Abstract
The antimicrobial activity of the intraurethrally administered probiotic Lactobacillus casei strain Shirota against Escherichia coli in a murine urinary tract infection (UTI) model was examined. UTI was induced by intraurethral administration of Escherichia coli strain HU-1 (a clinical isolate from a UTI patient, positive for type 1 and P fimbriae), at a dose of 1 × 106 to 2 × 106 CFU in 20 μl of saline, into a C3H/HeN mouse bladder which had been traumatized with 0.1 N HCl followed immediately by neutralization with 0.1 N NaOH 24 h before the challenge infection. Chronic infection with the pathogen at 106 CFU in the urinary tract (bladder and kidneys) was maintained for more than 3 weeks after the challenge, and the number of polymorphonuclear leukocytes and myeloperoxidase activity in the urine were markedly elevated during the infection period. A single administration of L. casei Shirota at a dose of 108 CFU 24 h before the challenge infection dramatically inhibited E. coli growth and inflammatory responses in the urinary tract. Multiple daily treatments with L. casei Shirota during the postinfection period also showed antimicrobial activity in this UTI model. A heat-killed preparation of L. casei Shirota exerted significant antimicrobial effects not only with a single pretreatment (100 μg/mouse) but also with multiple daily treatments during the postinfection period. The other Lactobacillus strains tested, i.e., L. fermentum ATCC 14931T, L. jensenii ATCC 25258T, L. plantarum ATCC 14917T, and L. reuteri JCM 1112T, had no significant antimicrobial activity. Taken together, these results suggest that the probiotic L. casei strain Shirota is a potent therapeutic agent for UTI.
Urinary tract infection (UTI) is the most common bacterial infection seen in clinical practice. Human UTI comprises disease entities such as acute pyelonephritis with renal parenchymal involvement, cystitis limited to the urinary bladder, and asymptomatic bacteruria. Enterobacteriaceae such as Escherichia coli, which are normal inhabitants of human intestines, account for the vast majority of these uncomplicated infections (37, 65). Appropriate hygiene and cleanliness of the genital area are therefore recommended for prevention of UTI. On the other hand, studies have shown a correlation between a loss or disruption of the normal genital microflora, in particular Lactobacillus species, and an increased incidence of genital and bladder infections (57). Preclinical and clinical reports have focused on lactobacillus strains, their possible prophylactic effects against experimental E. coli infection, and the use of these strains for the prevention of human urogenital infections (7, 12, 17, 59, 60).
Suitable animal experimental models are required for appropriate preclinical studies of UTIs. Hagberg et al. were the first to show that mice could be challenged intravesically (by introducing pathogens directly into the bladder) without further manipulations of the urinary tract (18), and the murine model of ascending pyelonephritis has served as an excellent tool for defining the roles of individual virulence factors in the pathogenesis of UTI (18, 23, 25, 26, 28, 61). It should be noted, however, that the inoculum doses used in murine models are very high (108 CFU). Furthermore, high bladder infection levels reportedly persisted over the 14-day study period only in C3H/HeJ and C3H/OuJ mice, which are lipopolysaccharide (LPS) nonresponder strains, while strains such as C3H/HeN, C57BL/6, BALB/c, DBA.1, DBA.2, and AKR showed progressive resolution of bladder infections over a 14-day period (23, 24). Therefore, an appropriate model in which chronic UTI can be induced with a lower inoculum of E. coli, regardless of differences in genetic backgrounds, is needed.
In the present report, we first describe an improved murine chronic infection model of UTI, in which the infection was induced by traumatization of the bladder mucosa with inorganic acid and subsequent neutralization, followed by a single infusion of only 1 × 106 to 2 × 106 CFU of E. coli into the bladder. Chemical pretreatment of the bladder cavity ensured persistent infection without induction of systemic infection, and chronic infection was equally inducible in C3H/HeN and C3H/HeJ strains, which have been shown to differ in susceptibility to UTI (23). Using the improved murine urethral infection model, we investigated the antimicrobial effects of intravesically administered Lactobacillus casei strain Shirota, which is a well-documented probiotic strain (40). Intraurethral treatment with L. casei Shirota (108 CFU/day) inhibited pathogen growth in the urinary tract and suppressed infection-induced inflammatory responses. The characteristics of this antimicrobial activity included (i) a heat-killed (HK) preparation of L. casei Shirota effectively lowering levels of infectious bacteria and (ii) effectiveness of treatment during the postinfection period. These results suggest that the probiotic L. casei strain Shirota is potentially useful for both preventive and therapeutic treatment of UTI.
MATERIALS AND METHODS
Pathogens.
Three E. coli strains were used. Two strains of E. coli (HU-1 and HU-2) isolated individually from urine cultures of patients with clinical symptoms were kindly provided by Toshihiko Mayumi, School of Medicine, Nagoya University, Nagoya, Japan. One strain (RI-1) was isolated from a rabbit intestine. The following characteristics of the bacterial strains were assessed: type I fimbriae (48), by mannose-sensitive agglutination of guinea pig erythrocytes; P fimbriae, by mannose-resistant agglutination of human type O erythrocytes (48); production of hemolysin, by qualitative evaluation using 5% sheep blood agar plates; capsule formation, by capsule stain (6). For the challenge experiments, E. coli strains were cultured overnight in brain heart infusion broth (Difco Laboratories, Detroit, Mch.). After being washed with phosphate-buffered saline (PBS) by centrifugation, bacterial cells were resuspended in PBS and adjusted to approximately 5 × 107 to 10 × 107 CFU/ml by comparison to McFarland turbidity standards confirmed by enumeration using the spread plate technique.
Murine model of UTI.
Specific-pathogen-free female C3H/HeN and C3H/HeJ mice at 7 weeks of age were purchased from Japan SLC, Inc., Shizuoka, Japan. The mice were housed under barrier-sustained conditions, with temperature (25 ± 0.5°C), humidity (55 ± 5%), and light (14 h of light and 10 h of darkness) automatically controlled, and were kept in polypropylene cages (CLEA Japan, Inc., Tokyo, Japan) with stainless steel lids and sterilized bedding. The mice were maintained on an MF diet (Oriental Yeast Co., Ltd., Tokyo, Japan) and sterilized water (126°C for 30 min), which contained Cl2 at a final concentration of 1.5 ppm (1.5 μg/ml).
The mice were anesthetized by administration of sodium pentobarbital (0.05 mg/g of body weight). After sterilization of the periurethral area with 70% ethanol, a sterile 24-gauge Teflon catheter (outer diameter, 0.7 mm; length, 19 mm; Becton Dickinson Infusion Therapy System, Inc., Sandy, Utah) was inserted into the bladder through the urethra. Before inoculation of bacteria, the bladder mucosa was traumatized by infusing 100 μl of 0.1 N HCl solution for 45 s, followed by neutralization with 100 μl of 0.1 N KOH and flushing with sterile saline through a tuberculin syringe (8). A 20-μl inoculum containing 1 × 106 to 2 × 106 organisms was then infused into the bladder through a catheter over 30 s through a microsyringe (GASTIGHT syringe; Hamilton Company, Reno, Nev.) 24 h after the bladder mucosa traumatization.
Lactobacilli.
L. casei strain Shirota, L. fermentum ATCC 14931T, L. jensenii ATCC 25258T, L. plantarum ATCC 14917T, and L. reuteri JCM 1112T were used. Each lactobacillus strain was cultivated in MRS broth (Difco) at 37°C for 24 h, washed with distilled water, and resuspended in saline at a concentration of 5 × 109 to 10 × 109 CFU/ml. For preparation of an HK bacterial suspension, the harvested cells of lactobacilli suspended in distilled water at a concentration of 5 × 109 to 10 × 109 CFU/ml were heated at 100°C for 30 min and then cooled on ice. A 20-μl inoculum of the lactobacillus suspension was infused into the bladder through the catheter over 30 s using a microsyringe. As treatment prior to the infection with E. coli, mice received a single administration of lactobacilli 15 min after the chemical treatment. As postinfection treatments, mice received once-daily intravesical inoculations of HK L. casei Shirota starting on day 1, 4, or 7 after the E. coli inoculation. Mice in the control group received saline instead of the lactobacillus suspension on the same schedule as the lactobacillus-treated groups.
Bacteriological analysis.
To determine the number of viable bacteria in organs, six to eight mice per group were killed after being anesthetized with ether. Bladder and kidneys were removed aseptically, placed in grinding tubes containing 1 and 5 ml of saline, respectively, and homogenized with a Teflon grinder. After serial dilution of the homogenates with saline, 50-μl portions were spread onto the following media. DHL agar (Nissui Seiyaku, Tokyo, Japan) was used for selective isolation of E. coli. LLV agar (72) and MRS agar (Difco) were used for selective isolation of inoculated L. casei Shirota and other lactobacillus strains, respectively. The plates were incubated aerobically for 24 h (DHL) or anaerobically for 48 h (LLV and MRS). Results are expressed as the mean number of bacteria in the entire organ ± the standard deviation (SD). The lower limit of bacterial detection by this procedure was 100 CFU (kidney) or 20 CFU (bladder).
Adhesive properties of lactobacilli. (i) Adhesion to MBT-2 cells.
MBT-2, a mouse transitional cell carcinoma of the bladder (64), was used to test lactobacillus adhesion. Confluent monolayers of MBT-2 cells in Dulbecco's modified Eagle's minimal essential medium (DMEM; GIBCO-BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS; GIBCO-BRL) were prepared in two-chamber slides (Lab-Tek chamber slide; Nalge Nunc International, Naperville, Ill.). The slides were washed three times with 1 ml of sterile PBS before the assay. Two milliliters of HK lactobacillus strains, prepared as described above, at concentrations ranging from 5.0 × 107 to 5.5 × 108 CFU/ml in the medium, were added to each chamber of the two-chamber slide and incubated at 37°C in an atmosphere of 5% air and 95% CO2, with gentle rocking. After incubation for 60 min, the monolayers were washed three times with sterile PBS (pH 7.2), fixed with methanol, stained with Giemsa stain (Sigma Chemicals, St. Louis, Mo.), and examined microscopically. The assay was conducted in duplicate, and the number of bacteria adhering to 100 MBT-2 cells was counted for 10 randomly selected microscopic fields in each well. Results are expressed as the number of bacteria bound per 100 MBT-2 cells versus the concentration of bacteria added (CFU per milliliter).
(ii) Platelet aggregometry.
Peripheral blood was drawn from the antecubital veins of two healthy volunteers. The fresh blood was immediately mixed with 0.1 M trisodium citrate (9:1 vol/vol). Platelet-rich plasma (PRP) was prepared by centrifugation of the citrated blood sample at 100 × g at 22°C for 10 min; platelet-poor plasma (PPP) was purified by further centrifugation at 2,350 × g at 22°C for 10 min (21). The PRP was then adjusted with the PPP to an optical density at 660 nm (OD660) of 0.35 ± 0.01. Platelet aggregation was carried out in a recording aggregometer (NKK hematracer 2; Nico Bioscience, Inc., Tokyo, Japan) with light transmission through PPP representing 100% aggregation and that through PRP representing 0% aggregation. Platelet viability was confirmed by addition of the agonist ADP to PRP to a final concentration of 20 μM. The optimal ratio of bacteria to platelets was determined by varying the CFU per milliliter added to PRP and calculating the ratio yielding the maximum percentage aggregation. Bacteria were then examined for their ability to induce platelet aggregation by the addition of 25 μl of a bacterial suspension (109 CFU/ml) to 0.25 ml of PRP and PPP, preincubated at 37°C for 5 min. A lag phase longer than 25 min was assumed to represent negative aggregation. ADP was added to the strains negative for aggregation to confirm platelet function. All aggregations were carried out in duplicate, utilizing different batches of platelets, and the results given are averages (percent). Platelet aggregation was monitored for 25 min.
(iii) Fibronectin binding. Fibronectin from human plasma (Sigma) was labeled with 125I by the method of Markwell (41). The binding assay was carried out essentially as described by Willcox and Knox (68). Bacteria were washed three times in either PBS (pH 7.3) or 0.1 M citrate-phosphate buffer (pH 5.4), each containing 1% (wt/vol) bovine serum albumin (BSA; Sigma) and 0.05% (vol/vol) Tween 20. The cells, resuspended in buffer at a concentration of 109 CFU/ml, were (0.5 ml) placed in a microcentrifuge tube followed by 1 μg of 125I-labeled fibronectin. After incubation of the tubes at 37°C for 30 min, cells were pelleted and washed once by centrifugation at 5,000 × g for 10 min with PBS, followed by washing with 0.1 M citrate-phosphate buffer containing both BSA and Tween 20. The supernatant was removed, and both total radiolabel counts and radiolabel counts associated with the bacterial pellet were taken. The degree of binding was expressed as the amount of radiolabel associated with the pellet divided by the total amount of radiolabel added. Any radioactivity associated with the control tubes containing fibronectin only was subtracted from the test score. Radioactivity recovery was generally greater than 90% of that applied.
(iv) Adhesion to polystyrene. Adhesion to polystyrene was assayed as described previously (20). Briefly, bacterial-cell suspensions (100 μl) were added to three wells of a polystyrene microplate (ICN Pharmaceutics, Inc., Costa Mesa, Calif.), with one well containing PBS as the blank, and were left for 15 min at room temperature. The plate was then rinsed with distilled water horizontally to the flow of water, then stained with crystal violet for 15 min and dried. The OD660 values of the wells were then read on a model 3550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, Calif.). Negative polystyrene adhesion values arose from the control wells binding more crystal violet dye than the experimental wells.
(v) Salivary aggregation. Salivary aggregation was determined by spectrophotometric assay (69). Fresh whole saliva was centrifuged at 10,000 × g for 20 min, and triplicate volumes (0.5 ml) were mixed with an equal volume of bacterial-cell suspension. The OD660 was measured after incubation at 22°C for 0 to 2 h. Controls for the bacterial-cell suspension with no saliva were also run. The change in optical density of the triplicate samples, relative to the bacterial controls, gave the average aggregation by saliva. Negative aggregation values arose where gravitational deposition of bacteria occurred in the control tubes.
Inflammatory responses in urine.
After infection with E. coli, urine was collected from individual anesthetized mice. The number of leukocytes in urine was examined microscopically using a hemocytometer (62). For morphologic analysis, fresh urine samples were centrifuged once at 1,000 × g, and the pellet was resuspended in Hanks' balanced salt solution (GIBCO-BRL) supplemented with 10% FBS at a concentration of 105 cells/ml. The cells were spun onto glass slides in a Cytospin 11 centrifuge (Shandon Scientific Ltd., Runcorn, United Kingdom) at 100 × g for 5 min. The slides were air dried, stained with Giemsa stain (Sigma), and then inspected by light microscopy.
Myeloperoxidase (MPO) activity in urine was measured as that solubilized with hexadecyl trimethylammonium bromide (36). In brief, urination was achieved in nine mice in different groups under anesthesia, and 20-μl portions from three mice in the same group were combined. Then 50-μl portions of the combined urine were sonicated on ice and mixed with the same amount of enzyme substrate buffer (50 mM phosphate buffer [pH 6.0]) containing O-dianisidine hydrochloride (Sigma) and hydrogen peroxide at final concentrations of 0.167 mg/ml and 0.0005%, respectively. Changes in the absorbance at 455 nm were measured in relation to a substrate blank. Results are expressed as means and SDs of three samples (from nine mice).
Histopathological examinations.
Mice were dissected on day 4 after challenge infection with E. coli. Each bladder and kidney were divided longitudinally and fixed overnight in 10% neutral buffered formalin. Paraffin-embedded sections stained with hematoxylin and eosin or Gram stain were examined by light microscopy by a pathologist blinded to the infecting organism.
Statistical analysis.
Statistical differences between the control group and the treated group were evaluated with Fisher's exact probability test for the incidence of infection and Student's t test for other benchmarks. A P value of <0.05 was considered significant.
RESULTS
Improved murine model of E. coli UTI.
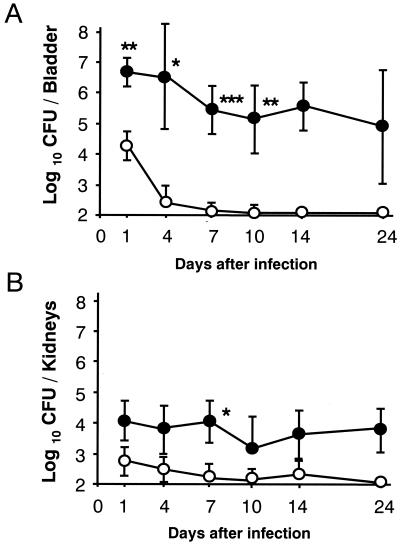
Without pretraumatization of the bladder, infusion of E. coli strain HU-1 at an inoculum size of 2 × 106 CFU into the bladder induced a local transitory infection in the mice, which terminated within a week after the challenge (Fig. 1). The intensity and duration of infection were dramatically increased by chemical treatment of the bladder 24 h before the bacterial challenge. A previous argument concerning the influence of inoculum size on possible reflux of the inoculated bacteria to the kidneys (27) prompted us to adopt an inoculum size of 20 μl, as well as to use a microsyringe for infusion, in order to minimize reflux. The viable count in the kidneys on day 1 after instillation was less than 1/100 of that in the bladder, suggesting that reflux of the pathogen by infusion treatment is minimal in this model. No viable counts were detected in the liver throughout the experimental period, suggesting that the infection was restricted to the urinary tract rather than becoming systemic (data not shown). The severity of UTI in the C3H/HeJ strain was greater than that in the C3H/HeN strain during both the initial phase (day 4) and the chronic phases (days 14 and 28 [Table 1]). Although the numbers of leukocytes in the urine before infection were under detectable levels in both strains (data not shown), increases in the number of leukocytes infiltrating the urine were detected in both strains at the chronic phases (Table 1), and more than 98% of the leukocytes were found by Giemsa staining to be neutrophils (data not shown).
FIG. 1.
A murine chronic UTI model. C3H/HeN mice were divided into two groups. Traumatization of the bladder was performed as described in Materials and Methods for one group (●), and another group (control) (○) was mock treated with saline. E. coli strain HU-1 at a dose of 2 × 106 CFU was infused into the bladders of anesthetized mice. The counts of viable bacteria in the bladder (A) and kidneys (B) were determined on days 1, 4, 7, 10, 14, and 24 after the challenge. Results are expressed as the means and SDs for six mice. Significant differences between the untreated controls and the treated group: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
TABLE 1.
Induction of UTI with E. coli in C3H/HeN and C3H/HeJ mice
| Days postinfectiona | Log10 viable bacteria in bladder (mean ± SD)b
|
Leukocyte concn in urine (104 cells/ml)c
|
||
|---|---|---|---|---|
| C3H/HeN | C3H/HeJ | C3H/HeN | C3H/HeJ | |
| 4 | 5.0 ± 0.1 | 7.0 ± 0.6 | NT | NT |
| 14 | 3.9 ± 0.6 | 5.3 ± 1.3 | 50 ± 18 | 42 ± 35 |
| 28 | 3.6 ± 1.1 | 5.1 ± 1.5 | 29 ± 3 | 61 ± 17 |
Mice were infected with E. coli strain HU-1 at an inoculum of 2 × 106 CFU and dissected on days 4, 14 and 28.
For eight mice per group.
Mean ± SD. Three mice per group were used. NT, not tested.
Preventive effect of intraurethrally administered L. casei Shirota on UTIs in mice.
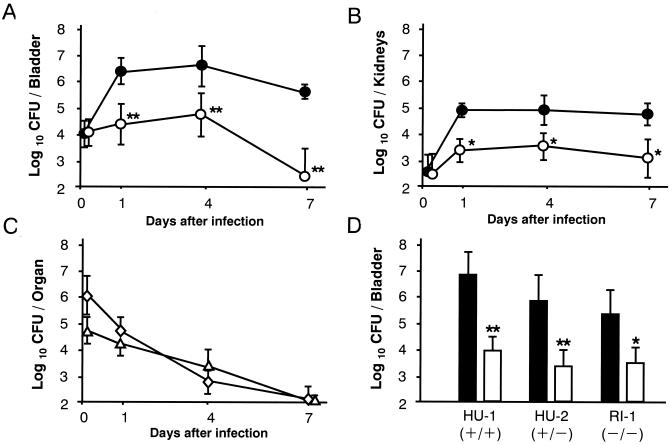
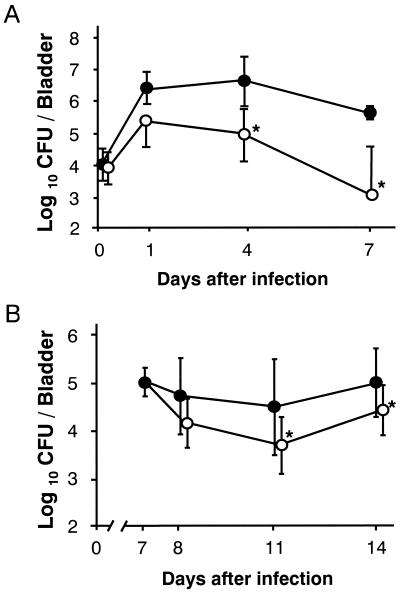
A single infusion of L. casei Shirota at a dose of 108 CFU 24 h before infection markedly inhibited E. coli growth in the urinary tract. Viable counts of E. coli in the bladder decreased to less than 1/100 of those in the control group at 24 h and approached the lower detection limit on day 7 after the challenge infection (Fig. 2A). Renal infection also subsided significantly with L. casei Shirota treatment (Fig. 2B). The total viable count of L. casei Shirota detected in the bladder and kidneys immediately after infection was about 1/100 of the inoculum and decreased logarithmically, reaching undetectable levels by day 7 (Fig. 2C). No viable E. coli or L. casei Shirota organisms were detected in the liver (data not shown). Three strains of E. coli, which have different T and P fimbrial expression patterns (all of them were hemolysin negative and not capsulated), showed somewhat different intensities of pathogenicity in this infection model. The pathogenicity of strain HU-1, which expresses both types of fimbriae, was strongest, while strain RI-1, which expresses neither type, showed significantly less pathogenicity (Fig. 2D). L. casei Shirota exerted potent antimicrobial activity against all three E. coli strains, regardless of the differences in fimbrial expression patterns.
FIG. 2.
Preventive effect of L. casei Shirota against chronic UTI. (A through C) Either L. casei Shirota at a dose of 1.2 × 108 CFU in 20 μl of saline (○) or saline alone (control) (●) was infused into the bladder 15 min after traumatization, and E. coli strain HU-1 at a dose of 1.8 × 106 CFU was infused into the bladder 24 h later. On days 0 (just after infection), 1, 4, and 7 after the challenge infection, eight mice per period were dissected for bacteriological determination in the bladder (A) and kidneys (B). (A and B) changes in viable E. coli counts, (C) changes in viable L. casei Shirota counts in the bladder (◊) and kidneys (▵). (D) Mice pretreated with saline (solid bars) or L. casei Shirota at a dose of 108 CFU 24 h earlier (open bars) were infected intravesically with E. coli strain HU-1 (2.1 × 106 CFU), HU-2 (2.5 × 106 CFU), or RI-1 (2.2 × 106 CFU) and dissected for bacterial examination 24 h after infection. Results are expressed as the means and SDs for eight mice. Plus symbols and minus symbols in parentheses in panel D indicate whether or not the strain expresses type 1 fimbriae (first symbol) and P fimbriae (second symbol). Significant differences between untreated controls and the treated group: ∗, P < 0.05; ∗∗, P < 0.01.
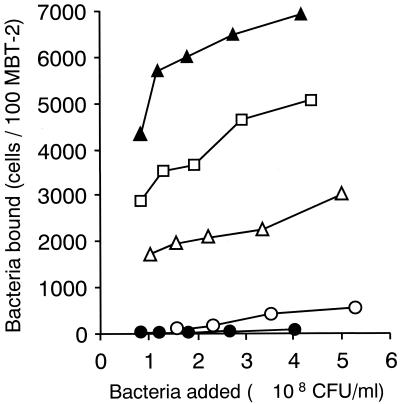
In order to assess whether or not the antimicrobial activity shown by L. casei Shirota was shared by the other lactobacillus strains, four strains which have different adhesive properties were tested. The results, shown in Table 2, clearly demonstrated differences in antimicrobial activity among the strains tested and showed that the type strains, e.g., L. fermentum, L. jensenii, L. plantarum, and L. reuteri, did not exert significant antimicrobial activity. The results also clearly showed that strains with strong adhesive properties, such as adhesion to murine bladder epithelial cells (Fig. 3), platelet aggregation, fibronectin binding, hydrophobicity, and salivary aggregation (Table 2), do not necessarily have strong antimicrobial activity. Studies concerning the influence of the inoculum dose on antimicrobial activity have shown that an L. casei Shirota inoculum of 107 CFU exerted less pronounced antimicrobial activity, while more than 108 CFU exerted potent antimicrobial activity. In contrast, L. fermentum at doses ranging from 107 to 109 CFU had no significant antimicrobial activity (data not shown).
TABLE 2.
Comparison of antimicrobial activity and adhesive activities among different Lactobacillus strains
| Lactobacillusa | Log10 difference in bacterial countb | Adhesive propertyc
|
||||
|---|---|---|---|---|---|---|
| Fibronectin binding (%)
|
Platelet aggregation (lag phase, min) | Polystyrene adhesion (%) | Salivary aggregation (%) | |||
| pH 7.3 | pH 5.4 | |||||
| L. casei strain Shirota | 2.2∗ | 0 | 1 | 0 (25<) | −5 | 0 |
| L. fermentum ATCC 14931T | 0.2 | 6 | 77 | 0 (25<) | 0 | −5 |
| L. jensenii ATCC 25258T | −0.4 | 1 | 8 | 0 (25<) | 5 | 75 |
| L. plantarum ATCC 14917T | −0.6 | 37 | 83 | 103 (13) | 1 | −8 |
| L. reuteri JCM 1112T | 0.5 | 8 | 48 | 0 (25<) | 33 | 27 |
Each strain used was grown in MRS broth for 24 h at 37°C.
Viable cells of each strain at an inoculum (L. casei Shirota, 1.8 × 108 CFU; L. fermentum, 1.0 × 108 CFU; L. jensenii, 1.0 × 108 CFU; L. plantarum, 2.2 × 108 CFU; L. reuteri, 1.9 × 108 CFU) were instilled intravesically on day −1, and the mice were infected with E. coli strain HU-1 (2 × 106 CFU) intravesically on day 0. Mice were dissected 24 h after the infection. The antimicrobial activity of each Lactobacillus strain is expressed as the log10 difference in the bacterial count, calculated as the log 10 number of E. coli bacteria in the bladders of mice pretreated with saline minus the log10 number of E. coli bacteria in the bladders of mice pretreated with Lactobacillus strains. Six mice per group were used. ∗, P < 0.05.
Determined as described in Materials and Methods.
FIG. 3.
Adhesion of lactobacillus strains to MBT-2 cell cultures, presented as the number of bacteria bound per 100 MBT-2 cells versus the concentration of bacteria added (CFU per milliliter). Representative results from two separate experiments are shown. Symbols: ○, L. casei Shirota; ●, L. fermentum ATCC 14931T; ▵, L. jensenii ATCC 25258T; ▴, L. plantarum ATCC 14917T; □, L. reuteri JCM 1112T.
Effects of L. casei Shirota on inflammatory responses in the urinary tract during infection.
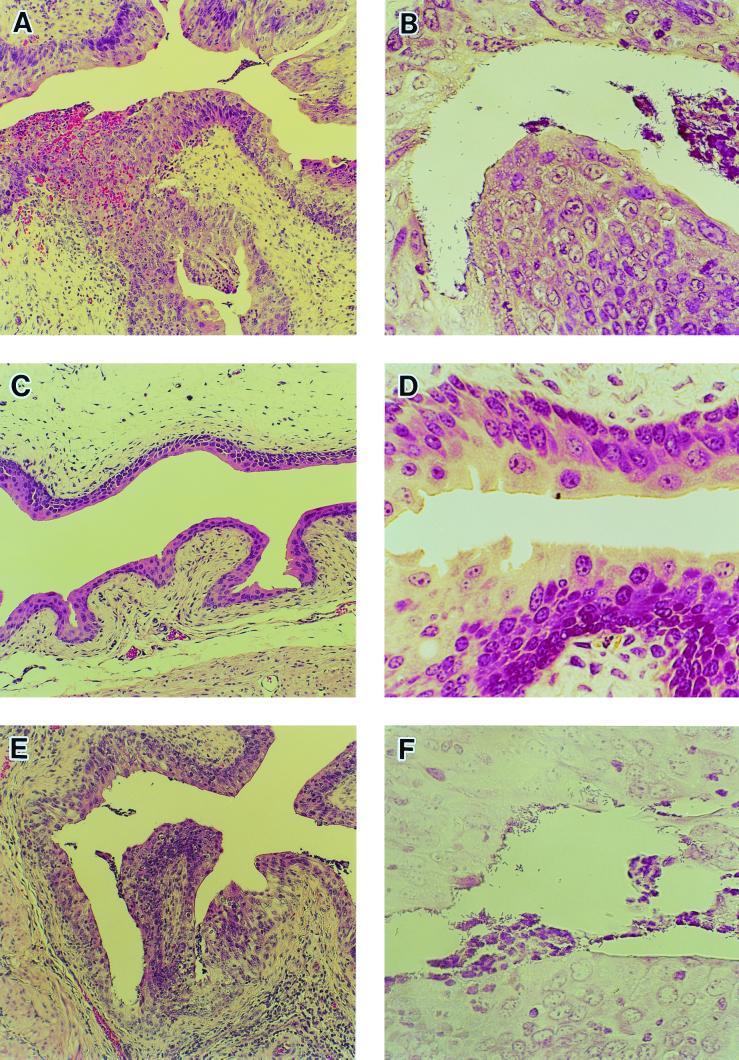
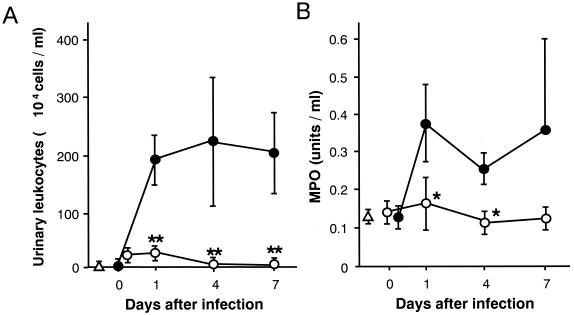
Histopathological analysis of the bladder clearly showed the inflammatory responses indicated by neutrophil infiltration and numerous gram-negative bacteria adhering to the mucosal layer in the bladders of both the infection control (Fig. 4A and B) and L. fermentum-treated (Fig. 4E and F) groups on day 4 after infection. Neither a notable inflammatory response nor mucosal damage was observed in the L. casei Shirota-treated group (Fig. 4C and D). Dramatic increases in the number of neutrophils infiltrating the urine of the control group were detected after infection, while far fewer neutrophils were detected in the urine of the L. casei Shirota-treated group (Fig. 5A). The MPO activity in urine also increased in the infection control group, while no significant increase in MPO activity was detected in the L. casei Shirota-treated group, throughout the experimental period (Fig. 5B).
FIG. 4.
Effects of L. casei Shirota on histopathological changes in the urinary tract during E. coli infection. Mice were challenged intravesically with E. coli strain HU-1 at a dose of 2 × 106 CFU and were dissected for histopathological examination of the bladder 4 days later. Either saline (A and B), L. casei Shirota at a dose of 1.6 × 108 CFU (C and D), or L. fermentum ATCC 14931T at a dose of 1.0 × 108 CFU (E and F) was infused into the bladder 24 h before the challenge infection. Panels A, C, and E were stained with hematoxylin and eosin. Magnification, ×108. Panels B, D, and F were Gram stained. Magnification, ×432.
FIG. 5.
Inhibition of infection-induced inflammatory responses by L. casei Shirota. Mice pretreated intravesically with saline (●) or L. casei Shirota at a dose of 1.6 × 108 CFU (○) were infected intravesically with E. coli strain HU-1 at an inoculum of 2 × 106 CFU 24 h later. Urination was obtained immediately after the challenge infection and on days 1, 4, and 7 from nine mice in each group per period. The number of leukocytes was counted (A), and MPO activity in the urine was determined (B). Results are expressed as the means and SDs for three samples (from nine mice). Δ, normal control. Significant differences between untreated controls and the treated group: ∗, P < 0.05; ∗∗, P < 0.01.
Antimicrobial activity of L. casei Shirota with postinfection treatment.
Because UTI with E. coli was sustained for more than 3 weeks after the challenge infection (Fig. 1; Table 1), L. casei Shirota treatment was initiated during the postinfection period to assess its therapeutic potential (Table 3). Multiple treatments of mice with L. casei Shirota significantly reduced viable E. coli counts in the bladder during the postinfection period.
TABLE 3.
Antimicrobial activity of L. casei Shirota by daily administration during the postinfection period
| Groupa | Log10 viable E. coli organisms in bladder (mean ± SD)b
|
||
|---|---|---|---|
| Days 1–11 | Days 4–11 | Days 7–11 | |
| Control | 6.6 ± 0.9c | 4.8 ± 0.7 | 4.8 ± 0.9 |
| L. casei Shirota | 4.2c | 3.9 ± 0.7∗ | 4.3 ± 0.6 |
Mice were treated with saline alone (control) or L. casei Shirota (1.2 × 108 to 1.6 × 108 CFU) once daily starting on day 1, 4, or 7 and continuing to day 11.
Mice were infected with E. coli strain HU-1 at an inoculum of 2 × 106 CFU on day 0 and dissected on day 12 after infection. Six to eight mice per group were used. ∗, P < 0.05.
The incidence or infection was 8 of 8 for the control group and 2 of 7 for the L. casei Shirota-treated group, respectively (P < 0.05 by Fisher's exact probability test).
Antimicrobial activity of an HK preparation of L. casei Shirota.
As shown in Fig. 2, the antimicrobial effect of L. casei Shirota with a single infusion was maintained for a week after E. coli inoculation despite the dramatic decrease in viable L. casei Shirota counts. An HK preparation of L. casei Shirota was then assessed for antimicrobial activity in order to ascertain whether the metabolic activity of the lactobacillus is required for the antimicrobial activity (Fig. 6). HK L. casei Shirota at a dose of 100 μg per mouse, which is nearly equivalent to 108 CFU, clearly showed antimicrobial activity to be exerted in a fashion similar to that of the live preparation (Fig. 6A). HK L. casei Shirota showed antimicrobial activity with multiple treatments during the postinfection period (Fig. 6B).
FIG. 6.
Antimicrobial activity of an HK preparation of L. casei Shirota. (A) Preventive activity. E. coli strain HU-1 at an inoculum of 1.8 × 106 CFU was infused 24 h after treatment with HK L. casei Shirota (100 μg/mouse), and viable E. coli counts were determined on days 0 (immediately after the challenge), 1, 4, and 7. (B) Therapeutic activity of HK L. casei Shirota with postinfection administration. Mice that had been infected intravesically with E. coli strain HU-1 at an inoculum of 2.0 × 106 CFU received 7 daily intravesical infusions of saline or HK L. casei Shirota at a dose of 100 μg/mouse starting on day 7 after the infection, and the mice were dissected for bacteriological examination on days 7 (controls only), 8, 11, and 14 after the challenge infection. Symbols: ●, saline-treated control, ○, group treated with HK L. casei Shirota. Results are expressed as the means and SDs for eight mice. ∗, P < 0.05 for differences between untreated controls, and treatment groups.
DISCUSSION
The advantage of the infection model used herein is that a relatively smaller inoculum, only 1 × 106 to 2 × 106 CFU, induces chronic UTI (Fig. 1; Table 1). In contrast, inoculum doses of more than 108 CFU were needed to induce UTI in the experimental models employed in prior studies (18, 23–25, 26, 28, 61). Hopkins et al. reported that genetically distinct inbred mice differ in initial susceptibility to an E. coli UTI and in their ability to resolve the infection. Significant UTIs were induced in the majority of murine strains evaluated, and these infections gradually resolved with the exception of two LPS nonresponder strains, C3H/HeJ and C3H/OuJ (23, 24). The present results showed UTI infection to persist throughout the 28-day study period in the C3H/HeN strain (Fig. 1; Table 1), which was reported to undergo progressive UTI resolution in a previous study. These results were apparently attributable to pretreatment of the bladder mucosa before infusion of the pathogen, because infections without pretreatment resolved within a week (Fig. 1A). Histopathological examination revealed chemical treatment of the bladder to induce inflammatory hyperplasia of the mucosa (Fig. 4), which may create conditions conducive to E. coli infection, such as increased expression of extracellular matrix (ECM). It is well known that fimbriae, such as type 1, Pap, and S, bind to ECM molecules such as fibronectin, laminin, and type IV collagen (for reviews, see references 55 and 67).
Characteristics of the antimicrobial activity of L. casei Shirota in the murine chronic UTI model include (i) a heat-killed preparation of the probiotic strain being effective against UTI (Fig. 6) and (ii) effectiveness of treatment during the postinfection period (Table 3; Fig. 6). Both of these characteristics appear to be quite important for safe and practical use of L. casei Shirota as a therapeutic agent for UTI patients. The mechanism by which L. casei Shirota exerts such unique and practical antimicrobial activity against UTI is still unclear from the results obtained in the present study. It is unlikely that the bactericidal substances produced by lactobacilli, such as lactic acid, hydrogen peroxide (15, 16), and several kinds of bacteriocin (3, 5, 46), contribute to the antimicrobial activity of L. casei Shirota. This is because viable counts of the strain in the urinary tract decreased dramatically after infection with E. coli, and a heat-killed preparation of L. casei Shirota exerts potent antimicrobial activity. Moreover, L. casei Shirota has been found not to produce hydrogen peroxide at detectable levels even under aerobic culture conditions (data not shown). Therefore, antimicrobial mechanisms other than those driven by bacterial metabolites appear to be mainly responsible for the results obtained in this experimental model.
Type 1 fimbriae are expressed by many members of the Enterobacteriaceae, and experimental evidence suggests that they mediate adherence in the bladder and thus probably contribute to the pathogenesis of lower UTI (9, 14, 38). On the other hand, numerous epidemiological studies have indicated that uropathogenic E. coli strains are much more likely to express P fimbriae than are fecal isolates of E. coli (13, 14). Indeed, the prevalence of P fimbriae among E. coli strains appears to correlate with the severity and anatomical location of UTI. Approximately 80% of acute pyelonephritis isolates have P fimbriae, while only about 30% of cystitis isolates are P fimbriated (13). As L. casei Shirota exerted antimicrobial activity against three E. coli strains despite their different fimbrial expressions (Fig. 2D), the mechanism of the antimicrobial activity appears to be unrelated to inhibition of the fimbria-mediated pathogenesis of E. coli. Recent reports have shown that there are differences in adhesion to intestinal epithelial cells (Caco-2 cells) among lactobacillus strains (35, 39, 60, 66), and that indigenous lactobacilli isolated from the urethral surfaces of healthy women block the adherence of gram-negative uropathogenic bacteria to uroepithelial cells from women without a history of UTI (59, 60). We, however, have found that L. casei Shirota does not have adhesive properties such as fibronectin binding, salivary aggregation, and platelet aggregation in vitro, while the ineffective strains, such as L. fermentum, L. jensenii, L. plantarum, and L. reuteri, show strong adhesive properties (Table 2). Moreover, L. casei Shirota exerted a much lower adhesive activity to a murine bladder epithelial cell line, MBT-2, than the ineffective strains, such as L. jensenii and L. plantarum (Fig. 3). Although the mechanisms underlying the antimicrobial activity of lactobacilli are believed to involve the production of inhibitory substances and competitive exclusion (57, 58), the present results suggest that these assumptions may not hold true for the antimicrobial activity of L. casei Shirota against UTI in this murine model. On the other hand, L. casei Shirota has been shown to have higher adhesion affinity to Caco-2 cells, intestinal mucus, and ileostomy glycoproteins than another probiotic, L. rhamnosus GG, while the adhesion of L. casei Shirota at saturating cell concentrations was much lower than that for L. rhamnosus GG (39, 66). Therefore, further studies are required to establish whether the inhibition of E. coli adhesion to urinary tract epithelial cells may be involved in the mechanism of protection.
The magnitude of local inflammation elicited by bacteria in the urinary tract accounts for most of the clinical features of UTI (37, 65). Evidence from murine models suggests that the inflammatory response at the initial phase of infection (within 24 h of infection) is essential for clearance of bacteria from the urinary tract (19, 62). It has been shown that uropathogenic E. coli stimulates local production of proinflammatory cytokines and chemokines in the urinary tract. In studies of mice with experimental UTI and in human volunteers deliberately colonized with E. coli, there were marked increases in the levels of interleukin-6 (IL-6) and IL-8 (2, 10, 22). Moreover, it has been shown that uroepithelial cells, upon exposure to E. coli, secrete cytokines such as IL-1α, IL-6, and IL-8 (1). However, the maintenance of augmented inflammatory responses indicated by the dramatic increases in neutrophils and MPO activity in the urine during E. coli infection (Table 1; Fig. 5) appears to show vain host responses aimed at eliminating the pathogen in the chronic infection model. The exaggeration and protraction of host defense responses in the UTI model may instead cause tissue injury and maladaptive repair, leading to a sustained infection. There are reports indicating that virulent strains of E. coli can utilize cytokines such as IL-1β, IL-2, and granulocyte-macrophage colony-stimulating factor (GM-CSF) to enhance their extracellular and intracellular growth (11, 30, 56).
Inflammatory responses in the urinary tract were markedly inhibited in the L. casei Shirota-treated group (Figs. 4, 5), suggesting that inhibition of the growth of pathogenic bacteria resulted in the suppression of subsequent infection-induced inflammatory responses. L. casei Shirota reportedly exerts antitumor (4, 32, 45, 54) and antimicrobial (47, 52, 53, 70) activities in clinical and preclinical studies. Furthermore, nonspecific augmentation of components of the innate immune system, such as macrophages (34, 47, 63) and natural killer cells (33), has been thought to play important roles in these activities. Strains such as L. fermentum ATCC 14931T and L. plantarum ATCC 14917T have been reported to have much weaker activities than L. casei Shirota (63, 71). There are also reports indicating the importance of cell-mediated immune responses in UTI resolution (29, 49, 50). Morin et al. reported that treatment of mice with staphylococcal enterotoxin B, a superantigen, leads to enhanced UTI resolution through a mechanism that may include direct stimulation of effector cells in the bladder and the actions of cytokines such as IL-1, IL-6, GM-CSF, and tumor necrosis factor alpha (49). Jones-Carson et al. reported that knockout mice with γδ T-cell or gamma interferon deficiencies were more susceptible to UTI than immunocompetent mice and mice with immunodeficiencies in IL-10, IL-4, inducible nitric oxide synthase, or antibody production (29). Taken together, these results raise the possibility that local activation of the innate antimicrobial activity by L. casei Shirota may facilitate inhibition of pathogen growth in the urinary tract.
On the other hand, L. casei Shirota has been shown to exert potent preventive activity in a wide variety of inflammatory disease models such as autoimmune diabetes (43), chronic rheumatoid arthritis (31), and allergic bronchial asthma (44). The mechanisms underlying the anti-inflammatory activity of L. casei Shirota have therefore been recognized as being exerted via improvement of disrupted immune responses in the disease state (42, 51). Further investigation is required to determine whether the administration of L. casei Shirota in the bladder potentiates the innate protective immune responses during UTI.
ACKNOWLEDGMENTS
We thank Kazumi Uchida and Shoichi Kado for performing the histopathological analyses. The skillful assistance of Kensuke Shimizu and Tomomi Suzuki in animal experiments is gratefully acknowledged.
REFERENCES
- 1.Agace W W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agace W, Hedges S R, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Investig. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson R E, Daeschel M A, Hassan H M. Antibacterial activity of plantaricin SIK-83, a bacteriocin produced by Lactobacillus plantarum. Biochimie. 1988;70:381–390. doi: 10.1016/0300-9084(88)90211-8. [DOI] [PubMed] [Google Scholar]
- 4.Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S The BLP Study Group. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. Eur Urol. 1995;27:104–109. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]
- 5.Barefoot S F, Klaenhammer T R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983;45:1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer M E. Visualization of the bacterial polysaccharide capsule. Curr Top Microbiol Immunol. 1990;150:129–157. doi: 10.1007/978-3-642-74694-9_7. [DOI] [PubMed] [Google Scholar]
- 7.Bruce A W, Reid G. Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol. 1988;34:339–343. doi: 10.1139/m88-062. [DOI] [PubMed] [Google Scholar]
- 8.Chin J L, Kadhim S A, Batislam E, Karlik S J, Garcia B M, Nickel J C, Morales A. Mycobacterium cell wall: an alternative to intravesical bacillus Calmette-Guérin (BCG) therapy in orthotopic murine bladder cancer. J Urol. 1996;156:1189–1193. doi: 10.1016/s0022-5347(01)65748-3. [DOI] [PubMed] [Google Scholar]
- 9.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Svanborg Edén C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis M, Campbell D, Gregg E O. Interleukin-2 and granulocyte-macrophage colony-stimulating factor stimulate growth of a virulent strain of Escherichia coli. Infect Immun. 1991;59:1853–1856. doi: 10.1128/iai.59.5.1853-1856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Ruiz C S, de Bocanera M E L, de Macías M E N, de Ruiz Holgado A A P. Effect of lactobacilli and antibiotics on E. coli urinary infections in mice. Biol Pharm Bull. 1996;19:88–93. doi: 10.1248/bpb.19.88. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren R A, editors. Urinary tract infection: molecular pathogenesis and clinical management. Washington, D.C.: American Society for Microbiology; 1996. pp. 135–174. [Google Scholar]
- 14.D'Orazio S E F, Collins C M. Molecular pathogenesis of urinary tract infections. Curr Top Microbiol Immunol. 1997;225:137–164. doi: 10.1007/978-3-642-80451-9_8. [DOI] [PubMed] [Google Scholar]
- 15.Eschenbach D A, Davick P R, Williams B L, Klebanoff S J, Young-Smith K, Critchlow C M, Holmes K K. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontaine E A, Taylor-Robinson D. Comparison of quantitative and qualitative methods of detecting hydrogen peroxide produced by human vaginal strains of lactobacilli. J Appl Bacteriol. 1990;69:326–331. doi: 10.1111/j.1365-2672.1990.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 17.Friedlander A, Druker M M, Schachter A. Lactobacillus acidophilus and vitamin B complex in the treatment of vaginal infection. Panminerva Med. 1986;28:51–53. [PubMed] [Google Scholar]
- 18.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraoka M, Hang L, Frendéus B, Godaly G, Burdick M, Strieter R, Svanborg C. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 20.Harty D W S, Knox K W. An in vitro study of adhesion of various Lactobacillus species. Microbiol Ecol Health Dis. 1991;4:19–28. [Google Scholar]
- 21.Harty D W S, Patrikakis M, Hume E B H, Oakey H J, Knox K W. The aggregation of human platelets by Lactobacillus species. J Gen Microbiol. 1993;139:2945–2951. doi: 10.1099/00221287-139-12-2945. [DOI] [PubMed] [Google Scholar]
- 22.Hedges S, Anderson P, Lidin-Janson G, de Man P, Svanborg C. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect Immun. 1991;59:421–427. doi: 10.1128/iai.59.1.421-427.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopkins W J, Gendron-Fitzpatrick A, Balish E, Uehling D T. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect Immun. 1998;66:2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins W J, Gendron-Fitzpatrick A, McCarthy D O, Haine J E, Uehling D T. Lipopolysaccharide-responder and nonresponder C3H mouse strains are equally susceptible to an induced Escherichia coli urinary tract infection. Infect Immun. 1996;64:1369–1372. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins W J, Hall J A, Conway B P, Uehling D T. Induction of urinary tract infection by intraurethral inoculation with Escherichia coli: refining the murine model. J Infect Dis. 1995;171:462–465. doi: 10.1093/infdis/171.2.462. [DOI] [PubMed] [Google Scholar]
- 26.Johnson D E, Lockatell C V, Russell R G, Hebel J R, Island M D, Stapleton A, Stamm W E, Warren J W. Comparison of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect Immun. 1998;66:3059–3065. doi: 10.1128/iai.66.7.3059-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J R. Reflux in the mouse model of urinary tract infection. Infect Immun. 1998;66:6063–6064. doi: 10.1128/iai.66.12.6063-6064.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J R, Berggren T, Manivel J C. Histopathologic-microbiologic correlates of invasiveness in a mouse model of ascending unobstructed urinary tract infection. J Infect Dis. 1991;165:299–305. doi: 10.1093/infdis/165.2.299. [DOI] [PubMed] [Google Scholar]
- 29.Jones-Carson J, Balish E, Uehling D T. Susceptibility of immunodeficient gene-knockout mice to urinary tract infection. J Urol. 1999;161:338–341. [PubMed] [Google Scholar]
- 30.Kanangat S, Meduri G U, Tolley E A, Patterson D R, Meduri C U, Pak C, Griffin J P, Bronze M S, Schaberg D R. Effect of cytokines and endotoxin on the intracellular growth of bacteria. Infect Immun. 1999;67:2834–2840. doi: 10.1128/iai.67.6.2834-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998;63:635–644. doi: 10.1016/s0024-3205(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 32.Kato I, Yokokura T, Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27:611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 33.Kato I, Yokokura T, Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol. 1984;28:209–217. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 34.Kato I, Yokokura T, Mutai M. Induction of tumoricidal peritoneal exudate cells by administration of Lactobacillus casei. Int J Immunopharmacol. 1985;7:103–109. doi: 10.1016/0192-0561(85)90015-3. [DOI] [PubMed] [Google Scholar]
- 35.Kirjavainen P V, Tuomola E M, Crittenden R G, Ouwehand A C, Harty D W S, Morris L F, Rautelin H, Playne M J, Donohue D C, Salminen S J. In vitro adhesion and platelet aggregation properties of bacteremia-associated lactobacilli. Infect Immun. 1999;67:2653–2655. doi: 10.1128/iai.67.5.2653-2655.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krawisz J E, Sharon P, Stenson W F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 37.Kunin C M. Urinary tract infections in females. Clin Infect Dis. 1994;18:1–12. doi: 10.1093/clinids/18.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesion-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y K, Lim C Y, Teng W L, Ouwehand A C, Tuomola E M, Salminen S. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl Environ Microbiol. 2000;66:3692–3697. doi: 10.1128/aem.66.9.3692-3697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y K, Nomoto K, Salminen S, Gorbach S L. Role of probiotics in health and diseases. In: Lee Y K, Nomoto K, Salminen S, Gorbach S L, editors. Handbook of probiotics. New York, N.Y: John Wiley & Sons, Inc.; 1999. pp. 67–146. [Google Scholar]
- 41.Markwell M A K. A new solid-state reagent to iodinate proteins. Anal Biochem. 1982;125:427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki T. Immunomodulation by treatment with Lactobacillus casei strain Shirota. Int J Food Microbiol. 1998;41:133–140. doi: 10.1016/s0168-1605(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, Yokokura T. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997;105:643–649. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. The effect of oral feeding of Lactobacillus casei strain Shirota on immunoglobulin E production in mice. J Dairy Sci. 1998;81:48–53. doi: 10.3168/jds.S0022-0302(98)75549-3. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki T, Yokokura T, Mutai M. Antitumor effect of intrapleural administration of Lactobacillus casei in mice. Cancer Immunol Immunother. 1988;26:209–214. doi: 10.1007/BF00199931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGroarty J A, Reid G. Detection of a lactobacillus substance that inhibits Escherichia coli. Can J Microbiol. 1988;34:974–978. doi: 10.1139/m88-171. [DOI] [PubMed] [Google Scholar]
- 47.Miake S, Nomoto K, Yokokura T, Yoshikai Y, Mutai M, Nomoto K. Protective effect of Lactobacillus casei on Pseudomonas aeruginosa infection in mice. Infect Immun. 1985;48:480–485. doi: 10.1128/iai.48.2.480-485.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mobley H L T, Green D M, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morin M D, Hopkins W J. Treatment of mice with staphylococcal enterotoxin B enhances resolution of an induced Escherichia coli urinary tract infection and stimulates production of proinflammatory cytokines. Infect Immun. 1998;66:2466–2470. doi: 10.1128/iai.66.6.2466-2470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulvey M A, Schilling J D, Martinez J J, Hultgren S J. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomoto K. Immunoregulatory functions of probiotics. Biosci Microflora. 2000;19:1–8. [Google Scholar]
- 52.Nomoto K, Yokokura T, Mitsuyama M, Yoshikai Y, Nomoto K. Prevention of indigenous infection of mice with Escherichia coli by nonspecific immunostimulation. Antimicrob Agents Chemother. 1992;36:361–367. doi: 10.1128/aac.36.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogawa M, Shimizu K, Nomoto K, Takahashi M, Watanuki M, Tanaka R, Tanaka T, Hamabata T, Yamasaki S, Takeda Y. Protective effect of Lactobacillus casei strain Shirota on Shiga toxin-producing Escherichia coli O157:H7 infection in infant rabbits. Infect Immun. 2001;69:1101–1108. doi: 10.1128/IAI.69.2.1101-1108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okawa T, Kita M, Arai T, Iida K, Dokiya T, Takegawa Y, Hosokawa Y, Yamazaki K, Hashimoto S. Phase II randomized clinical trial of LC 9018 concurrently used with radiation in the treatment of carcinoma of the uterine cervix. Cancer. 1989;64:1769–1776. doi: 10.1002/1097-0142(19891101)64:9<1769::aid-cncr2820640902>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 55.Patti J M, Allen B L, McGavin M J, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 56.Porat R, Clark B D, Wolff S M, Dinarello C A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 57.Redondo-Lopez V, Cook R L, Sobel J D. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 58.Reid G, Bruce A W, McGroarty J A, Cheng K J, Costerton J W. Is there a role for lactobacilli in prevention of urogenital and intestinal infections? Clin Microbiol Rev. 1990;3:335–344. doi: 10.1128/cmr.3.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reid G, Chan R C Y, Bruce A W, Costerton J W. Prevention of urinary tract infection in rats with an indigenous Lactobacillus casei strain. Infect Immun. 1985;49:320–324. doi: 10.1128/iai.49.2.320-324.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reid G, Cook R L, Bruce A W. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J Urol. 1987;138:330–335. doi: 10.1016/s0022-5347(17)43137-5. [DOI] [PubMed] [Google Scholar]
- 61.Schaeffer A J, Schwan W R, Hultgren S J, Dunkan J L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shahin R D, Engberg I, Hagberg L, Svanborg Edén C. Neutrophil requirement and bacterial clearance correlated with LPS responsiveness in local Gram-negative infection. J Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 63.Shimizu T, Nomoto K, Yokokura T, Mutai M. Role of colony-stimulating activity in antitumor activity of Lactobacillus casei in mice. J Leukoc Biol. 1987;42:204–212. doi: 10.1002/jlb.42.3.204. [DOI] [PubMed] [Google Scholar]
- 64.Soloway M S. Intravesical and systemic chemotherapy of murine bladder cancer. Cancer Res. 1977;37:2918–2929. [PubMed] [Google Scholar]
- 65.Stamm W E, McKevitt M, Roberts P L, White N J. Natural history of recurrent urinary tract infections in women. Rev Infect Dis. 1991;13:77–84. doi: 10.1093/clinids/13.1.77. [DOI] [PubMed] [Google Scholar]
- 66.Tuomola E M, Salminen S J. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol. 1998;41:45–51. doi: 10.1016/s0168-1605(98)00033-6. [DOI] [PubMed] [Google Scholar]
- 67.Westerlund B, Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 68.Willcox M D P, Knox K W. Surface-associated properties of Streptococcus milleri group strains and their potential relation to pathogenesis. J Med Microbiol. 1990;31:259–270. doi: 10.1099/00222615-31-4-259. [DOI] [PubMed] [Google Scholar]
- 69.Wyatt J E, Handley P S. Aggregation of Streptococcus sanguis biotypes I and II by parotid saliva: a comparison between peritrichously fibrillar and tufted strains. Microbios. 1987;51:113–123. [PubMed] [Google Scholar]
- 70.Yokokura T, Nomoto K, Shimizu T, Nomoto K. Enhancement of hematopoietic response of mice by subcutaneous administration of Lactobacillus casei. Infect Immun. 1986;52:156–160. doi: 10.1128/iai.52.1.156-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokokura T, Kato I, Matsuzaki T, Mutai M, Satoh H. Antitumor activity of Lactobacillus casei YIT 9018 (LC9018)—effect of administration route. Jpn J Chemother. 1984;11:2427–2433. [PubMed] [Google Scholar]
- 72.Yuki N, Watanabe K, Mike A, Tagami Y, Tanaka R, Ohwaki M, Morotomi M. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: selective isolation from faeces and identification using monoclonal antibodies. Int J Food Microbiol. 1999;48:51–57. doi: 10.1016/s0168-1605(99)00029-x. [DOI] [PubMed] [Google Scholar]