Abstract
Introduction
Radical cystectomy (RC) is the historic gold standard treatment for muscle-invasive bladder cancer (MIBC), but trimodal therapy (TMT) has emerged as a valid therapeutic option for select patients. Given that prospective clinical trials have been difficult to perform in this area, our aim was to compare these two primary treatment strategies using decision analytic methods.
Method
A two-dimensional Markov microsimulation model was constructed using TreeAge Pro to compare RC and TMT for patients with newly diagnosed MIBC. A comprehensive literature search was used to populate model probabilities and utilities. Our primary outcome was quality-adjusted life expectancy (QALE). Secondary outcomes included crude life expectancy (LE) and bladder cancer recurrences. The simulated patient for our model was an adult with MIBC (pT2-4 N0 M0) who was a candidate for either RC or TMT.
Results
A total of 500 000 patients were simulated. TMT resulted in an estimated mean QALE of 7.48 vs. 7.41 for RC. However, the average LE for patients treated with TMT was lower compared with RC (10.20 vs. 10.74 years). A sensitivity analysis evaluating the impact of age showed that younger patients treated with RC had greater QALE and longer LE than those treated with TMT; inverse findings were observed for elderly patients. Overall, 39.4% of patients treated with TMT experienced a bladder recurrence.
Conclusions
RC results in a longer LE compared to TMT (0.54 years), but with a lower QALE (−0.07 years). The preferred treatment strategy varied with patient age.
Introduction
Bladder cancer represents a significant source of morbidity and mortality worldwide. Nearly 430 000 diagnoses of bladder cancer are made each year, leading to approximately 165 000 deaths.1 Radical cystectomy (RC) has historically been accepted as the gold standard treatment for muscle-invasive bladder cancer (MIBC), supported by a large body of long-term evidence.2,3 However, RC is associated with significant risks of postoperative morbidity and even mortality. 4 Due to the risks associated with RC and the appeal of bladder preservation, trimodal therapy (TMT), including debulking transurethral resection of the tumour, followed by concurrent radio-sensitizing chemotherapy and external beam radiation, has emerged as a valid treatment option, albeit in select patients.5
Evaluating the two modalities directly has been challenging. Retrospective studies that include the early years of TMT adoption are likely impacted by indication bias, making conclusions regarding efficacy difficult to draw. The only randomized clinical trial in this space was closed early due to poor accrual because of issues with perceived lack of equipoise and patient reluctance to randomization.6
Our group has previously compared the oncological outcomes between patients treated with RC or TMT using a propensity score matched-cohort analysis and found that TMT yielded survival outcomes similar to those of matched patients who underwent RC.7 However, little literature has been published evaluating the quality-of-life impact from the two treatment types. Since these interventions and their downstream sequalae are complex, involving both benefits and harms to health, distillation of the relevant information to an overall estimation can contribute to better decision-making. 8 Decision models are an accepted tool used to guide clinical decision-making and models have previously been used in the field of urologic oncology.9,10 Therefore, the purpose of this study is to directly compare the effectiveness of TMT vs. RC for patients with MIBC using decision analytic techniques.
Methods
Model overview
We constructed a two-dimensional Markov microsimulation model with trackers using TreeAge Pro 2019 (TreeAge Software Inc., Williamstown, MA, U.S.) to compare treatment strategies for patients with newly diagnosed MIBC. A Markov model simulates patients over time and allows for transitions between various health states as disease progresses. Two management strategies were modelled — TMT vs. RC. Our primary outcome was quality-adjusted life expectancy (QALE). Secondary outcomes included crude life expectancy (LE), overall survival (OS), distant recurrence rates, and bladder cancer diagnoses in the TMT arm over a lifetime time horizon. The Markov cycle length mimicked the clinical experience: every three months in active treatment and during the first year of followup, then every six months for the second and third year, and then yearly moving forward if patients had no evidence of recurrence. If evidence of recurrence developed, they returned to a cycle length of three months. Within-cycle correction with a 1.5% discount rate was used to account for bias arising from discrete-time Markov models.11,12
Base case
The base case for our model was an adult patient with MIBC (pT2-4 N0 M0) appropriate for either RC or TMT. Distributions representative of the typical MIBC population were used to simulate real patients seen in clinical practice with individual-level sampling for age, gender, and reconstruction type. Distributions for patient-level variables are shown in Appendix S1 (available at cuaj.ca)
Model structure
Fig. 1 depicts the Markov state transition diagrams for both strategies. In both arms, patients may be treated with neoadjuvant chemotherapy (NAC) and experience adverse events, progression, or death, impacting their ability to complete chemotherapy.
Fig. 1.
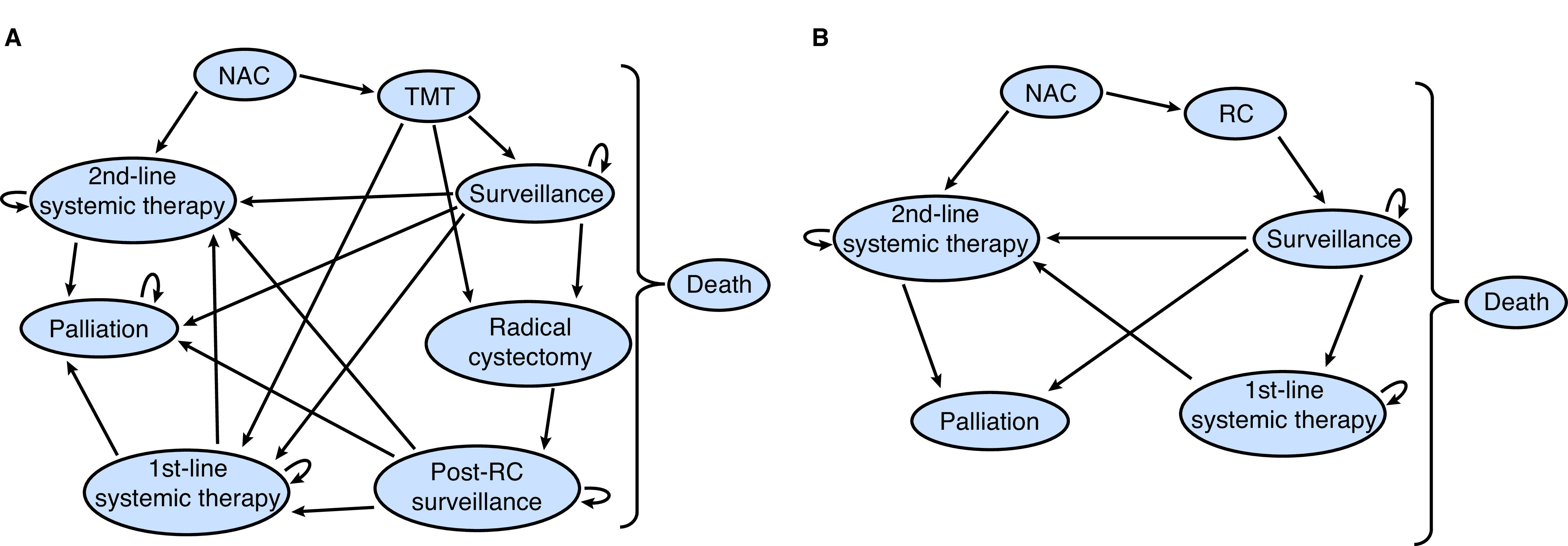
Model schematics depicting state transitions for (A) TMT; and (B) RC. MIBC: muscle-invasive bladder cancer; NAC: neoadjuvant chemotherapy; RC: radical cystectomy; TMT: trimodal therapy.
Patients in the TMT arm (Fig. 1A) could have a complete or incomplete response to therapy (requiring salvage cystectomy or systemic treatment). Following TMT, patients entered the surveillance phase, where they could develop a local recurrence, treatment-related complication (minor and major, based on Common Terminology Criteria for Adverse Events [CTCAE] grading), distant recurrence, or death. The bladder cancer recurrence could be non-muscle-invasive (NMIBC) (high-grade [HG] or low-grade [LG]) or muscle-invasive. Patients diagnosed with MIBC or recurrent HG NMIBC (>1 recurrence) underwent a salvage cystectomy. Further details regarding the modelling of complications can be found in Appendix S2 (available at cuaj.ca).
In the RC arm (Fig. 1B) and for patients undergoing salvage cystectomy in the TMT arm, patients could experience perioperative complications or mortality. Complications were similarly modelled as minor and major (based on Clavien-Dindo grading), which increased perioperative mortality rates.13,14 Following treatment, patients entered a post-cystectomy surveillance state. With each cycle, each patient had a risk of distant recurrence, short- or long-term postoperative complications, and death.
If patients in either cohort developed a distant, metastatic recurrence, they could be treated with either first-line (cisplatin-based) chemotherapy or second-line therapy. Eligibility for first-line chemotherapy was modelled based on the probability of a simulated patient having adequate renal function for cisplatin (defined as glomerular filtration rate [GFR] ≥60 mL/min), which decreased with age.15 Patients ineligible for cisplatin were treated with gemcitabine/carboplatin.16 Second-line therapy was modelled as pembrolizumab in keeping with the inclusion criteria from the KEYNOTE-045 trial.17 Patients could also transition into a palliative state (best supportive care). Assumptions made in the development of the model are detailed in the Appendix S3 (available at cuaj.ca).
Model parameters
Transition probabilities were determined from a comprehensive MEDLINE literature search as of March 1, 2019, supplemented by hand search of references from retrieved studies, review articles, previous decision analyses, and expert consultation (Table 1). If there were multiple data-points obtained for a given probability, we chose the value that was from the publication of the best methodological grade and represented the modelled cohort most accurately. In order to more closely approximate real-life events, equations representing survival and cumulative incidence curves from the published literature were calculated; these were then used to create per-cycle probabilities for key transition probabilities (Appendix S4; available at cuaj.ca).
Table 1.
Model probabilities
| Variable | Probability | Reference |
|---|---|---|
| Neoadjuvant chemotherapy | ||
| Starting proportion of patients in NAC | 36%^ | Krabbe et al, 201518; Kulkarni et al, 20177 |
| Death on chemotherapy | 1.1% | Winquist et al, 200419 |
| Completing NAC | 90.3% | Zargar et al, 201520 |
| Adverse event | 36.7% | Neidersüss-Beke et al, 201721 |
| Progression on NAC | 3.0% | Galsky et al 201522 |
| HR for distant recurrence if completed NAC | 0.78 | ABC meta-analysis collaboration, 200523 |
| Radical cystectomy | ||
| Perioperative mortality | 2.4% | Wallace et al, 201824 |
| Postoperative complication (grade III/IV) | 68% (22%) | Parekh et al, 201825 |
| Complication on surveillance | 40% at 2 years* | Shimko et al, 201126 |
| Composite long-term complication | 10% over 1.1 years** | Shimko et al, 201126 |
| Distant recurrence | 38% at 5 years* | Nuhn et al, 201227 |
| Trimodal therapy | ||
| Complication on treatment (major) | 55% (15.5%) | Tunio et al, 201228 |
| Complete response | 75.3% | Fahmy et al, 201829 |
| Immediate salvage cystectomy | 31.8% | Calibrated value |
| Complication on surveillance | 39% over 31 months** | Efstathiou et al, 200930 |
| Major complication on surveillance | 9.58% over 22.1 months** | Efstathiou et al, 200930; Rodel et al, 200231 |
| Bladder cancer recurrence | 60% over 10 years | Calibrated value |
| Secondary malignancy | 0.7% over 75 months** | Zelefsky et al, 201232 |
| Distant recurrence on surveillance | 28.8% at 5 years | Calibrated value |
| Complication post-salvage cystectomy (grade III/IV) | 69% (16%) | Eswara et al, 201233 |
| Perioperative mortality from salvage cystectomy | 2.2% | Eswara et al, 201233 |
| Composite long-term complication post-salvage cystectomy | 20% at 1 year* | Knap et al, 200434 |
| Distant recurrence post-salvage cystectomy | ||
| Immediate salvage cystectomy | 22.4% at 2 years* | Eswara et al, 201233 |
| Delayed salvage cystectomy | 16.14% at 2 years* | Eswara et al, 201233 |
| Systemic therapy | ||
| Eligibility for first-line chemotherapy | 28% overall – age adjusted | Dash et al, 200615 |
| Survival on first-line cisplatin-based chemotherapy (carboplatin-based) | 50% over 14 months** (50% over 9.3 months**) | Von der Maase et al, 200535; De Santis et al, 201216 |
| Progression on first-line cisplatin-based chemotherapy (carboplatin-based) | 50% over 7.7 months** (50% over 5.8 months**) | Von der Maase et al, 200535; De Santis et al, 201216 |
| Receipt of second-line systemic therapy after progression on first-line | 39.2% | Wang et al, 201736 |
| Survival on second-line systemic therapy: | ||
| Pembrolizumab | 50% over 10.3 months** | Bellmunt et al, 201717 |
| Survival on palliative therapy | 50% over 5.3 months** | Smith et al, 201437 |
| Baseline mortality rates | ||
| Non-bladder-specific cancer-related mortality | 0.7% (adjusted based on gender & age) per year | Calibrated value |
| HR female | 0.78 | Williams et al, 201738 |
| HR age 70–74 | 1.08 | Williams et al, 201738 |
| HR age 75–79 | 1.30 | Williams et al, 201738 |
| HR age≥80 | 1.76 | Williams et al, 201738 |
Utilities were obtained using the Tufts-New England Medical Center Cost Effectiveness Analysis registry (https://cevr.tuftsmedicalcenter.org/databases/cea-registry) and using a manual search of published urology decision models (Table 2). In cases where exact probabilities or utilities were not available, our search expanded to include other cancer sites and expert opinion. Transitional penalties to account for the inconvenience of procedures and potential short-term complications (e.g., transurethral resection of bladder tumor, chemotherapy, and operative complications) were subtracted from a given health state’s baseline utility.
Table 2.
Model utilities
| Variable | (Dis)utility | Reference |
|---|---|---|
| Neoadjuvant chemotherapy | ||
| NAC treatment state | 0.64 | Stevenson et al, 201439 |
| Adverse event | −0.17 | Stevenson et al, 201439 |
| Radical cystectomy | ||
| RC postoperative state | 0.8 | Kulkarni et al, 20079 |
| Major perioperative complication requiring return to OR | −0.25 | Stevenson et al, 201439 |
| Major perioperative complication | −0.2 | Stevenson et al, 201439 |
| Minor perioperative complication | −0.06 | Truzzi et al, 201840 |
| Cystectomy (ileal conduit) surveillance state | 0.84 | Royce et al, 201941 |
| Neobladder surveillance state | 0.88 | Expert opinion |
| Long-term complication in surveillance state | 0.88* | Joshi et al, 200342 |
| Short-term complication in surveillance state | −0.06 | Truzzi et al, 201840 |
| Trimodal therapy (TMT) | ||
| TMT treatment state | 0.8 | Expert opinion |
| TMT surveillance state | 0.91 | Royce et al, 201941 |
| Major treatment complication | −0.274 | Tam et al, 201343 |
| Minor treatment/surveillance complication | −0.06 | Truzzi et al, 201840 |
| BCG | −0.02 | Kulkarni et al, 20079 |
| TURBT | −0.1 | Kulkarni et al, 20079 |
| GI complication requiring | 0.8* | Expert opinion |
| OR | ||
| Salvage cystectomy utilities | 0.8* | |
| Secondary malignancy | 0.84* | Ayvaci et al, 201344 |
| Progression | ||
| First-line therapy | 0.6 | Kulkarni et al, 20079 |
| Second-line therapy | 0.5 | Aguiar et al, 201745 |
| Palliative therapy | 0.3 | Kulkarni et al, 20079 |
| Death | 0 | |
Applied as a multiplicative factor to the current state utility.
BCG: bacillus Calmette-Guérin; GI: gastrointestinal; NAC: neoadjuvant therapy; OR: operating room; RC: radical cystectomy; TURBT: transurethral resection of the bladder tumor.
Model calibration
We calibrated the baseline non-cancer mortality rate using two- and 10-year survival in the RC arm to better model OS with MIBC. Calibrations in the TMT arm were completed for the probabilities of proceeding to immediate cystectomy following incomplete response to TMT, developing a distant or bladder recurrence. These uncertain probabilities were calibrated against the salvage cystectomy rate and two- and 10-year survival in TMT. Further details are available in Appendix S5 (available at cuaj.ca).
Model validation
Internal model validity was assured through assessment of results’ face validity, placement of internal trackers, and ensuring logical model flow through the stages. We assessed external validity by evaluating our model’s ability to reproduce OS rates, disease-specific survival (DSS) rates, and absolute benefit derived from NAC in both the TMT and RC arms by comparing model-generated estimates with those published that were separate from those used to generate model probabilities.
Sensitivity analyses
Sensitivity analyses were used to assess how the change in one variable affected the overall outcome of the model. Scenario-based sensitivity analyses were used to evaluate the impact of NAC use and age on the primary outcome. One-way sensitivity analyses where the variable of interest can vary across the range of clinically plausible values were completed on the surveillance utility values.
Results
A total of 500 000 patients were simulated. Based on our base case analysis, TMT was the preferred treatment pathway, with an estimated QALE of 7.48 vs. 7.41 for RC. The non-quality-of-life-adjusted crude LE for patients treated with TMT was 10.20 years vs. 10.74 years with RC.
The model’s OS rates at one, five, and 15 years for TMT and RC were 90.2%, 58.8%, 24.1%, and 93.5%, 56.9%, and 26.7%, respectively (Table 3).
Table 3.
Overall survival of the simulated results and validation cohorts
DSS rates at five years were 69.5% in TMT and 65.7% in RC. Our validation cohort had DSS rates of 76.6% for TMT and 73.2% for RC.7
Secondary outcomes of interest were analyzed. In the TMT arm, 6.3% of patients did not complete therapy. Overall, 39.4% of patients experienced a bladder recurrence; 66.9% were NMIBC. The overall rate of salvage cystectomy was 26.6%; the two- and five-year salvage cystectomy rates were 11.2% and 17.9%, respectively. Over the course of the simulation, 31.8% of patients in the TMT arm had a distant recurrence.
In the RC arm, the perioperative mortality rate was 2.24%. Distant recurrence occurred in 41.3% of patients during the simulation. The overall incidence of complications during surveillance in the TMT arm was 44.3% and 38.9% in the RC arm.
Sensitivity analysis
Impact of NAC
As the use of NAC prior to TMT or RC is not universal, scenario-based analyses were undertaken to explore the impact of 100% use of NAC in both arms. If all patients received NAC in the TMT arm, the five-year OS was 60.4% compared to 57.9% if none of the patients received it. This represents an absolute OS benefit of 2.5%. In the RC arm, if 100% of patients received NAC, the five-year OS was 59.2% and 55.6% if none of the patients received chemotherapy. The absolute OS benefit was 3.6%. The absolute OS benefit from published meta-analyzed data is 5%.23
Impact of age
The impact of age on QALE and crude LE was investigated using scenario-based analyses. The starting age distribution was replaced with distinct age thresholds. This analysis showed that younger patients treated with RC had both greater QALE and longer crude survival than those treated with TMT. However, for elderly patients, the inverse was true (Table 4).
Table 4.
Scenario based analysis varying starting age
| Starting age | TMT (QALE/LY) | RC (QALE/LY) |
|---|---|---|
| 45 | 8.26/11.56 | 8.45/12.87 |
| 55 | 8.10/11.20 | 8.13/12.17 |
| 65 | 7.68/10.45 | 7.57/11.08 |
| 75 | 6.67/8.97 | 6.41/9.13 |
| 80 | 6.03/8.08 | 5.69/8.00 |
| 85 | 5.58/7.43 | 5.19/7.26 |
LY: life years; RC: radical cystectomy; TMT: trimodal therapy; QALE: quality-adjusted life expectancy.
Impact of utilities
One-way sensitivity analyses were completed around surveillance utility values for TMT and RC. Decreasing the TMT surveillance state utility from 0.91 to 0.899 results in a change in the preferred pathway; in the RC arm, increasing the surveillance state utility for non-neobladder patients to 0.848 from 0.84 results in a change in the preferred treatment modality with respect to QALE (Appendix S6; available at cuaj.ca).
Discussion
This Markov microsimulation comparing two treatment modalities for patients with newly diagnosed MIBC revealed that TMT resulted in a net gain of 0.07 quality-adjusted life years (QALYs) compared with RC. The quality-unadjusted life years, however, reveal that patients treated with TMT have an average life expectancy of 10.20 compared to 10.74 years for those treated with RC (a net benefit for RC of 0.54 years).
As a composite measure, QALYs encompass OS and health-related quality of life. In oncology decision analyses, the clinical interpretation of a meaningful change in QALYs can be challenging.48 In this setting, where TMT leads to a gain of one quality-adjusted life month (QALM) in the setting of a six-month crude life expectancy decrease, the gain in QALM is of questionable clinical significance. Our sensitivity analysis demonstrates that the model is exquisitely sensitive to changes in patient preference for both TMT and RC surveillance states.
In this decision analysis, the impact of age on the ultimate treatment choice was investigated. When patients are younger (≤55 years old), they derive greater QALYs and unadjusted life years from RC than they do from TMT because the impact of a longer follow-up results in the need for salvage procedures (i.e., greater oncological control from RC) and the occurrence of secondary malignancies in the TMT group. Whereas when patients are ≥81 years old, the inverse is true; the elderly have a longer unadjusted life expectancy and experience greater QALYs when treated with TMT, in large part because of the avoidance of postoperative mortality after RC. In the intermediate ranges of age (64–80), the results are mixed. TMT results in greater QALYs but RC leads to more unadjusted life years gained. Therefore, discussions about individual patient priorities are especially important in these age ranges. Since age and comorbidity are often correlated, we would expect similar findings in patients with high and low comorbidity states (i.e., TMT favored for highly comorbid patients regardless of age and RC favored for patients with few comorbidities). As the literature is conflicted with respect to whether octogenarians face an increased risk of perioperative mortality,49–52 all patients were modelled to have the same perioperative risk of morbidity and mortality. If the true risks for elderly patients are, in fact, higher than those in younger age cohorts, our findings would be further reinforced.
It is worth noting that not all patients with MIBC are ideal candidates for TMT and the selection of these eligible patients is of utmost importance. Patients with preserved bladder function with a unifocal tumor less than 7 cm in size, at maximum unilateral hydronephrosis, and the absence of multifocal carcinoma in situ represent the best candidates when comparing oncological outcomes.
External validity of the model was evaluated by comparing our OS results to those from studies not used in the generation of our analysis. Overall, the generated OS results fall within 7% of the literature results; importantly, our results follow the appropriate trend within the RC and TMT arms themselves and in relation to each other. Despite level 1 evidence to support the use of NAC in MIBC, there is consistent underuse.53,54
Our model illustrates that when every patient is given NAC prior to definitive management (compared to when 0% receive NAC), an absolute OS benefit is achieved from 2.5–3.6% at five years. While this is slightly lower than the estimates of effect generated by the meta-analyzed data, the OS from that meta-analysis was 45–50%, which is lower than contemporary data.23 As a result, they have more room for benefit to be derived from NAC and so these estimates of benefit from NAC are in largely in keeping with the meta-analyzed data.
Randomized clinical trials in this setting have been difficult to perform, as evidenced by the SPARE trial, which closed due to slow accrual.55 Given these circumstances, decision models are an increasingly accepted tool to guide clinical decision-making in the field of urologic oncology when trials are not available or possible. Similar models have been developed to guide management in prostate cancer56 and recurrent HG NMIBC.9
Our analysis is the most robust evaluation of TMT vs. RC for the treatment of MIBC to date. Royce and others have previously examined this research question with a decision analysis and demonstrated that TMT resulted in 0.59 more QALYs than RC but with identical unadjusted life years.41 Our analysis, however, employs a more detailed modelling approach, necessary to ensure that patient characteristics and treatment options are realistic and reflective of the population and their disease experience. For example, we consider patient-level sampling and variability; the potential for complications to develop during the treatment, perioperative, and surveillance phases; and multiple lines of systemic therapy and palliation during the clinical course. These nuances, along with a clinically appropriate cycle length, ensure that the experience is reflective of those from real-world patients. The difference in modelling details helps to explain the difference in QALYs between treatments produced in their paper compared to ours.
Our study demonstrates that the choice between TMT and RC is extremely preference-sensitive, with a small shift in preference/utility changing the recommendation from TMT to RC, or vice versa. As a result, incorporating cost within the model is unlikely to yield further benefit, as the resulting incremental cost-effectiveness ratio (ICER) would become very unstable as the difference in effectiveness approaches zero. Given this, we believe the selection of treatment should be based on individual patient factors and their preference in a clinical setting.
Due to the nature of literature comparing the two treatment modalities, our study has some limitations. Stratifying patients by pathological details (presence of carcinoma in situ, hydronephrosis, clinical tumor stage) would add more granularity to help decide which treatment is best suited for which patient, but insufficient data were available. Moreover, due to the inconsistencies in reporting comorbidities between radiation and surgical papers, the inclusion of comorbidity status was not possible, although age may represent a surrogate. Also, many of our input parameters were obtained from retrospective studies. Although bias is inherent in these studies, we chose values from the highest-quality studies where possible. Since much of our current knowledge and clinical practice as it pertains to TMT and RC stem from retrospective studies, our confidence in the model inputs should not be undermined by the retrospective nature of the data.
Conclusions
We demonstrated that in patients with MIBC who are a candidate for either therapy, RC provides slightly longer unadjusted OS compared to TMT (0.54 years) but with slightly less quality of life (−0.07 QALYs) of questionable clinical significance. Differences in treatment preference were dependent on age, with a larger survival benefit seen in younger patients treated with RC secondary to improved oncological control. NAC, with either TMT or RC, provides a meaningful OS benefit.
Supplementary Information
Footnotes
Appendix available at cuaj.ca
This paper has been peer-reviewed.
Competing interests: Dr. Sridhar has been an advisory board member for Astellas, AstraZeneca, Bayer, BMS, Immunomedex, Janssen, Merck, Pfizer, Roche, and Seagen. Dr. Catton has been an advisory board member for Abbvie and Bayer; and has received payment/honoraria from Abbvie and TerSera for preparing and presenting educational materials. Dr. Chung has received grants and/or honoraria from Abbvie, Boston Scientific, TerSera, and Verity; and has participated in clinical trials supported by ICON and Medivation. Dr. Zlotta has been an advisory board member for AstraZeneca, Ferring, Janssen, Merck, Sanofi, and Verity. Dr. Fleshner has received honoraria, advisory consulting, and speaker bureau fees from Abbvie, Astellas, Janssen, Merck, and Sanofi; holds stock in Verity Pharma; and has participated in clinical trials supported by Astellas, Bayer, and Janssen. Dr. Kulkarni has been an advisory board member for Astellas, Bristol Myers Squibb, Ferring, Janssen, Merck, Roche, Theralase, and Verity; has received grants and/or honoraria from Abbvie, Ferring, Sanofi, and TerSera; and has participated in clinical trials supported by Astra Zeneca, Bristol Myers Squibb, Janssen, Merck, Pfizer, Seagen, Theralase, and Verity. The remaining authors do not report any competing personal or financial interests related to this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU international consultation on bladder cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: Update of the EAU guidelines. Eur Urol. 2011;59:1009–18. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–74. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Ploussard G, Daneshmand S, Efstathiou JA, et al. Critical analysis of bladder-sparing with trimodal therapy in muscle-invasive bladder cancer: A systematic review. Eur Urol. 2014;66:120–37. doi: 10.1016/j.eururo.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE – a randomized feasibility study of selective bladder preservation vs. radical cystectomy. BJU Int. 2017;120:639–50. doi: 10.1111/bju.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy vs. bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35:2299–305. doi: 10.1200/JCO.2016.69.2327. [DOI] [PubMed] [Google Scholar]
- 8.Neumann PJ, Ganiats TG, Russell LB, et al. Cost-effectiveness in health and medicine. 2nd Edition ed. New York: Oxford University Press; 2017. [DOI] [Google Scholar]
- 9.Kulkarni GS, Finelli A, Fleshner NE, et al. Optimal management of high-risk T1G3 bladder cancer: A decision analysis. PLoS Med. 2007;4:e284. doi: 10.1371/journal.pmed.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaassen Z, Li K, Kassouf W, et al. Contemporary cost-consequence analysis of blue light cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. Can Urol Assoc J. 2017;11:173–81. doi: 10.5489/cuaj.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naimark DM, Bott M, Krahn M. The half-cycle correction explained: Two alternative pedagogical approaches. Med Decis Making. 2008;28:706–12. doi: 10.1177/0272989X08315241. [DOI] [PubMed] [Google Scholar]
- 12.CADTH Guidelines for the economic evaluation of health technologies. 4th edition ed. Canadian Agency for Drugs and Technologies in Health; 2017. [Accessed November 18, 2021]. Published March 2017. Available at: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. [Google Scholar]
- 13.Borgi J, Rubinfeld I, Ritz J, et al. The differential effects of intermediate complications with postoperative mortality. Am Surg. 2013;79:261–6. doi: 10.1177/000313481307900324. [DOI] [PubMed] [Google Scholar]
- 14.Lyon TD, Boorjian SA, Shah PH, et al. Comprehensive characterization of perioperative reoperation following radical cystectomy. Urol Oncol. 2019;37:292e11–7. doi: 10.1016/j.urolonc.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 16.De Santis M, Bellmunt J, Mead G, et al. Randomized phase 2/3 trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–26. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krabbe LM, Westerman ME, Margulis V, et al. Changing trends in utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer. Can J Urol. 2015;22(4):7865–75. [PubMed] [Google Scholar]
- 19.Winquist E, Kirchner TS, Segal R, et al. Genitourinary cancer disease site group CCOPiE-bCPGI. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: A systematic review and meta-analysis. J Urol. 2004;171:561–9. doi: 10.1097/01.ju.0000090967.08622.33. [DOI] [PubMed] [Google Scholar]
- 20.Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67:241–9. doi: 10.1016/j.eururo.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niedersüss-Beke D, Puntus T, Kunit T, et al. Neoadjuvant chemotherapy with gemcitabine plus cisplatin in patients with locally advanced bladder cancer. Oncology. 2017;93:36–42. doi: 10.1159/000463389. [DOI] [PubMed] [Google Scholar]
- 22.Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–93. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- 23.Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 24.Wallace B, Breau RH, Cnossen S, et al. Age-stratified perioperative mortality after urological surgeries. Can Urol Assoc J. 2018;12:256–9. doi: 10.5489/cuaj.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomized, phase 3, non-inferiority trial. Lancet. 2018;391:2525–36. doi: 10.1016/S0140-6736(18)30996-6. [DOI] [PubMed] [Google Scholar]
- 26.Shimko MS, Tollefson MK, Umbreit EC, et al. Long-term complications of conduit urinary diversion. J Urol. 2011;185:562–7. doi: 10.1016/j.juro.2010.09.096. [DOI] [PubMed] [Google Scholar]
- 27.Nuhn P, May M, Sun M, et al. External validation of postoperative nomograms for prediction of all-cause mortality, cancer-specific mortality, and recurrence in patients with urothelial carcinoma of the bladder. Eur Urol. 2012;61:58–64. doi: 10.1016/j.eururo.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 28.Tunio MA, Hashmi A, Qayyum A, et al. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: Single-institution experience. Int J Radiat Oncol Biol Phys. 2012;82:e457–62. doi: 10.1016/j.ijrobp.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 29.Fahmy O, Khairul-Asri MG, Schubert T, et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol. 2018;36:43–53. doi: 10.1016/j.urolonc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89–03, 95–06, 97–06, 99–06. J Clin Oncol. 2009;27:4055–61. doi: 10.1200/JCO.2008.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061–71. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Zelefsky MJ, Pei X, Teslova T, et al. Secondary cancers after intensity-modulated radiotherapy, brachytherapy and radical prostatectomy for the treatment of prostate cancer: incidence and cause-specific survival outcomes according to the initial treatment intervention. BJU Int. 2012;110:1696–701. doi: 10.1111/j.1464-410X.2012.11385.x. [DOI] [PubMed] [Google Scholar]
- 33.Eswara JR, Efstathiou JA, Heney NM, et al. Complications and long-term results of salvage cystectomy after failed bladder sparing therapy for muscle-invasive bladder cancer. J Urol. 2012;187:463–8. doi: 10.1016/j.juro.2011.09.159. [DOI] [PubMed] [Google Scholar]
- 34.Knap MM, Lundbeck F, Overgaard J. Early and late treatment-related morbidity following radical cystectomy. Scand J Urol Nephrol. 2004;38:153–60. doi: 10.1080/00365590310020060. [DOI] [PubMed] [Google Scholar]
- 35.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Mishina S, Takai S, et al. Systemic treatment patterns with advanced or recurrent non-small cell lung cancer in Japan: A retrospective hospital administrative database study. Clin Ther. 2017;39:1146–60. doi: 10.1016/j.clinthera.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Smith AB, Deal AM, Woods ME, et al. Muscle-invasive bladder cancer: Evaluating treatment and survival in the National Cancer Data Base. BJU Int. 2014;114:719–26. doi: 10.1111/bju.12601. [DOI] [PubMed] [Google Scholar]
- 38.Williams SB, Huo J, Chu Y, et al. Cancer and all-cause mortality in bladder cancer patients undergoing radical cystectomy: Development and validation of a nomogram for treatment decision-making. Urology. 2017;110:76–83. doi: 10.1016/j.urology.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevenson SM, Danzig MR, Ghandour RA, et al. Cost-effectiveness of neoadjuvant chemotherapy before radical cystectomy for muscle-invasive bladder cancer. Urol Oncol. 2014;32:1172–7. doi: 10.1016/j.urolonc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Truzzi JC, Teich V, Pepe C. Can hydrophilic coated catheters be beneficial for the public healthcare system in Brazil? – a cost-effectiveness analysis in patients with spinal cord injuries. Int Braz J Urol. 2018;44:121–31. doi: 10.1590/s1677-5538.ibju.2017.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royce TJ, Feldman AS, Mossanen M, et al. Comparative effectiveness of bladder-preserving trimodality therapy versus radical cystectomy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2019;17:23–31e3. doi: 10.1016/j.clgc.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Joshi HB, Stainthorpe A, MacDonagh RP, et al. Indwelling ureteral stents: Evaluation of symptoms, quality of life, and utility. J Urol. 2003;169:1065–9. doi: 10.1097/01.ju.0000048980.33855.90. ;discussion 9. [DOI] [PubMed] [Google Scholar]
- 43.Tam VC, Ko YJ, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20:e90–106. doi: 10.3747/co.20.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayvaci MU, Shi J, Alagoz O, et al. Cost-effectiveness of adjuvant FOLFOX and 5FU/LV chemotherapy for patients with stage II colon cancer. Med Decis Making. 2013;33:521–32. doi: 10.1177/0272989X12470755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguiar PN, Jr, Perry LA, Penny-Dimri J, et al. The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol. 2017;28:2256–63. doi: 10.1093/annonc/mdx305. [DOI] [PubMed] [Google Scholar]
- 46.Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term outcomes after bladder-preserving trimodality therapy for patients with muscle-invasive bladder cancer: An updated analysis of the Massachusetts General Hospital experience. Eur Urol. 2017;71:952–60. doi: 10.1016/j.eururo.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Zehnder P, Studer UE, Skinner EC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: A comparative study. J Urol. 2011;186:1261–8. doi: 10.1016/j.juro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Garau M, Shah KK, Mason AR, et al. Using QALYs in cancer: A review of the methodological limitations. Pharmacoeconomics. 2011;29:673–85. doi: 10.2165/11588250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Elsayed AS, Aldhaam NA, Brownell J, et al. Perioperative and oncological outcomes of robot-assisted radical cystectomy in octogenarians. J Geriatr Oncol. 2020;11:727–30. doi: 10.1016/j.jgo.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Froehner M, Koch R, Hubler M, et al. Predicting 90-day and long-term mortality in octogenarians undergoing radical cystectomy. BMC Urol. 2018;18:91. doi: 10.1186/s12894-018-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haden TD, Prunty MC, Jones AB, et al. Comparative perioperative outcomes in septuagenarians and octogenarians undergoing radical cystectomy for bladder cancer – do outcomes differ? Eur Urol Focus. 2018;4:895–9. doi: 10.1016/j.euf.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Savin Z, Herzberg H, Schreter E, et al. Radical cystectomy and perioperative chemotherapy in octogenarians with bladder cancer. Can Urol Assoc J. 2021;15:E465–70. doi: 10.5489/cuaj.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 54.Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: A sign of changing tides. Eur Urol. 2015;67:165–70. doi: 10.1016/j.eururo.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huddart RA, Hall E, Lewis R, et al. Life and death of spare (selective bladder preservation against radical excision): Reflections on why the spare trial closed. BJU Int. 2010;106:753–5. doi: 10.1111/j.1464-410X.2010.09537.x. [DOI] [PubMed] [Google Scholar]
- 56.Wallis CJD, Morton G, Jerath A, et al. Adjuvant vs. salvage radiotherapy for patients with adverse pathological findings following radical prostatectomy: A decision analysis. MDM Policy Pract. 2017;2:2381468317709476. doi: 10.1177/2381468317709476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


