Abstract
Background
The aim of the present systematic review was to evaluate the clinical effect of vitamin C on bone healing after bone fracture or bone reconstruction procedures.
Material and Methods
In October 2020, Cochrane Library, Scopus and PubMed-Medline databases were searched without restrictions to identify animal and human studies that fulfilled the eligibility criteria. Outcome measures were bone healing time, bone gain (mm), bone density and adverse events. The risk of bias assessment of the selected studies was evaluated by means of Cochrane Collaboration’s Tool for randomized clinical trials, while randomized clinical animal trials were assessed according to SYRCLE’s tool. Additionally, quality of reporting animal studies were assessed according to ARRIVE guidelines.
Results
Out of the 248 articles that yielded the initial search, 11 papers about the effect of ascorbic acid on bone healing were selected. In most of the animal studies, vitamin C seemed to accelerate bone formation owing to an enhanced osteoblastic proliferation and differentiation and its antioxidant function when pro-oxidant substances were added. It was not possible to observe this phenomenon in human studies.
Conclusions
Although additional well-performed animal and human studies are required, vitamin C seems to accelerate bone regeneration without adverse events. However, it is not possible to recommend a specific dose or route of administration of vitamin C to improve the bone healing process in humans as there was great heterogeneity among the included studies.
Key words:Vitamin C, Fracture healing, Bone regeneration, Bone mineral density, Implants.
Introduction
Vitamin C (vit C) or ascorbic acid (chemical name: 2,3-didehydro-L-threo-hexane-1,4-lactone) is a water-soluble vitamin obtained from natural or synthetic sources that plays an important role in many biological reactions (1). This vitamin is synthesized from glucose in the liver of most mammalian species, but not in humans or other animal groups (non-human primates, guinea pigs and bats) (2).
An imbalance between the production of reactive oxygen species (free radicals) and antioxidant substances can lead to cell damage and be the cause of various conditions (2). However, vitamin C, as an electron donor, can eliminate hydroxyl and superoxide radicals and, therefore, prevent cell damage by protecting the capillary endothelium and circulating cells (2-4). On the other hand, the importance of vitamin C in bone metabolism is also remarkable, since it is related to the hydroxylation of collagen (5-7), and to the expression of no-collagenic proteins such as alkaline phosphatase, osteonectin and osteocalcin (8). Besides, vit C promotes the expression of genes related to chondrocytes differentiation and is involved in osteoblastogenesis and osteoclastogenesis (4). Indeed, Urban et al. (9) showed that the addition of vit C in concentrations up to 200 g/ml in cell cultures had a positive effect on osteoblast proliferation and also increased type-I collagen synthesis.
Severe vitamin C deficiency results in scurvy, a disease that is characterized by weakening of collagenous structures, resulting in poor wound healing and impaired immunity (10). Currently, it is difficult to find this condition among the population, however, there are some groups that have a higher requirement of vit C, such as the elderly, alcoholics, smokers and diabetics (10,11). In addition, preclinical and clinical studies have shown that vitamin C deficiency causes a delay in tissue healing and inhibits collagen synthesis (1,12). Furthermore, this deficiency has been linked to an increased risk of osteoporosis and fractures due to decreased bone formation (4).
Bone defects can heal totally or partially depending on local or systemic factors (13,14). When spontaneous bone regeneration is not achieved, additional measures are needed, such as specific surgical techniques and materials (13,15). Several articles have shown that vit C can improve bone healing during regeneration procedures, however, this topic has not yet been systematically reviewed. Thus, a systematic review of animal and human studies investigating the efficacy of the use of vit C as a supplement to a bone healing procedure may add new information.
The purpose of this study was to evaluate the current knowledge on the efficacy of vit C in bone regeneration, as well as to stablish a protocol of dosage and posology of vit C to improve bone healing process.
Material and Methods
The present systematic review was performed according to the statements of “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) (16).
- Eligibility criteria
The focus questions to be addressed were:
How can systemic or oral administration of vit C influence the bone healing process in terms of speed and quality? Are there any differences compare to the bone healing process without Vit C administration? Accordingly, articles that fulfilled the following eligibility criteria were selected (PICO parameters):
(P) Population: Patients or animals treated with vit C.
(I) Intervention: Bone healing using systemic or oral administration of vit C.
(C) Comparison: Bone healing without the use systemic or oral administration of vit C.
(O) Outcomes: For both animal and human studies bone healing time, histomorphometry (bone gain (mm) bone density (HU)) and number of adverse effects were assessed. Additionally, gene/cytokines expression was only assessed in animal studies.
Inclusion criteria were human and animal studies that evaluated the effect of systemically or oral administered vitamin C in terms of bone healing. Studies that evaluated the effect of different vitamins simultaneously were excluded. No restrictions were applied regarding the language and the year of publication.
- Information sources and search strategy
A systematic search in the Cochrane Library, Scopus and PubMed-MEDLINE databases was conducted in October 2020. The following search strategy was used:
1) PubMed-MEDLINE: ("ascorbic acid" [MH] OR "acid, ascorbic" [TIAB] OR "L-ascorbic acid" [TIAB] OR "acid, L-ascorbic" [TIAB] OR "vitamin C" [TIAB]) AND ("bone regeneration" [MH] OR "osteoconduction" [TIAB] OR "bone transplantation" [MH] OR "bone grafting" [TIAB] OR "guided tissue regeneration" [MH] OR "bone remodeling" [MH] OR "fracture healing" [MH] OR "osseointegration" [MH]).
2) Scopus: TITLE-ABS-KEY((“ascorbic acid" OR "vitamin C") AND ("bone regeneration" OR "fracture healing" OR "osseointegration")).
3) Cochrane Library: (“ascorbic acid" OR "vitamin C") AND ("bone regeneration" OR "fracture healing" OR "osseointegration").
Additionally, a cluster search and a manual search of articles published during the last 10 years in "Journal of Clinical Periodontology", "Journal of Periodontal Research", "Clinical Oral investigations", "Journal of Oral and Maxillofacial Surgery" "Medicina Oral Patología Oral y Cirugía Bucal", "Oral Surgery Oral Medicine Oral Pathology Oral Radiology", "Journal of Dentistry", "The International Journal of Oral and Maxillofacial Implants" and "Clinical Oral Implants Research" were carried out. Grey literature was also explored through the Bielefeld Academic Search Engine (BASE).
- Selection process of studies
The selection of the studies was made by two independent reviewers (K.B-G. and J.T-S.). After removing duplicates and screening the remaining articles reading by their title and by their abstract, the studies that fulfilled the eligibility criteria were selected. A third reviewer (M.Á.S-G) with broad experience in systematic reviews resolved any disagreement during the article selection process. Cohen’s kappa was calculated to measure the level of agreement between the two reviewers.
- Data collection process and synthesis of the results
A qualitative synthesis was performed using data extraction Tables. The following information was retrieved from the selected articles: name of the authors, year of publication, study design, number of participants, description of experimental groups, type of bone defect, vitamin C dosage, exposition route and administration frequency, follow-up time and outcomes variables (healing time, bone gain measured in mm, adverse effects). If necessary, authors of the selected studies were contacted for clarification missing or incomplete data.
Since high heterogeneity was found among the selected studies, a quantitative synthesis was not carried out.
- Risk of bias and quality assessment of the included studies
Risk of bias and quality assessment of the included studies was conducted by two independent reviewers (K.B-G and J.T-S). A third reviewer (M.Á.S-G) resolved any disagreements.
Randomized clinical trials (RCT) were evaluated by means of “Cochrane Handbook for Systematic Reviews of Interventions” (17). The following items were classified in low, unclear or high risk of bias: random sequence generation, allocation concealment, patient blinding, outcome blinding, incomplete outcome data and selective reporting. Additionally, the “SYRCLE tool for assessing the risk of bias of animal intervention studies” was used to assess the risk of bias of randomized clinical animal trial (RCAT) studies (18). The following items were classified in low, unclear or high risk of bias: sequence generation, baseline characteristics, allocation concealment, random housing, blinding of the intervention to caregivers and researchers, random outcome assessment, blinding of outcome assessor, incomplete outcome data, selective outcome reporting and other sources of bias. On the other hand, quality of reporting animal studies were assessed according to ARRIVE guidelines (19) for in vivo experiments and assigned predefined grades (study design, sample size, inclusion and exclusion criteria, randomization, blinding, outcome measures, statistical methods, experimental animals, experimental procedures and results). Any disagreement during this step was resolved thanks to one independent investigator (M.Á.S-G).
Results
- Study selection
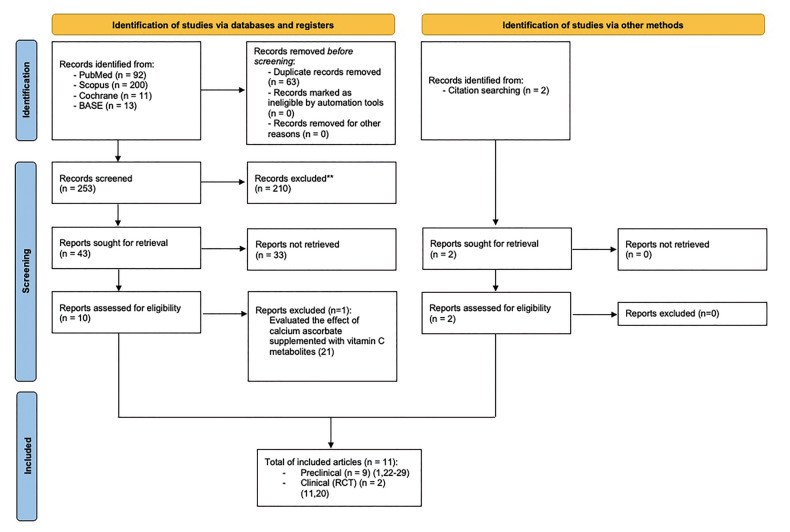
The initial search yielded 253 studies after eliminating duplicates. After discarding 210 studies by reading their title and 33 by reading the abstract. the full-text of 12 articles was assessed for elegibility (1,11,20-29). Only one study was excluded because evaluated the effect of calcium ascorbate supplemented with vit C metabolites (21). Finally, 11 studies, written in English, were included in the present systematic review; nine animal studies (1,22-29), and two randomized clinical trials (11,20). The level of agreement between the two reviewers was 93.75% with a Cohen’s kappa statistic of 0,84.
Fig. 1 shows the flow-chart of the study selection process.
Figure 1.
Flow-chart of the review process following PRISMA statements.
- Risk of bias and quality assessment of the included studies
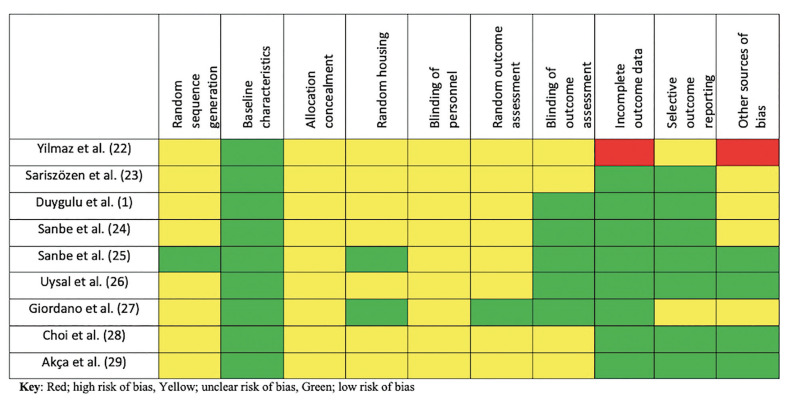
Regarding animal studies, one study has a high risk of bias and low quality of reporting (22) because it has attrition bias, another has an unclear risk of bias and low quality of reporting (27), other two studies have an unclear risk of bias and an unclear quality of reporting (23,28) and five studies have an unclear risk of bias but high quality of reporting (1,24-26,29) (Fig. 2).
Figure 2.
Assessment of quality and risk of bias of included animal studies.
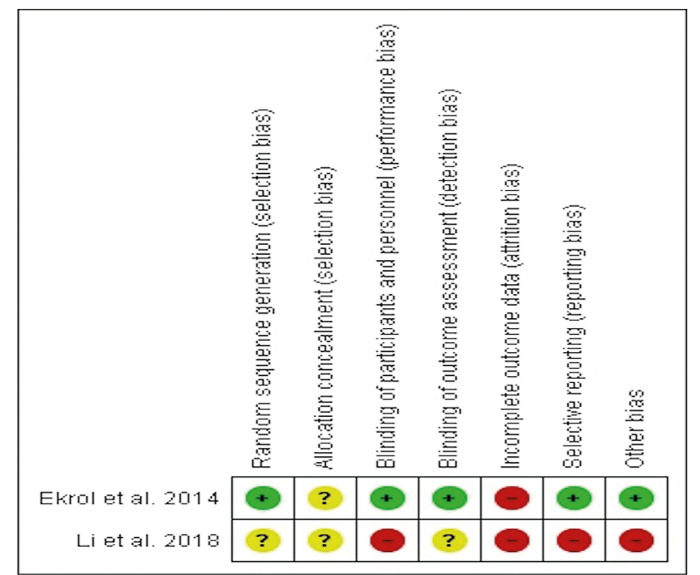
Regarding human studies, both were classified as having high risk of bias. The study of Ekrol et al. (11) because it had an attrition bias, whereas the study of Li et al. (20) had a high risk of bias due mainly to performance, attrition and reporting bias (Fig. 3).
Figure 3.
The Cochrane collaboration’s tool for assessing risk of bias for randomized.
- Qualitative synthesis
None of the included studies assessed bone gain or bone quality outcomes.
Animal studies
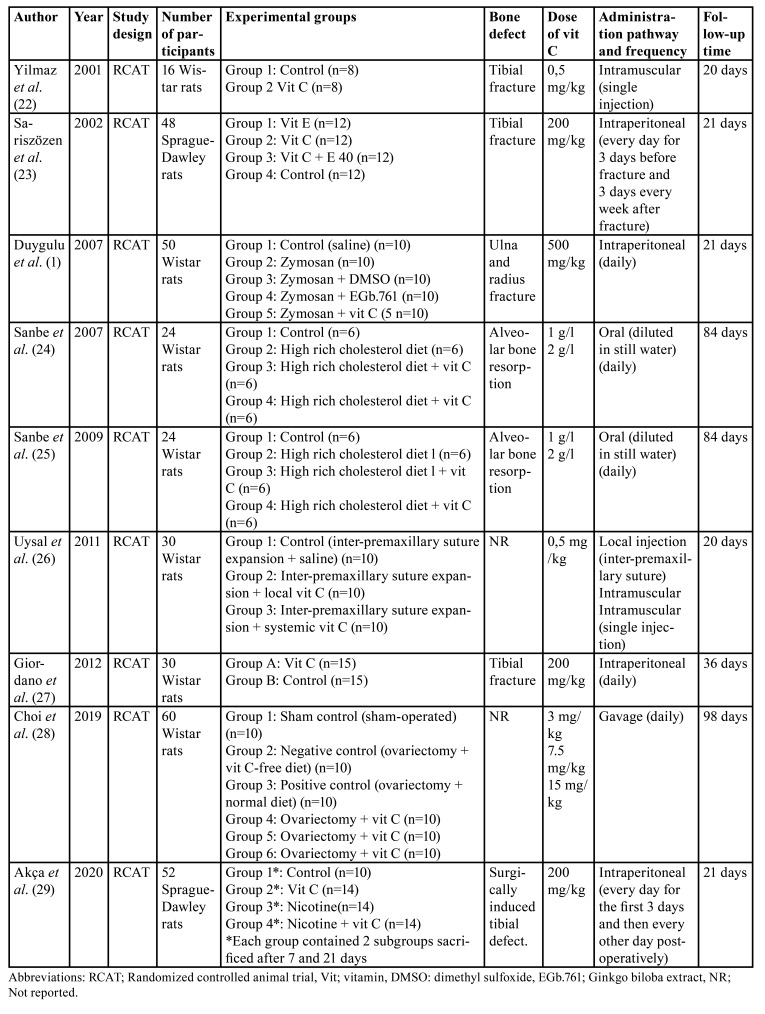
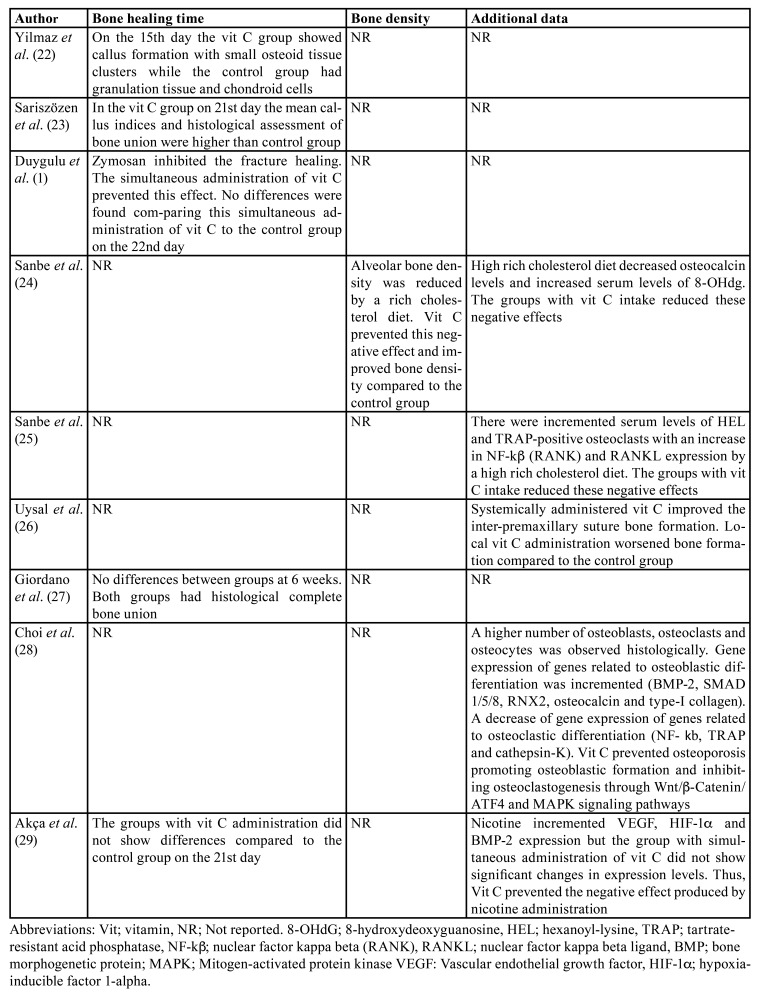
The nine animal studies included comprised 334 rats over a follow-up period ranging from 20 to 98 days. All the studies evaluated the effect of vitamin C in the following clinical scenarios: on bone healing after tibial fracture (22,23,27), on bone defects healing with the addition of pro-oxidant substances (zymosan and nicotine) (1,29), on alveolar bone resorption in rodents fed cholesterol-rich diets (24,25), in bone formation during the expansion of the inter-premaxillary suture (26) and in osteogenic differentiation and osteoclast formation in ovariectomized rats (a useful animal model for evaluating the effect of osteoporotic treatments on the skeletal system) (28) (Table 1).
Table 1. Description of the included animal studies.
In Table 2 is depicted the main results of the included animal studies. Two of them (22,23) showed that vit C administration accelerated bone healing after fracture when administered systemically at doses ranging from 0,5 mg/kg to 200 mg/kg. This effect was also observed when pro-oxidant substances were added (1), but a daily dose of 500 mg/kg was necessary. In fact, Akça et al. (29) found that vit C reversed the negative effects produced by nicotine administration on bone healing. In this line, two papers from the same group (24,25) found that vit C diluted in water at concentrations of 1g/l and 2g/l reversed the negative effects on alveolar bone produced by a cholesterol-rich diet (decreased bone density and osteocalcin levels higher expression of hexanoyl-lysine, 8-hydroxideoxyguanosin, nuclear factor kappa beta and RANKL). Conversely, the study by Giordano et al. (27) did not show any benefit from vit C administration at a dose of 200 mg/kg on bone healing after tibial fracture.
Table 2. Outcomes of the included animal studies.
Regarding the route of administration, Uysal et al. (26) observed that during inter-premaxillary suture expansion, the systemic administration of vit C had significantly better results than local administration of vit C, both at a dose of 0.5 mg/kg. On the other hand, Choi et al. (28) observed that oral administration of vit C at doses of 3 mg/kg, 7.5 mg/kg and 15 mg/kg improved bone mineral density and increased the expression of genes involved with osteoblastic differentiation (BMP-2, SMAD 1/5/8, RNTF-2, osteocalcin and COL1) and decreased the expression of genes related to osteoclastic differentiation (RANK, RANKL, TRAP and cathepsin-K) in ovariectomized rats.
Human studies
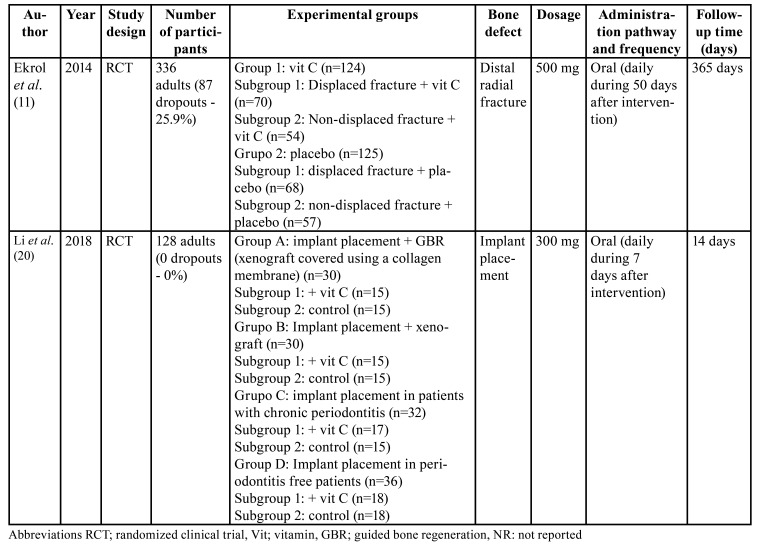
Regarding the human studies, two randomized clinical trials met the inclusion criteria. These studies comprised 464 patients during a follow-up period ranging from 14 to 365 days (Table 3).
Table 3. Description of the included human studies.
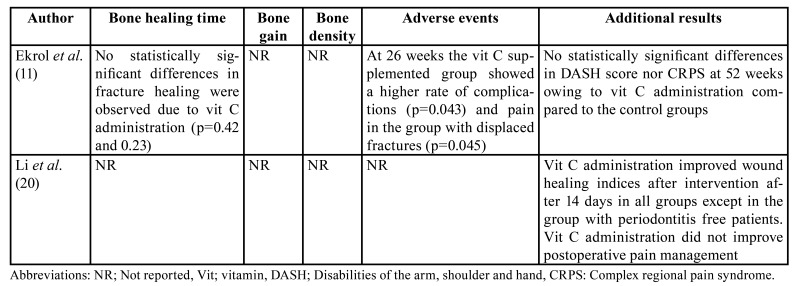
In Table 4 is depicted the main results of the included human studies. Ekrol et al. (11) studied the effect of vit C by oral supplementation at daily dose of 500 mg/day for 50 days in 336 patients with displaced and non-displaced distal radial fractures, of whom 87 (25,9%) dropped out within the follow-up period. No statistically differences in , terms of bone healing were observed (11). On the other hand, the study by Li et al. (20) evaluated the effect of oral administration of vit C for 7 days at dose a 300 mg/day after dental implant placement in different clinical scenarios in 128 patients (no patient dropped out). After 14 weeks of follow-up, they observed that vit C improved soft tissue healing (P < 0.05) after dental implant placement in patients who undergoing guided bone regeneration procedures. However, there were no benefits after vit C supplementation in terms of pain management. - Bone healing or implant success were not assessed in the present study.
Table 4. Outcomes of the included human studies.
Since high heterogeneity was found among the selected studies, a quantitative synthesis was not carried out.
Discussion
According to the results of the included animal studies, the administration of vit C seems to improve bone healing and bone formation, as vit C may modulate osteoblastogenesis and osteoclastogenesis, and also has an antioxidant function. However, data extracted from the included RCTs did not show any additional benefits of oral vit C supplementation on either bone healing or bone regeneration.
Different results were found regarding bone healing speed among the included animal studies. This fact could be due to the ability of rodents to synthesize vit C from a normal diet (30). This makes it necessary to know exactly the dietary pattern of the animals. Thus, whereas the rodents in the study by Giordano et al. (27) had unlimited access to food, the feeding pattern followed in the studies by Yilmaz et al. (22) and Sariszözen et al. (23) was not exactly explained.
Uysal et al. (26) described that locally injected vit C had a negative impact on bone formation after expanding the inter-premaxillary suture. The authors explained that this could be due to an alteration in apoptotic regulation related to bone healing (26). However, the lack of further studies evaluating the local effect of vit C makes it impossible to know if this is an isolated phenomenon or if it is really due to this route of administration. Nevertheless, the authors observed that systemic administration of vit C obtained good results in terms histomorphometry of bone formation (26).
A minimum intake of 75 mg/day for adult women and 90 mg/day for adult men of vit C has been recommended in The United States of America and Canada (4). Additionally, an increase of this intake values is recommended in 15 mg/day for pregnant women, 50 mg/day if lactating, and 35 mg/day for smokers (4).
Smokers are constantly exposed to a source of prooxidant substances and reactive oxygen species that lead to an increased requirement of vit C (3). However, taking into account that nicotine also has a negative effect on osteoblastic proliferation (31) and that the vit C requirement is higher in smokers (2,4), we believe that vit C supplementation should be indicated in these patients, especially when they undergo bone regeneration procedures of the jaws or after traumatic injuries. In our review, two studies (1,29) induced the formation of free radicals and reactive oxygen species by administration of zymosan and nicotine. The authors observed that vit C acts as an antioxidant by scavenging these free radicals (1-3), which explains the good bone healing reported in these studies. In this line, Tomofujiet al. (32) evaluated Wistar rats fed a cholesterol-rich diet and demonstrated that this type of diet can initiate and increase bone loss around the teeth.However, it seems that, as demonstrated in the studies of Sanbeet al. (24,25), vit C prevents the negative effects produced by this type of diet.This is mainly due to the inhibition of lipid peroxidation and an increase in osteoblastic proliferation and differentiation genes, as well as a decrease in osteoclastic prolifer- ation and differentiation genes.
Regarding human studies, Ekrol et al. (11) did not observe an improved bone healing due to vit C administration, however, they pointed out that the benefits of vit C administration may only be observable in vit C-deficient populations On the other hand, Li et al. (20) suggested that vit C improves surgical wound healing after dental implant placement, but the study did not evaluate the effect of vit C on bone regeneration by radiography or histology.
The scientific literature describes some adverse effects associated with vit C supplementation, such as diarrhea and abdominal pain, with high dose in a single administration. Hyperuricosuria has been described in vit C concentrations higher than 3 g, hyperoxaluria in concentrations higher than 1 g, hyperoxalemia in patients treated with hemodialysis when administered intravenously repeatedly in doses of 1 g and hemolysis in patients with phosphate-6-glucose dehydrogenase deficiency administered intravenously or orally when the concentration is higher than 6 g in a single dose (33). In our review, none of the selected studies reported any of the adverse events mentioned above.
Regarding the use of other vitamins to aid bone formation and healing, vitamins D and E have also been studied. Carinci et al. (34) studied vitamins C and E to evaluate their effect on preosteoblast gene expression. Vitamin E showed no effect, whereas vitamin C modified preosteoblast genes by increasing cell growth, metabolism, morphogenesis and cell communication. Similarly, one of the studies included in this review used vit E on bone healing (23), and showed no additional benefit, not even associated with vit C. On the other hand, the use of vit D supplementation can be useful in patients with osteoporosis as vit D has a crucial role on bone mineralization. In fact, vit D deficit has been associated to a worse dental implant osteointegration and an increased risk of early implant failure (35). However, as with vit C, further studies are still required to confirm the clinical effect of their oral supplementation.
Finally, there are several limitations related to the present systematic review that should be mentioned. First, most of the included studies were animal studies which may represent a problem in the external validation of their results. Furthermore, only two RCTs with low quality related to vit C administration could be included. Another limitation was the heterogeneity found among the selected articles, which prevents us from comparing the results obtained with respect to bone defect, different bone regeneration techniques and populations with higher vit C requirements (elderly, diabetics and smokers). To overcome these limitations, it is necessary to perform well-designed RCTs to determine whether vit C supplementation in standardized models of bone defects implies any benefit in the speed of healing or in the quality of bone obtained in patients without dietary vit C deficiency. An experimental animal model with a cranial bone defect of critical size could be useful to compare various groups (test and control), in terms of histomorphometry, at different time points and vitamin C doses. The defect proposed by Higuchy et al. (36) or by Kustro et al.(37) or especially by Liu et al. (38) because of its dimension, will help to confirm the null hypothesis of the present work.
There is enough evidence that vitamin C has good results at the in vitro level regarding osteoblast differentiation and maturation and, it is possible to think that it could be a very easy way to improve bone and soft tissue healing conditions, without increasing morbidity and cost of a treatment.
Bone tissue regeneration and dental implant placement are increasing, especially among elderly patients. If our hypothesis is confirmed, recommending vit C supplementation during the bone healing period could be an effective, inexpensive and easy-to-implement treatment. These supplements could be especially useful in smoking patients, and hypercholesterolemic patients due to the requirement of antioxidants.
The techniques used in oral and implant surgery are constantly improving using different materials, some of them experimental or expensive, and it seems that there is no room for simplicity and, in certain cases, less could be more.
Conclusions
Although additional well-performed animal and human studies are required, vitamin C seems to accelerate bone regeneration without adverse events. However, it is not possible to recommend a specific dose or route of administration of vitamin C to improve the bone healing process in humans as there was great heterogeneity among the included studies.
Acknowledgments
Ackcnowledgements This study was carried out by the research group “Odontologic and Maxillofacial Pathology and Therapeutics” of the Biomedical Investigation Institute of Bellvitge (IDIBELL).
Authors contributions Kevin Barrios-Garay and Jorge Toledano-Serrabona: Acquisition, analysis and interpretation of the data, drafting of the article; approval of the final version of the manuscript and agreement to be accountable for all aspects of the work. Cosme Gay-Escoda: Drafting of the article; critical review of the manuscript; approval of the final version of the manuscript and agreement to be accountable for all aspects of the work. Mª Ángeles Sánchez-Garcés: Conception and design of the study; interpretation of the data; drafting of the article; approval of the final version of the manuscript and agreement to be accountable for all aspects of the work.
Conflicts of interest The authors declare that they do not have any conflict of interest.
Funding This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1.Aghajanian P, Hall S, Wongworawat M, Mohan S. The roles and mechanisms of actions of vitamin C in bone: New developments. J Bone Miner Res. 2015;30:1945–55. doi: 10.1002/jbmr.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duygulu F, Yakan B, Karaoglu S, Kutlubay R, Karahan OI, Ozturk A. The effect of zymosan and the protective effect of various antioxidants on fracture healing in rats. Arch Orthop Trauma Surg. 2007;127:493–501. doi: 10.1007/s00402-007-0395-7. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Kim JS, Shin HS, Keen CL. Influence of smoking on markers of oxidative stress and serum mineral concentrations in teenage girls in Korea. Nutrition. 2003;19:240–3. doi: 10.1016/s0899-9007(02)01002-x. [DOI] [PubMed] [Google Scholar]
- 4.Buettner GR. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-yocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz RI. Procollagen secretion meets the minimum requirements for the rate-controlling step in the ascorbate induction of procollagen synthesis. J Biol Chem. 1985;260:3045–9. [PubMed] [Google Scholar]
- 6.Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T, Kivirikko KI. Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris: Formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J. 1997;16:6702–12. doi: 10.1093/emboj/16.22.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007;6:1675–81. doi: 10.4161/cc.6.14.4474. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–46. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 9.Urban K, Höhling HJ, Lüttenberg B, Szuwart T, Plate U. An in vitro study of osteoblast vitality influenced by the vitamins C and E. Head Face Med. 2012;8:1–10. doi: 10.1186/1746-160X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1–25. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekrol I, Duckworth AD, Ralston SH, Court-Brown CM, McQueen MM. The influence of vitamin C on the outcome of distal radial fractures. J Bone Joint Surg Am. 2014;96:1451–9. doi: 10.2106/JBJS.M.00268. [DOI] [PubMed] [Google Scholar]
- 12.Moores J. Vitamin C: A wound healing perspective. Br J Community Nurs. 2013;S6:S8–11. doi: 10.12968/bjcn.2013.18.sup12.s6. [DOI] [PubMed] [Google Scholar]
- 13.Benic GI, Hämmerle CHF. Horizontal bone augmentation by means of guided bone regeneration. Periodontol 2000. 2014;66:13–40. doi: 10.1111/prd.12039. [DOI] [PubMed] [Google Scholar]
- 14.Donos N, Dereka X, Mardas N. Experimental models for guided bone regeneration in healthy and medically compromised conditions. Periodontol 2000. 2015;68:99–121. doi: 10.1111/prd.12077. [DOI] [PubMed] [Google Scholar]
- 15.Thrivikraman G, Athirasala A, Twohig C, Boda K, Bertassoni L. Biomaterials for craniofacial bone Regeneration. Dent Clin North Am. 2018;61:1835–56. doi: 10.1016/j.cden.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:1–12. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Tang L, Lin YF, Xie GF. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin Implant Dent Relat Res. 2018;20:793–8. doi: 10.1111/cid.12647. [DOI] [PubMed] [Google Scholar]
- 21.Rowe DJ, Ko S, Tom XM, Silverstein SJ, Richards DW. Enhanced production of mineralized nodules and collagenous proteins in vitro by calcium ascorbate supplemented with vitamin C metabolites. J Periodontol. 1999;70:992–9. doi: 10.1902/jop.1999.70.9.992. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121:426–8. doi: 10.1007/s004020100272. [DOI] [PubMed] [Google Scholar]
- 23.Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30:309–13. doi: 10.1177/147323000203000312. [DOI] [PubMed] [Google Scholar]
- 24.Sanbe T, Tomofuji T, Ekuni D, Azuma T, Tamaki N, Yamamoto T. Oral administration of vitamin C prevents alveolar bone resorption induced by high dietary cholesterol in rats. J Periodontol. 2007;78:2165–70. doi: 10.1902/jop.2007.070181. [DOI] [PubMed] [Google Scholar]
- 25.Sanbe T, Tomofuji T, Ekuni D, Azuma T, Irie K, Tamaki N. Vitamin C intake inhibits serum lipid peroxidation and osteoclast differentiation on alveolar bone in rats fed on a high-cholesterol diet. Arch Oral Biol. 2009;54:235–40. doi: 10.1016/j.archoralbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Uysal T, Amasyali M, Olmez H, Enhos S, Karslioglu Y, Gunhan O. Effect of vitamin C on bone formation in the expanded inter-premaxillary suture. Early bone changes. J Orofac Orthop. 2011;72:290–300. doi: 10.1007/s00056-011-0034-3. [DOI] [PubMed] [Google Scholar]
- 27.Giordano V, Albuquerque RP, do Amaral NP, Chame CC, de Souza F, Apfel MIR. Supplementary vitamin C does not accelerate bone healing in a rat tibia fracture model. Acta Ortop Bras. 2012;20:10–2. doi: 10.1590/S1413-78522012000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HK, Kim G J, Yoo H S, Song DH, Chung K H, Lee K J. Vitamin C activates osteoblastogenesis and inhibits osteoclastogenesis via Wnt/β-catenin/ATF4 signaling pathways. Nutrients. 2019;11:1–20. doi: 10.3390/nu11030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akça EK, Atalay B, Öner B. Histopathologic and immunohistochemical investigation of the effects of vitamin C on bone healing in rats exposed to nicotine. J Oral Maxillofac Surg. 2020;78:194.e1–14. doi: 10.1016/j.joms.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Gabbay KH, Bohren KM, Morello R, Bertin T, Liu J, Vogel P. Ascorbate synthesis pathway: Dual role of ascorbate in bone homeostasis. J Biol Chem. 2010;285:19510–20. doi: 10.1074/jbc.M110.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HH, Lee SE, Chung WJ, Choi Y, Kwack K, Kim SW. Stabilization of hypoxia-inducible factor-1α is involved in the hypoxic stimuli-induced expression of vascular endothelial growth factor in osteoblastic cells. Cytokine. 2002;17:14–27. doi: 10.1006/cyto.2001.0985. [DOI] [PubMed] [Google Scholar]
- 32.Tomofuji T, Kusano H, Azuma T, Ekuni D, Yamamoto T, Watanabe T. Effects of a high-cholesterol diet on cell behavior in rat periodontitis. Practitioner. 2005;249:752–6. doi: 10.1177/154405910508400813. [DOI] [PubMed] [Google Scholar]
- 33.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. J Am Med Assoc. 1999;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 34.Carinci F, Pezzetti F, Spina AM, Palmieri A, Laino G, De Rosa A. Effect of vitamin C on pre-osteoblast gene expression. Arch Oral Biol. 2005;50:481–96. doi: 10.1016/j.archoralbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Nastri L, Moretti A, Migliaccio S, Paoletta M, Annunziata M, Liguori S. Do dietary supplements and nutraceuticals have effects on dental implant osseointegration? A scoping review. Nutrients. 2020;12:268. doi: 10.3390/nu12010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higuchi T, Kinoshita A, Takahashi K, Oda S, Ishikawa I. Bone Regeneration by Recombinant Human Bone Morphogenetic Protein-2 in Rat Mandibular Defects. An Experimental Model of Defect Filling. J Periodontol. 1999;70:1026–31. doi: 10.1902/jop.1999.70.9.1026. [DOI] [PubMed] [Google Scholar]
- 37.Kustro T, Kiss T, Chernohorskyi D, Chepurnyi Y, Helyes Z, Kopchak A. Quantification of the mandibular defect healing by micro-CT morphometric analysis in rats. J Cranio-Maxillofacial Surg. 2018;46:2203–13. doi: 10.1016/j.jcms.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Guo Y, Zhang L, Wang X, Liu R, Huang P. A standardized rat burr hole defect model to study maxillofacial bone regeneration. Acta Biomater. 2019;86:450–64. doi: 10.1016/j.actbio.2018.12.049. [DOI] [PubMed] [Google Scholar]