cycle ergometer | NR | NR | NR | NR |
| Gama Lordello et al.[9], 2020 | Cycle ergometer of upper and lower limbs. After drain removal, progressive activity for orthostatism, sitting on the chair, and walking in the ICU corridor | NR | Twice daily | 10 minutes | NR |
| Mendes et al.[35], 2010 | Active-assisted exercises | Exercise HR = HR rest + 20 bpm | Once daily | NR | 2 to 4 METs |
| STEP 1: 5 × 10 repetitions in Fowler’s position | |||||
| STEP 2: 2 × 15 repetitions in sitting position | |||||
| STEP 3: 3 × 15 repetitions in sitting position | |||||
| STEP 4: 3 × 15 repetitions in sitting position + 10 minutes of walking | |||||
| STEP 5: 3 × 15 repetitions in orthostatism + 10 minutes of walking + climbing 4 floor of stairs | |||||
| Pantoni et al.[14], 2016 | CPAP (10-12 cmH2O) during exercises | Exercise HR = HR rest + 20 bpm | Twice daily | NR | 2 to 4 METs |
| 1st POD: upper and lower extremity exercises | |||||
| 3rd POD: active exercises and 5 minutes of walking | |||||
| 4th POD: active exercises and 10 minutes of walking | |||||
| 5th POD: 10 minutes of walking and stair training | |||||
| Silva et al.[15], 2017 | Sitting out of bed at 1st POD, active exercises, and progressive walking | NR | NR | 30 minutes | NR |
| Stein et al.[16], 2009 | 1st POD: hip and knee flexion (2 × 15 repetitions), upper limbs active exercises (flexion and abduction up 90° - 2 × 10 repetitions), knees and wrist flexion and extension (3 minutes each) | NR | NR | NR | Walked distance |
| 2nd POD: marching on the spot after mediastinal drain removal (3 × 1 to 3 minutes) | |||||
| 3rd POD: walking - 100 to 200 meters | |||||
| 4th POD: walking - 200 to 300 meters | |||||
| 5th POD: walking - 300 to 400 meters and climbing 15 steps | |||||
| 6th POD: walking - 500 to 600 meters and climbing 15 steps | |||||
| Tariq et al.[17], 2017 | Immediate postoperative period: sitting on the edge of bed (assisted by the physiotherapist) for 5 minutes. Orthostatism (1-2 minutes), marching on the spot (10 steps), and sitting on the chair (90 minutes) | NR | NR | NR | ≤ 3 METs |
| Ximenes et al.[2], 2015 | Resistance exercises and progressive walking | Borg (does not specify value) | ICU: twice daily Ward: once daily | 30 minutes | Positioning (45° on bed, sitting on the edge of bed and orthostatism) |
| Windmoller et al.[23], 2020 | Cycle ergometer with CPAP (10 cmH2O) (once daily, from 2nd to 4th POD) + exercises: | Resting HR + 30 bpm | Twice daily | 20 to 30 minutes | 2-6 METs |
| STEP 1: supine - active exercises | |||||
| STEP 2: sitting - active exercises | |||||
| STEP 3: orthostatism - passive stretching of lower limbs and walking (35 to 60 meters) | |||||
| STEP 4: orthostatism - passive stretching of lower limbs and active stretching of upper limbs, walking (60 to 100 meters), and climbing 1 floor of stairs | |||||
| STEP 5: orthostatism - active stretching of upper and lower limbs, active exercises, walking (100 to 150 meters), and climbing up/down 1 floor of stairs | |||||
| STEP 6: orthostatism - active stretching of upper and lower limbs, active exercises, walking (150 to 200 meters), and climbing up/down 2 floors of stairs | |||||
| STEP 7: orthostatism - active stretching of upper and lower limbs, active exercises, walking (> 200 meters), and climbing up/down 3 floors of stairs | |||||
| Zanini et al.[22],2019 | Group 1: active exercises (shoulders, hips, knees, and ankle flexions), IMT, progressive walking, and conventional therapy | Borg: 11/20 | Twice daily | NR | Active mobilization: series and repetition Walking: distance |
| Group 2: active exercises (shoulders, hips, knees, and ankle flexions), progressive walking, and conventional therapy | |||||
| Group 3: IMT and conventional therapy |
CPAP=continuous positive airway pressure; HR=heart rate; ICU=intensive care unit; IMT=inspiratory muscle training; METs=metabolic equivalent of task; NR=not reported; POD=postoperative day
The most frequently assessed outcomes in the studies were functional capacity, using the six-minute walk test, and respiratory muscle strength. The main results of the included studies are described in Table 4.
Table 4.
Main outcomes of the included studies.
| Author | Main outcomes |
|---|---|
| Borges et al.[13], 2016 | Functional capacity was maintained in the intervention group. A significant difference in functional capacity was also found in intergroup analyses at hospital discharge. |
| Cacau et al.[7], 2013 | Intervention group showed lower reduction in functional performance, decreased pain score, higher energy level, shorter hospital length of stay, and higher 6MWT distance. |
| Herdy et al.[14], 2008 | Intervention group had shorter time to endotracheal extubation, decreased incidence of pleural effusion, atelectasis, pneumonia, and atrial fibrillation or flutter, and reduced hospital length of stay. |
| Hirschhorn et al.[15], 2007 | Intervention group had significantly higher 6MWT distance at hospital discharge. |
| Hojskov et al.[16], 2019 | No significant differences between groups in 6MWT. Anxiety and depression were decreased in intervention group. |
| Lordello et al.[9], 2020 | No significant difference was found in the total number of steps between the groups. However, self-reports indicated better motivation in the intervention group. |
| Mendes et al.[17], 2010 | Intervention group presented significantly higher parasympathetic HRV values, global power, non-linear HRV indexes and mean RR. Higher values of mean HR, LF (sympathetic activity), and the LF/HF (global sympathovagal balance) were found in control group. |
| Pantoni et al.[14], 2016 | Intervention group had increased exercise time, better thoracoabdominal coordination, increased ventilation during walking, increased SpO2 values at the end of walking, and reduced dyspnea rate. |
| Silva et al.[15], 2017 | Reduction of MIP in both groups, while the maximum expiratory pressure did not reduce in the intervention group. There was no change in the MRC and decrease in spirometry values in both groups at hospital discharge. |
| Stein et al.[16], 2009 | Intervention group maintained MIP measured at 7 and 30 days postoperatively, while it was significantly reduced in the control group. 6MWT distance was higher 7 days after cardiac surgery in intervention group. VO2 peak at day 30 was also higher in the intervention group. |
| Tariq et al.[17], 2017 | In the intervention group, there was an improvement in dyspnea, respiratory rate, and oxygen saturation. |
| Ximenes et al.[2], 2015 | Intervention group maintained functional capacity at hospital discharge measured by 6MWT, while control group had a significant decrease. |
| Windmoller et al.[23], 2020 | Functional capacity decreased in both groups, without significant difference in the intervention group. ICU length of stay was lower in the intervention group. In both groups there was a decrease in maximal inspiratory and expiratory pressures, as well as in the 1-min sit-to-stand test on the fourth postoperative day compared to the preoperative period. |
| Zanini et al.[22], 2019 | The 6MWT distance on the sixth postoperative day was significantly higher in groups which included early ambulation and upper and lower limbs exercise, remaining higher at 30 days post-discharge. Peak VO2 on day 30 was also higher in in the same groups. All groups achieved similar recovery of lung function |
6MWT=6-minute walk test; HF=high frequency; HR=heart rate; HRV=heart rate variability; ICU=intensive care unit; LF=low frequency; MIP=maximal inspiratory pressure; MRC=Medical Research Council; RR=R-R intervals; SpO2=saturation of peripheral oxygen; VO2=oxygen uptake
The methodological quality of the studies was evaluated using the Cochrane tool of risk of bias, described in Figure 2. Random sequence generation, allocation concealment, and selective results have a low proportion of risk of bias. On the other hand, a high proportion of high risk of bias for blinding and other types of bias were found.
Fig. 2.
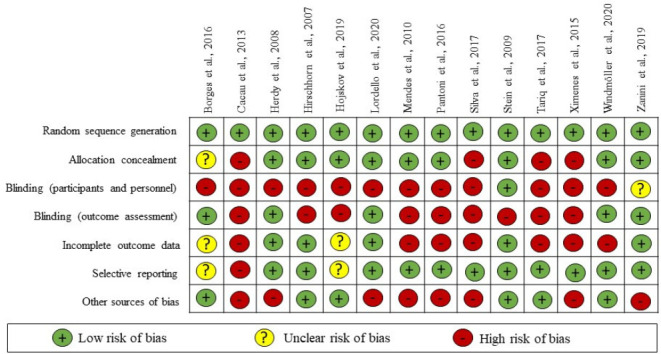
Assessment of risk of bias of the included studies.
DISCUSSION
Cardiac surgery leads to exercise capacity decreases in early stages of rehabilitation programs, when compared to patients undergoing less invasive or non-cardiac interventions[13,14]. Such changes are associated with severity of the disease, high prevalence of comorbidities[15], duration of muscle deconditioning[16], incisional pain[17], chest drain, and extracorporeal circulation[18].
Therefore, it is common to observe decline in functional performance during the ICU stay[19]. Functional capacity decrease comparing pre and postoperative periods of cardiac surgery was reported by several studies[2,20-22].
Despite this, spontaneous restoration of functional capacity was observed, including in patients who did not participate in any research protocol[23]. In specific conditions, such as the elderly, lower training volume and longer recovery periods may be sufficient[24].
Due to individual particularities, a structured therapy including mode, intensity, frequency, and duration based on individualized assessments is fundamental for a proper prescription[25] and consequently long-term functional outcomes after hospital discharge[26].
Early mobilization in cardiac surgery is performed in the first hours after the surgical procedure as soon as the patient presents clinical conditions for the intervention[27]. In the studies included in this review, the time to start mobilization was four hours after extubation to the first postoperative day.
According to Stiller et al.[27] and Bourding et al.[28], early mobilization after cardiac surgery promotes several benefits, including improved ventilation, ventilation/perfusion ratio, respiratory muscle strength, and functional capacity. The systematic reviews of Kanejima et al.[11] and Guerra et al.[29] also demonstrated positive effects on functional capacity, being considered safe and feasible in critically ill patients. On the other hand, Santos et al.[26] suggests that early mobilization, evaluated in short term, does not promote significant changes in functional capacity.
Different results may be justified by divergences about early mobilization concepts[27]. It is important to highlight that the variety of studies with different starting points difficult the prescription, as it is essential to define the moment of initiation to avoid risks to the patient due to very early or late mobilization[30].
Additionally, the term mobilization also covers several therapies. Most types of modalities found were protocols of progressive mobilization, including active exercises, sitting out of bed and walking[31-34], only walking protocols[35,36], and early sitting out of bed[21,22]. Not all these therapies require instruments for their realization.
Other studies included instruments, such as the cycle ergometer[9,13,23], virtual reality[7], and resistance exercises with shin pads and dumbbells[2]. The cycle ergometer is considered a viable strategy for those with restriction to walk[12]. The practice of resistance exercises in this population is restricted due to incisional precautions, but it is known that this modality optimizes cardiovascular function and peripheral muscle strength[2,29] and promotes reduction of inflammation, cognitive dysfunction, and sarcopenia[37].
In a systematic review, Ramos dos Santos et al.[26] observed that the groups submitted to early mobilization presented lower rates of postoperative complications, improvement of functional capacity, and reduction of hospital stay in comparison with control groups without treatment. However, when comparing different mobilization protocols, there was no superiority of any intervention.
Regarding intensity, most studies[2,7,9,13,16,19-21] did not use objective criteria. Hirschhorn et al.[18] applied the modified Borg scale with target of three to four points, equivalent to moderate to low exercise intensity[38], while Zanini et al.[22] performed exercises aiming level 11 on the Borg scale from six to 20 points. This intensity corresponds to light exercises, in which participants feel that the effort is “very light”[39].
In other studies, heart rate (HR) change from 20 to 30 bpm above baseline HR was used to determine exercise intensity according to guidelines from the American College of Sports Medicine (or ACSM) for patients who do not have a stress test performed. Increased exercise intensity considered the patient’s perceived effort, signs and symptoms, and normal physiological response[40].
Another way to determine the exercise intensity is the reserve HR (maximum HR - resting HR)[41]. However, this is based on the maximum HR achieved in an effort test that can quantify the anaerobic thresholds and thus determine the prescription of adequate exercise. This type of test is not performed in early postoperative period.
Subjectively, one can also consider the speech test or Talk Test, with the perception of the ventilation itself, that is, the exercises are performed in intensity that feels the most panting breath, however, without a degree of tachypnea that prevents the patient from completing a phrase[42]. None of the studies analyzed used this way of determining intensity.
The definition of intensity is fundamental to determine the continuity or suspension of therapy. In phase I of the cardiac rehabilitation, low-intensity exercises should predominate, aiming the best possible physical and psychological conditions to patient hospital discharge[43,44]. However, it is important to highlight that the increase in exercise intensity is associated with enhanced cardiac output and oxygen consumption, resulting from increased muscle oxygen consumption. Thus, such physiological changes may be associated with greater gains in peripheral muscle strength[45].
The frequency of interventions found was once to twice daily, lasting 10 to 30 minutes. The South American Guidelines for Cardiovascular Prevention and Rehabilitation[46] recommended duration between 40 and 60 minutes daily. However, there is no consensus about the appropriate duration of therapy during phase I of cardiovascular rehabilitation.
Another important point in the prescription is the criteria for progression. There are protocols that demonstrate progression in steps that evolve according to patient recovery[47,48] and others with progressive therapeutic strategies[49-51]. Winkelman et al.[52] described a protocol in which each step is determined by activities with frequency and intensity corresponding to a given energy expenditure (2 to 4 metabolic equivalents of task) until hospital discharge. This form of progression was also used in several studies[7,14,17,18,21,22].
The volume of therapy[15,23], time[13], or evolution in positioning were identified as determining factors for progression. Regardless of the form of progression, it is important to consider the functional capacity, clinical condition, use of medications, age, and objectives of the program. Moreover, in the early periods it is essential to respect the adaptation to exercise and later evolve with progression, especially in those who are reestablishing themselves from an acute event, such as cardiac surgery[45].
In general, the therapy prescription is not clearly defined for patients in phase I of cardiac rehabilitation, as there is no standardization on the “dosage” of the therapy. According to the South American Guidelines for Cardiovascular Rehabilitation[43], in this phase, the focus is patient education and low-intensity exercises, which include from passive mobilization to light walks with individual progressions. However, although the intensity is mild, it is important to respect the criteria of the prescription to guarantee reproducibility and efficacy of therapy, thus respecting the bases of exercise physiology.
In addition, in most of the protocols studied, it is common to perceive the same therapy for all patients. However, it is important to highlight that physical exercise, as well as drug prescription, should be individualized, aiming to maximize the benefits and minimize risks[45].
Functional loss in the postoperative period is an expected complication if no therapeutic intervention is performed. So, interventions performed in this period aim to maintain functionality during the hospital stay. Divergent data were observed in the studies included in this review. Maintenance of the functional capacity in the intervention group, comparing post and preoperative periods, was found in several studies[2,7,13,15,23], while others found decrease[16,22] or similar values[9]. Concerning to respiratory function, respiratory muscle strength was decreased[13,22] or maintained[19,20] in both intervention and control groups, while lower incidence of respiratory complications[14] was found in the intervention group . The protocols presented by Ximenes et al.[2], Cacau et al.[7], Borges et al.[36], Stein et al.[16], and Zanini et al.[22] presented the best results in the most relevant outcomes.
Despite all the restrictions of the prescription of early mobilization in the postoperative period of cardiac surgery, due to the severity of the patient in phase I of cardiovascular rehabilitation, its benefits in this population are known. Thus, the question arises: would a mobilization program carefully prescribed for patients in the postoperative period of cardiac surgery be able to optimize outcomes?
CONCLUSION
During the hospitalization phase, the prescription of early mobilization is not a frequent concern, since there are few studies specifically targeting the most appropriate type, intensity, frequency, duration, and progression. In addition, the protocols are generalist and not individual, as recommended by the physiological bases of exercise prescription. As for the studies included in the review, low-intensity exercises are used, through progressive mobilization, once to twice daily, during 10 to 30 minutes.
Authors' roles & responsibilities
| MGBB | Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published |
| DLB | Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published |
| MOR | Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published |
| LSSL | Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published |
| KCMM | Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published |
| VJSN | Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published |
Footnotes
No financial support.
This study was carried out at the Hospital Universitário da Universidade Federal of Maranhão (UFMA), São Luís, Maranhão, Brazil.
No conflict of interest.
REFERENCES
- 1.Brick AV, Souza DSR de, Braile DM, Buffolo E, Lucchese FA, Silva FP de V, et al. Diretrizes da cirurgia de revascularização miocárdica valvopatias e doenças da aorta. Arq Bras Cardiol. 2004;82(suppl 5):1–20. doi: 10.1590/S0066-782X2004001100001. [DOI] [PubMed] [Google Scholar]
- 2.Ximenes NN, Borges DL, Lima RO, Barbosa e Silva MG, Silva LN, Costa Mde A, et al. Effects of resistance exercise applied early after coronary artery bypass grafting: a randomized controlled trial. Braz J Cardiovasc Surg. 2015;30(6):620–5. doi: 10.5935/1678-9741.20150077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camargo JBG, Cavenaghi OM, Mello JRC, de Brito MVC, Ferreira LL. Mobilidade funcional de pacientes críticos em terapia intensiva: um estudo piloto. Rev Aten Saúde. 2020;18(63):14–20. doi: 10.13037/ras.vol18n63.6101. [DOI] [Google Scholar]
- 4.Laizo A, Delgado FEF, Rocha GM. Complications that increase the time of hospitalization at ICU of patients submitted to cardiac surgery. Braz. J. Cardiovasc. Surg. 2010;25(2):166–71. doi: 10.1590/S0102-76382010000200007. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Pissolato J, Fleck CS. Mobilização precoce na unidade de terapia intensiva adulta. Fisioter Bras. 2018;19(3):377–84. [Google Scholar]
- 6.Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, et al. Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung. 2014;43(1):19–24. doi: 10.1016/j.hrtlng.2013.11.003. Erratum in: Heart Lung. 2014;43(4):388. doi:10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Cacau Lde A, Oliveira GU, Maynard LG, Araújo Filho AA, WM Jr Silva, Cerqueria Neto ML, et al. The use of the virtual reality as intervention tool in the postoperative of cardiac surgery. Rev Bras Cir Cardiovasc. 2013;28(2):281–9. doi: 10.5935/1678-9741.20130039. [DOI] [PubMed] [Google Scholar]
- 8.Fontes Cerqueira TC, Cerqueira Neto ML, Cacau LAP, Oliveira GU, Silva Júnior WMD, Carvalho VO, et al. Ambulation capacity and functional outcome in patients undergoing neuromuscular electrical stimulation after cardiac valve surgery: a randomised clinical trial. Medicine (Baltimore) 2018;97(46):e13012. doi: 10.1097/MD.0000000000013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gama Lordello GG, Gonçalves Gama GG, Lago Rosier G, Viana PADC, Correia LC, Fonteles Ritt LE. Effects of cycle ergometer use in early mobilization following cardiac surgery: a randomized controlled trial. Clin Rehabil. 2020;34(4):450–9. doi: 10.1177/0269215520901763. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanejima Y, Shimogai T, Kitamura M, Ishihara K, Izawa KP. Effect of early mobilization on physical function in patients after cardiac surgery: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(19):7091. doi: 10.3390/ijerph17197091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. New Jersey: Wiley Online Library; 2008. [Google Scholar]
- 13.Mendes RG, Simões RP, De Souza Melo Costa F, Pantoni CB, Di Thommazo L, Luzzi S, et al. Short-term supervised inpatient physiotherapy exercise protocol improves cardiac autonomic function after coronary artery bypass graft surgery--a randomised controlled trial. Disabil Rehabil. 2010;32(16):1320–7. doi: 10.3109/09638280903483893. [DOI] [PubMed] [Google Scholar]
- 14.Pantoni CB, Di Thommazo-Luporini L, Mendes RG, Caruso FC, Mezzalira D, Arena R, et al. Continuous positive airway pressure during exercise improves walking time in patients undergoing inpatient cardiac rehabilitation after coronary artery bypass graft surgery: a RANDOMIZED CONTROLLED TRIAL. J Cardiopulm Rehabil Prev. 2016;36(1):20–7. doi: 10.1097/HCR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 15.Silva LN, Marques MJS, Lima RS, Fortes JVS, Silva MGB, Baldez TEP, et al. Retirada precoce do leito no pós operatório de cirurgia cardíaca: Repercussões cardiorrespiratórias e efeitos na força muscular respiratória e periférica, na capacidade funcional e função pulmonar. ASSOBRAFIR Ciênc. 2017;8(2):25–39. doi: 10.47066/2177-9333/ac.27867. [DOI] [Google Scholar]
- 16.Stein R, Maia CP, Silveira AD, Chiappa GR, Myers J, Ribeiro JP. Inspiratory muscle strength as a determinant of functional capacity early after coronary artery bypass graft surgery. Arch Phys Med Rehabil. 2009;90(10):1685–91. doi: 10.1016/j.apmr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Tariq MI, Khan AA, Khalid Z, Farheen H, Siddiqi FA, Amjad I. Effect of early ≤ 3 mets (metabolic equivalent of tasks) of physical activity on patient's outcome after cardiac surgery. J Coll Physicians Surg Pak. 2017;27(8):490–4. [PubMed] [Google Scholar]
- 18.Hirschhorn AD, Richards D, Mungovan SF, Morris NR, Adams L. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery--a randomised controlled trial. Heart Lung Circ. 2008;17(2):129–38. doi: 10.1016/j.hlc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Højskov IE, Moons P, Egerod I, Olsen PS, Thygesen LC, Hansen NV, et al. Early physical and psycho-educational rehabilitation in patients with coronary artery bypass grafting: a randomized controlled trial. J Rehabil Med. 2019;51(2):136–43. doi: 10.2340/16501977-2499. [DOI] [PubMed] [Google Scholar]
- 20.Hansen D, Dendale P, Berger J, Meeusen R. Rehabilitation in cardiac patients: what do we know about training modalities? Sports Med. 2005;35(12):1063–84. doi: 10.2165/00007256-200535120-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Corbi G, Bosimini E, Cobelli F, Furgi G, Giannuzzi P, et al. Cardiac rehabilitation in the elderly: patient selection and outcomes. Am J Geriatr Cardiol. 2006;15(1):22–7. doi: 10.1111/j.1076-7460.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- 22.Zanini M, Nery R, Lima J, Buhler R, Silveira R, Stein R. Effects of different rehabilitation protocols in inpatient cardiac rehabilitation after coronary artery bypass graft surgery: a randomized clinical trial. J Cardiopulm Rehabil Prev. 2019;39(6):19–25. doi: 10.1097/HCR.0000000000000431. http://doi:10.1097/HCR.0000000000000431 . [DOI] [PubMed] [Google Scholar]
- 23.Windmöller P, Bodnar ET, Casagrande J, Dallazen F, Schneider J, Berwanger SA, et al. Physical exercise combined with CPAP in subjects who underwent surgical myocardial revascularization: a randomized clinical trial. Respir Care. 2020;65(2):150–7. doi: 10.4187/respcare.06919. [DOI] [PubMed] [Google Scholar]
- 24.Lavie CJ, Milani RV, Marks P, de Gruiter H. Exercise and the heart: risks, benefits, and recommendations for providing exercise prescriptions. Ochsner J. 2001;3(4):207–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Achttien RJ, Staal JB, van der Voort S, Kemps HM, Koers H, et al. Exercise-based cardiac rehabilitation in patients with coronary heart disease: a practice guideline. Neth Heart J. 2013;21(10):429–38. doi: 10.1007/s12471-013-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos Dos Santos PM, Aquaroni Ricci N, Aparecida Bordignon Suster É, de Moraes Paisani D, Dias Chiavegato L. Effects of early mobilisation in patients after cardiac surgery: a systematic review. Physiotherapy. 2017;103(1):1–12. doi: 10.1016/j.physio.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Stiller K, Phillips AC, Lambert P. The safety of mobilization and its effect on hemodynamic and respiratory status of intensive care patients. Physiother Theor Pract. 2004;20(3):175–85. doi: 10.1080/09593980490487474. [DOI] [Google Scholar]
- 28.Bourdin G, Barbier J, Burle JF, Durante G, Passant S, Vincent B, et al. The feasibility of early physical activity in intensive care unit patients: a prospective observational one-center study. Respir Care. 2010;55(4):400–7. [PubMed] [Google Scholar]
- 29.Guerra ML, Singh PJ, Taylor NF. Early mobilization of patients who have had a hip or knee joint replacement reduces length of stay in hospital: a systematic review. Clin Rehabil. 2015;29(9):844–54. doi: 10.1177/0269215514558641. [DOI] [PubMed] [Google Scholar]
- 30.Busch JC, Lillou D, Wittig G, Bartsch P, Willemsen D, Oldridge N, et al. Resistance and balance training improves functional capacity in very old participants attending cardiac rehabilitation after coronary bypass surgery. J Am Geriatr Soc. 2012;60(12):2270–6. doi: 10.1111/jgs.12030. Erratum in: J Am Geriatr Soc. 2013;61(3):479. doi:10.1111/jgs.12030. [DOI] [PubMed] [Google Scholar]
- 31.Herdy AH, Marcchi PLB, Vila A, Tavares C, Collaco J, Niebauer J, Ribeiro JP. Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: a randomized controlled trial. Am J Phys Med Rehabil. 2008;87(9):714–719. doi: 10.1097/PHM.0b013e3181839152. https://doi:10.1097/PHM.0b013e3181839152 . [DOI] [PubMed] [Google Scholar]
- 32.Ades PA, Savage PD, Brawner CA, Lyon CE, Ehrman JK, Bunn JY, et al. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113(23):2706–12. doi: 10.1161/CIRCULATIONAHA.105.606624. [DOI] [PubMed] [Google Scholar]
- 33.Meyer T, Lucía A, Earnest CP, Kindermann W. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters--theory and application. Int J Sports Med. 2005;26(Suppl 1):S38–48. doi: 10.1055/s-2004-830514. [DOI] [PubMed] [Google Scholar]
- 34.Eder B, Hofmann P, von Duvillard SP, Brandt D, Schmid JP, Pokan R, et al. Early 4-week cardiac rehabilitation exercise training in elderly patients after heart surgery. J Cardiopulm Rehabil Prev. 2010;30(2):85–92. doi: 10.1097/HCR.0b013e3181be7e32. [DOI] [PubMed] [Google Scholar]
- 35.Mendes RG, Simões RP, De Souza Melo Costa F, Pantoni CB, Di Thommazo L, Luzzi S, et al. Short-term supervised inpatient physiotherapy exercise protocol improves cardiac autonomic function after coronary artery bypass graft surgery--a randomised controlled trial. Disabil Rehabil. 2010;32(16):1320–7. doi: 10.3109/09638280903483893. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N, Corbi G, Bosimini E, Cobelli F, Furgi G, Giannuzzi P, et al. Cardiac rehabilitation in the elderly: patient selection and outcomes. Am J Geriatr Cardiol. 2006;15(1):22–7. doi: 10.1111/j.1076-7460.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- 37.Borges DL, Silva MG, Silva LN, Fortes JV, Costa ET, Assunção RP, et al. Effects of aerobic exercise applied early after coronary artery bypass grafting on pulmonary function, respiratory muscle strength, and functional capacity: a randomized controlled trial. J Phys Act Health. 2016;13(9):946–51. doi: 10.1123/jpah.2015-0614. [DOI] [PubMed] [Google Scholar]
- 38.Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet. 1999;354:1229–33. doi: 10.1016/s0140-6736(98)10063-6. https://doi:10.1016/s0140-6736(98)10063-6 . [DOI] [PubMed] [Google Scholar]
- 39.Burneto AF, Paulin E, Yamaguti WPS. Comparação entre a escala modificada de Borg e a escala de Borg modificada análago visual aplicadas em pacientes com dispnéia. Rev Bras Ciênc Mov. 1989;3(1):34–40. [Google Scholar]
- 40.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 41.Santos PMR, Ricci NA, Suster ÉAB, Paisani DM, Chiavegato LD. Effects of early mobilisation in patients after cardiac surgery: a systematic review. Physiotherapy. 2017;103(1):1–12. doi: 10.1016/j.physio.2016.08.003. https://doi:10.1016/j.physio.2016.08.003 . [DOI] [PubMed] [Google Scholar]
- 42.Royse CF, Saager L, Whitlock R, Ou-Young J, Royse A, Vincent J, et al. Impact of methylprednisolone on postoperative quality of recovery and delirium in the steroids in cardiac surgery trial: a randomized, doubleblind, placebo-controlled substudy. Anesthesiology. 2017;126(2):223–33. doi: 10.1097/ALN.0000000000001433. https://doi:10.1097/ALN.0000000000001433 . [DOI] [PubMed] [Google Scholar]
- 43.Sebastian LA, Reeder S, Williams M. Determining target heart rate for exercising in a cardiac rehabilitation program: a retrospective study. J Cardiovasc Nurs. 2015;30(2):164–71. doi: 10.1097/JCN.0000000000000154. Erratum in: J Cardiovasc Nurs. 2015;30(3):221. doi:10.1097/JCN.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 44.Herdy AH, López-Jiménez F, Terzic CP, Milani M, Stein R, Carvalho T, et al. Diretriz Sul Americana de prevenção e reabilitação cardiovascular. Arq Bras Cardiol. 2014;103(2Supl1):1–31. doi: 10.36660/abc.20200407. [DOI] [PubMed] [Google Scholar]
- 45.Dehart-Beverley M, Foster C, Porcari JP, Fater DCW, Mikat RP. Relationship between the talk test and ventilatory threshold. Clin Exerc Physiol. 2000;2(1):34–8. [Google Scholar]
- 46.Castro CLB, Araujo CGS. Princípios da prescrição do exercício físico e critérios para realização sob supervisão médica. Rev SOCERJ. 2000;3(4):198–200. [Google Scholar]
- 47.Forton K, Motoji Y, Deboeck G, Faoro V, Naeije R. Effects of body position on exercise capacity and pulmonary vascular pressure-flow relationships. J Appl Physiol (1985) 2016;121(5):1145–50. doi: 10.1152/japplphysiol.00372.2016. [DOI] [PubMed] [Google Scholar]
- 48.Regenga MM. Fisioterapia em Cardiologia: da Unidade de Terapia Intensiva à Reabilitação. São Paulo: Roca; 2000. p. 417p. [Google Scholar]
- 49.Umeda IIK. Manual de Fisioterapia na Reabilitação Cardiovascular. São Paulo: Manole; 2005. [Google Scholar]
- 50.Babu AS, Noone MS, Haneef M, Naryanan SM. Protocol-guided phase-1 cardiac rehabilitation in patients with ST-elevation myocardial infarction in a rural hospital. Heart Views. 2010;11(2):52–6. doi: 10.4103/1995-705X.73209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dias CM, Vieira Rde O, Oliveira JF, Lopes AJ, Menezes SL, Guimarães FS. Three physiotherapy protocols: effects on pulmonary volumes after cardiac surgery. J Bras Pneumol. 2011;37(1):54–60. doi: 10.1590/s1806-37132011000100009. [DOI] [PubMed] [Google Scholar]
- 52.Winkelmann ER, Dallazen F, Bronzatti AB, Lorenzoni JC, Windmöller P. Analysis of steps adapted protocol in cardiac rehabilitation in the hospital phase. Rev Bras Cir Cardiovasc. 2015;30(1):40–8. doi: 10.5935/1678-9741.20140048. [DOI] [PMC free article] [PubMed] [Google Scholar]