Tubuloglomerular feedback (TGF) is a negative response between the tubules and arterioles in the kidneys. During the past few decades, the regulatory mechanisms of the TGF response have been extensively studied with in vivo micropuncture and in vitro microperfusion of the isolated juxtaglomerular apparatus, which has significantly advanced our understanding about TGF responses. The TGF response is initiated by an increase in NaCl at macula densa cells, which are specialized epithelial cells at the end of the thick ascending limbs. Increased NaCl delivery activates macula densa luminal Na+-K+-2Cl− cotransporter, which stimulates basolateral Cl− efflux that results in cell depolarization, activates the luminal Na+/H+ exchanger that alkalinizes the macula densa, and alters cell volume and intracellular Ca2+ concentrations. One or more of these factors induces the release of ATP from the basolateral membrane of the macula densa. ATP released from the macula densa directly acts on afferent arterioles or is broken down to form adenosine that constricts the afferent arterioles. This coordinated process is termed the TGF response (1).
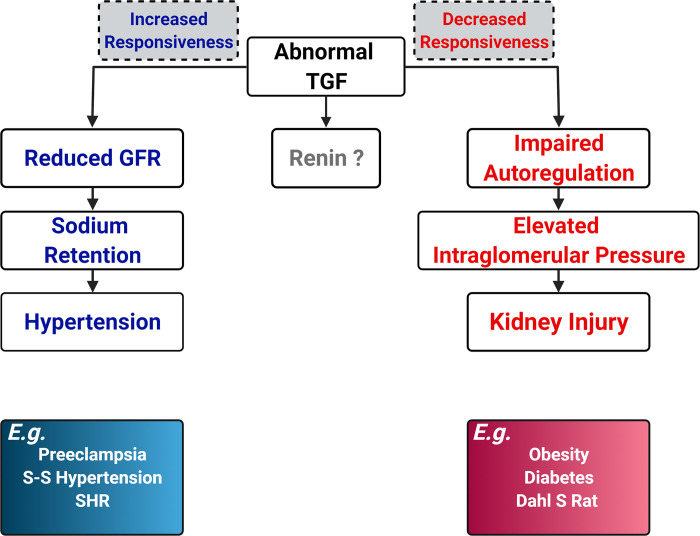
The TGF response is usually considered to have three functions. One function is to stabilize NaCl delivery to the distal nephron by a tonic regulation on single-nephron glomerular filtration rate (GFR). The second function is to maintain renal autoregulation by working together with the myogenic response. The third function is to regulate renin release. These functions are not separate pathways but rather are highly coordinated and integrated mechanisms with common targets, the afferent and efferent arterioles. For the first function, the TGF response is not only a short-term stabilizer in the maintenance of constant NaCl delivery to distal nephrons but also plays an essential role in the control of Na+ excretion, volume hemostasis, and blood pressure in both physiological and pathological situations. Alterations in TGF responsiveness or TGF resetting change Na+ and water excretion by regulating GFR and NaCl delivery to the tubules. During the past 10 yr, several studies have demonstrated the long-term significance of TGF responsiveness. Splice variants α, β, and γ of nitric oxide synthase 1 (NOS1) have been identified in macula densa cells (2). Following a high-salt intake, the NOS1β-mediated TGF response helps to maintain blood pressure within a normal range by promoting GFR elevation and Na+ excretion (3). Together with Na+-glucose transporter (SGLT)1, macula densa NOS1 promotes glomerular hyperfiltration in type 1 and type 2 diabetes via a SGLT1-NOS1-TGF pathway (4). This mechanism may integrate with the SGLT2 pathway promoting the development of diabetic hyperfiltration and kidney injury. A recent study has provided direct evidence that SGLT2 inhibition reduces glomerular capillary pressure, which is dependent on an intact TGF system (5). In addition, the macula densa NOS1β-mediated TGF response also dominates the sex dimorphism found in salt-sensitive and ANG II-induced hypertension (6). Normal pregnancy is characterized by systemic vasodilation that leads to a 45%–50% increase in renal blood flow and GFR, and a 5–10-mmHg decrease in blood pressure. The TGF response has been found to play an essential role in hemodynamic and volume adaptations (7). Impaired macula densa NOS1 activity enhances TGF responsiveness, which induces salt-sensitive hypertension (3), hypertension in diabetes (8), and gestational hypertension (7). For the second function, the TGF and myogenic response are two major components of renal autoregulatory function, which is essential to maintain constant interglomerular pressure and protect against glomerular injury from alterations in arterial pressure (9). Typical examples are spontaneously hypertensive rats and Dahl salt-sensitive rats. Spontaneously hypertensive rats have relatively intact autoregulatory function and thereby have less kidney injury in the presence of hypertension. In contrast, Dahl salt-sensitive rats exhibit impaired renal autoregulation and develop severe kidney injury during hypertension. Regarding the third function, it has still not been clarified about the role of the TGF response in renin release (Fig. 1).
Figure 1.
Possible consequences of an abnormal tubuloglomerular feedback (TGF) response. Dahl S rat, Dahl salt-sensitive rat; GFR, glomerular filtration rate; SHR, spontaneously hypertensive rat; S-S hypertension, salt-sensitive hypertension.
Obesity is a global pandemic and a risk factor for kidney injury. However, the TGF response in obesity has not been investigated. In an article recently published in the American Journal of Physiology-Renal Physiology, Monu et al. (10) examined the TGF response in Sprague-Dawley rats fed a high-fat diet (HFD) or normal-fat diet (NFD). The TGF response and tubular pressure were measured with micropuncture. After 16 wk of HFD feeding, the TGF response was attenuated by >50% compared with rats fed the NFD. Stop flow pressure measured at a perfusion rate of 0 nL/min was taken as a surrogate for intraglomerular pressure, which was significantly higher in rats fed the HFD compared with the NFD. Meanwhile, mean arterial pressure increased by ∼15 mmHg and was accompanied with notable glomerulosclerosis after 16 wk of HFD feeding.
This study provided the first evidence that HFD-induced obesity impairs renal autoregulation and induces kidney injury. It is unlikely that the altered TGF response contributes to the development of hypertension, since the changes in the TGF response were inverse to the directions that promote an increase in blood pressure. The hypertension and kidney injury induced by a HFD seems to be mediated by separate mechanisms, which warrant further investigation. Several open questions about the TGF response could be considered for future studies. The mechanisms underlying the HFD impairing the TGF response need to be further examined, including changes in gene expression as well as functions of macula densa cells and afferent and efferent arterioles. The corresponding changes in the myogenic response need to be determined. The similarities and differences in alterations in the TGF response and kidney injury need to be determined in obese animals with or without insulin resistance, diabetes, or hypertension. More importantly, it is essential to determine whether or how the animal findings can be extrapolated to humans. For example, not all people with obesity or HFD develop kidney injury or hypertension.
GRANTS
This work was partially supported by National Heart, Lung, and Blood Institute Grants HL142814 and HL137987.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
R.L. drafted manuscript; edited and revised manuscript; approved final version of manuscript.
REFERENCES
- 1.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 65: 501–529, 2003. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 2.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD Jr, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 298: F1465–F1471, 2010. doi: 10.1152/ajprenal.00650.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J Am Soc Nephrol 27: 2346–2356, 2016. doi: 10.1681/ASN.2015050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Cai J, Cui Y, Jiang S, Wei J, Kim YC, Chan J, Thalakola A, Le T, Xu L, Wang L, Jiang K, Wang X, Wang H, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. Role of the macula densa sodium glucose cotransporter type 1-nitric oxide synthase 1-tubuloglomerular feedback pathway in diabetic hyperfiltration. Kidney Int 101: 541–550, 2022. doi: 10.1016/j.kint.2021.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson SC, Vallon V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am J Physiol Renal Physiol 320: F761–F771, 2021. doi: 10.1152/ajprenal.00552.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Qu L, Wei J, Jiang S, Xu L, Wang L, Cheng F, Jiang K, Buggs J, Liu R. A new mechanism for the sex differences in angiotensin II-induced hypertension: the role of macula densa NOS1β-mediated tubuloglomerular feedback. Am J Physiol Renal Physiol 319: F908–F919, 2020. doi: 10.1152/ajprenal.00312.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei J, Zhang J, Jiang S, Xu L, Qu L, Pang B, Jiang K, Wang L, Intapad S, Buggs J, Cheng F, Mohapatra S, Juncos LA, Osborn JL, Granger JP, Liu R. Macula densa NOS1β modulates renal hemodynamics and blood pressure during pregnancy: role in gestational hypertension. J Am Soc Nephrol 32: 2485–2500, 2021. doi: 10.1681/ASN.2020070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Wang X, Cui Y, Jiang S, Wei J, Chan J, Thalakola A, Le T, Xu L, Zhao L, Wang L, Jiang K, Cheng F, Patel T, Buggs J, Vallon V, Liu R. Knockout of macula densa neuronal nitric oxide synthase increases blood pressure in db/db mice. Hypertension 78: 1760–1770, 2021. doi: 10.1161/HYPERTENSIONAHA.121.17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monu SR, Wang H, Potter DL, Liao TD, Ortiz PA. Decreased tubuloglomerular feedback response in high-fat diet-induced obesity. Am J Physiol Renal Physiol 322: F429–F436, 2022. doi: 10.1152/ajprenal.00307.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]