Abstract
Introduction
Active surveillance (AS) of small renal masses (SRM) is increasingly recognized as a safe option. A recent U.S. study found that half of patients receiving treatment on AS were for preference, but these findings may not be generalizable to other jurisdictions and healthcare models. We aimed to investigate AS failure rates and causes among a contemporary biopsy-evaluated cohort in Canada.
Methods
A retrospective review was performed of SRM patients on AS undergoing treatment at our tertiary care center (1999–2018). All patients had undergone renal biopsy and been diagnosed with renal cell carcinoma (RCC). Demographic and clinical parameters surrounding the decision to treat were extracted from chart review. Indications for treatment were dichotomized into clinical (radiographical) progression or preference. Qualitative assessment of clinic notes confirmed treatment indication. Ethics approval was obtained.
Results
A total of 38 SRM-RCC patients who underwent treatment on AS were identified, of which 29 had been on AS ≥1 year. Most (75.9%) were male and the mean age beginning AS was 65.9±9.0 years. Most patients had clear-cell RCC with low-grade disease. Seventeen of 29 (58.6%) patients experienced clinical progression after 3.82 (2.57–7.16) years, whereas preference accounted for 12/29 (41.4%) after 2.22 (1.69–3.53) years (time-to-treatment p=0.032). The longest duration on AS was 14.2 years prior to clinical progression. No patients had metastatic progression before treatment.
Conclusions
Two-fifths of patients received treatment for preference and at a much higher rate vs. clinical progression. These findings suggest a clinical gap where effective patient counselling prior to and during AS may improve adherence.
Introduction
Small renal masses (SRM), defined as incidentally discovered enhancing renal masses less than 4 cm in maximal diameter with imaging consistent with T1aN0M0 renal cell carcinoma (RCC), have become increasingly common due to widespread imaging.1,2 Numerous treatment approaches, including surgical resection, tissue ablation, and active surveillance (AS), have all been proven to be effective in the management of SRMs.3–5 AS in particular is gaining increasing recognition as an acceptable and safe option in patients, especially in older and/or comorbid individuals.6–8
Of patients placed on AS, whom are serially monitored through radiographic and clinical followup, approximately 1/3 of patients progressed to delayed treatment in a recent systematic review by Gupta et al.9 Of these, half did so for radiographic progression, including large overall size or rapid growth rate (clinically appropriate), and half (51.9%) were shown to be due to patient or physician preference in the absence of disease progression (potentially preventable/modifiable). Similarly, the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry published last year demonstrated concordant results: 22/46 (47.8%) patients undergoing delayed treatment were for patient preference.10
However, many of the patients included in these studies did not have biopsy histology readily available; and their findings may not be generalizable to other jurisdictions and healthcare models with different practice patterns, counselling, and treatment indications. We aimed to investigate AS failure rates and causes among a contemporary biopsy-evaluated cohort in Canada.
Methods
A retrospective review was performed of SRM-RCC patients on AS undergoing treatment at our tertiary care centre over 20 years (1999–2015 with followup to 2018). This cohort of AS patients has been previously published as part of the Renal Cell Carcinoma Consortium of Canada (RC4) phase 2 AS trial11 and Princess Margaret Cancer Centre (PMCC) AS cohort,12 and represent the subset of patients belonging to our center (PMCC). Our center has six urologic oncologists and has excellent access to interventional radiology, including routine renal rounds. In brief, all patients within this cohort had undergone percutaneous renal mass biopsy (RMB) and been diagnosed with RCC, excluding those with benign or non-diagnostic biopsies.
Demographic, clinical, and tumor characteristics (including age at AS enrollment, sex, initial tumor size, and biopsy histology and grade) were extracted from chart review surrounding the decision to treat their SRM. For our primary outcome, indications for treatment were dichotomized as either for clinical (radiographic) progression or preference (e.g., anxiety). Progression was defined as SRM growth >0.5 cm/year for two years, absolute size >4 cm, or volume doubling <1 year, and was assessed in the time (up to two years, dependent on criteria) immediately preceding treatment. Qualitative assessment of clinic notes confirmed treatment indication in all patients. Additionally, treatment outcomes, including treatment type (nephrectomy or renal frequency ablation [RFA]), time to treatment, and final histology and grade (if surgically managed), were recorded. Patients who remained on AS <1 year prior to treatment were included in sensitivity analyses only, as these patients represented a heterogenous group of very fast progressors and those not truly interested in AS. Once patients reached progression criterion thresholds, it was standard practice to recommend treatment; however, the individual counselling, informed discussions, and decisions for AS, managing anxiety, and treatment were at the discretion of the patient and treating physician.
Descriptive statistics were completed on all variables using Student’s t-test, Wilcoxon rank-sum test, Chi-squared test, or Fisher’s exact test as appropriate for continuous and categorical data. Treatment survival distributions by indication (clinical progression or preference) were descriptively plotted using the Kaplan-Meier product-limit method. These were intended for graphical representation only, and no comparative testing was completed (as treatment indications signify outcome measures, not exposures). A p-value <0.05 was used to indicate statistical significance for two-tailed comparison. All analyses were completed using SAS Statistical Software. Ethics approval was obtained after institutional review.
Results
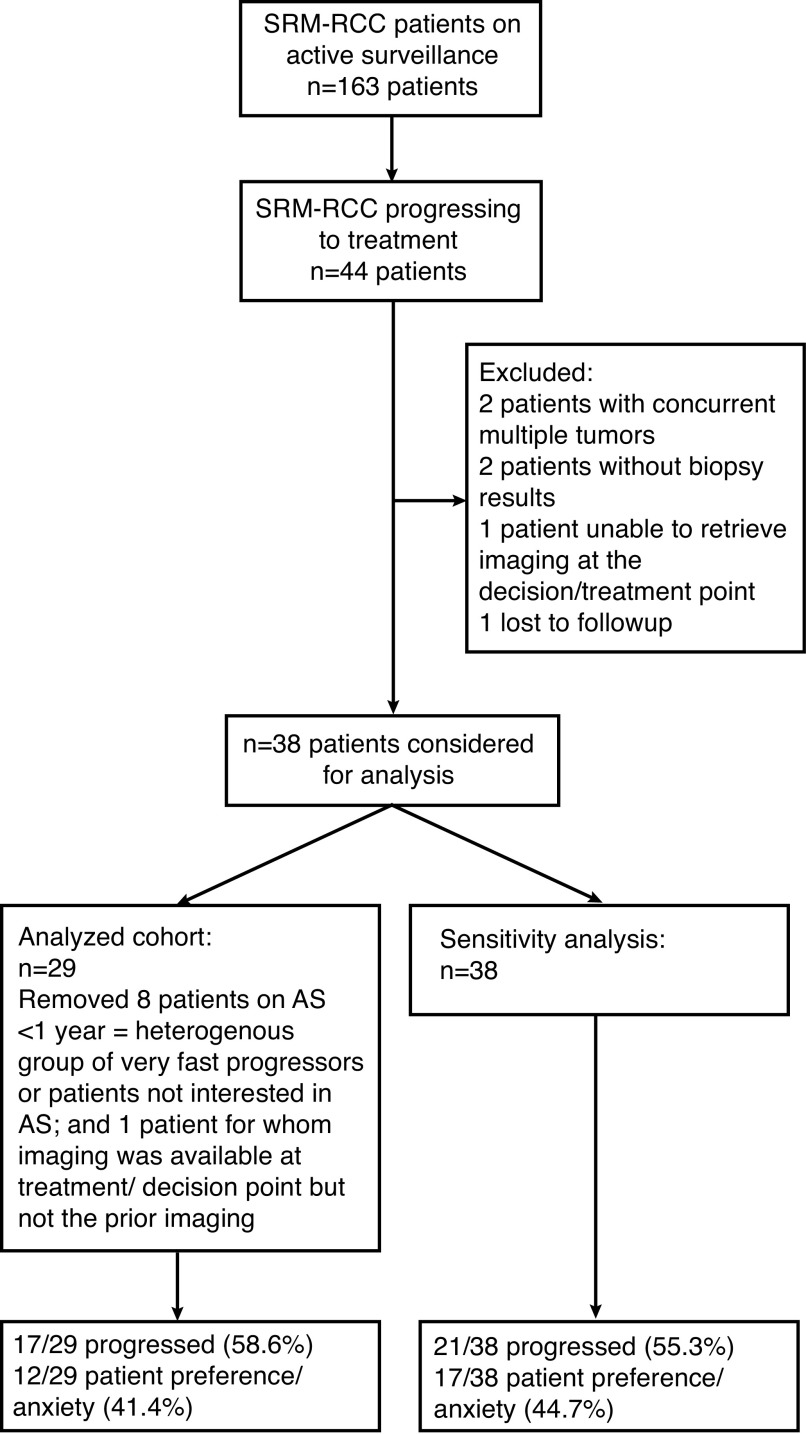
A total of 163 SRM-RCC patients on AS were identified, of which 44 underwent treatment while on AS. After excluding patients with concurrent multiple tumors, incomplete biopsy or imaging results, or loss to followup, 38 patients with SRM-RCC on AS and 29 on AS for ≥1 year were included (Fig. 1). All patients underwent treatment with either nephrectomy or RFA during followup and had biopsy-proven malignant histology.
Fig. 1.
Flow chart of small renal mass (SRM)-renal cell carcinoma (RCC) patients progressing onto treatment. Progression defined as growth >0.5 cm/year for two years, absolute size >4 cm, volume doubling <1 year. AS: active surveillance.
Demographic and clinical variables are available in Table 1. Twenty-two male (75.9%) and seven (24.1%) female patients were identified with a mean age at AS initiation of 65.9±9.0 years. The youngest patient was 46.7 years old and the oldest patient was 83.0 years old. Most patients had clear-cell RCC pathology on biopsy (72.4%), with low-grade (grade 1–2: 95.8%) disease. The average tumor size at presentation was 2.50±0.63 cm.
Table 1.
Demographic and clinical variables for treated active surveillance patients
| Demographic and clinical variables | Patients treated for preference (n=12) | Patients treated for clinical progression (n=17) | Combined (n=29) | p for preference vs. progression |
|---|---|---|---|---|
| Age (mean ± SD) | 62.6±9.3 years | 68.2±8.3 years | 65.9±9.0 years | 0.10 |
| Sex (% male) | 8 (66.7%) | 14 (82.4%) | 22 (75.9%) | 0.40 |
| Initial tumor presentation size (mean ± SD) | 2.38±0.68 cm | 2.58±0.59 cm | 2.50±0.63 cm | 0.41 |
| Biopsy histology | 7 clear-cell RCC (58.3%) 1 clear-cell papillary RCC (8.3%) 1 papillary type 1 (8.3%) 2 papillary type 2 (16.7%) 1 papillary NOS (8.3%) |
14 clear-cell RCC (82.4%) 1 papillary type 1 (5.9%) 1 papillary type 2 (5.9%) 1 mixed (papillary type 1, clear cell RCC; 5.9%) |
21 clear-cell RCC (72.4%) 1 clear-cell papillary RCC (3.5%) 2 papillary type 1 (6.9%) 3 papillary type 2 (10.3%) 1 mixed (papillary type 1, clear-cell RCC; 3.5%) 1 papillary NOS (3.5%) |
0.41 |
| Maximum biopsy grade | 4 grade 1 (50.0%) 3 grade 2 (37.5%) 1 grade 3 (12.5%) 4 missing |
8 grade 1 (50.0%) 8 grade 2 (50.0%) 1 missing |
12 grade 1 (50.0%) 11 grade 2 (45.8%) 1 grade 3 (4.2%) 5 missing |
0.46 |
| Reasons for clinical progression | N/A | 12 growth >0.5 cm/year for 2 years (70.6%) 13 absolute size >4 cm (76.5%) 8 volume doubling <1 year (47.1%)* |
N/A | N/A |
Totals exceed 100% as patients may meet more than one criterion.
NOS: not otherwise specified; RCC: renal cell carcinoma; SD: standard deviation.
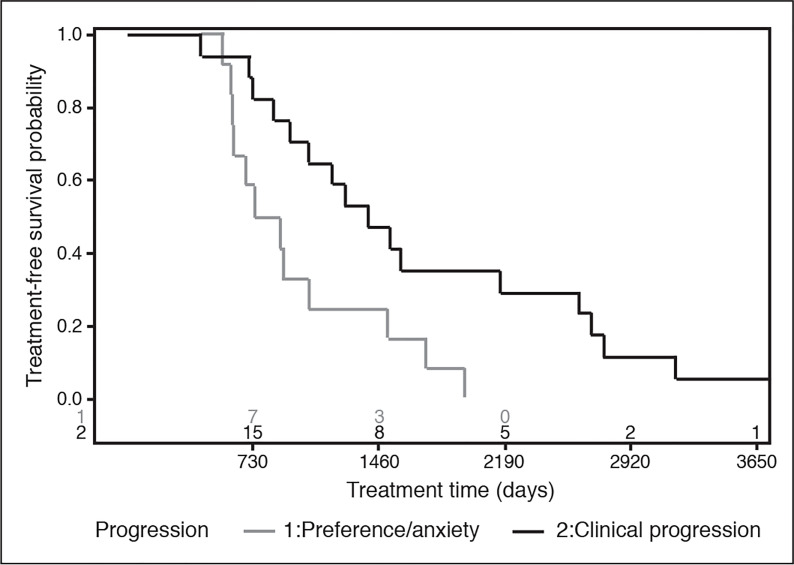
Of 29 treated patients, 18 (62.1%) elected for nephrectomy (five radical nephrectomy, 13 partial nephrectomy) and 11 (37.9%) for RFA. Using progression criteria, 17/29 (58.6%) patients experienced clinical progression vs. 12/29 patients (41.4%) undergoing treatment for preference (Table 2). Patients with progression frequently met multiple criteria of absolute size (13/17, 76.5%), growth rate (12/17, 70.6%), and volume doubling (8/17, 47.1%). The time to treatment was significantly shorter for patients being treated for preference vs. clinical progression: 2.22 (1.69–3.53) years vs. 3.82 (2.57–7.16) years, respectively (p=0.032). The longest duration that a patient was on AS was 14.2 years prior to clinical progression. The time to treatment for patients progressing for either clinical progression or preference is graphically depicted in Fig. 2. There were no significant differences in final surgical histology or grade between groups. All patients treated with nephrectomy for preference had pT1a lesions, whereas five patients with progression were pT1b (41.7%) and two were pT3b (16.7%). No patients had metastatic progression prior to treatment; one patient developed recurrence to the renal bed and subsequent metastatic disease following radical nephrectomy.
Table 2.
Treatment outcomes for active surveillance patients by progression vs. preference
| Treatment outcomes | Patients treated for preference (n=12) | Patients treated for clinical progression (n=17) | Combined (n=29) | p for preference vs. progression |
|---|---|---|---|---|
| Time to treatment, median (IQR) | 2.22 (1.69–3.53) years | 3.82 (2.57–7.16) years | 2.89 (2.00–4.75) years | 0.032 |
| Treatment type | 6 nephrectomy (50.0%) | 12 nephrectomy (70.6%) | 18 nephrectomy (62.1%) | 0.44 |
| 6 RFA (50.0%) | 5 RFA (29.4%) | 11 RFA (37.9%) | ||
| Final nephrectomy histology | 3 clear-cell RCC (50.0%) | 10 clear-cell RCC (83.3%) | 13 clear-cell RCC (72.2%) | 0.27 |
| 3 papillary type 1 (50.0%) | 2 papillary type 1 (16.7%) | 5 papillary type 1 (27.8%) | ||
| Nephrectomy stage | 6 pT1a (100%) | 5 pT1a (41.7%) | 11 pT1a (61.1%) | 0.07 |
| 5 pT1b (41.7%) | 5 pT1b (27.8%) | |||
| 2 pT3b (16.7%) | 2 pT3b (11.1%) | |||
| Nephrectomy grade | 2 grade 1 (33.3%) | 2 grade 1 (16.7%) | 4 grade 1 (22.2%) | 0.82 |
| 3 grade 2 (50.0%) | 6 grade 2 (50.0%) | 9 grade 2 (50.0%) | ||
| 1 grade 3 (16.7%) | 4 grade 3 (33.3%) | 5 grade 3 (27.8%) |
IQR: interquartile range; RCC: real cell carcinoma; RFA: renal frequency ablation.
Fig. 2.
Overlaid treatment survival distributions for patients treated secondary to clinical progression and preference. These represent conditional probabilities of patients who ultimately received treatment (for either indication). As treatment indications signify outcome measures (not exposures), no comparative analysis is completed.
Qualitative chart review demonstrated agreement in 27/29 (93%) cases, with one patient crossing over each category (one met progression criteria but deferred treatment until worsening clinical anxiety, and one had a single measurement of rapid growth but did not meet criteria). Results were similar when patients on surveillance <1 year were included (17/38, 44.7% treatment for preference).
Discussion
In this study, we queried our institutional database of SRM patients on AS in Toronto, Canada. Despite being biopsy (histology)-informed, we found that the indications for AS failure appeared to be similar to those recently reported in American cohort studies,10 with almost 40–50% of patients choosing to progress onto treatment despite clinical stability. Furthermore, despite having similar characteristics as those who ultimately clinically progressed, the time to treatment for patients being treated for preference was almost half of that for progression. This alone was a surprising finding among a contemporary, biopsy-informed, Canadian cohort, and warrants further investigation.
There are multiple potential explanations for this relatively high proportion of preference-driven treatment, both among Americans and Canadians. Uncertainties about the disease and safety of AS may be important drivers of patient and clinician anxiety.10,13 Indeed, while metastases remain rare in SRM patients on AS, sporadic cases of rapid clinical progression illustrate our incomplete understanding of the disease biology and natural history beyond histology.14 In this study, we elected to focus our investigation on patients with >1 year on AS (precluding some of the very fast progressors). However, analyses with and without these patients demonstrated consistent findings, with a high proportion of patients being treated for preference regardless, suggesting that other factors may have an important role. Along these lines, another reason may represent individual uncertainty about their own overall health: patients (and their clinicians) may be hesitant to delay treatment if they worry that they will not be as fit for intervention at a later date. Ultimately, the relationship between patient/clinician anxiety and treatment is likely to be complex, with numerous inter-related and confounding elements; a conceptual directed acyclic graph (DAG) for the causal relationship between patient/clinician anxiety and treatment is included in Supplementary Fig. 1 (available at cuaj.ca).
The psycho-oncological effects and stigma around cancer should also be considered. One important difference between surgery and AS is the notion that the patient still “has cancer” when they are on AS, as opposed to being “cancer-free” after surgery or ablation. There is a wealth of literature demonstrating that following diagnosis, patients’ quality of life declines, even when asymptomatic.15,16 In particular, a cancer diagnosis and illness uncertainty was found to predict general and cancer-specific quality of life, intrusive thoughts, and avoidance behaviors in a study of SRM patients by Matin et al.16 Similarly, from the treating physician’s perspective, taking action may also be seen as preferable to surveillance (i.e., commission bias), and can be accentuated based on previous (negative) experiences with surveillance approaches in other circumstances (i.e., recall bias). These effects on care provision appear to extend medico-legally as well; the most common reason for over-treatment in the U.S. is fear of malpractice, according to a nationwide study.17 In the case of SRM, surgery also remains the standard-of-care in many guideline statements.7,18
Finally, it is worthwhile to consider the role of RMB, both as it pertains to the diagnostic uncertainty of RMB and whether RMB itself contributes to patient anxiety. There remains much controversy about the use of biopsy, and whether it is representative of the true pathophysiology in each patient: false-negatives, false-positives, and tumor heterogeneity are all important issues, in addition to a 14% non-diagnostic rate with RMB.13,19 Because of this diagnostic uncertainty, both physicians and patients alike may err on the side of caution, that being extirpative surgery or tissue ablation, even after RMB. Further research is needed to improve the diagnostic certainty of RMBs and other genetic or histological biomarkers to reassure patients of their risk of disease progression when opting for AS.
A second criticism is RMB-associated anxiety; in a recent study by Goldberg et al, patients with a malignant biopsy were found to have the highest psychological distress in those selecting AS vs. treatment.20 Intuitively, these match the findings of our cohort, explaining the high incidence of non-progression-driven treatment in SRM-RCC patients. Notably, however, their psychological distress regression estimates contrasted with those of RMB or surveillance individually (non-significant to borderline significant), suggesting that this effect is not driven by the performance of biopsy alone or AS itself, rather by how patients respond to AS for a malignant diagnosis.20 This context underscores the aforementioned psycho-oncological effects and stigma surrounding cancer (despite excellent RCC-specific survival), and thus, the imperative for improved patient counselling.
Moving forward, learning from an analogous example in urology, patients on AS for prostate cancer are subject to similar long-term surveillance with clinical and biochemical followup and relatively low metastatic risk.21 In contrast however, very few (5–10%) prostate cancer patients progress onto treatment for preference without clinical indication, and comfort with this modality has increased with time.22,23 This insight provides a valuable opportunity to learn beyond the SRM literature; in one study measuring anxiety and distress for AS prostate cancer patients, the larger the role that the physician played in the treatment decision, the more doubts the patients had regarding their own treatment.24 Furthermore, standardized counselling addressing patient- and physician-level biases has been shown to increase acceptance of AS treatment modalities for prostate cancer.25 Incorporation of these shared decision-making strategies, training, and standardization should be a focus for SRMs as well. Decision aids for the management of SRM26 are further examples of advances to the provision of standardized and evidence-based information to aid patients and their caregivers in the decision-making process and address these biases.
Limitations
Our study was limited by its small sample size, precluding further analysis of the predictors of non-clinically indicated AS failure. However, these features would likely need to be evaluated in a prospective setting, along with patient interviews, to garner a better understanding, as no clear demographic differences were evident and our study is hypothesis-generating in this respect. Furthermore, despite the limited number of total patients, this still represents one of the largest studies of the indications for delayed intervention, which account for a minor proportion of patients on AS, and of which only a fraction are biopsy-evaluated. This is further strengthened by the chart review confirming the treatment intent and indication in our cohort, and long duration of followup.
Conclusions
In our retrospective series, two-fifths of patients elected to transition to treatment for preference, and at a much higher rate vs. clinical progression for growth or absolute tumour size. Although the causes are likely multifactorial, representing patient, disease, physician, and psycho-oncological factors, these findings point to a clinical gap where effective patient counselling prior to and during AS may improve adherence.
Supplementary Information
Footnotes
See related commentary on page 102
Appendix available at cuaj.ca
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Volpe A. The role of active surveillance of small renal masses. Int J Sur. 2016;36:518–24. doi: 10.1016/j.ijsu.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: Analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Withington J, Neves JB, Barod R. Surgical and minimally invasive therapies for the management of the small renal mass. Curr Urol Rep. 2017;18:61. doi: 10.1007/s11934-017-0705-8. [DOI] [PubMed] [Google Scholar]
- 4.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: A systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate, or observe: The small renal mass dilemma — a meta-analysis and review. J Urol. 2008;179:1227–33. doi: 10.1016/j.juro.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 6.Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35:668–80. doi: 10.1200/jco.2016.69.9645. [DOI] [PubMed] [Google Scholar]
- 7.Jewett MA, Rendon R, Lacombe L, et al. Canadian guidelines for the management of small renal masses (SRM) Can Urol Assoc J. 2015;9:160–3. doi: 10.5489/cuaj.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung DC, Finelli A. Active surveillance in small renal masses in the elderly: A literature review. Eur Urol Focus. 2017;3:340–51. doi: 10.1016/j.euf.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M, Blute ML, Jr, Su ML, et al. Delayed intervention of small renal masses on active surveillance. J Kidney Cancer VHL. 2017;4:24–30. doi: 10.15586/jkcvhl.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta M, Alam R, Patel HD, et al. Use of delayed intervention for small renal masses initially managed with active surveillance. Urol Oncol. 2019;37:18–25. doi: 10.1016/j.urolonc.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: Progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Finelli A, Cheung DC, Al-Matar A, et al. Small renal mass surveillance: Histology-specific growth rates in a biopsy-characterized cohort. Eur Urol. 2020;78:460–7. doi: 10.1016/j.eururo.2020.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Golan S, Lotan P, Tapiero S, et al. Diagnostic needle biopsies in renal masses: Patient and physician perspectives. Eur Urol Focus. 2018;4:749–53. doi: 10.1016/j.euf.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med. 2010;362:624–34. doi: 10.1056/NEJMcp0910041. [DOI] [PubMed] [Google Scholar]
- 15.Parker PA, Alba F, Fellman B, et al. Illness uncertainty and quality of life of patients with small renal tumors undergoing watchful waiting: A 2-year prospective study. Eur Urol. 2013;63:1122–7. doi: 10.1016/j.eururo.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matin SF. Kidney cancer: Quality-of-life outcomes in patients with small renal masses. Nature reviews. Urology. 2016;13:443–4. doi: 10.1038/nrurol.2016.124. [DOI] [PubMed] [Google Scholar]
- 17.Lyu H, Xu T, Brotman D, et al. Overtreatment in the United States. PloS One. 2017;12:e0181970. doi: 10.1371/journal.pone.0181970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finelli A, Ismaila N, Russo P. Management of small renal masses: American Society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2017;13:276–8. doi: 10.1200/JOP.2016.019620. [DOI] [PubMed] [Google Scholar]
- 19.Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: Systematic review of the literature. J Urol. 2016;195:1340–7. doi: 10.1016/j.juro.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg H, Ajaj R, Caceres JOH, et al. Psychological distress associated with active surveillance in patients younger than 70 with a small renal mass. Urol Oncol. 2020;38:603e17–25. doi: 10.1016/j.urolonc.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Briganti A, Fossati N, Catto JWF, et al. Active surveillance for low-risk prostate cancer: The European Association of Urology position in 2018. Eur Urol. 2018;74:357–68. doi: 10.1016/j.eururo.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Venderbos LD, van den Bergh RC, Roobol MJ, et al. A longitudinal study on the impact of active surveillance for prostate cancer on anxiety and distress levels. Psycho-oncology. 2015;24:348–54. doi: 10.1002/pon.3657. [DOI] [PubMed] [Google Scholar]
- 23.Marzouk K, Assel M, Ehdaie B, et al. Long-term cancer specific anxiety in men undergoing active surveillance of prostate cancer: Findings from a large prospective cohort. J Urol. 2018;200:1250–5. doi: 10.1016/j.juro.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Bergh RC, Essink-Bot ML, Roobol MJ, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115:3868–78. doi: 10.1002/cncr.24446. [DOI] [PubMed] [Google Scholar]
- 25.Ehdaie B, Assel M, Benfante N, et al. A systematic approach to discussing active surveillance with patients with low-risk prostate cancer. Eur Urol. 2017;71:866–71. doi: 10.1016/j.eururo.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAlpine K, Breau RH, Stacey D, et al. Shared decision-making for the management of small renal masses: Development and acceptability testing of a novel patient decision aid. Can Urol Assoc J. 2020;14:385–91. doi: 10.5489/cuaj.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.