Summary
Sarcoplasmic reticulum (SR) is a specialized tubular network, which not only maintains the intracellular concentration of Ca2+ at a low level but is also known to release and accumulate Ca2+ for the occurrence of cardiac contraction and relaxation, respectively. This subcellular organelle is composed of several phospholipids and different Ca2+-cycling, Ca2+-binding and regulatory proteins, which work in a coordinated manner to determine its function in cardiomyocytes. Some of the major proteins in the cardiac SR membrane include Ca2+-pump ATPase (SERCA2), Ca2+-release protein (ryanodine receptor), calsequestrin (Ca2+-binding protein) and phospholamban (regulatory protein). The phosphorylation of SR Ca2+-cycling proteins by protein kinase A or Ca2+-calmodulin kinase (directly or indirectly) has been demonstrated to augment SR Ca2+-release and Ca2+-uptake activities and promote cardiac contraction and relaxation functions. The activation of phospholipases and proteases as well as changes in different gene expressions under different pathological conditions have been shown to alter the SR composition and produce Ca2+-handling abnormalities in cardiomyocytes for the development of cardiac dysfunction. The post-translational modifications of SR Ca2+-cycling proteins by processes such as oxidation, nitrosylation, glycosylation, lipidation, acetylation, sumoylation, and O-GlcNacylation have also been reported to affect the SR Ca2+-release and uptake activities as well as cardiac contractile activity. The SR function in the heart is also influenced in association with changes in cardiac performance by several hormones including thyroid hormones and adiponectin as well as by exercise-training. On the basis of such observations, it is suggested that both Ca2+-cycling and regulatory proteins in the SR membranes are intimately involved in determining the status of cardiac function and are thus excellent targets for drug development for the treatment of heart disease.
Keywords: Cardiac sarcoplasmic reticulum, Ca2+-pump ATPase, Ca2+-release protein, Ca2+-cycling proteins, Cardiac phospholamban, Ca2+-regulatory proteins
Introduction
Since the observations of Sydney Ringer describing the involvement of Ca2+ in cardiac contraction, the role of Ca2+ in heart function, metabolism and structure has been a subject of extensive investigations (Ringer 1883, Nayler 1963, Ebashi and Endo 1968, Langer 1968, Katz 1970, Carafoli 1973, Fabiato and Fabiato 1979, Ebashi 1976, Dhalla et al. 1982, Dhalla et al. 1984, Eisner et al. 2000, Bers 2002, Eisner at al. 2017, Mackrill and Shiels 2020, Marty and Faure 2016, Synetos et al. 2016). It is now clear that not only the extracellular Ca2+ is required for maintaining the integrity of myocardial cell membrane but a small amount of Ca2+-influx into cardiomyocytes is also essential for the occurrence of cardiac contraction. Various subcellular organelles such as sarcoplasmic reticulum (SR), sarcolemma, mitochondria and nucleus have been shown to maintain the intracellular concentration of Ca2+ at a low level (Lehninger et al. 1967, Haugaard et al. 1969, Dhalla et al. 1970, Dhalla et al. 1977, Reddish et al. 2017, Santulli et al. 2015, Primeau et al. 2018, Stammers et al. 2015, Zhihao et al. 2020). Raising and lowering the concentration of intracellular Ca2+ upon depolarization and repolarization of cardiomyocyte have been demonstrated to be associated with cardiac contraction and relaxation processes, respectively. Furthermore, instability of Ca2+-handling mechanisms in cardiomyocyte has been linked to the pathogenesis of cardiac arrhythmias (Ter Keurs and Boyden 2007, Landstrom et al. 2017, Greiser 2017, Dobrev and Wehrens 2017) whereas the occurrence of Ca2+-overload has been reported to produce myocardial cell damage and cardiac dysfunction (Zimmerman and Hulsmann 1966, Dhalla 1976, Alto and Dhalla 1979, Santulli et al. 2015). In fact, it has become evident that abnormalities in intracellular Ca2+ handling are involved in the development of impaired heart function.
By virtue of its ability to release and accumulate Ca2+, SR is to known to be intimately associated with cardiac and skeletal muscle contraction and relaxation processes (Inesi 1972, Martonosi 1972, MacLennan and Holland 1975, Dhalla et al. 1982). It is noteworthy that the function of SR for accumulating Ca2+ in an energy-dependent manner was discovered in skeletal muscle about sixty years ago (Hasselbach and Makinose 1962, Ebashi and Lipmann 1962, Hasselbach 1964, Ebashi and Ebashi 1962) whereas electrophysiological, biophysical and biochemical studies have provided evidence for Ca2+-release from SR (Lee et al. 1966, Fabiato 1983, Dhalla et al. 1983, Nabauer et al. 1989, Bers 2004, Santulli et al. 2017b, Guerrero-Hernandez et al. 2020). The involvement of SR Ca2+-transport in muscular contraction is evident from the observations that the activity of SR for Ca2+-handling in skeletal muscle is much greater than that in cardiac or smooth muscles (Adachi 2010, Frank et al. 2003, MacLennan and Holland 1975, Yoshida et al. 2005, Ganguly et al. 1986, Ganguly et al. 1983). Furthermore, the SR Ca2+-transport activity in the left ventricle is higher than that in the right ventricle (Afzal and Dhalla 1992, Dhalla et al. 1980). It should be mentioned that several observations from different animals have revealed that both heart function and cardiac SR activities are species-dependent (Lüss et al. 1999, Dhalla et al. 1980, Afzal and Dhalla 1992, Singal et al. 1986, Sulakhe and Dhalla 1971, Dhalla et al. 1984, Heyliger et al. 1985). Depressed cardiac function due to aging and myocardial infarction has also been shown to be associated with decreased SR Ca2+-transport activity (Knyushko et al. 2005, Jahng et al. 2015, Dhalla et al. 2012, Dhalla et al. 2009). Thus changes in SR Ca2+-release and Ca2+-uptake activities can be seen to play a critical role in determining the status of cardiac performance in health and disease.
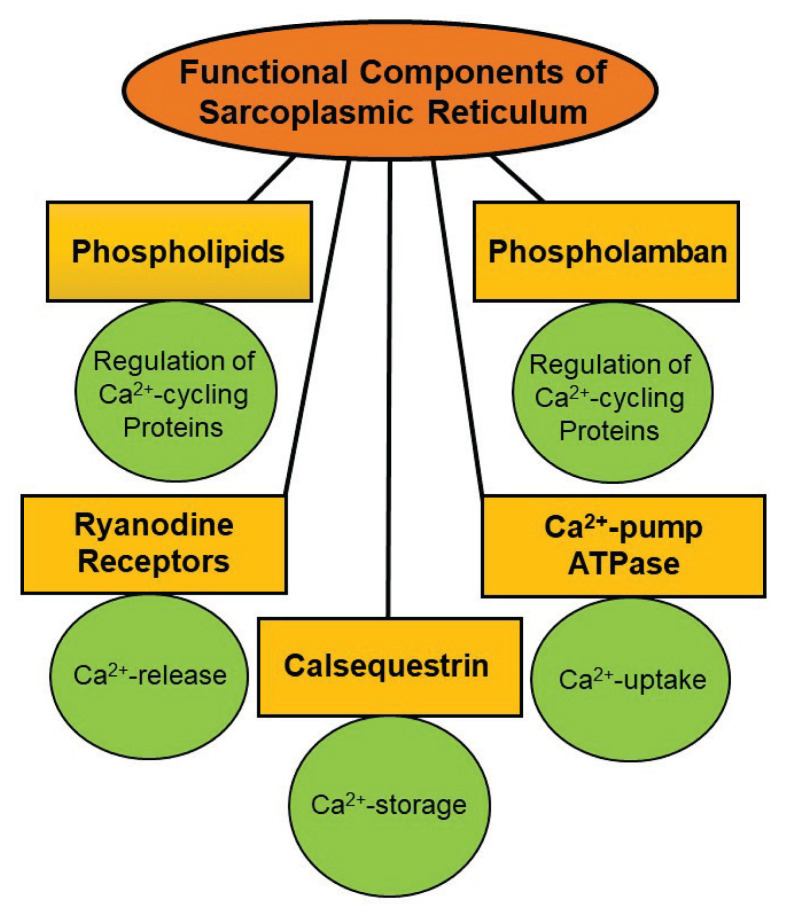
Several proteins and phospholipids have been identified as structural components of the SR membrane (Martonosi et al. 1968, Martonosi and Halpin 1971, MacLennan et al. 1973, MacLennan et al. 1974, MacLennan 1970). Some of the major components in the SR membrane and their functions in the myocardium are shown in Figure 1. It was demonstrated that SR Ca2+-transport activities are regulated by different mechanisms involving several endogenous proteins and phospholipids as well as protein kinases in the cytoplasm (Martonosi et al. 1971, Katz et al. 1975, Tada et al. 1978, LePeuch et al. 1979, Netticadan et al. 1999). This review is intended to provide an updated synthesis of the existing literature regarding structure-function of different major protein components of the cardiac SR membrane with particular focus on their relationship with SR Ca2+-pump system. The regulation of cardiac SR Ca2+-transport system by some protein kinases will be described to emphasize the role of protein phosphorylation in modulating cardiac contractile function. Various mechanisms which are known to modify the function of some SR proteins for Ca2+-transport activities will be discussed to show the involvement of Ca2+-handling abnormalities in cardiac dysfunction in heart disease. In addition, the influence of some hormones such as thyroid hormones and adiponectin as well as exercise on cardiac SR activities will be outlined to gain further information regarding structure-function relationships with Ca2+-cycling and regulatory proteins. Since there are several similarities between cardiac muscle and skeletal muscle SR organelles with respect to their functional and biochemical aspects, some of the work for structural components of the skeletal muscle SR has also been included in this review.
Fig. 1.
Some of the major components of the sarcoplasmic reticulum and their functions in cardiomyocytes.
Structural and functional aspects of SR
The SR is a tabular network in both skeletal and cardiac muscles. It is a specialized form of the smooth muscle endoplasmic reticulum (ER), which is responsible for the maintenance of intracellular Ca2+ homeostasis as well as Ca2+ storage (Sommer 1982, Altshuler et al. 2012, Prins and Michalak 2011, Rossi et al. 2008, Doroudgar and Glembotski 2013, Lam and Galione 2013). From the structural point of view, SR is divided into two well-characterized regions: the terminal cisternae, from where Ca2+ ions are released, and the longitudinal tubules, which accumulates Ca2+ ions (Beard et al. 2004). Cardiac and skeletal muscle cells also contain transverse tubules (T-tubules), which are the extensions of the cell membrane and are closely associated with the terminal cisternae, the primary site of Ca2+ storage and release (Hong and Shaw 2017). The longitudinal SR (LSR) are thinner projections, that run between the terminal cisternae and junctional SR (JSR), and are the location for Ca2+ ion accumulation. The LSR tubules envelop the myofibrils and the dyadic junctions between terminal cisternae and t-tubules have a specific location at the Z-lines whereas terminal cisternae are a specific form of JSR. Structural variability of dyads has been reported to relate with Ca2+-release in cardiomyocyte (Novotova et al. 2020). It is pointed out that a small amount of Ca2+ entering upon depolarization of cardiomyocytes has been shown to be sufficient for opening Ca2+-sensitive channels in the SR and causing a marked release of Ca2+ into the cytoplasm. This Ca2+ then binds with troponin and promotes the interaction of actin and myosin for the occurrence of myofibril contraction. On the other hand, repolarization of cardiomyocyte is associated with lowering the intracellular concentration of Ca2+, mainly due to accumulation in the SR and this then results in myofibril relaxation. These events in cardiac excitation, contraction and relaxation processes involving the SR Ca2+-release and uptake have been described elsewhere (Dhalla et al. 1982, Berridge et al. 2003, Eisner et al. 2017, Bovo et al. 2019, Mckillop and Geeves 1991, McKillop and Geeves 1993, Jones et al. 1998). It is pointed out that we have described the following 13 SR proteins, which are involved in SR Ca2+-release, Ca2+-uptake, Ca2+-binding and regulation of Ca2+-cycling. Since some of these proteins play multiple roles, their description has not followed any specific order:
Sarcoplasmic reticulum ATPase (SERCA, SR Ca2+-pump ATPase) plays a major role in Ca2+ signalling (Jiao et al. 2009), and is involved in various aspects of cell function (Clapham et al. 2007), such as transcription (Flavell and Greenberg 2008), apoptosis, exocytosis, signal transduction (Dodd et al. 2010), and cell motility (Qi et al. 2007). SERCA is responsible for the movement of Ca2+ against concentration gradient between the SR and the cytosol. There are three different genes coding for 3 SERCA isoforms, which are spliced alternatively into 11 variants (SERCA1a–1b, −2a–2c, and −3a–3f) (Altshuler et al. 2012; Brandl et al. 1987). It has been demonstrated that SR contains Ca2+-pump ATPases in its membrane that are responsible for pumping Ca2+ ions into the tubular network as these are required because Ca2+ ions from the cytoplasm cannot passively pass into the SR (Voss et al. 1994). These Ca2+-pumps have several forms with SERCA2a mainly found in cardiac and skeletal muscle (Martonosi 1996, Satoh et al. 2011, Lamboley et al. 2013). It may be noted that SERCA is composed of 13 subunits, M1–M10, A, P and N; the sM1–M10 subunits are located in the SR membrane and are responsible for binding Ca2+ ions whereas the A, P and N subunits are located on the outer surface of the membrane and are responsible for ATP binding (Primeau et al. 2018). Smejtek and coworkers (2014) have reported that SR is considered as a complex biomembrane due to the presence of Ca2+-ATPase in the APN domain. Protein components of the LSR and terminal cisternae are quite different, representing the functional specialization of these membrane elements, which are Ca2+-uptake and release, respectively; the major component of the LSR, is SERCA which is responsible for pumping the released Ca2+ back into the SR (Van Petegem 2012).
When two Ca2+ ions and one molecule of ATP bind to the cytosolic side of the Ca2+-pump ATPase, the pump opens because ATP hydrolyses releases a phosphate group. Consequently, the released phosphate group then binds to the pump, causing a conformational change, allowing the cytosolic side of the pump to open and permitting two Ca2+ ions to enter. The cytosolic side of the pump then closes and releasing Ca2+ ions into the SR (Toyoshima and Nomura 2002, Le Peuch et al. 1983). It has been shown that SERCA 2 gene knockout disrupted SR function and this disruption was associated with heart failure in mice (Bers 2002, Louch et al. 2010). Additionally, it has been reported that cardiomyocyte-specific gene knockout resulted in mice that remained alive for 10 weeks following the gene knockout (Louch et al. 2010, Andersson et al. 2009a). It was also observed that 4 weeks after the gene knockout, SERCA2 protein content was reduced to <5%, without any changes in cardiac function, and it was only after 7 weeks that these mice started developing severe cardiac dysfunction (Andersson et al. 2009b). Accordingly, some defect in SERCA2 was suggested to result in the development of cardiac contractile dysfunction.
Ryanodine receptor (RyR) is another major protein in the SR membrane and is responsible for the release of Ca2+ from the intracellular stores during excitation-contraction (Wehrens and Marks 2003). This protein is the largest known ion channel, which exists in three isoforms (RyR 1, 2 and 3) (Lanner et al. 2010). It consist of four identical subunits, which interact with and are regulated by phosphorylation, redox modifications, and other various small proteins (Meissner 2010). It has been shown that complete absence of RyR2 in cardiomyocytes of knockout mouse model is lethal due to the lack of SR Ca2+ release (Kushnir et al. 2010). Mutations in RyR2, the predominant form in the heart muscle, are associated with human disorders such as catecholaminergic polymorphic ventricular tachycardia while mutations in RyR1 underlie diseases such as central core disease and malignant hyperthermia (Priori et al. 2002, Nakai et al. 1990, Valdivia 2007). Thus SR Ca2+-release is not only known to play a critical role in inducing cardiac contraction but alterations in its signaling and function are also considered to be involved in the genesis of cardiac arrhythmias and fibrillation (Landstrom et al. 2017, Greiser 2017, Debrev and Wehrens 2017).
The structure-function relationships for SR Ca2+-release channels (both RyR1 and RyR2) under a wide variety of physiological and pathological situations have been described recently (Santulli et al. 2017b, Santulli et al. 2018, Meissner 2017, Sheard et al. 2019). While RyR1 has been reported to be associated with intracellular Ca2+-leak and induction of cardiomyopathy (Chen and Kudryashev 2020), RyR2 is mainly concerned with Ca2+-release for the generation of cardiac contractile force (Lascano et al. 2017). The mechanisms for opening the RyR and its functional role in cardiac excitation-contraction coupling have also been discussed elsewhere (Santulli et al. 2017a, Van Petegem 2016). The sensitivity of RyR has been shown to govern the stability and synchrony of Ca2+-release during the process of excitation-contraction coupling in the heart (Wescott et al. 2016). Several investigators have studied the molecular and cellular control for the regulation of SR Ca2+-release channels. In this regard, it is pointed out that SR Ca2+-release is not only regulated by phosphorylation and dephosphorylation (Yamaguchi 2020, Terentyev and Hamilton 2016) but is also modulated by glycation and oxidation (Ruiz-Meana et al. 2019, Zima and Mazurek 2016). SR Ca2+-release is also controlled by SR luminal Ca2+ as well as protein-protein interaction (Jones et al. 2017, Rani et al. 2016, Seidel et al. 2015) and its magnitude is dependent upon the RyR cluster size (Galice et al. 2018). Different recent studies have revealed the role of RyR in the genesis of arrhythmias and fibrillation (Dridi et al. 2020, Campbell et al. 2020, Alsina et al. 2019). Excessive release of SR Ca2+ induced by inositol triphosphate has been demonstrated to produce arrhythmias (Blanch et al. 2018) whereas that induced by CAMKinase associated phosphorylation has been shown to produce cardiac dysfunction (Sepulveda et al. 2020, Sepulveda et al. 2017). Thus targeting RyR and associated pathological SR Ca2+-release has been considered to have a great impact for the treatment of arrhythmias and heart failure (Connell et al. 2020).
Phospholamban (PLB) is present in the LSR and it is involved in regulating the activity of the SR Ca2+-pump in the heart. Over the years, numerous investigators have demonstrated that PLB is an inhibitor protein for the SERCA2 function in the heart; the mechanism of inhibition is accomplished by PLB binding to SERCA, decreasing its affinity to Ca2+ and preventing Ca2+ uptake into the SR. Accordingly, failure to remove Ca2+ from the cytosol, prevents the cardiac relaxation and consequently decrease the muscle contraction (Simmerman and Jones 1998). Nevertheless, hormones such as adrenaline and noradrenaline can prevent PLB from inhibiting SERCA (Dhalla et al. 2019). When these hormones bind to the beta 1 adrenoceptor on the cell membrane, they increase the formation of cAMP, activate protein kinase A (PKA), phosphorylate PLB, prevent the inhibition of SERCA and thus promote cardiac muscle relaxation (Le Peuch et al. 1983, Moccia et al. 2019). Slack and coworkers (1997) showed that PLB is a key determinant of relaxation in slow-twitch skeletal muscle under basal conditions and during isoproterenol stimulation, supporting its important role in the regulation of SERCA activity. In fact, PLB knockout models have revealed that the ratio of PLB to SERCA2a may be a significant determinant of the regulation of the cardiac contraction–relaxation cycle (Frank et al. 2003, Periasamy et al. 1999).
Inositol-Trisphosphate Receptor (InsP3R) is a Ca2+ release channel found widely in the ERSR system in almost all cells. The function of InsP3R is to facilitate Ca2+ release from the intracellular Ca2+ stores upon binding of Inositol trisphosphate (IP3) and resulting in Ca2+ signals that control various physiological processes in the cell (Foskett et al. 2007). Furthermore, InsP3R is a Ca2+ selective cation channel that is regulated by cytoplasmic Ca2+ in addition to InsP3. Its interaction with other ER/SR proteins contribute to the specificity and speed of Ca2+ signaling pathways (Yamazaki et al. 2011, Zhou et al. 2014). It has been reported that genetic hypertension in rats is related to the increase in IP3 concentrations as well as InsP3R-IP3 binding affinity in smooth muscle cells (Narayanan et al. 2012). Although the presence of InsP3Rs has also been detected in cardiomyocyte SR membranes, extensive work needs to be carried out to establish their functional significance under both physiological and pathological conditions. Nonetheless, InsP3R has been reported to play some role in the interaction between SR and mitochondria (Dia et al. 2020).
Calsequestrin is another protein which is primarily located in the junctional SR and terminal cisternae, in close association with the Ca2+ release channel (Lamboley et al. 2013). This protein is capable of binding a large amount of Ca2+, reducing free Ca2+ in the SR and serving as a Ca2+-store in the SR; calsequestrin is thus, considered as a Ca2+ buffering system (Perni et al. 2013, Treves et al. 2009). Furthermore, Wang and coworkers (2019) reported that calsequestrin binds to the ER luminal domain of inositol-requiring enzyme 1 (IRE1α), inhibiting its dimerization and suppressing the activation of IRE1α at the junctional SR. By using a transgenic mice model, it has been reported that calsequestrin is both a storage and a Ca2+-signaling cascade regulatory protein in the myocardium (Jones et al. 1998). It should be mentioned that calsequestrin is considered to be a regulator of RyR2 activity and its malfunction either as a Ca2+-buffer or as a regular may lead to the genesis of arrhythmias. The structure-function of calsequestrin as well as its Ca2+-binding properties are also well described (Wang and Michalak 2020, Loescher et al. 2019, Woo et al. 2020).
Sarcalumenin (SAR) is a minor glycoprotein in the LSR membrane which is encoded by the SAR gene. and is partially responsible for Ca2+ buffering in the lumen of SR. Interestingly, alternative splicing of the same transcript results in two variants of SAR. The large transcript of SAR has a low affinity and high capacity Ca2+ binding protein whereas the shorter product lacks the Ca2+-binding domain. Because of the presence of SAR in close vicinity of SERCA protein, it is considered to be involved in the regulation of the SERCA activity (Leberer et al. 1990). Some studies with SAR knockout animal models have revealed that SAR plays a crucial role in the maintenance of cardiac function under physiological stresses, by regulating Ca2+ transport activity into the SR (Yoshida et al. 2005, Jiao et al. 2009).
Junctin and Junctate are integral membrane proteins of cardiac and skeletal muscle SR, with moderate affinity, high capacity for Ca2+ binding (Treves et al. 2000). It should be pointed out that aspartyl beta-hydroxylas, junctin and junctate are three different single gene products, generated by alternative splicing (Feriotto et al. 2005). Both junctin and junctate are involved in the regulation of intracellular Ca2+ concentrations (Delbono et al. 2007, Dinchuk et al. 2000, Ha et al. 2007). Furthermore, knockout and transgenic mice model studies have shed light on the function of these proteins; it has been suggested that altered expression of junctin or junctate can modify the cellular Ca2+ handling and disturb the balanced activity of other Ca2+ regulatory proteins (Treves et al. 2004). It should be mentioned that junctin is predominantly a RyR regulator whereas junctate in cardiomyocytes is connected with other membrane proteins such as InsP3R and SERCA (Kwon and Kim 2009, Stamboulian et al. 2005). Junctin together with triadin has also been shown to play a role in preventing arrhythmias (Wleklinski et al. 2020) However, the exact contribution of these Ca2+-storage site in the SR membrane remains to be established.
Nexilin (NEXN) is an essential component of the junctional membrane complex which is necessary for optimal Ca2+ transients and is required for initiation and formation of T-tubules (Hassel et al. 2009). Recently, NEXN has been shown to play a role in the maintenance of the tranverse-axial tubular system (Spinozzi et al. 2020). Even though, the exact role of NEXN in cardiac function and disease is not well understood, it has been identified as an actin-binding protein (Liu et al. 2019). Aherrahrou and coworkers (2016) have evaluated the functional role of NEXN by using a constitutive NEXN knockout mouse model, and have reported that the absence of NEXN may result in premature death of mice due to dilated cardiomyopathy.
Amphiphysin 2 and Mitsugumin29 (Mg 29) are proteins that play a major role in T-tubules formation; amphiphysin 2 induces membrane curvature and tubulations, which is similar to the sarcolemma T-tubules (Lee et al. 2002). Knockout of amphiphysin 2 is associated with abnormal T-tubules formation and excitation-contraction coupling defects (Razzaq et al. 2001). Since amphiphysin 2 has been shown to be involved in the formation of dyads, it can be seen to play a role in the excitation-contraction coupling (Guo et al. 2021, De La Mata et al. 2019). On the other hand, Mg29 is a structural protein that is thought to be exclusively expressed in skeletal muscle. Mg29 is a member of the synaptophysin family, which is involved in the fusion of synaptic vesicles with the cell membrane. Mg29 knockout animal model showed structural abnormalities in both the SR and T-tubules, suggesting that it plays a role in assembly and/or docking of such membrane systems (Komazaki et al. 2001). Mg29 interacts with transient receptor potential canonical 3. However, Mg29 mRNA is not expressed in mouse heart as it was untraceable by various immunoblotting techniques. Nonetheless, Mg29 mRNA is expressed in human heart at a very low level. Interestingly, it was shown that the gene expression of Mg29 is significantly upregulated in animal models of heart failure (Woo et al. 2015, Correll et al. 2017). Thus the exact functional role of both amphiphysin 2 and Mg 29 with respect to the structural integrity of SR membrane needs to be determined in future studies.
Junctophilins are a family of integral membrane proteins which provide a structural bridge between the sarcolemma and SR. These proteins are attached to the JSR by a transmembrane domain in the C-terminus and contact the plasma membrane through lipid-interacting motifs in their N-terminus (Takeshima et al. 2000). There are several junctophilin isoforms, with isoform 2 being expressed in the heart (Nishi et al. 2000, Munro et al. 2016). As was demonstrated in knockout animal models, ablation of junctophilin resulted in a less contractile force after electrical stimulation and showed abnormal sensitivity to extracellular Ca2+ and altered triad formation as well (Ito et al. 2001, Komazaki et al. 2002). Since the heterologous expression of junctophilins resulted in the development of junctional-like assemblies between the ER and the plasma membrane (Takeshima et al. 2000, Komazaki et al. 2002), junctophilins are considered to play an important role in triad organization and stabilization (Nakeda et al. 2018). It should be noted that the interaction of junctophilin-2 with L-type Ca2+-channel is important for dyad assembly and intracellular Ca2+ dynamics (Gross et al. 2021, Poulet et al. 2021).
TRIC-A/SRP-27 is a trimeric intracellular cation-selective channel (TRIC-A) or (SRP-27). This SR protein is expressed in excitable tissues, particularly in fast twitch skeletal muscles (Yazawa et al. 2007, Bleunven et al. 2008). It has been reported that its expression level peaks after 2 months of post-natal development (Zhou et al. 2007, Treves et al. 2009). Treves and coworkers (2009) have also reported that mice lacking TRIC/SRP-27 are viable and display no overt phenotype. In contrast, other investigators have observed that TRIC-A knockout mice have impaired ER/SR Ca2+ release in several cell types and developed hypertension (Yamazaki et al. 2011). Evidence has also been presented to suggest that TRIC channels mediate counter potassium movements to facilitate physiological Ca2+ release from intracellular Ca2+ stores and can be seen to provide a counter-current for SR/ER Ca2+ release; thus it may also function as accessory proteins that directly modulate the RyR/IP3 receptor channel functions (Zhou et al. 2014). Recent information on cardiac muscle has revealed the interaction of TRIC-A with RyR2 for handling and storage of Ca2+ in the SR tubules (Zhou et al. 2020, Zhou et al. 2021).
Regulation of SR Ca2+-ATPase protein
Due to its essential role in regulating Ca2+ handling and contractility of the heart muscle, the SERCA2a protein has been extensively investigated. The SERCA protein is embedded in the SR membrane and consists of three parts namely, a cytoplasmic face, transmembrane helices (that harbor Ca2+ binding sites), and luminal loops (Periasamy et al. 2007, Tupling et al. 2004, Periasamy et al. 2008). The cytoplasmic face of SERCA consists of a phosphorylation domain, a nucleotide domain and an actuator domain. Each domain plays a special role that governs the function of SERCA as a pump. While ATP hydrolysis takes place at the interface between the phosphorylation and the nucleotide domains, the actuator domain provides the hub for Ca2+ translocation (Stammers et al. 2015, Toyoshima and Inesi 2004). The importance of SERCA function attributes to its essential role in controlling and regulating the handling of Ca2+. ATP hydrolysis facilitates transfer of two Ca2+ molecules into the lumen of SR. Therefore, ATP hydrolysis is essential, because it provides the required energy for pumping Ca2+ against the concentration gradient, as a much higher concentration gradient is present across the SR membrane (Toyoshima 2009, Smith et al. 2013).
Regulation of SERCA function by gene transcription and alternative splicing
The gene which encodes the SERCA Ca2+-ATPase for catalyzing the hydrolysis of ATP associated with the translocation of Ca2+ from the cytosol to the SR lumen, is located on human chromosome 12 (Papp et al. 1993, Otsu et al. 1993). During gene expression, alternative splicing of the SERCA gene results in three different mRNAs species that encode for SERCA2a, SERCA2b, and SERCA2c proteins (Zarain-Herxberg 2006, Gelebert et al. 2003). These SERCA proteins are expressed in different body tissues at various rates; SERCA2a is expressed preferentially in cardiac muscle, SERCA2b is expressed in small quantities in muscle cells (Gelebert et al. 2003). The SERCA2c isoform is also expressed in cardiac myocytes, but it has a lower affinity for cytosolic Ca2+ than SERCA2a and the turnover rate is comparable to that of SERCA2b (Dode et al. 2003, Wuytack et al. 2002, Dally et al. 2006).
The transcription of ATP2a2 gene is regulated by several transcriptional factors that bind to the promotor region. Mitochondrial transcription factors A (TFAM) and B2 (TFB2M) were shown to regulate the transcription of the SERCA2 gene in the myocardium by binding to specific promoter regions (Fujino et al. 2012, Watanabe et al. 2011). It was demonstrated that the expression of mRNA for SERCA2a in the myocardium is correlated with the expression of both TFAM and TFB2M (Watanabe et al. 2011). The overexpression of TFAM and TFB2M was shown to increase the transcriptional activity by 2-folds, in addition to protecting against the stress related decrease in mRNA levels of SERCA2a. The importance of factors such as TFAM and TFB2M was found to be crucial in regulating the transcription of the SERCA gene, as the expression of TFAM was downregulated in the diabetic heart and cardiac failure due to myocardial infarction (Kang et al. 2007, Choi et al. 2001, Ikeuchi et al. 2005, Suarej et al. 2003). Furthermore, the Specificity Protein 1 (SP1), another transcriptional factor, was observed to play a role in decreasing the SERCA2a mRNA level due to pressure overload (Brady et al. 2003, Takizawa et al. 2003).
Role of phospholamban in the regulation of SERCA2a
In cardiac myocytes, SERCA2a activity is inhibited by the SR protein, phospholamban (PLN) (Asahi et al. 2003). PLN binds to SERCA2a when the cytosolic Ca2+ is low, causing a decrease in SERCA2a affinity for Ca2+ (Ha et al. 2007, Periasamy and Huke 2001, Periasamy et al. 2008). Nevertheless, when Ca2+ is high, the inhibitory effect is removed, due to the activation of Ca2+/calmodulin kinase (CaMKII), which phosphorylates PLN (Mattiazzi and Kranias 2011). PLN is similarly regulated by protein kinase A (PKA), which is activated due to β-adrenergic stimulation (Drummond and Severson 1979, MacLennan and Kranias 2003), which eventually phosphorylates PLN and prevents the inhibition of SERCA2a and thus augments cardiac muscle contraction and relaxation (MacLennan and Kranias 2003, Koss et al. 1998). The activity of SERCA2a has been reported to increase significantly when PLN is phosphorylated by either CAMKII or PKA; this change enhances the velocity of relaxation and contributes to the relaxant effects of high intramuscular Ca2+ and β-adrenergic stimulation as well (MacLennan and Kranias 2003, Koss et al. 1998). It is pointed out that the regulation of cardiac SERCA2a is accomplished by either increasing the gene expression of PLN protein or the phosphorylation of PLN. It has been demonstrated that the expression of PLN in murine ventricular myocytes is much higher than that in atrial myocytes (Koss et al. 1998) as well as PLN expression is significantly less in human right atrium when compared to that in the right ventricular (Lüss et al. 1999). Although, variations in the level of PLN expression can be seen to contribute to the increased contractile activity in isolated human right ventricule in comparison to right atrium, the exact reason for this difference is far from clear.
Role of sarcolipin in the regulation of SERCA2a
Sarcolipin (SLN) has been shown to attenuate Ca2+ sensitivity by binding to SERCA2a and inhibiting its activity in the myocardium by lowering its affinity for Ca2+ (Asahi et al. 2003, MacLennan and Kranias 2003). It has been suggested that SLN is an uncoupler of SERCA pump activity and increase ATP hydrolysis resulting in heat production (Shaikh et al. 2015). When SLN is phosphorylated by serine/threonine kinase 16 (STK16), it facilitates the relaxant effects of β-adrenergic stimulation by promoting the separation of SLN from SERCA2a (Babu et al. 2007, Gramolini et al. 2006). This observation was supported by studies on PLN knockout mice, overexpressing SLN. It was demonstrated that the inhibitory effect of SLN on SERCA was alleviated when β-adrenergic agonist was administered (Babu et al. 2007, Gramolini et al. 2006). Furthermore, SERCA2a inhibition by SLN was evident from the reduction of cell shortening in rat myocytes overexpressing SLN after adenoviral gene transfer (Asahi et al. 2004). On the other hand, it was reported that SLN mRNA is highly expressed in murine and human atrium but not in human ventricle (Babu et al. 2005, Minamisawa et al. 2003). Moreover, SLN expression is poorly regulated in patients suffering from cardiovascular disease (Odermatt et al. 1997). Interestingly, a substantial increase in the levels of both SLN mRNA and protein was observed in left ventricular samples obtained from patients following surgery for mitral valve regurgitation (Zheng et al. 2014). Such evidence may indicate that SLN offers further control over Ca2+ handling in human myocytes, mainly in areas like the atrium where PLN is not present and in settings where Ca2+ concentrations are elevated (Sahoo et al. 2013).
Role of thyroid hormone in the regulation of SERCA2a
Thyroid hormone plays a critical role in the regulation of SERCA2a gene expression in the heart (Nagai et al. 1989, Ojamaa et al. 2000). It has been shown that administration of thyroid hormone causes significant increase in the mRNA levels in cardiomyocytes of experimental animals (Rohrer and Dillmann 1988, Arai et al. 1991, Kinugawa et al. 2001) which, results in accelerated uptake of Ca2+ into the SR, enhanced relaxation time, and improved force production in the heart. It may be noted that the mRNA levels for SR Ca2+-pump ATPase and RyR2 as well as for myosin heavy chain (β-MHC) were increased in hearts from hypertrophied animals. On the other hand, it has been reported that there occurs a decrease in the mRNA of levels encoding SERCA2a and RyR2 as well as for α-MHC in hearts of hypothyroid animal models (Rohrer and Dillmann 1988, Kinugawa et al. 2001, Ji et al. 2000, Reed et al. 2000). A substantial increase in both mRNA and protein levels for SERCA2a was reported in hearts of hyperthyroid animals (Kinuzawa et al. 2001, Kiss et al. 1994), and a marked decrease in hearts of hypothyroid animals (Kiss et al. 1994). Such an effect of thyroid hormone on SERCA2a in hearts has been shown to be the result of transcriptional regulation, which is facilitated by distinct thyroid hormone-response elements (Hartong et al. 1994, Zarain-Herzberg et al. 1994). Additionally, the thyroid hormone, triiodothyronine, is involved in the regulation of PLN (Kiss et al. 1994). It has been demonstrated in experimental animal models under conditions of hyperthyroidism and hypothyroidism that the levels of PLN mRNA and protein are markedly reduced and increased, respectively (Kimura et al. 1994, Chang et al. 1997). Moreover, such change in the expression of PLN was reported to boost the effects of thyroid hormone on the functionality of SERCA2a, enhancing the uptake of Ca2+ in hyperthyroidism and reducing cardiac performance in chronic hypothyroidism (Kimura et al. 1994, Chang et al. 1997).
Role of adiponectin in the regulation of SERCA2a
Adiponectin is a hormone peptide of adipocytes (Ahima 2006), the reduction of which plays an essential role in obesity-related cardiovascular disorders (Fortuño et al. 2003, Hug and Lodish 2005). Besides its function as an antioxidant and cardioprotective agent, it is also involved in the regulation of SERCA2a function (Pischon et al. 2004, Zhang et al. 2013, Shibata et al. 2012, Villarreal-Molina and Antuna-Puente 2012). It was suggested that the cardio-protection effect of adiponectin occurs by alleviating the stress of endoplasmic reticulum, thus enabling the recovery of SERCA2a function (Zhang et al. 2013). It has been shown in animal models of myocardial ischemia/reperfusion and cardiomyocyte hypoxia/reoxygenation that intravenous administration of adiponectin causes restoration of the function of SERCA2a (Guo et al. 2013). Moreover, a substantial increase in the levels of mRNA of SERCA2a in H9C2 cells was demonstrated when cardiomyocytes were cultured in an adiponectin-enriched medium for 60 minutes in comparison to those in adiponectin-depleted medium (Jahng et al. 2015, Boddu et al. 2014). While the exact mechanism of activation of SERCA2a by adiponectin is not known, it could be due to the enhancement of PLN phosphorylation, thus relieving its inhibition of SERCA2a. In fact, adiponectin administration in rats has been reported to increase PLN phosphorylation in the left ventricle (Guo et al. 2013). Therefore, the increase in PLN phosphorylation alleviates inhibition and increases Ca2+ sequestering action of the SERCA. Furthermore, it is believed that cardioprotective effect of adiponectin is achieved through the signaling of the regulatory enzyme of energy homeostasis, adenosine monophosphate-activated protein kinase (AMPK). It should be noted that AMPK is responsible for the metabolic regulation of adiponectin (Gonon et al. 2008), which has been shown to alter SERCA1a protein levels and SERCA2a mRNA in mice muscle (Morissette et al. 2014).
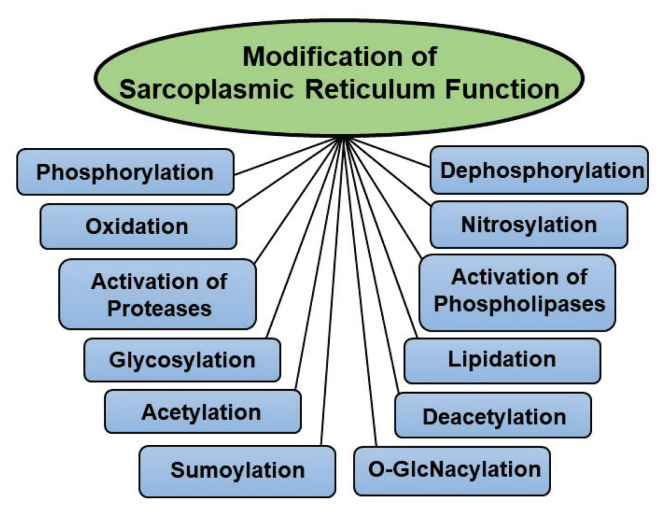
Post-translational modifications of SERCA2a
In addition to PLN and SLN phosphorylations, which regulate SERCA2a function, numerous posttranslational events are known to participate in the modification of SR protein function. Although both SERCA2a and RyR2 are modified by different posttranslational events by similar mechanism, only these affecting the SERCA2a function are given in Figure 2. These events include different processes such as nitrosylation, acetylation, sumoylation, glycosylation, O-GlcNAcylation, and glutathionylation. It is pointed out that nitrosylation or nitration is the addition of a nitro group to proteins, which has been reported to increase due to aging (Knyushko et al. 2005). The aging hearts were found to reveal much higher levels of nitrotyrosine in comparison to control hearts (Knyushko et al. 2005). Furthermore, nitrotyrosine levels in SR were almost 2 folds higher in the ischemic hearts perfused with high glucose than those perfused with normal levels of glucose (Tang et al. 2010). It has been reported that nitration of SERCA2a and RyR proteins occurs through polyol pathway, which plays a central role in oxidative stress due to hyperglycemia as seen in diabetes (Tang et al. 2010, Arun and Nalini, 2002). A significant negative correlation was found between SERCA2a as well as RyR tyrosine nitration and maximal SR acitvities. Although nitration of the SERCA2a was confirmed in experimental animal models of chronic disease, its role in modifying the activity of SERCA2a in humans is poorly understood. Nonetheless, it is believed that nitration of certain tyrosine residues may cause distortion in helical interactions and thus restricts the coordinated movement of membrane helices, which is required for optimal SERCA function (Kynyushko et al. 2005).
Fig. 2.
Modification of cardiac sarcoplasmic reticulum function by some post-translational events.
Sumoylation of SERCA2a is achieved by binding of a small ubiquitin-related modifier type 1 protein, SUMO1 to SERCA2a lysine residue (Kho et al. 2015). A significant decrease in SUMO1 in heart failure, has been reported to accompany a decrease in SERCA2a sumoylation (Kho et al. 2015). A SUMO1 injection in the pressure overload animal model of heart failure was found to enhance cardiac performance and SERCA2a activity (Kho et al. 2011, Kho et al. 2015). These studies indicate that sumoylation has cardioprotective properties in the heart. It should be mentioned that downregulation of SUMO1 by a hairpin RNA caused a significant decrease in the level of SERCA2a protein (Kho et al. 2011). Moreover, the transfer of SUMO1 gene was shown to reinstate mRNA expression of SERCA2a, cause enhancement in the left ventricular ejection fraction and maintain the activity of SERCA2a in an animal model of ischemic heart disease (Kho et al. 2011). It was suggested that inhibition of acetylation of certain lysine residues of SERCA2a and increase in its sensitivity to ATP underlies the action of sumoylation in maintaining SERCA2a function (Park and Oh, 2013). On the other hand, it is believed that acetylation and deacetylation play a role in the acute modification of SERCA2a activity (Park and Oh 2013, Sack 2012). Although, SERCA2a acetylation and deacetylation are not well defined, some evidence has been presented to show that these processes could play a role in intracellular Ca2+ handling in cardiac myocytes (Grillon et al. 2012). In diabetes, when glucose levels are high, an increase in SERCA2a glycosylation occur, which has been reported to cause a significant decrease in SERCA2a activity (Clark et al. 2003, Belke et al. 2004). A substantial decrease in both SERCA2a mRNA and proteins as well as a considerable increase in PLN have been attributed to the increase in glycosylation (Belke et al. 2004, Hamm et al. 2016, Calligaris et al. 2013).
When O-linked N-acetylglucosamine is incorporated into threonine or serine residues, this process is called O-GlcNAcylation, which is known to modify the levels of SERCA2a protein and result in the disruption of Ca2+ cycling (Medford et al. 2013, Bennett et al. 2013). In fact, Clark and coworkers (2003) have reported a significant decrease in SERCA2a protein levels associated with longer Ca2+ decay in cardiac myocytes of newborn rat. Additionally, it was shown that O-GlcNAcylation controls SERCA2a gene expression in hearts, which is facilitated by the transcription factor SP1 and is involved in the transcription of many heart function regulatory genes (Dellow et al. 2001, Belke 2011, Johnsen et al. 2013). A significant reduction in SP1specific O-GlcNAcylation in hearts of swim-trained mice has been reported (Belke 2011). Furthermore, O-GlcNAcylation was also observed to alter the SERCA2a function by phosphorylation of PLN (Hu et al. 2005, Watson et al. 2010). A significant decrease in total PLN protein level and an increase in phosphorylated PLN has been demonstrated by decreasing the cellular O-GlcNAcylation by adenoviral overexpression of the enzyme O-GlycNAcase (Hu et al. 2005). These studies seem to support the view that O-GlcNAcylation affects SERCA2a function in the heart, either by affecting the SERCA2a activity directly or by prompting the PLN phosphorylation.
It is noteworthy that both oxidative stress and nitrosative stress are known to adversely affect the process of glutathionylation where disulphide bonds are formed between cysteine and glutathione in SR proteins (Ghezzi 2005), to increase SERCA activity and boost Ca2+ uptake (Lancel et al. 2009, Tong et al. 2008, Jardim-Messeder et al. 2012). Under normal conditions, the SERCA2a sulfhydryl modifications are reversible, but in the case of atherosclerosis, SERCA2a cysteine becomes oxidized irreversibly, to prevent glutathionylation and activation of SERCA function (Tong et al. 2008, Jardim-Messeder et al. 2012, Adachi 2010). Thus, alterations in the process of glutathionylation of SERCA2a in addition to other post-translational events can be seen to play a major role in inducing Ca2+-handling abnormalities in cardiomyocytes during the development of cardiac dysfunction in heart disease.
Modification of SERCA2a gene expression by miRNAs
MicroRNAs (miRNAs) are small, noncoding RNAs with important functions in development, cell differentiation and apoptosis, gene expression post-translationally, degradation of mRNAs and preventing the translation process (Ambros 2004, Karakikes et al. 2013). Since the 3-prime untranslated region (3′-UTR) of the SERCA2a gene harbors the recognition site for miRNA, it has been suggested that miRNAs alter heart function by regulating SERCA2a gene expression. In this regard, a significant increase in miRNA expression was found cause a substantial reduction in SERCA2a protein and was associated with a progressive reduction in fractional shortening (Montgomery et al. 2011). On the other hand, a significant increase in the expression of SERCA2a protein was observed when mice were injected with antisense oligonucleotide against miRNA (Zsebo et al. 2014, Wahlquist et al. 2014). In fact, miRNA knockout mice showed long cytosolic Ca2+ decay and lower SR Ca2+ load, in comparison to wild-type mice (Huang et al. 2013).
Modification of SERCA2a gene expression by exercise
It has been reported that aerobic exercise improves SR Ca2+ uptake and contractility of cardiomyocyte in some models of cardiovascular disorders (Natali et al. 2002, Wisløff et al. 2001, Kemi and Wisløff 2010). In fact, it was shown that aerobic exercise for 8-weeks in mice caused 30% increase in SR Ca2+ uptake; this event was explained by an increase in the protein content of SERCA2a (Kemi et al. 2008). Furthermore, 8-weeks of strenuous exercise increased the expression of SERCA2a protein by approximately 40% in the left ventricle, but when exercise was stopped, this increase was reversed within a period of one month (Carneiro-Junior et al. 2005). However, it is pointed out that the mechanism of increased SERCA2a activity due to exercise are not well understood. Although, exercise is a burden on the homeostasis of energy metabolism which causes an increase in the number and function of mitochondria, the synthesis of mitochondria requires activation of gene expression which cannot be achieved without the activation of certain transcriptional factors, such as TFAM and TFB2M; both these factors were involved in the regulation of SERCA2a (Watanabe et al. 2011). It is also pointed out that the effect of repeated muscular contractions on the activation of TFAM and TFB2M is controversial because some reports on repeated contractions have reported activation of TFAM and TFB2M (Gleyzer et al. 2005) whereas others have shown that exercise has no effect on TFAM and TFB2M in skeletal muscles (Norrbom et al. 2004). Nonetheless, upregulation of TFAM or TFB2M has been reported to occur due to exercise (Theilen et al. 2017, Norrbom et al, 2010, Lumini-Oliveira et al. 2011). For instance, it has been shown that when diabetic rats showing both reduced SR Ca2+ uptake and TFAM expression, were subjected to strenuous exercise for 14-weeks, an attenuation of the decrease in TFAM was observed (Lumini-Oliveira et al. 2011). Furthermore, athletes were found to have significantly higher TFB2M mRNA in comparison to less active individuals and the blood flow restricted training for 10 days in comparison to control showed an upsurge in the basal TFB2M levels (Norrbom et al. 2010). Therefore, the enhancement in SERCA2a function and Ca2+ handling following aerobic exercise can be explained by an increase in induction of TFAM or TFB2M expression.
Different regulatory factors and posttranslational events have also been considered to explain the overexpression of SERCA2a due to exercise. It may be noted that exercise has been reported to regulate the activity of SERCA2a by phosphorylating PLN, as well as by directly modifying the expression of SERCA2a protein (Bupha-Intr et al. 2009). Additionally, it was shown that exercise causes a significant increase in SERCA2a protein without affecting total PLN protein and thus inducing a decrease in PLN to SERCA2a ratio (Kemi et al. 2007). Furthermore, an enhancement in the inotropic and lusitropic sensitivity to β-adrenergic stimulation was demonstrated as a response to PLN phosphorylation in hypertensive rats subjected to exercise (MacDonnell et al. 2005). It was also shown that when aged mice were subjected to exercise, SERCA activity and SR Ca2+ uptake were normalized (Bupha-Intr et al. 2009) and it was concluded that exercise would be expected to improve SR Ca2+ uptake due to the relief of the inhibitory effects of PLN. On the other hand, the enhancement in SERCA2a activity due to exercise was shown to occur by post-translational modifications when mice were subjected to exercise for 6 weeks and a significant reduction in cellular O-GlcNAcylation was noted (Bennett et al. 2013, Belke 2011). Upon comparing mice selected for a high running capacity to those with low running capacity, a decrease in O-GlcNAcylated SERCA2a to total SERCA2a ratio was reported (Johnsen et al. 2013). Such effect was facilitated by SP1 transcription factor, an O-GlcNAcylation target, which is known to alter the activity of some cardiac genes (Belke, 2011). It emphasized that the role of other posttranslational modifications, such as acetylation, glutathionylation and sumoylation in the overexpression of SERCA2a due to exercise remains to be investigated.
Concluding Remarks
From the foregoing discussion, it is evident that SR plays a critical role not only in determining the status of heart function but also in maintaining the intracellular concentration of Ca2+ at a low level. Such functions of SR are carried out by the presence of Ca2+-cycling proteins, ryanodine receptors and Ca2+-pump ATPase (SERCA2a), which are directly involved in Ca2+-release and Ca2+-accumulation for the occurrence of cardiac contraction and relaxation, respectively. Furthermore, the regulation of SR Ca2+-transport activities is carried out by different Ca2+-regulatory proteins such as phospholamban (mainly) and InsP3R as well as different phospholipids. In addition to several structural proteins, some Ca2+-binding and Ca2+-buffering proteins such as calsequestrin and SAR have been demonstrated to determine the SR function. Extensive work has revealed that phosphorylation of phospholamban by protein kinase A or CaM Kinase relieves its inhibitory effect on Ca2+-cycling proteins in the SR membrane, promotes Ca2+-transport activities and augments cardiac function. On the other hand, activation of different proteases and phospholipases as well as changes in gene expression results in depressing the Ca2+-release and Ca2+-pump activities, induce myocardial cell damage and impair cardiac performance. The functions of SR Ca2+-cycling and Ca2+-regulatory proteins are also markedly altered by several post-translational modifications involving various reactions such as oxidation, nitrosylation, acetylation, lipidation and glycosylation; these alterations are associate with cardiac dysfunction. It should be emphasized that the occurrence of oxidative stress and Ca2+-handling abnormalities are now known to result in the development of SR defects and subsequent myocardial derangements. Thus different components of the SR membrane as well as several processes involved in the modification of their functions are considered to be excellent targets for drug development to improve heart function under a wide variety of pathophysiological conditions.
Acknowledgements
The infrastructure support for the preparation of this article was provided by St. Boniface Hospital Albrechtsen Research Centre. Dr. Mohamad Nusier was a visiting professor from the Jordan University of Science and Technology. Thanks are also due to Ms. Andrea Opsima for typing this manuscript. The authors received no funding for this work.
Abbreviations
- AMPK
Adenosine Monophosphate-Activated Protein Kinase
- ER
Endoplasmic Reticulum
- IP3
Inositol Trisphosphate
- InsP3R
Inositol-Trisphosphate Receptor
- JSR
Junctional Sarcoplasmic Reticulum
- LSR
Longitudinal Sarcoplasmic Reticulum
- MHC
Myosin Heavy Chain
- NEXN
Nexilin
- PLB or PLN
Phospholamban
- RyR
Ryanodine Receptor
- SAR
Sarcalumenin
- SLN
Sarcolipin
- SR
Sarcoplasmic Reticulum
- SERCA
Sarcoplasmic Reticulum ATPase
- T-Tubules
Transverse Tubules
- TRIC-A/SRP-27
Trimeric Intracellular Cation-Selective Channel
Footnotes
This paper is dedicated to the 70th anniversary of the founding of Physiologia Bohemoslovaca (currently Physiological Research)
At the time of 70th Anniversary of Physiological Research, this article is dedicated to honor the leadership of Dr. Josef Zicha and Prof. Dr. Bohuslav Ostadal in promoting the journal.
Conflict of Interest
There is no conflict of interest.
References
- ADACHI T. Modulation of vascular sarco/endoplasmic reticulum calcium ATPase in cardiovascular pathophysiology. Adv Pharmacol. 2010;59:165–195. doi: 10.1016/S1054-3589(10)59006-9. [DOI] [PubMed] [Google Scholar]
- AFZAL N, DHALLA NS. Differential changes in left and right ventricular SR calcium transport in congestive heart failure. Am J Physiol. 1992;263(3 Pt 2):H868–H874. doi: 10.1152/ajpheart.1992.262.3.H868. [DOI] [PubMed] [Google Scholar]
- AHERRAHROU Z, SCHLOSSAREK S, STOELTING S, KLINGER M, GEERTZ B, WEINBERGER F, KESSLER T, AHERRAHROU R, MORETH K, BEKEREDJIAN R, HRABE DE ANGELIS M, JUST S, ROTTBAUER W, ESCHENHAGEN T, SCHUNKERT H, CARRIER L, EDRMANN J. Knock-out of nexilin in mice leads to dilated cardiomyopathy and endomyocardial fibroelastosis. Basic Res Cardiol. 2016;111:1–10. doi: 10.1007/s00395-015-0522-5. [DOI] [PubMed] [Google Scholar]
- AHIMA RS. Metabolic actions of adipocyte hormones: focus on adiponectin. Obesity (Silver Spring) 2006;14(Suppl 1):9S–15S. doi: 10.1038/oby.2006.276. [DOI] [PubMed] [Google Scholar]
- ALSINA KM, HULSURKAR M, BRADENBURG S, KOWNATZI-DANGER D, LENZ C, URLAUB H, ABU0THA I, KAMLER M, CHIANG DY, LAHIRI SK, REYNOLDS JO, QUICK AP, SCOTT L, JR, WORD TA, GELVES MD, HECK AJR, LI N, DOBREV D, LEHNHART SE, WEHRENS XHT. Loss of protein phosphate 1 regulatory subunit PPP1R3A promotes atrial fibrillation. Circulation. 2019;140:681–693. doi: 10.1161/CIRCULATIONAHA.119.039642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTO LE, DHALLA NS. Myocardial cation contents during induction of calcium paradox. Amer J Physiol. 1979;237:H713–H719. doi: 10.1152/ajpheart.1979.237.6.H713. [DOI] [PubMed] [Google Scholar]
- ALTSHULER I, VAILLANT JJ, XU S, CRISTESCU ME. The evolutionary history of sarco (endo) plasmic calcium ATPase (SERCA) PLoS One. 2012;7:e52617. doi: 10.1371/journal.pone.0052617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBROS V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- ANDERSSON KB, BIRKELAND JA, FINSEN AV, LOUCH WE, SJAASTAD I, WANG Y, CHEN J, MOLKENTIN JD, CHIEN KR, SEJERSTED OM, CHRISTENSIN G. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol. 2009b;47:180–187. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- ANDERSSON KB, FINSEN AV, SJALAND C, WINER LH, SJAASTAD I, ODEGAARD A, LOUCH WE, WANG Y, CHEN J, CHIEN KR, SEJERSTED OM, CHRISTENSEN G. Mice carrying a conditional Serca2flox allele for the generation of Ca2+ handling-deficient mouse models. Cell Calcium. 2009a;46:219–225. doi: 10.1016/j.ceca.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAI M, OTSU K, MACLENNAN DH, ALPERT NR, PERIASAMY M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 1991;69:266–276. doi: 10.1161/01.res.69.2.266. [DOI] [PubMed] [Google Scholar]
- ARUN N, NALINI N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant foods Hum Nutr. 2002;57:41–52. doi: 10.1023/a:1013106527829. [DOI] [PubMed] [Google Scholar]
- ASAHI M, OTSU K, NAKAYAMA H, HIKOSO S, TAKEDA T, GRAMOLINI AO, TRIVIERA MG, OUDIT GY, MORITA T, KUSAKARI Y, HIRANO S, HONGO K, HIROTANI S, YAMAGUCHI O, PETERSON A, BACKX PH, KURIHARA S, HORI M, MACLENNAN DH. Cardiac-specific overexpression of sarcolipin inhibits sarco (endo) plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci. 2004;101:9199–9204. doi: 10.1073/pnas.0402596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASAHI M, SUGITA Y, KURZYDLOWSKI K, DE LEON S, TADA M, TOYOSHIMA C, MacLENNAN DH. Sarcolipin regulates sarco (endo) plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. Proc Natl Acad Sci. 2003;100:5040–5045. doi: 10.1073/pnas.0330962100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABU GJ, BHUPATHY P, TIMOFEYEV V, PETRASHEVSKAYA NN, REISER PJ, CHIAMVIMONVAT N, PERIASAMY M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci. 2007;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABU GJ, ZHENG Z, NATARAJAN P, WHEELER D, JANSSEN PM, PERIASAMY M. Overexpression of sarcolipin decreases myocyte contractility and calcium transient. Cardiovasc Res. 2005;65:177–186. doi: 10.1016/j.cardiores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- BEARD NA, LAVER DR, DULHUNTY AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- BELKE DD, SWANSON EA, DILLMANN WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53:3201–3208. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- BELKE DD. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol. 2011;111:157–162. doi: 10.1152/japplphysiol.00147.2011. [DOI] [PubMed] [Google Scholar]
- BENNETT CE, JOHNSEN VL, SHEARER J, BELKE DD. Exercise training mitigates aberrant cardiac protein O-GlcNAcylation in streptozotocin-induced diabetic mice. Life Sci. 2013;92:657–663. doi: 10.1016/j.lfs.2012.09.007. [DOI] [PubMed] [Google Scholar]
- BERRIDGE MJ, BOOTMAN MD, RODERICK HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- BERS DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- BERS DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- BLEUNVEN C, TREVES S, JINYU X, LEO E, RONJAT M, DE WAARD M, KERN G, FLUCHER BE, ZORZATO F. SRP-27 is a novel component of the supramolecular signalling complex involved in skeletal muscle excitation-contraction coupling. iochem J. 2008;411:343–349. doi: 10.1042/BJ20070906. [DOI] [PubMed] [Google Scholar]
- BODDU NJ, THEUS S, LUO S, WEI JY, RANGANATHAN G. Is the lack of adiponectin associated with increased ER/SR stress and inflammation in the heart? Adipocyte. 2014;3:10–18. doi: 10.4161/adip.26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVO E, NIKOLAIENKO R, BHAYANI S, KAHN D, CAO Q, MARTIN JL, KUO IY, ROBIA SL, ZIMA AV. Novel approach for quantification of endoplasmic reticulum Ca2+ transport. Am J Physiol - Hear Circ Physiol. 2019;316:H1323–H1331. doi: 10.1152/ajpheart.00031.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY M, KOBAN MU, DELLOW KA, YACOUB M, BOHELER KR, FULLER SJ. Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes. Cardiovasc Res. 2003;60:347–354. doi: 10.1016/s0008-6363(03)00529-7. [DOI] [PubMed] [Google Scholar]
- BRANDL CJ, DELEON S, MARTIN DR, MACLENNAN DH. Adult forms of the Ca2+ ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem. 1987;262:3768–3774. [PubMed] [Google Scholar]
- BUPHA-INTR T, LAOSIRIPISAN J, WATTANAPERMPOOL J. Moderate intensity of regular exercise improves cardiac SR Ca2+ uptake activity in ovariectomized rats. J Appl Physiol. 2009;107:1105–1112. doi: 10.1152/japplphysiol.00407.2009. [DOI] [PubMed] [Google Scholar]
- CALLIGARIS SD, LECANDA M, SOLIS F, EZQUER M, GUTIERREZ J, BRANDAN E, LEIVA A, SOBREVIA L, CONGET P. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL HM, QUICK AP, ABU-TAHA I, CHIANG DY, KRAMM CF, WORD TA, BRANDENBURG S, HULSURKAR M, ALSINA KM, LIU HB, MARTIN B, UHLENKAMP D, MOORE OM, LAHIRI SK, CORRADINI E, KAMLER M, HECK AJR, LEHNART SE, DOBREV DV, WEHRENS XHT. Loss of SPEG inhibitory phosphorylation of ryanodine receptor type-2 promotes atrial fibrillation. Circulation. 2020;142:1159–1172. doi: 10.1161/CIRCULATIONAHA.120.045791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARAFOLI E. The transport of calcium by mitochondria. Problems and perspectives. Biochimie. 1973;55:755–762. doi: 10.1016/s0300-9084(73)80028-8. [DOI] [PubMed] [Google Scholar]
- CARNEIRO-JÚNIOR MA, PRÍMOLA-GOMES TN, QUINTÃO-JÚNIOR JF, DRUMMOND LR, LAVORATO VN, DRUMMOND FR, FELIX LB, OLIVEIRA EM, CRUZ JS, NATALI AJ, MILL JG. Regional effects of low-intensity endurance training on structural and mechanical properties of rat ventricular myocytes. J Appl Physiol. 2013;115:107–115. doi: 10.1152/japplphysiol.00041.2013. [DOI] [PubMed] [Google Scholar]
- CHANG KC, FIGUEREDO VM, SCHREUR JH, KARIYA K, WIENER MW, SIMPSON PC, CAMACHO SA. Thyroid hormone improves function and Ca2+ handling in pressure overload hypertrophy. Association with increased sarcoplasmic reticulum Ca2+-ATPase and alpha-myosin heavy chain in rat hearts. J Clin Invest. 1997;100:1742–1749. doi: 10.1172/JCI119699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI YS, KIM S, PAK YK. Mitochondrial transcription factor A (mtTFA) and diabetes. Diabetes Res Clin Pract. 2001;54(Suppl 2):S3–S9. doi: 10.1016/s0168-8227(01)00330-8. [DOI] [PubMed] [Google Scholar]
- CLAPHAM DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- CLARK RJ, MCDONOUGH PM, SWANSON E, TROST SU, SUZUKI M, FUKUDA M, DILLMANN WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- CONNELL P, WORD TA, WEHRENS XHT. Targeting pathological leak of ryanodine receptors: preclinical progress and the potential impact on treatments of cardiac arrhythmias and heart failure. Expert Opin Ther Targets. 2020;24:25–36. doi: 10.1080/14728222.2020.1708326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRELL RN, LYNCH JM, SCHIPS TG, PRASAD V, YORK AJ, SARGENT MA, BROCHET DX, MA J, MOLKENTIN JD. Mitsugumin 29 regulates t-tubule architecture in the failing heart. Sci Rep. 2017;7(1):5328. doi: 10.1038/s41598-017-05284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLY S, BREDOUX R, CORVAZIER E, ANDERSEN JP, CLAUSEN JD, DODE L, FANCHAOUY M, GELEBART P, MONCEAU V, DEL MONTE F, GWATHMEY JK, HAJJAR R, CHAABANE C, BOBE R, RAIES A, ENOUF J. Ca2+-ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c) Biochem J. 2006;395:249–258. doi: 10.1042/BJ20051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA MATA A, TAJADA S, O’DWYER S, MATSUMOTO C, DIXON RE, HARIHARAN N, MMORENO CM, SANTANA LF. BIN1 induces the formation of T-tubules and adult-like Ca2+ release units in developing cardiomyocytes. Stem Cells. 2019;37:54–64. doi: 10.1002/stem.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELBONO O, XIA J, TREVES S, WANG ZM, JIMENEZ-MORENO R, PAYNE AM, MESSI ML, BRIGUET A, SCHAERER F, NISHI M, TAKESHIMA H, ZORZATO F. Loss of skeletal muscle strength by ablation of the sarcoplasmic reticulum protein JP45. Proc Natl Acad Sci U S A. 2007;104:20108–20113. doi: 10.1073/pnas.0707389104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLOW KA, BHAVSAR PK, BRAND NJ, BARTON PJR. Identification of novel, cardiac-restricted transcription factors binding to a CACC-box within the human cardiac troponin I promoter. ardiovasc Res. 2001;50:24–33. doi: 10.1016/s0008-6363(01)00204-8. [DOI] [PubMed] [Google Scholar]
- DHALLA NS. Involvement of membrane systems in heart failure due to intracellular calcium overload and deficiency. J Mol Cell Cardiol. 1976;8:661–667. doi: 10.1016/0022-2828(76)90008-0. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, ALTO LE, HEYLIGER CE, PIERCE GN, PANAGIA V, SINGAL PK. Sarcoplasmic reticular Ca2+-pump adaptation in cardiac hypertrophy due to pressure overload in pigs. Eur Heart J. 1984;5(Suppl F):323–328. doi: 10.1093/eurheartj/5.suppl_f.323. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, GANGULY PK, BHULLAR SK, TAPPIA PS. Role of catecholamines in the pathogenesis of diabetic cardiomyopathy. Can J Physiol Pharmacol. 2019;97:815–819. doi: 10.1139/cjpp-2019-0044. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, MCNAMARA DB, SULAKHE PV. Excitation-contraction coupling heart. V. contribution of mitochondria and sarcoplasmic reticulum in the regulation of calcium connection in the heart. Cardiology. 1970;55:178–191. doi: 10.1159/000169281. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, PIERCE GN, PANAGIA V, SINGAL PK, BEAMISH RE. Calcium movements in relation to heart function. Basic Res Cardiol. 1982;77:117–139. doi: 10.1007/BF01908167. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, RANGI S, BABICK AP, ZIEROTH S, ELIMBAN V. Cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev. 2012;17:671–681. doi: 10.1007/s10741-011-9278-7. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, SAINI-CHOHAN HK, RODRIGUEZ-LEYVA D, ELIMBAN V, DENT MR, TAPPIA PS. Subcellular remodelling may induce cardiac dusfunction in congestive heart failure. Cardiovasc Res. 2009;81:429–438. doi: 10.1093/cvr/cvn281. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, SINGAL PK, PANAGIA V, HARROW JAC, ANAND-SRIVASTAVA MB, BEAMSIH RE. Progress and problems in understanding the involvement of calcium in heart function. Can J Physiol Pharmacol. 1984;62:867–873. doi: 10.1139/y84-146. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, SULAKHE PV, LAMERS JMJ, GANGULY PK. Characterization of Ca2+ release from the cardiac sarcoplasmic reticulum. Gen Physiol Biophys. 1983;2:339–351. [PubMed] [Google Scholar]
- DHALLA NS, SULAKHE PV, LEE SL, SINGAL PK, VARLEY KG, YATES JC. Subcellular Ca2+ transport in different areas of dog heart. Can J Physiol Pharmacol. 1980;58:360–367. doi: 10.1139/y80-062. [DOI] [PubMed] [Google Scholar]
- DHALLA NS, ZIEGELHOFFER A, HARROW JAC. Regulatory role of membrane systems in heart function. Can J Physiol Pharmacol. 1977;55:1211–1234. doi: 10.1139/y77-167. [DOI] [PubMed] [Google Scholar]
- DIA M, GOMEZ L, THIBAULT H, TESSIER N, LEON C, CHOUABE C, DUCREUX S, GALLO-BONA N, TUBBS E, BENDRIDI N, CHANON S, AYMERIC L, BELMUDES L, COUTE Y, KURDI M, OVIZE M, RIEUSSET J, PAILLARD M. Reduced reticulum-mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res Cardiol. 2020;115:74. doi: 10.1007/s00395-020-00835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINCHUK JE, HENDERSON NL, BURN TC, HUBER R, HO SP, LINK J, O’NEIL KT, FOCHT RJ, SCULLY MS, HOLLIS JM, HOLLIS GF, FRIEDMAN PA. Aspartyl β-hydroxylase (Asph) and an evolutionarily conserved isoform of Asph missing the catalytic domain share exons with junctin. J Biol Chem. 2000;275:39543–39554. doi: 10.1074/jbc.M006753200. [DOI] [PubMed] [Google Scholar]
- DOBREV D, WEHRENS XHT. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol. 2017;595:4001–4008. doi: 10.1113/JP273048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODD AN, KUDLA J, SANDERS D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- DODE L, ANDERSEN JP, LESLIE N, DHITAVAT J, VILSEN B, HOVNANIAN A. Dissection of the functional differences between sarco (endo) plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J Biol Chem. 2003;278:47877–47889. doi: 10.1074/jbc.M306784200. [DOI] [PubMed] [Google Scholar]
- DOROUDGAR S, GLEMBOTSKI CC. New concepts of endoplasmic reticulum function in the heart: Programmed to conserve. J Mol Cell Cardiol. 2013;55:85–91. doi: 10.1016/j.yjmcc.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRIDI H, KUSHNIR A, ZALK R, YUAN Q, MELVILLE Z, MARKS AR. Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target. Nat Rev Cardiol. 2020;17:732–747. doi: 10.1038/s41569-020-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUMMOND GI, SEVERSON DL. Cyclic nucleotides and cardiac function. Circ Res. 1979;44:145–153. doi: 10.1161/01.res.44.2.145. [DOI] [PubMed] [Google Scholar]
- EBASHI S, EBASHI F. Removal of calcium and relaxation in actomyosin systems. Nature. 1962;194:378–379. doi: 10.1038/194378a0. [DOI] [PubMed] [Google Scholar]
- EBASHI S, ENDO M. Ca ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- EBASHI S, LIPMANN F. Adenosine triphosphate-linked concentrate ion of calcium ions in a particulate function of rabbit muscle. J Cell Biol. 1962;14:389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI S. Excitation-contraction coupling. Annu Rev Physiol. 1976;38:293–313. doi: 10.1146/annurev.ph.38.030176.001453. [DOI] [PubMed] [Google Scholar]
- EISNER DA, CALDWELL JL, KISTAMAS K, TRAFFORD AW. Calcium and Excitation-Contraction Coupling in the Heart. Circ Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISNER DA, CHOI HS, DIAZ ME, O’NEILL SC, TRAFFORD AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- FABIATO A, FABIATO F. Calcium and cardiac excitation–contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- FABIATO A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- FERIOTTO G, FINOTTI A, VOLPE P, TREVES S, FERRARI S, ANGELELLI C, ZORZATO F, GAMBARI R. Myocyte enhancer factor 2 activates promoter sequences of the human A H-J-J locus, encoding aspartyl-hydroxylase, junctin, and junctate. Mol Cell Biol. 2005;25:3261–3275. doi: 10.1128/mcb.25.8.3261-3275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVELL SW, GREENBERG ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTUÑO A, RODRIGUEZ A, GÓMEZ-AMBROSI J, FRUHBECK G, DIEZ J. Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003;59:51–60. doi: 10.1007/BF03179868. [DOI] [PubMed] [Google Scholar]
- FOSKETT JK, WHITE C, CHEUNG KH, MAK DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK KF, BOLCK B, ERDMANN E, SCHWINGER RHG. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res. 2003;57:20–27. doi: 10.1016/s0008-6363(02)00694-6. [DOI] [PubMed] [Google Scholar]
- FUJINO T, IDE T, YOSHIDA M, ONITSUKA K, TANAKA A, HATA Y, NISHIDA M, TAKEHARA T, KANEMARU T, KITAJIMA N, TAKAZAKI S, KUROSE H, KANG D, SUNAGAWA K. Recombinant mitochondrial transcription factor A protein inhibits nuclear factor of activated T cells signaling and attenuates pathological hypertrophy of cardiac myocytes. Mitochondrion. 2012;12:449–458. doi: 10.1016/j.mito.2012.06.002. [DOI] [PubMed] [Google Scholar]
- GALICE S, XIE Y, YANG Y, SATO D, BERS DM. Size matters: ryanodine recepot cluster size affects arrhythmogenic sarcoplasmic reticulum calcium release. J Am Heart Assoc. 2018;7:e008724. doi: 10.1161/JAHA.118.008724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANGULY PK, MATHUR S, GUPTA MP, BEAMISH RE, DHALLA NS. Calcium pump activity of sarcoplasmic reticulum in diabetic rat skeletal muscle. Am J Physiol. 1986;251(5 Pt 1):E515–E523. doi: 10.1152/ajpendo.1986.251.5.E515. [DOI] [PubMed] [Google Scholar]
- GANGULY PK, PIERCE GN, DHALLA KS, DHALLA NS. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983;244:E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- GELEBART P, MARTIN V, ENOUF J, PAPP B. Identification of a new SERCA2 splice variant regulated during monocytic differentiation. Biochem Biophys Res Commun. 2003;303:676–684. doi: 10.1016/s0006-291x(03)00405-4. [DOI] [PubMed] [Google Scholar]
- GHEZZI P. Oxidoreduction of protein thiols in redox regulation. Biochem Soc Trans. 2005;33:1378–1381. doi: 10.1042/BST20051378. [DOI] [PubMed] [Google Scholar]
- GLEYZER N, VERCAUTEREN K, SCARPULLA RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONON AT, WIDEGREN U, BULHAK A, SALEHZADEH F, PERSSON J, SJOQUIST PO, PERNOW J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- GRAMOLINI AO, TRIVIERI MG, OUDIT GY, KISLINGER T, LI W, PATEL MM, EMILI A, KRANIAS EG, BACKX PH, MACLENNAN DH. Cardiac-specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. roc Natl Acad Sci. 2006;103:2446–2451. doi: 10.1073/pnas.0510883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREISER M. Calcium signalling silencing in atrial fibrillation. J Physiol. 2017;595:4009–4017. doi: 10.1113/JP273045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRILLON JM, JOHNSON KR, KOTLO K, DANZIGER RS. Non-histone lysine acetylated proteins in heart failure. Biochim Biophys Acta. 2012;1822:607–614. doi: 10.1016/j.bbadis.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS P, JOHNSON J, ROMERO CM, EATON DM, POULET C, SANCHEZ-ALONSO J, LUCARELLI C, ROSS J, GIBB AA, GARBINCIUS JF, LAMBERT J, VAROL E, YANG Y, WALLNER M, FELDSOTT EA, KUBO H, BERRETTA RM, YU D, RIZZO V, ELROD J, SABRI A, GORELIK J, CHEN X, HOUSER SR. Interaction of the joining region in junctophilin-2 with the L-type Ca2+ channel is pivotal for cardiac dyad assembly and intracellular Ca2+ dynamics. Circ Res. 2021;128:92–114. doi: 10.1161/CIRCRESAHA.119.315715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERRERO-HERNANDEZ A, SANCHEZ-VAZQEUZ VH, MARTINEZ-MARTINEZ E, SANDOVAL-VASQUEZ L, PEREZ-ROSAS NC, LOPEX-FARIAS R, DAGNINO-ACOSTA A. Sarco-endoplasmic reticulum calcium release model based on changes in the luminal calcium content. Adv Exp Med Biol. 2020;1131:337–370. doi: 10.1007/978-3-030-12457-1_14. [DOI] [PubMed] [Google Scholar]
- GUO J, BIAN Y, BAI R, LI H, FU M, XIAO C. Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca2+-ATPase activity and inhibiting endoplasmic reticulum stress. J Cardiovasc Pharmacol. 2013;62:143–153. doi: 10.1097/FJC.0b013e31829521af. [DOI] [PubMed] [Google Scholar]
- GUO J, TIAN Q, BARTH M, XIAN W, RUPPENTHAL S, SCHAEFERS HJ, CHEN Z, MORETTI A, LAUGWITZ KL, LIPP P. Human BIN1 isoforms grow, maintain and regenerate excitation-contraction couplons in adult rat and human stem cell-derived cardiomyocytes. Cardiovasc Res. 2021;21:cvab195. doi: 10.1093/cvr/cvab195. [DOI] [PubMed] [Google Scholar]
- HA KN, TRAASETH NJ, VERARDI R, ZAMOON J, CEMBRAN A, KARIM CB, THOMAS DD, VEGLIA G. Controlling the inhibition of the sarcoplasmic Ca2+-ATPase by tuning phospholamban structural dynamics. J Biol Chem. 2007;282:37205–37214. doi: 10.1074/jbc.M704056200. [DOI] [PubMed] [Google Scholar]
- HAMM NC, STAMMERS AN, SUSSER SE, HLYNSKY MW, KIMBER DE, KEHLER DS, DUHAMEL TA. Regulation of cardiac sarco (endo) plasmic reticulum calcium-ATPases (SERCA2a) in response to exercise. In: Chakraborti S, Dhalla NS, editors. Regulation of Ca2+-ATPases, V-ATPases and F-ATPases. Springer; 2016. pp. 187–206. [Google Scholar]
- HARTONG R, WANG N, KUROKAWA R, LAZAR MA, GLASS CK, APRILETTI JW, DILLMANN WH. Delineation of three different thyroid hormone-response elements in promoter of rat sarcoplasmic reticulum Ca2+ ATPase gene. Demonstration that retinoid X receptor binds 5’to thyroid hormone receptor in response element 1. J Biol Chem. 1994;269:13021–13029. [PubMed] [Google Scholar]
- HASSEL D, DAHME T, ERDMANN J, MEDER B, HUGE A, STOLL M, JUST S, HESS A, EHLERMANN P, WEICHENCHAN D, GRIMMLER M, LIPTAU H, HETZER R, REGITZ-ZAGROSEK V, FISCHER C, NURNBERG P, SCHUNKERT H, KATUS HA, ROTTBAUER W. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat Med. 2009;15:1281–1288. doi: 10.1038/nm.2037. [DOI] [PubMed] [Google Scholar]
- HASSELBACH W, MAKINOSE M. ATP and active transport. Biochem Biophys Res Commun. 1962;7:132–136. doi: 10.1016/0006-291x(62)90161-4. [DOI] [PubMed] [Google Scholar]
- HASSELBACH W. Relaxing factor and the relaxation of muscle. Prog Biophys Biophys Chem. 1964;14:167–222. [Google Scholar]
- HAUGAARD N, HAUGAARD ES, LEE NH, HORN RS. Possible role of mitochondria in regulation of cardiac contractility. Fed Proc. 1969;28:1657–1662. [PubMed] [Google Scholar]
- HEYLIGER CE, GANGULY PK, DHALLA NS. Sarcoplasmic reticular and mitochondrial calcium transport in cardiac hypertrophy. Can J Cardiol. 1985;1:401–408. [PubMed] [Google Scholar]
- HONG CS, KOWN SJ, KIM DH. Multiple functions of junctin and junctate, two distinct isoforms of aspartyl beta-hydroxylase. Biochem Biophys Res Commun. 2007;362:1–4. doi: 10.1016/j.bbrc.2007.07.166. [DOI] [PubMed] [Google Scholar]
- HONG TT, SHAW RM. Cardiac t-tubule microanatomy and function. Physiol Rev. 2017;97:227–252. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU Y, BELKE D, SUAREZ J, SWANSON E, CLARK R, HOSHIJIMA M, DILLMANN WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- HUANG Z-P, CHEN J, SEOK HY, ZHANG Z, KATAOKA M, HU X, WANG DZ. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res. 2013;112:1234–1243. doi: 10.1161/CIRCRESAHA.112.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUG C, LODISH HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005;5:129–134. doi: 10.1016/j.coph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- IKEUCHI M, MATSUSAKA H, KANG D, MATSUSHIMA S, IDE T, KUBOTA T, FUJIWARA T, HAMASAKI N, TAKESHITA A, SUNAGAWA K, TSUTSUI H. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–690. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- INESI G. Active transport of calcium ion in sarcoplasmic reticulum membranes. Annu Rev Biophys Bioeng. 1972;1:191–210. doi: 10.1146/annurev.bb.01.060172.001203. [DOI] [PubMed] [Google Scholar]
- ITO K, KOMAZAKI S, SASAMOTO K, YOSHIDA M, NISHI M, KITAMURA K, TAKESHIMA H. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J Cell Biol. 2001;154:1059–1067. doi: 10.1083/jcb.200105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHNG JWS, TURDI S, KOVACEVIC V, DADSON K, LI RK, SWEENEY G. Pressure overload-induced cardiac dysfunction in aged male adiponectin knockout mice is associated with autophagy deficiency. ndocrinology. 2015;156:2667–2677. doi: 10.1210/en.2015-1162. [DOI] [PubMed] [Google Scholar]
- JARDIM-MESSEDER D, CAMACHO-PEREIRA J, GALINA A. 3-Bromopyruvate inhibits calcium uptake by sarcoplasmic reticulum vesicles but not SERCA ATP hydrolysis activity. Int J Biochem Cell Biol. 2012;44:801–807. doi: 10.1016/j.biocel.2012.02.002. [DOI] [PubMed] [Google Scholar]
- JI Y, LALLI MJ, BABU GJ, XU Y, KIRKPATRICK DL, LIU LH, CHIAMVIMONVAT N, WALSH RA, SHULL GE, PERIASAMY M. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem. 2000;275:38073–38080. doi: 10.1074/jbc.M004804200. [DOI] [PubMed] [Google Scholar]
- JIAO Q, BAI Y, AKAIKE T, TAKESHIMA H, ISHIKAWA Y, MINAMISAWA S. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am J Physiol Heart Circ Physiol. 2009;297:H576–H582. doi: 10.1152/ajpheart.00946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIAO Q, BAI Y, AKAIKE T, TAKESHIMA H, ISHIKAWA Y, MINAMISAWA S. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am J Physiol - Heart Circ Physiol. 2009;297:H576–H582. doi: 10.1152/ajpheart.00946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSEN VL, BELKE DD, HUGHEY CC, HITTEL DS, HEPPLE RT, KOCH LG, BRITTON SL, SHEARER J. Enhanced cardiac protein glycosylation (O-GlcNAc) of selected mitochondrial proteins in rats artificially selected for low running capacity. hysiol Genomics. 2013;45:17–25. doi: 10.1152/physiolgenomics.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES LR, SUZUKI YJ, WANG W, KOBAYASHI YM, RAMESH V, FRANZINI-ARMSTRONG C, CLEEMANN L, MORAD M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest. 1998;101:1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG D, KIM SH, HAMASAKI N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- KARAKIKES I, CHAANINE AH, KANG S, MUKETE BN, JEONG D, ZHANG S, HAJJAR RJ, LEBECHE D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload–induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc. 2013;2:e000078. doi: 10.1161/JAHA.113.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ AM, TADA M, KIRCHBERGER MA. Control of calcium transport in the myocardium by the cyclic AMP-protein kinase system. Adv Cyclic Nucleotide Res. 1975;5:453–472. [PubMed] [Google Scholar]
- KATZ AM. Contractile proteins of the heart. Physiol Rev. 1970;50:63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- KEMI OJ, ELLINGSEN Ø, CECI M, GRIMALDI S, SMITH GL, CONDORELLI G, WISLOFF U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol. 2007;43:354–361. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMI OJ, ELLINGSEN O, SMITH GL, WISLOFF U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front Biosci. 2008;13:356–368. doi: 10.2741/2685. [DOI] [PubMed] [Google Scholar]
- KEMI OJ, WISLØFF U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol. 2010;199:425–439. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- KHO C, LEE A, JEONG D, OH JG, CHAANINE AH, KIZANA E, PARK WJ, HAJJAR RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHO C, LEE A, JEONG D, OH JG, GORSKI PA, FISH K, SANCHEZ R, DEVITA RJ, CHRISTENSEN G, DAHL R, HAJJAR RJ. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat Commun. 2015;6:1–11. doi: 10.1038/ncomms8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA Y, OTSU K, NISHIDA K, KUZUYA T, TADA M. Thyroid hormone enhances Ca2+ pumping activity of the cardiac sarcoplasmic reticulum by increasing Ca2+ ATPase and decreasing phospholamban expression. J Mol Cell Cardiol. 1994;26:1145–1154. doi: 10.1006/jmcc.1994.1133. [DOI] [PubMed] [Google Scholar]
- KINUGAWA K, MINOBE WA, WOOD WM, RIDGWAY EC, BAXTER JD, RIBEIRO RC, TAWADROUS MF, LOWES BA, LONG CS, BRISTOW MR. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation. 2001;103:1089–1094. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- KISS E, JAKAB G, KRANIAS EG, EDES I. Thyroid hormone-induced alterations in phospholamban protein expression. Regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circ Res. 1994;75:245–251. doi: 10.1161/01.res.75.2.245. [DOI] [PubMed] [Google Scholar]
- KNYUSHKO TV, SHAROV VS, WILLIAMS TD, SCHONEICH C, BIGELOW DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- KOMAZAKI S, ITO K, TAKESHIMA H, NAKAMURA H. Deficiency of triad formation in developing skeletal muscle cells lacking junctophilin type 1. FEBS Lett. 2002;524:225–229. doi: 10.1016/S0014-5793(02)03042-9. [DOI] [PubMed] [Google Scholar]
- KOMAZAKI S, NISHI M, TAKESHIMA H, NAKAMURA H. Abnormal formation of sarcoplasmic reticulum networks and triads during early development of skeletal muscle cells in mitsugumin29-deficient mice. Dev Growth Differ. 2001;43:717–723. doi: 10.1046/j.1440-169X.2001.00609.x. [DOI] [PubMed] [Google Scholar]
- KOSS KL, GRUPP IL, KRANIAS EG. The relative phospholamban and SERCA2 ratio: a critical determinant of myocardial contractility. Basic Res Cardiol 92 Suppl. 1997;1:17–24. doi: 10.1007/BF00794064. [DOI] [PubMed] [Google Scholar]
- KUSHNIR A, BETZENHAUSER MJ, MARKS AR. Ryanodine receptor studies using genetically engineered mice. FEBS Lett. 2010;584:1956–1965. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWON SJ, KIM DH. Characterizations of junctate-SERCA2a interaction in murine cardiomyocyte. Biochem Biophys Res Commun. 2009;390:1389–1394. doi: 10.1016/j.bbrc.2009.10.165. [DOI] [PubMed] [Google Scholar]
- LAM AKM, GALIONE A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim Biophys Acta. 2013;1833:2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- LAMBOLEY CR, MURPHY RM, MCKENNA MJ, LAMB GD. Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in type I and type II human skeletal muscle fibres. J Physiol. 2013;591:6053–6068. doi: 10.1113/jphysiol.2013.265900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEL S, ZHANG J, EVANGELISTA A, TRUCILLO MP, TONG X, SIWIK DA, COHEN RA, COLUCCI WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDSTROM AP, DOBREV D, WEHRENS XHT. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER GA. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968;48:708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- LANNER JT, GEORGIOU DK, JOSHI AD, HAMILTON SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCANO E, NEGRONI J, VILA PETROFF M, MATTIAZZI A. Impact of RyR2 potentiation on myocardial function. Am J Physiol Heart Circ Physiol. 2017;312:H1105–H1109. doi: 10.1152/ajpheart.00855.2016. [DOI] [PubMed] [Google Scholar]
- LE PEUCH CJ, HAIECH J, DEMAILLE JG. Concerted regulation of cardiac sarcoplasmic reticulum by cyclic adenosine monophosphate-dependent and calcium-calmodulin-dependent phosphorylation. Biochemistry. 1979;18:5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- LE PEUCH CJ, LE PEUCH DA, DEMAILLE JG. Covalent regulation of the cardiac sarcoplasmic reticulum calcium pump: Purification and properties of phospholamban, a substrate of cAMP-dependent protein kinase and Ca2+-calmodulin-dependent phospholamban kinase. Methods Enzymol. 1983;102:261–278. doi: 10.1016/S0076-6879(83)02027-3. [DOI] [PubMed] [Google Scholar]
- LEBERER E, TIMMS BG, CAMPBELL KP, MACLENNAN DH. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic reticulum. J Biol Chem. 1990;265:10118–10124. [PubMed] [Google Scholar]
- LEE E, MARCUCCI M, DANIELL L, PYPAERT M, WEISZ OA, OCHOA GC, FARSAD K, WENK MR, CAMILLI PD. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- LEE KS, LADINSKY H, CHOI SJ, KASUYA Y. Studies on the in vitro interaction of electrical stimulation and Ca++ movement in sarcoplasmic reticulum. J Gen Physiol. 1966;49:689–715. doi: 10.1085/jgp.49.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER AL, CARAFOLI E, ROSSI CS. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- LIU C, SPINOZZI S, CHEN JY, FANG X, PERKINS G, CATTANEO P, GUIMARAES-CAMBOA N, DALTON ND, PETERSON KL, WU T, OUYANG K, FU XD, EVANS SM, CHEN J. Nexilin is a new component of junctional membrane complexes required for cardiac T-tubule formation. Circulation. 2019;140:55–66. doi: 10.1161/circulationaha.119.039751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOESCHER CM, GIBSON LM, STEPHENSON DG. Dantrolene sodium increases calcium binding by human recombinant cardiac calsequestrin and calcium loading by sheep cardiac sarcoplasmic reticulum. Acta Physiol. 2019;226:e13261. doi: 10.1111/apha.13261. [DOI] [PubMed] [Google Scholar]
- LOUCH WE, HOUGEN K, MORK HK, SWIFT F, ARONSEN JM, SJAASTAD I, REIMS HM, ROALD B, ANDERSSON KB, CHRISTENSEN G, SEJERSTED OM. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following SERCA2 knockout. J Physiol. 2010;588:465–478. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUMINI-OLIVEIRA J, MAGALHÃES J, PEREIRA CV, MOREIRA AC, OLIVEIRA PJ, ASCENSAO A. Endurance training reverts heart mitochondrial dysfunction, permeability transition and apoptotic signaling in long-term severe hyperglycemia. Mitochondrion. 2011;11:54–63. doi: 10.1016/j.mito.2010.07.005. [DOI] [PubMed] [Google Scholar]
- LÜSS I, BOKNIK P, JONES LR, KIRCHHEFER U, KNAPP J, LINCK B, LÜSS H, MEISSNER A, MULLER FU, SCHMITZ W, VAHLENSIECK U, NUEMANN J. Expression of cardiac calcium regulatory proteins in atrium v ventricle in different species. J Mol Cell Cardiol. 1999;31:1299–1314. doi: 10.1006/jmcc.1999.0962. [DOI] [PubMed] [Google Scholar]
- MACDONNELL SM, KUBO H, CRABBE DL, RENNA BF, REGER PO, MOHARA J, SMITHWICK LA, KOCH WJ, HOUSER SR, LIBONATI JR. Improved myocardial β-adrenergic responsiveness and signaling with exercise training in hypertension. Circulation. 2005;111:3420–3428. doi: 10.1161/CIRCULATIONAHA.104.505784. [DOI] [PubMed] [Google Scholar]
- MACKRILL JJ, SHIELS AA. Evolution of excitation-contraction coupling. Adv Exp Med Biol. 2020;1131:281–320. doi: 10.1007/978-3-030-12457-1_12. [DOI] [PubMed] [Google Scholar]
- MACLENNAN DH. Purification and properties of an adenosine triphosphate for sarcoplasmic reticulum. J Biol Chem. 1970;245:4508–4518. [PubMed] [Google Scholar]
- MACLENNAN DH, HOLLAND PC. Calcium transport in sarcoplasmic reticulum. Annu Rev Biophys Bioeng. 1975;4:377–404. doi: 10.1146/annurev.bb.04.060175.002113. [DOI] [PubMed] [Google Scholar]
- MACLENNAN DH, KRANIAS EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- MACLENNAN DH, OSTWALD TJ, STEWART PS. Structural components of the sarcoplasmic reticulum membrane. Ann N Y Acad Sci. 1974;227:527–536. doi: 10.1111/j.1749-6632.1974.tb14415.x. [DOI] [PubMed] [Google Scholar]
- MACLENNAN DH, YIP CC, ILES GH, SEEMAN P. Isolation of sarcoplasmic reticulum proteins. Cold Spring Harbor Symp Quant Biol. 1973;37:469–477. [Google Scholar]
- MARTONOSI A, DONLEY JR, HALPIN RA. Sarcoplasmic reticulum. III. The role of phospholipids in the adenosine triphosphate activity and Ca++ transport. J Biol Chem. 1968;243:61–70. [PubMed] [Google Scholar]
- MARTONOSI A, DONLEY JR, PUCELL AG, HALPIN RA. Sarcoplasmic reticulum. XI. The mode of involvement of phospholipids in the hydrolysis of ATP by sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971;144:529–540. doi: 10.1016/0003-9861(71)90358-4. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A, HALPIN RA. Sarcoplasmic reticulum. X. The protein composition of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971;144:66–77. doi: 10.1016/0003-9861(71)90455-3. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A. Transport of calcium by the sarcoplasmic reticulum. In: Hokin LE, editor. metabolic pathways. Vol. 6. New York: Academic; 1972. pp. 317–349. [Google Scholar]
- MARTONOSI AN. Structure-function relationships in the Ca2+-ATPase of sarcoplasmic reticulum: Facts, speculations and questions for the future. Biochim Biophys Acta - Bioenerg. 1996;1275:111–117. doi: 10.1016/0005-2728(96)00059-X. [DOI] [PubMed] [Google Scholar]
- MARTY I, FAURE J. Excitation-contraction coupling alterations in myopathies. J Neuromuscul Dis. 2016;3:443–453. doi: 10.3233/JND-160172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTIAZZI A, KRANIAS EG. CaMKII regulation of phospholamban and SR Ca2+ load. Heart Rhythm. 2011;8:784–787. doi: 10.1016/j.hrthm.2010.11.035. oi: 10.1016/j.hrthm.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKILLOP DF, GEEVES MA. Regulation of the acto.myosin subfragment 1 interaction by troponin/tropomyosin: Evidence for control of a specific isomerization between two acto.myosin subfragment 1 states. Biochem J. 1991;279:711–718. doi: 10.1042/bj2790711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKILLOP DF, GEEVES MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDFORD HM, PORTER K, MARSH SA. Immediate effects of a single exercise bout on protein O-GlcNAcylation and chromatin regulation of cardiac hypertrophy. Am J Physiol Circ Physiol. 2013;305:H114–H123. doi: 10.1152/ajpheart.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISSNER G. Regulation of ryanodine receptor ion channels through posttranslational modifications. Curr Top Membr. 2010;66:91–113. doi: 10.1016/S1063-5823(10)66005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISSNER G. The structural basis of ryanodine receptor ion channel function. J Gen Physiol. 2017;149:1065–1089. doi: 10.1085/jgp.201711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMISAWA S, WANG Y, CHEN J, ISHIKAWA Y, CHIEN KR, MATSUOKA R. Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling. J Biol Chem. 2003;278:9570–9575. doi: 10.1074/jbc.m213132200. [DOI] [PubMed] [Google Scholar]
- MOCCIA F, LODOLA F, STADIOTTI I, PILATO CA, BELLIN M, CARUGO S, POMPILIO G, SOMMARIVA E, MAIONE AS. Calcium as a key player in arrhythmogenic cardiomyopathy: Adhesion disorder or intracellular alteration? Int J Mol Sci. 2019;20:3986. doi: 10.3390/ijms20163986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTGOMERY RL, HULLINGER TG, SEMUS HM, DICKINSON BA, SETO AG, LYNCH JM, STACK C, LATIMER PA, OLSON EN, VAN ROOIJ E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORISSETTE MP, SUSSER SE, STAMMERS AN, O’HARA KA, GARDINER PF, SHEPPARD P, MOFFATT TL, DUHAMEL TA. Differential regulation of the fiber type-specific gene expression of the sarcoplasmic reticulum calcium-ATPase isoforms induced by exercise training. J Appl Physiol. 2014;117:544–555. doi: 10.1152/japplphysiol.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO ML, JAYASINGHE ID, WANG Q, QUICK A, WANG W, BADDELEY D, WEHRENS XHT, SOELLER C. Junctophilin-2 in the nanoscale organisation and functional signalling of ryanodine receptor clusters in cardiomyocytes. J Cell Sci. 2016;129:4388–4398. doi: 10.1242/jcs.196873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NABAUER M, CALLEWAERT G, CLEEMANN L, MORAD M. Regulation of calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- NAGAI R, ZARAIN-HERZBERG A, BRANDL CJ, FUJII J, TADA M, MACLENNAN DH, ALPERT NR, PERIASAMY M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci. 1989;86:2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA T, KASHIHARA T, KOMATSU M, KOJIMA K, TAKESHITA T, YAMADA M. Physical interaction of junctophilin and the CaV1.1 C terminus is crucial for skeletal muscle contraction. Proc Natl Acad Sci USA. 2018;115:4507–4512. doi: 10.1073/pnas.1716649115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAI J, IMAGAWA T, HAKAMAT Y, SHIGEKAWA M, TAKESHIMA H, NUMA S. Primary structure and functional expression from cDN A of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990;271:169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- NARAYANAN D, ADEBIYI A, JAGGAR JH. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Circ Physiol. 2012;302:H2190–H2210. doi: 10.1152/ajpheart.01146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATALI AJ, WILSON LA, PECKHAM M, TURNER DL, HARRISON SM, WHITE E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol. 2002;541:863–875. doi: 10.1113/jphysiol.2001.013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAYLER WG. The significance of calcium ions in cardiac excitation and contraction. Am Heart J. 1963;65:404–411. doi: 10.1016/0002-8703(63)90016-4. [DOI] [PubMed] [Google Scholar]
- NETTICADAN T, TEMSAH R, OSADA M, DHALLA NS. Status of Ca2+/calmodulin protein kinase phosphorylation of cardiac SR proteins in ischemic-reperfusion. Am J Physiol. 1999;277:C384–C391. doi: 10.1152/ajpcell.1999.277.3.C384. [DOI] [PubMed] [Google Scholar]
- NISHI M, MIZUSHIMA A, NAKAGAWARA KI, TAKESHIMA H. Characterization of human junctophilin subtype genes. Biochem Biophys Res Commun. 2000;273:920–927. doi: 10.1006/bbrc.2000.3011. [DOI] [PubMed] [Google Scholar]
- NORRBOM J, SUNDBERG CJ, AMELN H, KRAUS WE, JANSSON E, GUSTAFSSON T. PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol. 2004;96:189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- NORRBOM J, WALLMAN SE, GUSTAFSSON T, RUNDQVIST H, JANSSON E, SUNDBERG CJ. Training response of mitochondrial transcription factors in human skeletal muscle. Acta Physiol. 2010;198:71–79. doi: 10.1111/j.1748-1716.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- NOVOTOVA M, ZAHRADNIKOVA A, JR, NICHTOVA Z, KRALOVA E, STANKOVICOVA T, ZAHRADNIKOVA A, ZAHRADNIK I. Structural variability of dyads relates to calcium release in rat ventricular myocytes. Sci Rep. 2020;10:8076. doi: 10.1038/s41598-020-64840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODERMATT A, TASCHNER PE, SCHERER SW, BEATTY B, KHANNA VK, CORNBLATH DR, CHAUDHRY V, YEE WC, SCHRANK B, KARPATI G, BREUNING MH, KNOERS N, MACLENNAN DH. Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated with SERCA1: absence of structural mutations in five patients with Brody disease. Genomics. 1997;45:541–553. doi: 10.1006/geno.1997.4967. [DOI] [PubMed] [Google Scholar]
- OJAMAA K, KENESSEY A, KLEIN I. Thyroid hormone regulation of phospholamban phosphorylation in the rat heart. Endocrinology. 2000;141:2139–2144. doi: 10.1210/endo.141.6.7514. [DOI] [PubMed] [Google Scholar]
- OTSU K, FUJII J, DIFILIPPANTONIO M, UPPENDER M, WARD DC, MacLENNAN DH. Chromosome mapping of five human cardiac and skeletal muscle sarcoplasmic reticulum protein genes. Genomics. 1993;17:507–509. doi: 10.1006/geno.1993.1357. [DOI] [PubMed] [Google Scholar]
- PAPP B, CORVAZIER E, MAGNIER C, KOVACS T, BOURDEAU N, LEVY-TOLEDANO S, BREDOUX R, LEVY B, POITEVIN P, LOMPRE AM. Spontaneously hypertensive rats and platelet Ca2+-ATPases: specific up-regulation of the 97 kDa isoform. Biochem J. 1993;295:685–690. doi: 10.1042/bj2950685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK WJ, OH JG. SERCA2a: a prime target for modulation of cardiac contractility during heart failure. BMB Rep. 2013;46:237. doi: 10.5483/bmbrep.2013.46.5.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERIASAMY M, BHUPATHY P, BABU GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- PERIASAMY M, BHUPATHY P, BABU GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- PERIASAMY M, HUKE S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001;33:1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- PERIASAMY M, KALYANASUNDARAM A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- PERIASAMY M, REED TD, LIU LH, JI Y, LOUKIANOV E, PAUL RJ, NIEMAN ML, RIDDLE T, DUFFY JJ, DOETSCHMAN T, LORENZ JN, SHULL GE. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco (endo) plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999;274:2556–2562. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- PERNI S, CLOSE M, FRANZINI-ARMSTRONG C. Novel details of calsequestrin gel conformation in situ. J Biol Chem. 2013;288:31358–31362. doi: 10.1074/jbc.M113.507749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISCHON T, GIRMAN CJ, HOTAMISLIGIL GS, RIFAI N, HU FB, RIMM EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- POULET C, SANCHEZ-ALONSO J, SWIATLOWSKA P, MOUY F, LUCARELLI C, ALVAREZ-LAVIADA A, GROSS P, TERRACCIANO C, HOUSER S, GORELIK J. Junctophilin-2 tethers t-tubules and recruits functional L-type calcium channels to lipid rafts in adult cardiomyocytes. 2021;117:149–161. doi: 10.1093/cvr/cvaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIMEAU JO, ARMANIOUS GP, FISHER ME, YOUNG HS. The sarcoendoplasmic reticulum calcium ATPase. Subcell Biochem. 2018;87:229–258. doi: 10.1007/978-981-10-7757-9_8. [DOI] [PubMed] [Google Scholar]
- PRINS D, MICHALAK M. Organellar calcium buffers. Cold Spring Harb Perspect Biol. 2011;3:1–16. doi: 10.1101/cshperspect.a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIORI SG, NAPOLITANO C, MEMMI M, COLOMBI B, DRAGO F, GASPARINI M, DESIMONE L, COLTORTI F, BLOISE R, KEEGAN R, CRUZ FILHO FES, VIGNATI G, BENATAR A, DELOGU A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- QI H, MORAN MM, NAVARRO B, CHONG JA, KRAPIVINSKY G, KRAPIVINSKY L, KIRICHOK Y, RAMSEY IS, QUILL TA, CLAPHAM DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANI S, PARK CS, SREENIVASAIAH PK, KIM DH. Characterization of Ca2+-dependent protein-protein interactions within the Ca2+ release units of cardiac sarcoplasmic reticulum. Mol Cells. 2016;39:149–155. doi: 10.14348/molcells.2016.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZZAQ A, ROBINSON IM, MCMAHON HT, SKEPPER JN, SU Y, ZELHOF AC, JACKSON AP, GAY NJ, O’KANE CJ. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 2001;15:2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDISH FN, MILLER CL, GORKHALI R, YANG JJ. Calcium dynamics mediated by the endoplasmic/sarcoplasmic reticulum and related diseases. Intl J Mol Sci. 2017;18:1024. doi: 10.3390/ijms18051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REED TD, BABU GJ, JI Y, ZILBERMAN A, VER HEYEN M, WUYTACK F, PERIASAMY M. The expression of SR calcium transport ATPase and the Na+/Ca2+ exchanger are antithetically regulated during mouse cardiac development and in hypo/hyperthyroidism. J Mol Cell Cardiol. 2000;32:453–464. doi: 10.1006/jmcc.1999.1095. [DOI] [PubMed] [Google Scholar]
- RINGER S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol (London) 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROHRER D, DILLMANN WH. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem. 1988;263:6941–6944. [PubMed] [Google Scholar]
- ROSSI D, BARONE V, GIACOMELLO E, CUSIMANO V, SORRENTINO V. The sarcoplasmic reticulum: An organized patchwork of specialized domains. Traffic. 2008;9:1044–1049. doi: 10.1111/j.1600-0854.2008.00717.x. [DOI] [PubMed] [Google Scholar]
- RUIZ-MEANA M, MINGUET M, BOU-TEEN D, MIRO-CASAS E, CASTANS C, CASTELLANO J, BONZON-KULICHENKO E, IGUAL A, RODRIGUEZ-LECOQ R, VAZQUEZ J, GARCIA-DORADO D. Ryanodine receptor glycation favors mitochondrial damage in the senescent heart. Circulation. 2019;139:949–964. doi: 10.1161/CIRCULATIONAHA.118.035869. [DOI] [PubMed] [Google Scholar]
- SACK MN. The role of SIRT3 in mitochondrial homeostasis and cardiac adaptation to hypertrophy and aging. J Mol Cell Cardiol. 2012;52:520–525. doi: 10.1016/j.yjmcc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHOO SK, SHAIKH SA, SOPARIWALA DH, BAL NC, PERIASAMY M. Sarcolipin protein interaction with sarco (endo) plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem. 2013;288:6881–6889. doi: 10.1074/jbc.M112.436915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTULLI G, LEWIS D, DES GEORGES A, MARKS AR, FRANK J. Ryanodine receptor structure and function in health and disease. Subcell Biochem. 2018;87:329–52. doi: 10.1007/978-981-10-7757-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTULLI G, LEWIS DR, MARKS AR. Physiology and pathophysiology of excitation-contraction coupling: the functional role of ryanodine receptor. J Muscle Res Cell Motil. 2017a;38:37–45. doi: 10.1007/s10974-017-9470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTULLI G, NAKASHIMA R, YUAN Q, MARKS AR. Intracellular calcium release channels: an update. J Physiol. 2017b;595:3041–3051. doi: 10.1113/JP272781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTULLI G, XIE W, REIKEN SR, MARKS AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATOH K, MATSU-URA T, ENOMOTO M, NAKAMURA H, MICHIKAWA T, MIKOSHIBA K. Highly cooperative dependence of sarco/endoplasmic reticulum calcium ATPase (SERCA) 2a pump activity on cytosolic calcium in living cells. J Biol Chem. 2011;286:20591–20599. doi: 10.1074/jbc.M110.204685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDEL M, LAI FA, ZISSIMOPOULOS S. Structural and functional interactions within ryanodine receptor. Biochem Soc Trans. 2015;43:377–383. doi: 10.1042/BST20140292. [DOI] [PubMed] [Google Scholar]
- SEPULVEDA M, BURGOS JI, CIOCCI PARDO A, GONZALEZ-ARBELAEZ L, MOSCA S, VILA PETROFF M. CaMKII-dependent ryanodine receptor phosphorylation mediates sepsis-induced cardiomyocyte apoptosis. J Cell Mol Med. 2020;24:9627–9637. doi: 10.1111/jcmm.15470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEPULVEDA M, GONANO LA, VIOTTI M, MORELL M, BLANCO P, LOPEZ ALARCON M, PEROBA RAMOS I, BASTOS CARVALHO A, MEDEI E, VILA PETROFF M. Calcium/calmodulin protein kinase-II-dependent ryanodine receptor phosphorylation mediates cardiac contractile. Crit Care Med. 2017;45:e399–e408. doi: 10.1097/CCM.0000000000002101. [DOI] [PubMed] [Google Scholar]
- SHAIKH SA, SAHOO SK, PERIASAMY M. Phospholamban and sarcoplipin: are they functionally redundant or distinct regulator of the sarco(endo)plasmic reticulum calcium ATPase? Mol Cell Cardiol. 2016;91:81–91. doi: 10.1016/j.yjmcc.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEARD TMD, KHARCHE SR, PINALI C, SHIELS HA. 3D ultrastructural organisation of calcium release units in the avian sarcoplasmic reticulum. J Exp Biol. 2019;222(Pt 7):jeb197640. doi: 10.1242/jeb.197640. [DOI] [PubMed] [Google Scholar]
- SHIBATA R, MUROHARA T, OUCHI N. Protective role of adiponectin in cardiovascular disease. Curr Med Chem. 2012;19:5459–5466. doi: 10.2174/092986712803833164. [DOI] [PubMed] [Google Scholar]
- SIMMERMAN HK, JONES LR. Phospholamban: Protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- SINGAL PK, LEE SL, GANGULY PK, PANAGIA V, DHALLA NS. Reversibility of ultrastructural, contractile function and Ca2+ transport changes in guinea pig hearts after global ischemia. Can J Physiol Pharmacol. 1986;64:1368–1375. doi: 10.1139/y86-232. [DOI] [PubMed] [Google Scholar]
- SLACK JP, GRUPP IL, LUO W, KRANIAS EG. Phospholamban ablation enhances relaxation in the murine soleus. Am J Physiol. 1997;273:C1–C6. doi: 10.1152/ajpcell.1997.273.1.C1. [DOI] [PubMed] [Google Scholar]
- SMEJTEK P, WORD RC, SATTERFIELD LE. Electrophoretic mobility of sarcoplasmic reticulum vesicles - Analytical model includes amino acid residues of A + P + N domain of Ca2+-ATPase and charged lipids. Biochim Biophys Acta - Biomembr. 2014;1838:766–775. doi: 10.1016/j.bbamem.2013.09.019. [DOI] [PubMed] [Google Scholar]
- SMITH IC, BOMBARDIER E, VIGNA C, TUPLING AR. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PLoS One. 2013;8:e68924. doi: 10.1371/journal.pone.0068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMMER JR. The anatomy of the sarcoplasmic reticulum in vertebrate skeletal muscle: Its implications for excitation contraction coupling. Zeitschrift fur Naturforsch - Sect C J Biosci. 1982;37:665–678. doi: 10.1515/znc-1982-7-816. [DOI] [PubMed] [Google Scholar]
- SPINOZZI S, LIU C, CHEN Z, FENG W, ZHANG L, OUYANG K, EVANS SM, CHEN J. Nexilin is necessary for maintaining the transverse-axial tubular system in adult cardiomyocytes. Circ Heart Fail. 2020;13:e006935. doi: 10.1161/CIRCHEARTFAILURE.120.006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMBOULIAN S, MOUTIN MJ, TREVES S, POCHON N, GRUNWALD D, ZORZATO F, DE WAARD M, RONJAT M, ARNOULT C. Junctate, an inositol 1,4,5-triphosphate receptor associated protein, is present in rodent sperm and binds TRPC2 and TRPC5 but nit TRPC1 channels. Dev Biol. 2005;286:326–337. doi: 10.1016/j.ydbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- STAMMERS AN, SUSSER SE, HAMM NC, HLYNSKY MW, KIMBER DE, KEHLER DS, DUHAMEL TA. The regulation of sarco (endo) plasmic reticulum calcium-ATPases (SERCA) Can J Physiol Pharmacol. 2015;93:843–854. doi: 10.1139/cjpp-2014-0463. [DOI] [PubMed] [Google Scholar]
- SUAREZ J, HU Y, MAKINO A, FRICOVSKY E, WANG H, DILLMANN WH. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. Am J Physiol Physiol. 2008;295:C1561–C1568. doi: 10.1152/ajpcell.00076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULAKHE PV, DHALLA NS. Excitation-contraction coupling in heart. VII. Calcium accumulation in subcellular particles in congestive heart failure. J Clin Invest. 1971;50:1019–1027. doi: 10.1172/JCI106573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYNETOS A, STATHOGIANNIS K, PAPANIKOLAOU A, DRAKOPOULOU M, TRANTALIS G, KAITOZIS O, LATSIOS G, GIANNOPOULOS G, DEFTEREOS S, TOUTOUZAS K, TOUSOULIS D. Therapeutic applications of calcium metabolism modulation in heart disease. Med Chem. 2016;12:177–183. doi: 10.2174/157340641202160209103313. [DOI] [PubMed] [Google Scholar]
- TADA M, YAMAMOTO T, TONOMURA Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978;58:1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- TAKESHIMA H, KOMAZAKI S, NISHI M, LINO M, KANGAWA K. Junctophilins: A novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- TAKIZAWA T, ARAI M, TOMARU K, KOITABASHI N, BAKER DL, PERIASAMY M, KURABAYASHI M. Transcription factor Sp1 regulates SERCA2 gene expression in pressure-overloaded hearts: a study using in vivo direct gene transfer into living myocardium. J Mol Cell Cardiol. 2003;35:777–783. doi: 10.1016/s0022-2828(03)00122-6. [DOI] [PubMed] [Google Scholar]
- TANG WH, KRAVTSOV GM, SAUERT M, TONG XY, HOU XY, WONG TM, CHUNG SK, MAN CHUNG SS. Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins. J Mol Cell Cardiol. 2010;49:58–69. doi: 10.1016/j.yjmcc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERENTYEV D, HAMILTON S. Regulation of sarcoplasmic reticulum Ca2+ release by serine-threonine phosphatases in the heart. J Mol Cell Cardiol. 2016;101:156–164. doi: 10.1016/j.yjmcc.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TER KEURS HEDJ, BOYDEN PA. Calcium and arrhythmias. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEILEN NT, KUNKEL GH, TYAGI SC. The role of exercise and TFAM in preventing skeletal muscle atrophy. J Cell Physiol. 2017;232:2348–2358. doi: 10.1002/jcp.25737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG X, YING J, PIMENTEL DR, TRUCILLO M, ADACHI T, COHEN RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOYOSHIMA C, INESI G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. doi: 10.1146/annurev.biochem.73.011303.073700. [DOI] [PubMed] [Google Scholar]
- TOYOSHIMA C, NOMURA H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature. 2002;418:605–611. doi: 10.1038/nature00944. [DOI] [PubMed] [Google Scholar]
- TOYOSHIMA C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta. 2009;1793:941–946. doi: 10.1016/j.bbamcr.2008.10.008. [DOI] [PubMed] [Google Scholar]
- TREVES S, FERIOTTO G, MOCCAGATTA L, GAMBARI R, ZORZATO F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. J Biol Chem. 2000;275:39555–39568. doi: 10.1074/jbc.M005473200. [DOI] [PubMed] [Google Scholar]
- TREVES S, FRANZINI-ARMSTRONG C, MOCCAGATTA L, ARNOULT C, GRASSO C, SCHRUM A, DUCREUX S, ZHU MX, MIKOSHIBA K, GIRARD T, SMIDA-REZGUI S, RONJAT M, ZORZATO F. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol. 2004;166:537–548. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVES S, VUKCEVIC M, MAJ M, THURNHEER R, MOSCA B, ZORZATO F. Minor sarcoplasmic reticulum membrane components that modulate excitation-contraction coupling in striated muscles. J Physiol. 2009;587:3071–3079. doi: 10.1113/jphysiol.2009.171876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUPLING AR, GRAMOLINI AO, DUHAMEL TA, KONDO H, ASAHI M, TSUCHIYA SC, BORRELLI MJ, LEPOCK JR, OSTU K, HORI M, MACLENNAN DH, GREEN HJ. HSP70 binds to the fast-twitch skeletal muscle sarco (endo) plasmic reticulum Ca2+-ATPase (SERCA1a) and prevents thermal inactivation. J Biol Chem. 2004;279:52382–52389. doi: 10.1074/jbc.M409336200. [DOI] [PubMed] [Google Scholar]
- VALDIVIA HH. One gene, many proteins: Alternative splicing of the ryanodine receptor gene adds novel functions to an already complex channel protein. irc Res. 2007;100:761–763. doi: 10.1161/01.RES.0000263400.64391.37. [DOI] [PubMed] [Google Scholar]
- VAN PETEGEM F. How to open a ryanodine receptor. Cell Res. 2016;26:1073–1074. doi: 10.1038/cr.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN PETEGEM F. Ryanodine receptors: Structure and function. J Biol Chem. 2012;287:31624–31632. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLARREAL-MOLINA MT, ANTUNA-PUENTE B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94:2143–2149. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- VOSS J, JONES LR, THOMAS DD. The physical mechanism of calcium pump regulation in the heart. Biophys J. 1994;67:190–196. doi: 10.1016/S0006-3495(94)80469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHLQUIST C, JEONG D, ROJAS-MUNOZ A, KHO C, LEE A, MITSUYAMA S, VAN MIL A, PARK WJ, SLUIJTER JPG, DOEVENDANS PAF, HAJJAR RJ, MERCOLA M. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–535. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q, GROENENDYK J, PASKEVICIUS T, QIN W, KOR KC, LIU Y, HIESS F, KNOLLMANN BC, CHEN SRW, TANG J, CHEN XZ, AGELLON LB, MICHALAK M. Two pools of IRE1α in cardiac and skeletal muscle cells. FASEB J. 2019;33:8892–8904. doi: 10.1096/fj.201802626r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q, MICHALAK M. Calsequestrin. Structure, function and evolution. Cell Calcium. 2020;90:102242. doi: 10.1016/j.ceca.2020.102242. [DOI] [PubMed] [Google Scholar]
- WATANABE A, ARAI M, KOITABASHI N, NIWANO K, OHYAMA Y, YAMADA Y, KATO N, KURABAYASHI M. Mitochondrial transcription factors TFAM and TFB2M regulate SERCA2 gene transcription. Cardiovasc Res. 2011;90:57–67. doi: 10.1093/cvr/cvq374. [DOI] [PubMed] [Google Scholar]
- WATSON LJ, FACUNDO HT, NGOH GA, AMEEN M, BRAINARD RE, LEMMA KM, LONG BW, PRABHU SD, XUAN YT, JONES SP. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEHRENS XHT, MARKS AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- WESCOTT AP, JARFI MS, LEDERER WJ, WILLIAMS GS. Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling. Mol Cell Cardiol. 2016;92:82–92. doi: 10.1016/j.yjmcc.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISLØFF U, LOENNECHEN JP, FALCK G, BEISVAG V, CURRIE S, SMITH G, ELLINGSEN O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res. 2001;50:495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- WLEKLINSKI MJ, KANNANKERIL PJ, KNOLLMAN BC. Molecular and tissue mechanisms of catecholaminergic polymorphic ventricular tachycardia. J Physiol. 2020;598:2817–2834. doi: 10.1113/JP276757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO JS, HWANG JH, HUANG M, AHN MK, CHO CH, MA J, LEE EH. Interaction between mitsugumin 29 and TRPC3 participates in regulating Ca2+ transients in skeletal muscle. Biochem Biophys Res Commun. 2015;464:133–139. doi: 10.1016/j.bbrc.2015.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO JS, JEONG SY, PARK JH, CHOI JH, LEE EH. Calsequestrin: a well-known but curios protein in skeletal muscle. Exp Mol Med. 2020;52:1908–1925. doi: 10.1038/s12276-020-00535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUYTACK F, RAEYMAEKERS L, MISSIAEN L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium. 2002;32:279–305. doi: 10.1016/s0143416002001847. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI N. Molecular insights into calcium dependent regulation of ryanodine receptor calcium release channels. Adv Exp Med Biol. 2020;1131:321–336. doi: 10.1007/978-3-030-12457-1_13. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI D, TABARA Y, KITA S, HANADA H, KOMAZAKI S, NAITOU D, MISHIMA A, NISHI M, YAMAMURA H, YAMAMOTO S, KAKIZAWA S, MIYACHI H, YAMAMOTO S, MIYATA T, KAWANO Y, KAMIDE K, OGIHARA T, HATA A, UMEMURA S, SOMA M, TAKAHASHI N, IMAIZUMI Y, MIKI T, IWAMOTO T, TAKESIMA H. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011;14:231–341. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- YAZAWA M, FERRANTE C, FENG J, MIO K, OGURA T, ZHANG M, LIN PH, PANZ KOMAZAKI S, KATO K, NISHI M, ZHAO X, WEISLEDER N, SATO C, MA J, TAKESHIMA H. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature. 2007;448:78–82. doi: 10.1038/nature05928. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M, MINAMISAWA S, SHIMURA M, KOMAZAKI S, KUME H, ZHANG M, MATSUMURA K, NISHI M, SAITO M, SAEKI Y, ISHIKAWA Y, YANAGISAWA T, TAKESHIMA H. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J Biol Chem. 2005;280:3500–3506. doi: 10.1074/jbc.M406618200. [DOI] [PubMed] [Google Scholar]
- ZARAIN-HERZBERG A, MARQUES J, SUKOVICH D, PERIASAMY M. Thyroid hormone receptor modulates the expression of the rabbit cardiac sarco (endo) plasmic reticulum Ca2+-ATPase gene. J Biol Chem. 1994;269:1460–1467. [PubMed] [Google Scholar]
- ZARAIN-HERZBERG A. Regulation of the sarcoplasmic reticulum Ca2+-ATPase expression in the hypertrophic and failing heart. Can J Physiol Pharmacol. 2006;84:509–521. doi: 10.1139/y06-023. [DOI] [PubMed] [Google Scholar]
- ZHANG Y, WANG XL, ZHAO J, WANG YJ, LAU WB, YUAN YX, GAO EH, KOCH WJ, MA XL. Adiponectin inhibits oxidative/nitrative stress during myocardial ischemia and reperfusion via PKA signaling. Am J Physiol Endocrinol Metab. 2013;305:E1436–E1443. doi: 10.1152/ajpendo.00445.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG J, YNACEY DM, AHMED MI, WEI CC, POWELL PC, SHANMUGAM M, GUPTA H, LLOYD SG, MCGIFFIN DC, SCHIROS CG, DENNEY TS, JR, BABU GJ, DELL’ITALIA LJ. Increased sarcolipin expression and adrenergic drive in humans with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation. Circ Heart Fail. 2014;7:194–202. doi: 10.1161/CIRCHEARTFAILURE.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHIHAO L, JINGYU N, LAN L, MICHAEL S, RUI G, XIYUN B, XIAOZHI L, GUANWEI F. SERCA2a: a key protein in the Ca2+ cycle of the heart failure. Heart Fail Rev. 2020;25:523–535. doi: 10.1007/s10741-019-09873-3. [DOI] [PubMed] [Google Scholar]
- ZHOU X, LI A, LIN PH, ZHOU J, MA J. TRIC-A regulates intracellular Ca2+ homeostasis in cardiomyocytes. Pflugers Arch. 2021;473:547–556. doi: 10.1007/s00424-021-02513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X, LIN P, YAMAZAKI D, PARK KH, KOMAZAKI S, CHEN SR, TAKESHIMA H, MA J. TRIC Channels and sarcoplasmic/endoplasmic reticulum calcium homeostasis. Circ Res. 2014;114:706–716. doi: 10.1161/CIRCRESAHA.114.301816.TRIC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X, PARK KH, YAMAZAKI D, LIN PH, NISHI M, MA Z, QIU L, MURAYAMA T, ZOU X, TAKESHIMA H, ZHOU J, MA J. TRIC-A channel maintains store calcium handling by interacting with type 2 ryanodine receptor in cardiac muscle. Circ Res. 2020;126:417–435. doi: 10.1161/CIRCRESAHA.119.316241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMA AV, MAZUREK SR. Functional impact of ryanodine receptor oxidation on intracellular calcium regulation in the heart. Rev Physiol Biochem Pharmacol. 2016;171:39–62. doi: 10.1007/112_2016_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN ANE, HULSMANN WC. Paradoxical influence of calcium ions on the permeability of the cell membrane of the isolated rat heart. Nature. 1966;211:646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]
- ZSEBO K, YAROSHINSKY A, RUDY JJ, WAGNER K, GREENBERG B, JESSUP M, HAJJAR RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]