Abstract
Herein, we have developed naturally-occurring Emodin, which is commercially available at low-cost, as a novel organic photocatalyst for the first time. Emodin was successfully employed in the selective oxidation of sulfides promoted by visible-light, delivering valuable sulfoxides with high efficiency. Mechanistic investigations suggested both single-electron transfer (SET) and energy transfer (EnT) pathways might be involved in the oxidation reaction.
Naturally-occurring Emodin was successfully employed in the selective oxidation of sulfides promoted by visible-light as a novel organic photocatalyst for the first time.
Visible-light-promoted reactions have attracted great interest from chemists over the past decade for the usage of sunlight as the renewable energy source.1 As a logical consequence, the development of novel photocatalysts plays an essential role in the field of photocatalysis.2 Among them, organo-photocatalysts, such as Rose bengal,3 Eosin Y,4 9,10-dicyanoanthracene (DCA),5etc, were more appealing due to the avoidance of toxic, expensive and environmentally unfriendly metals (Fig. 1).6 However, the investigations of organic photocatalysts were restricted in a limited number of well-developed skeletons. Thus, it is worth further developing more varieties of organo-photocatalysts with versatile frameworks.
Fig. 1. Selected examples for organic photocatalysts.
Meanwhile, nature generously provided plentiful natural products as high-efficient catalysts.7 Recently, we have successfully exploited cercosporin8 from plant pathogenic fungi Cercospora species as a novel photocatalyst in oxidation,9 cycloaddition10 and cross-coupling11 reactions. Take it into consideration that Emodin12 has the similar quinone skeleton with hydroxy groups and photostablity (see ESI†) as cercosporin, we rationalize naturally-occurring Emodin might have potential photophysical properties13 as a novel photoredox catalyst, which has yet not been reported to the best of our knowledge (Scheme 1a).
Scheme 1. Emodin-catalyzed selective oxidation of sulfides.
On the other hand, selective oxidation is a kind of fundamental reactions along with significant challenges because of the over-oxidation and the hazardous oxidizing agents employed in the reactions.14 With the growing environmental concerns, photocatalytic selective oxidation has been considered as an alternative method utilizing oxygen as green terminal oxidant and visible-light as renewable energy source.15 Based on our continuous interest in developing novel photocatalysts from natural products and their applications in photoredox-catalyzed reactions, herein, we will report the first example of Emodin-catalyzed selective oxidation of sulfides with the promotion of visible-light (Scheme 1b).
After systematic reaction condition evaluations, the selective oxidation of thioanisole was successfully achieved in 99% yield with Emodin (1.5 mol%) as the photocatalyst in methanol at room temperature under air atmosphere (Table 1, entry 1). Solvents screening revealed that protonic solvents (MeOH and EtOH) showed better results than others like DMF, DMSO, dioxane, THF and toluene (Table 1, entries 2–7). Increasing the amount of Emodin had little influence on the reaction, while decreasing the amount to 1 and 0.5 mol% would decelerate the reaction (Table 1, entries 8–10). Additionally, in this selective oxidation process, 5 W blue LED was proved better light source than corresponding CFL and green LED (Table 1, entries 11–12), which shows that when the maximum absorption wavelength of the photocatalyst is consistent with the emission spectrum of the light source (see ESI†), the catalytic efficiency is the highest. Unexpectedly, a reduced result was obtained using 30 W blue LED, which probably resulted from the side-reactions induced by high energy of the light source (Table 1, entry 13). Other organic photocatatlysts were also tested, Eosin Y has the similar photocatalytic efficiency compared to Emodin (Table 1, entry 14), while the photocatalytic activities of other organic catalysts (methylene blue, methyl orange and Rhodamine B) were obviously lower than that of Emodin (Table 1, entries 15–17).
Effect of reaction parametersa.

| ||
|---|---|---|
| Entry | Variation from the standard conditions | Yieldb (%) |
| 1 | None | 99 |
| 2 | EtOH instead of MeOH | 82 |
| 3 | DMSO instead of MeOH | 25 |
| 4 | DMF instead of MeOH | 41 |
| 5 | Dioxane instead of MeOH | 53 |
| 6 | THF instead of MeOH | 19 |
| 7 | Toluene instead of MeOH | 24 |
| 8 | Emodin (2%) instead of Emodin (1.5%) | 99 |
| 9 | Emodin (1%) instead of Emodin (1.5%) | 90 |
| 10 | Emodin (0.5%) instead of Emodin (1.5%) | 76 |
| 11 | 5 W CFL instead of 5 W blue LED | 71 |
| 12 | 5 W green LED instead of 5 W blue LED | 29 |
| 13 | 30 W blue LED instead of 5 W blue LED | 58 |
| 14 | Eosin Y instead of Emodin | 93 |
| 15 | Methylene blue instead of Emodin | 51 |
| 16 | Methyl orange instead of Emodin | None |
| 17 | Rhodamine B instead of Emodin | None |
Conducted with 1a (0.25 mmol), Emodin (1.5 mol%) in methanol (2 mL) at room temperature under air atmosphere for 6 h.
Isolated yield.
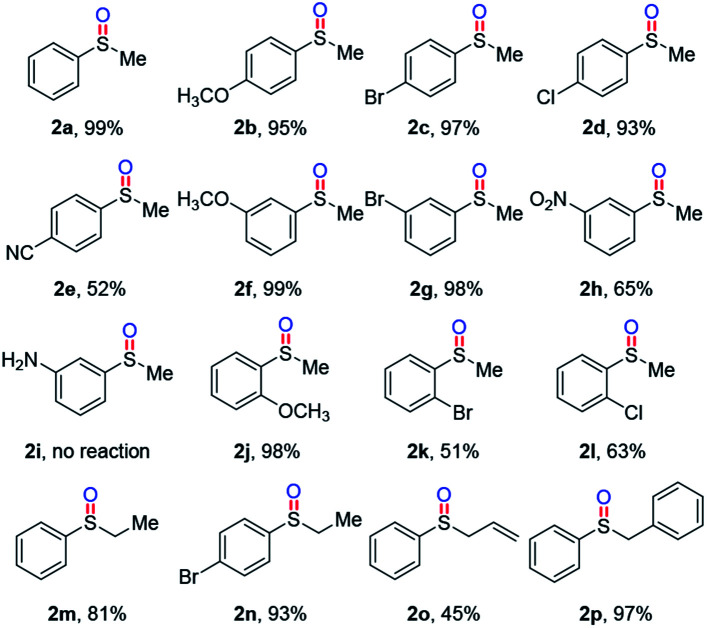
With the optimal reaction conditions, we next investigated the substrate scope of aryl alkyl sulfides, and the results were summarized in Table 2. Electron-donating group (–OMe) and halogen groups (–Br, –Cl) at the para-position of the benzene ring led to the oxidation products 2b–2d in 93–99% yields; electron-withdrawing group (–CN) led to 2e in moderate yield. Products 2f–2h with meta-substitutions were produced in 65–99% yields. Unfortunately, substrate with unprotected amino group (1i) was an unsuccessful example in the reaction. When methoxy group was substituted at the ortho-position of the benzene ring, 2j could be delivered in 98% yield; while 2k and 2l with bromine and chlorine atoms at the ortho-position showed poor efficiency probably due to the steric hindrance. Moreover, this oxidative reaction was tolerant of ethyl (2m, 2n), allyl (2o) and benzyl (2p) sulfides.
Substrate scope of aryl alkyl sulfidesa,b.

|
|---|
|
Conducted with 1 (0.25 mmol), Emodin (1.5 mol%) in methanol (2 mL) at room temperature under air atmosphere for 6–12 h.
Isolated yield.
Subsequently, other sulfides with two alkyl groups or two aryl groups were tested (Table 3). Benzyl sulfoxide 2q and n-butyl sulfoxide 2r were achieved in good to excellent yields (77–97%). What is more, symmetric (1s, 1t) and unsymmetrical (1u–1y) di-phenyl sulfides were also compatible in the oxidation reactions, producing corresponding diphenyl sulfoxides 2s–2y in 20–79% yields.
Substrate scope of other sulfidesa,b.

|
|---|

|
Conducted with 1 (0.25 mmol), Emodin (1.5 mol%) in methanol (2 mL) at room temperature under air atmosphere for 6–12 h.
Isolated yield.
Next, several control experiments were carried out to gain more insight into the mechanism of this novel Emodin-catalyzed oxidation reaction of sulfides. The reaction was completely inhibited without the catalyst or the light source; and the reaction was not affected when air was changed to oxygen, but failed if air was replaced by nitrogen, which revealing the roles of these factors (Scheme 2a). Furthermore, the radical and singlet oxygen inhibition experiments suggested that both O2˙− and 1O2 are responsible for the Emodin-catalyzed oxidation reaction of sulfides (Scheme 2b). According to the above-mentioned results and previous literature, we have proposed the reaction mechanism in Scheme 2c. First, the photocatalyst Emodin was excited with light irradiation to generate the excited species Emodin*. In the SET cycle, reductive quenching occurred to produce sulfide radical cation 3 along with the radical anion Emodin˙−. Subsequently, Emodin˙− was oxidized by oxygen to regenerate Emodin into the next catalytic cycle and achieve the reactive radical anion O2˙−, which further reacted with radical cation 3 to deliver methyl phenyl sulfoxide 2a. While in the EnT cycle, singlet oxygen 1O2 was produced to oxidize 1a to the product 2avia the intermediate 4, and photocatalyst Emodin was regenerated simultaneously.
Scheme 2. Mechanistic insights.
Conclusions
The natural product Emodin has been developed as a novel organic photocatalyst for the first time. Emodin-photocatalyzed selective oxidation reaction of sulfides with the promotion of visible-light proceeded in high efficiency and exhibited good functional group tolerance. Two possible mechanism including SET and Ent cycles were proposed according to the mechanistic investigations. Further investigations on Emodin-catalyzed photoredox reactions are currently underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02702b
Notes and references
- For selected reviews, see: ; (a) Ravelli D. Protti S. Fagnoni M. Chem. Rev. 2016;116:9850–9913. doi: 10.1021/acs.chemrev.5b00662. [DOI] [PubMed] [Google Scholar]; (b) Hopkinson M. N. Sahoo B. Li J.-L. Glorius F. Chem.–Eur. J. 2014;20:3874–3886. doi: 10.1002/chem.201304823. [DOI] [PubMed] [Google Scholar]; (c) Hari D. P. König B. Angew. Chem., Int. Ed. 2013;52:4734–4743. doi: 10.1002/anie.201210276. [DOI] [PubMed] [Google Scholar]; (d) Prier C. K. Rankic D. A. MacMillan D. W. C. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xuan J. Xiao W.-J. Angew. Chem., Int. Ed. 2012;51:6828–6838. doi: 10.1002/anie.201200223. [DOI] [PubMed] [Google Scholar]; (f) Narayanam J. M. R. Stephenson C. R. J. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]; (g) Yoon T. P. Ischay M. A. Du J. Nat. Chem. 2010;2:527–532. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see: ; (a) Glaser F. Wenger O. S. Coord. Chem. Rev. 2020;405:213129. doi: 10.1016/j.ccr.2019.213129. [DOI] [Google Scholar]; (b) Hockin B. M. Li C. Robertson N. Zysman-Colman E. Catal. Sci. Technol. 2019;9:889–915. doi: 10.1039/C8CY02336K. [DOI] [Google Scholar]; (c) Arias-Rotondo D. M. McCusker J. K. Chem. Soc. Rev. 2016;45:5803–5820. doi: 10.1039/C6CS00526H. [DOI] [PubMed] [Google Scholar]; (d) Sudha D. Sivakumar P. Chem. Eng. Process. 2015;97:112–133. doi: 10.1016/j.cep.2015.08.006. [DOI] [Google Scholar]; (e) Liao P. Carter E. A. Chem. Soc. Rev. 2013;42:2401–2422. doi: 10.1039/C2CS35267B. [DOI] [PubMed] [Google Scholar]
- For selected review, see: ; (a) Sharma S. Sharma A. Org. Biomol. Chem. 2019;17:4384–4405. doi: 10.1039/C9OB00092E. [DOI] [PubMed] [Google Scholar]; . For selected examples, see: ; (b) Singsardar M. Mondal S. Laru S. Hajra A. Org. Lett. 2019;21:5606–5610. doi: 10.1021/acs.orglett.9b01954. [DOI] [PubMed] [Google Scholar]; (c) Li P. Wang G.-W. Org. Biomol. Chem. 2019;17:5578–5585. doi: 10.1039/C9OB00790C. [DOI] [PubMed] [Google Scholar]; (d) Xin J.-R. He Y.-H. Guan Z. Org. Chem. Front. 2018;5:1684–1688. doi: 10.1039/C8QO00161H. [DOI] [Google Scholar]; (e) Pan Y. Kee C. W. Chen L. Tan C.-H. Green Chem. 2011;13:2682–2685. doi: 10.1039/C1GC15489C. [DOI] [Google Scholar]
- For selected examples, see: ; (a) Wu K. Wang L. Colón-Rodríguez S. Flechsig G.-U. Wang T. Angew. Chem., Int. Ed. 2019;58:1774–1778. doi: 10.1002/anie.201811004. [DOI] [PubMed] [Google Scholar]; (b) Zhao G. Wang T. Angew. Chem., Int. Ed. 2018;57:6120–6124. doi: 10.1002/anie.201800909. [DOI] [PubMed] [Google Scholar]; (c) Wang H. Li Y. Tang Z. Wang S. Zhang H. Cong H. Lei A. Acs Catal. 2018;8:10599–10605. doi: 10.1021/acscatal.8b02617. [DOI] [Google Scholar]; (d) Yadav A. K. Yadav L. D. S. Green Chem. 2016;18:4240–4244. doi: 10.1039/C6GC00924G. [DOI] [Google Scholar]; (e) Majek M. von Wangelin A. J. Angew. Chem., Int. Ed. 2015;54:2270–2274. doi: 10.1002/anie.201408516. [DOI] [PubMed] [Google Scholar]
- For selected examples, see: ; (a) Qin H.-T. Xu X. Liu F. Chemcatchem. 2017;9:1409–1412. doi: 10.1002/cctc.201700061. [DOI] [Google Scholar]; (b) Yang C. Yang J.-D. Li Y.-H. Li X. Cheng J.-P. J. Org. Chem. 2016;81:12357–12363. doi: 10.1021/acs.joc.6b02385. [DOI] [PubMed] [Google Scholar]; (c) Pandey G. Laha R. Angew. Chem., Int. Ed. 2015;54:14875–14879. doi: 10.1002/anie.201506990. [DOI] [PubMed] [Google Scholar]
- For selected reviews on organo-photocatalysts, see: ; (a) Rahman M. Z. Kibria M. G. Mullins C. B. Chem. Soc. Rev. 2020 doi: 10.1039/c9cs00313d. [DOI] [Google Scholar]; (b) Zhang Y. Schilling W. Das S. Chemsuschem. 2019;12:2898–2910. doi: 10.1002/cssc.201900414. [DOI] [PubMed] [Google Scholar]; (c) Li C. Xu Y. Tu W. Chen G. Xu R. Green Chem. 2017;19:882–899. doi: 10.1039/C6GC02856J. [DOI] [Google Scholar]; (d) Romero N. A. Nicewicz D. A. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]; (e) Fukuzumi S. Ohkubo K. Org. Biomol. Chem. 2014;12:6059–6071. doi: 10.1039/C4OB00843J. [DOI] [PubMed] [Google Scholar]
- For selected examples, see: ; (a) Kasar S. B. Thopate S. R. Curr. Org. Synth. 2018;15:110–115. doi: 10.2174/1570179414666170621080701. [DOI] [PubMed] [Google Scholar]; (b) Li W. Fedosov S. Tan T. Xu X. Guo Z. Appl. Biochem. Biotechnol. 2014;173:278–290. doi: 10.1007/s12010-014-0840-3. [DOI] [PubMed] [Google Scholar]; (c) Jiang H.-F. Ye J.-W. Qi C.-R. Huang L.-B. Tetrahedron Lett. 2010;51:928–932. doi: 10.1016/j.tetlet.2009.12.031. [DOI] [Google Scholar]
- For selected reviews on Emodin, see: ; (a) Tu Y. Wu Z. Tan B. Yang A. Fang Z. Oncol. Rep. 2019;42:1259–1271. doi: 10.3892/or.2019.7264. [DOI] [PubMed] [Google Scholar]; (b) Li L. Song X. Yin Z. Jia R. Li Z. Zhou X. Zou Y. Li L. Yin L. Yue G. Ye G. Lv C. Shi W. Fu Y. Microbiol. Res. 2016;186:139–145. doi: 10.1016/j.micres.2016.03.008. [DOI] [PubMed] [Google Scholar]; (c) Dong X. Fu J. Yin X. Cao S. Li X. Lin L. Huyiligeqi Ni J. Phytother. Res. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wei W.-T. Lin S.-Z. Liu D.-L. Wang Z.-H. Oncol. Rep. 2013;30:2555–2562. doi: 10.3892/or.2013.2741. [DOI] [PubMed] [Google Scholar]; (e) Izhaki I. New Phytol. 2002;155:205–217. doi: 10.1046/j.1469-8137.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- (a) Kuyama S. Tamura T. J. Am. Chem. Soc. 1957;79:5725–5726. doi: 10.1021/ja01578a038. [DOI] [Google Scholar]; (b) Kuyama S. Tamura T. J. Am. Chem. Soc. 1957;79:5726–5729. doi: 10.1021/ja01578a039. [DOI] [Google Scholar]; (c) Daub M. E. Ehrenshaft M. Annu. Rev. Phytopathol. 2000;38:461–490. doi: 10.1146/annurev.phyto.38.1.461. [DOI] [PubMed] [Google Scholar]; (d) Daub M. E. Phytopathology. 1987;77:1515–1520. doi: 10.1094/Phyto-77-1515. [DOI] [Google Scholar]
- Li J. Bao W. Tang Z. Guo B. Zhang S. Liu H. Huang S. Zhang Y. Rao Y. Green Chem. 2019;21:6073–6081. doi: 10.1039/C9GC02270H. [DOI] [Google Scholar]
- (a) Li J. Bao W. Zhang Y. Rao Y. Org. Biomol. Chem. 2019;17:8958–8962. doi: 10.1039/C9OB01946D. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y. Cao Y. Lu L. Zhang S. Bao W. Huang S. Rao Y. J. Org. Chem. 2019;84:7711–7721. doi: 10.1021/acs.joc.9b00545. [DOI] [PubMed] [Google Scholar]
- (a) Tang Z. Li J. Lin F. Bao W. Zhang S. Guo B. Huang S. Zhang Y. Rao Y. J. Catal. 2019;380:1–8. doi: 10.1016/j.jcat.2019.09.036. [DOI] [Google Scholar]; (b) Zhang S. Tang Z. Bao W. Li J. Guo B. Huang S. Zhang Y. Rao Y. Org. Biomol. Chem. 2019;17:4364–4369. doi: 10.1039/C9OB00659A. [DOI] [PubMed] [Google Scholar]
- (a) Wang L. Zhang Z. Ye B. Electrochim. Acta. 2006;51:5961–5965. doi: 10.1016/j.electacta.2006.03.082. [DOI] [Google Scholar]; (b) Li D. Jin B. J. Electrochem. 2017;23:347–355. [Google Scholar]
- For selected reviews on selective oxidation reactions, see: ; (a) Pu T. Tian H. Ford M. E. Rangarajan S. Wachs I. E. Acs Catal. 2019;9:10727–10750. doi: 10.1021/acscatal.9b03443. [DOI] [Google Scholar]; (b) Dai C. Zhang J. Huang C. Lei Z. Chem. Rev. 2017;117:6929–6983. doi: 10.1021/acs.chemrev.7b00030. [DOI] [PubMed] [Google Scholar]; (c) Wendlandt A. E. Stahl S. S. Angew. Chem., Int. Ed. 2015;54:14638–14658. doi: 10.1002/anie.201505017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu K. Ou H. Shi X. Dong X. Ma W. Wei J. Chinese J. Org. Chem. 2014;34:681–692. doi: 10.6023/cjoc201310019. [DOI] [Google Scholar]
- For selected reviews on photocatalytic selective oxidation, see: ; (a) Chen L. Tang J. Song L.-N. Chen P. He J. Au C.-T. Yin S.-F. Appl. Catal. B-Environ. 2019;242:379–388. doi: 10.1016/j.apcatb.2018.10.025. [DOI] [Google Scholar]; (b) Lang X. Zhao J. Chen X. Angew. Chem., Int. Ed. 2016;55:4697–4700. doi: 10.1002/anie.201600405. [DOI] [PubMed] [Google Scholar]; (c) Zhang N. Zhang Y. Pan X. Fu X. Xu Y. Sci. Sin. Chim. 2011;41:1097–1111. doi: 10.1360/032010-651. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





